Wood Distillate Mitigates Ozone-Induced Visible and Photosynthetic Plant Damage: Evidence from Ozone-Sensitive Tobacco (Nicotiana tabacum L.) BelW3
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Tobacco Plant Measurements
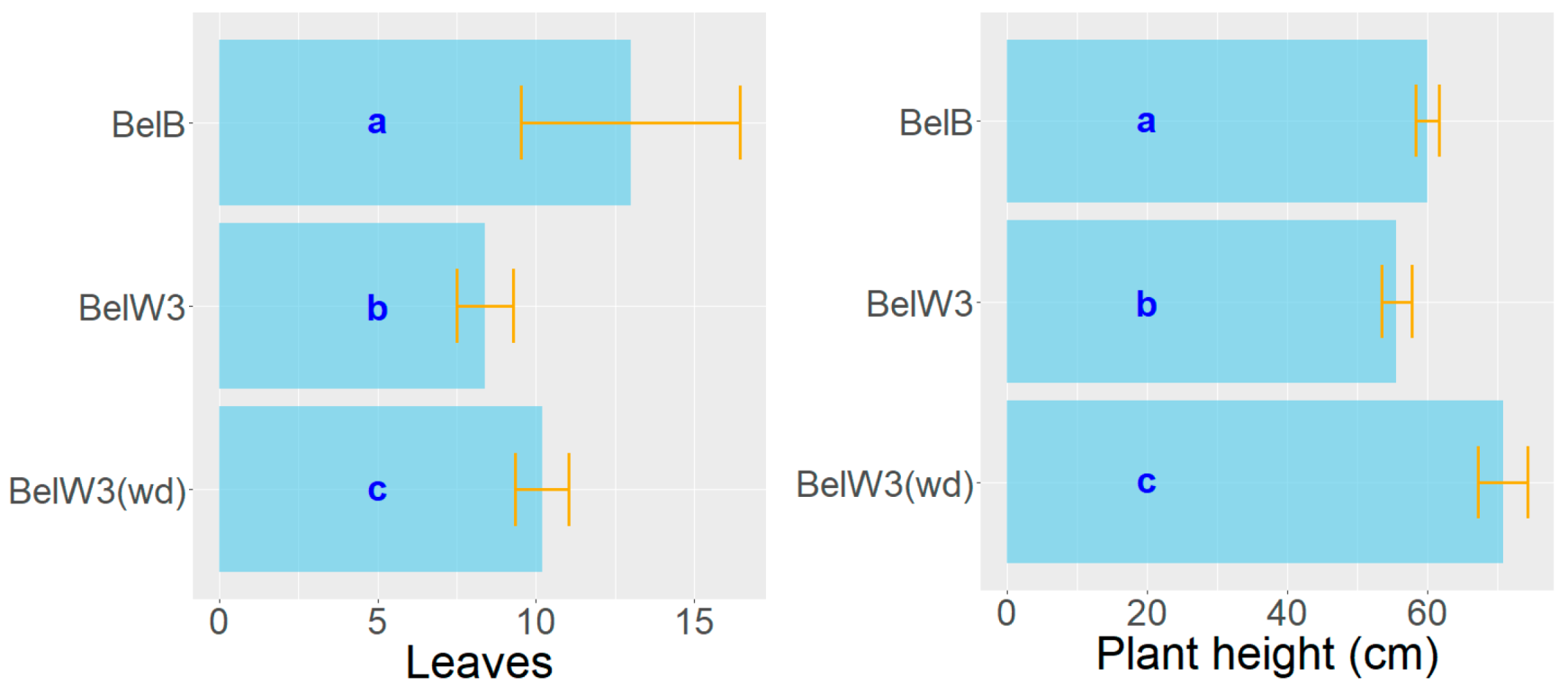
2.3. Measurements of Plant Height and Leaf Number
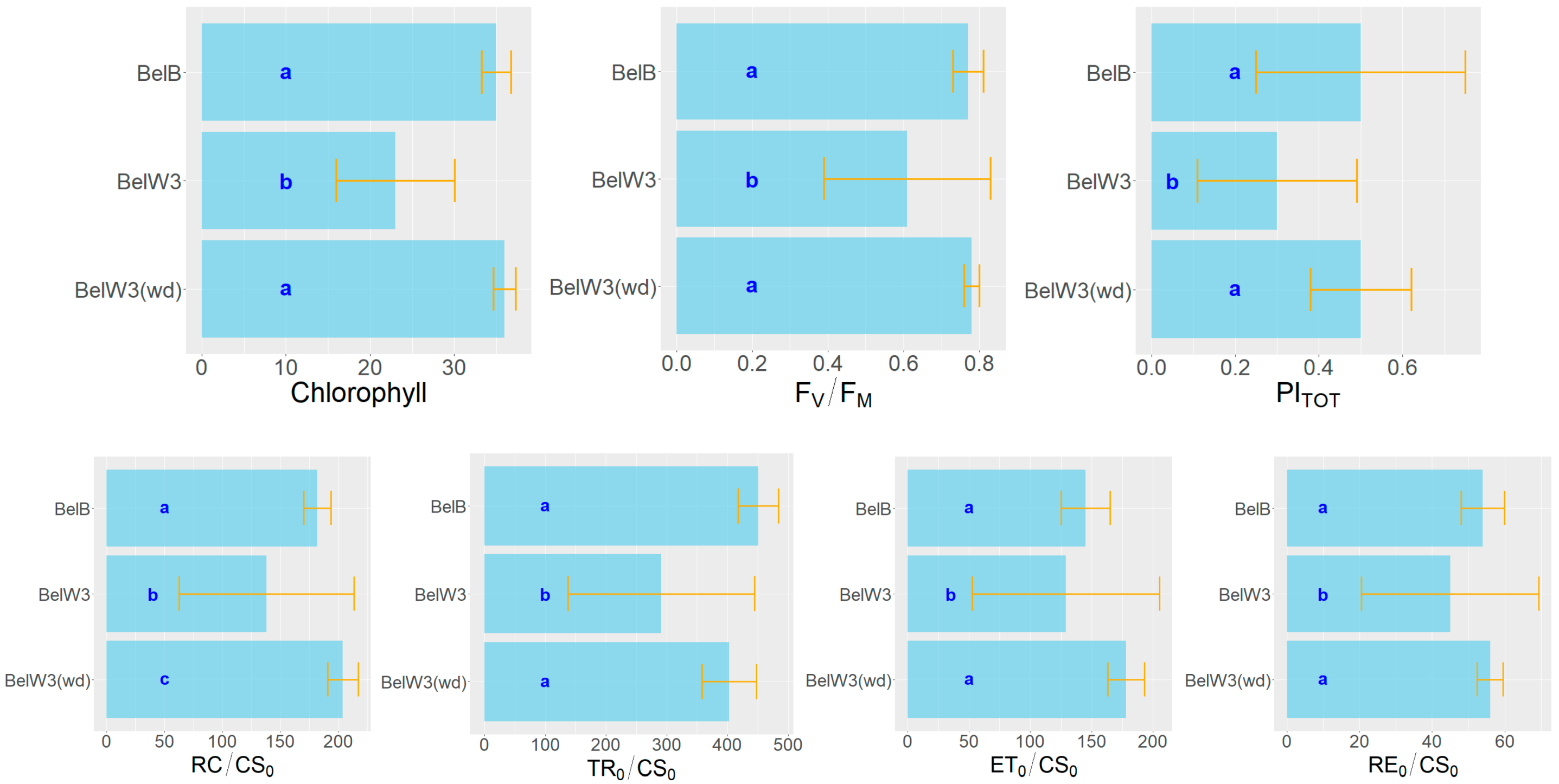
2.4. Measurements of Photosynthetic System Functionality
2.5. Statistical Analysis
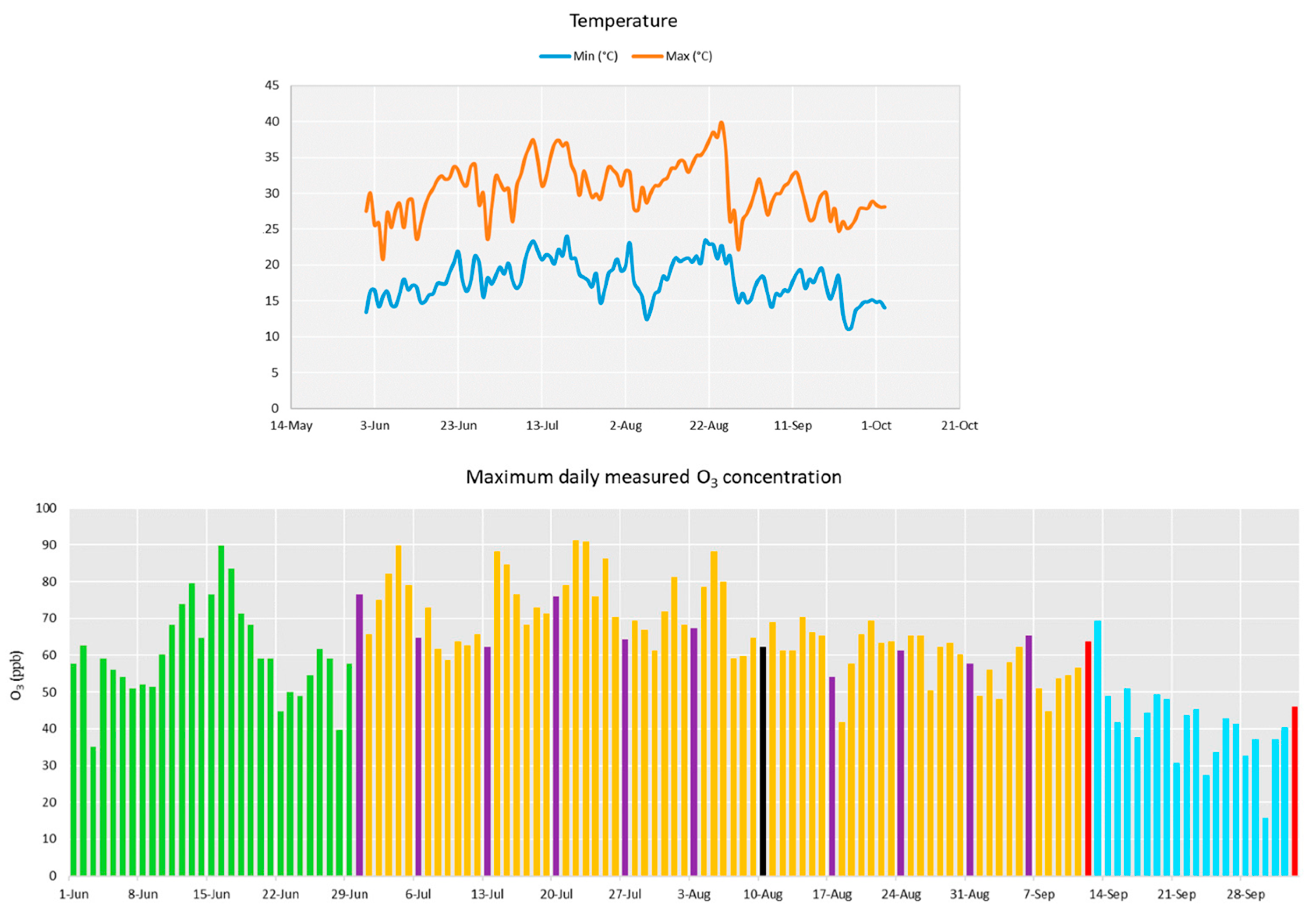
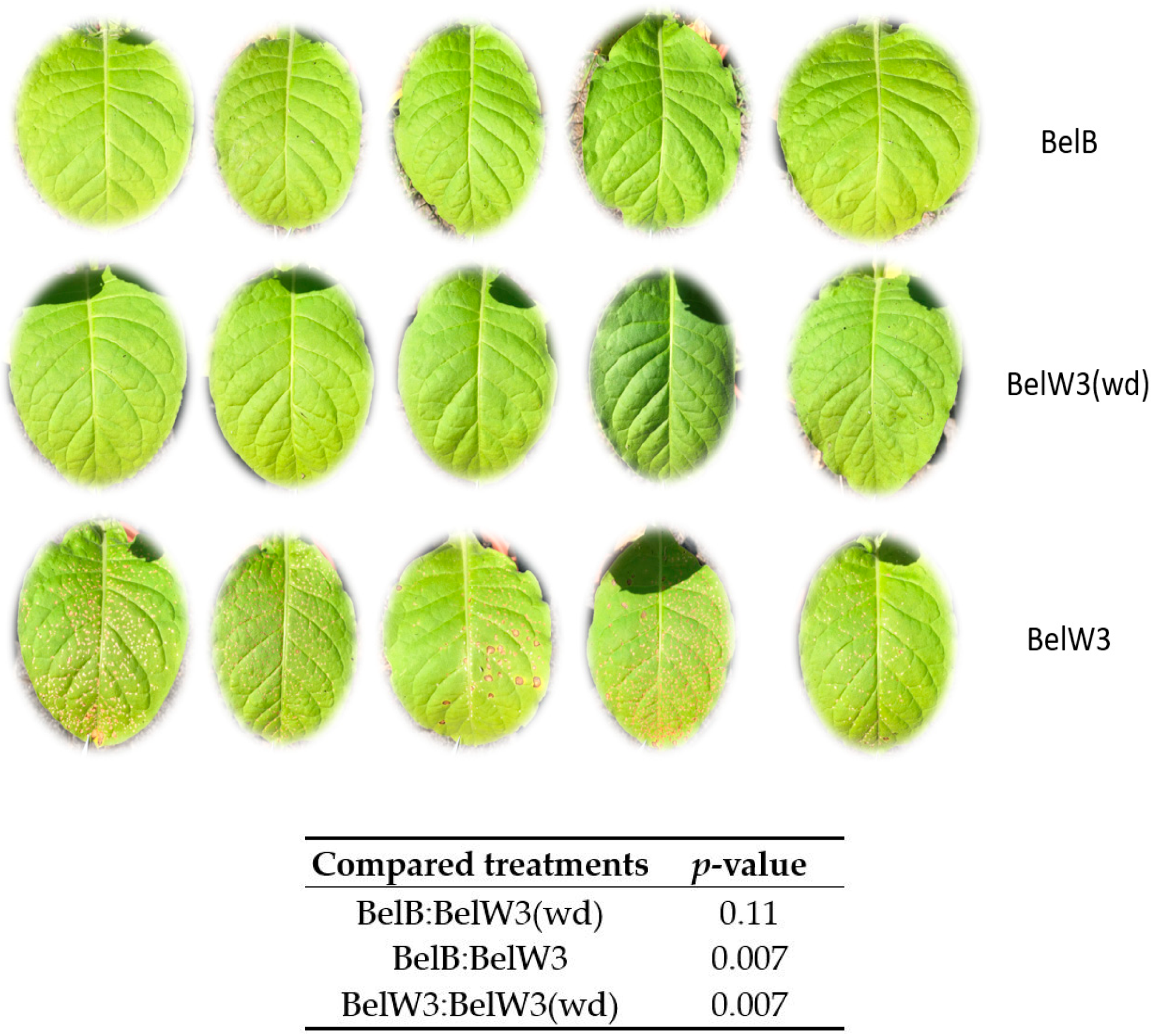
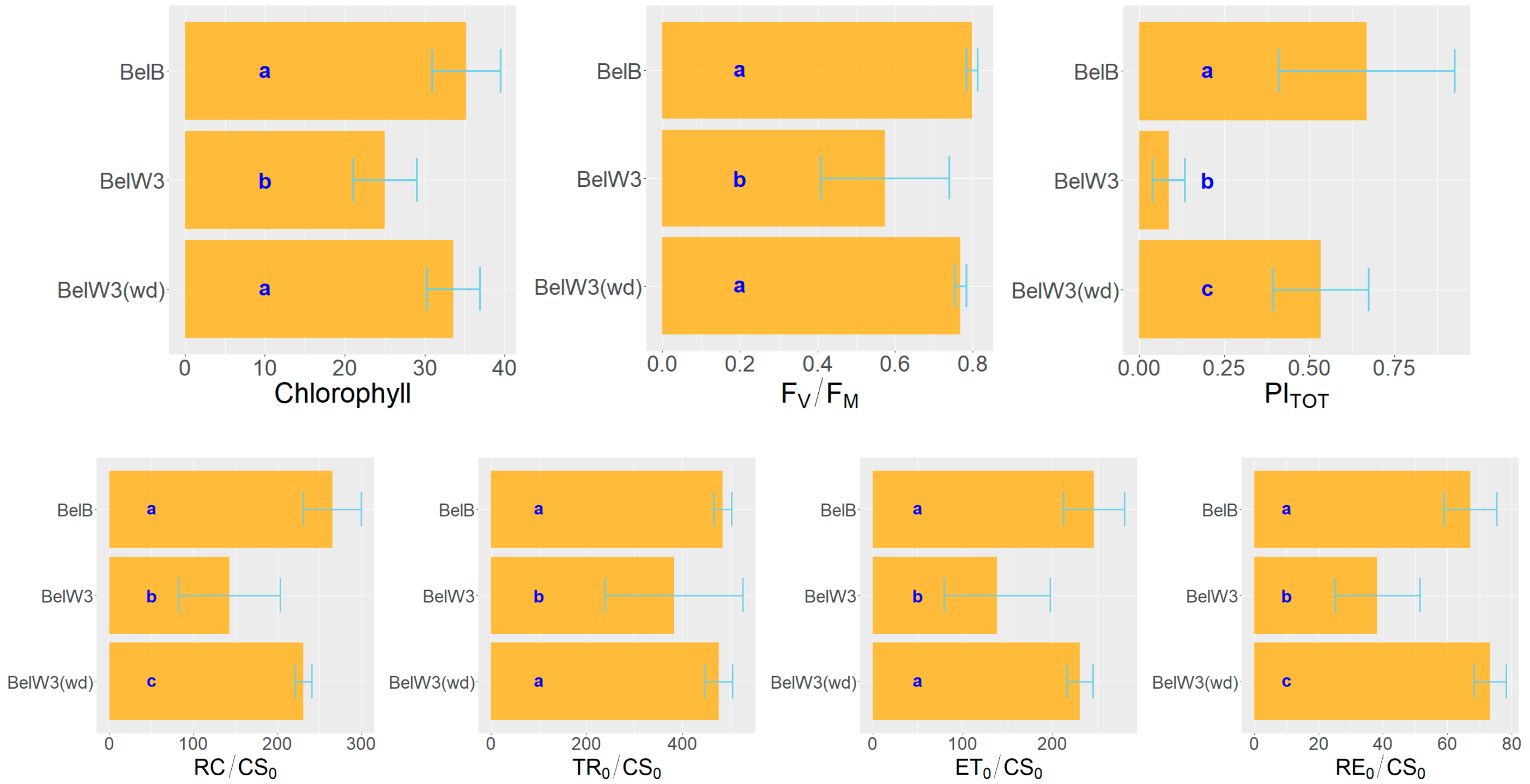
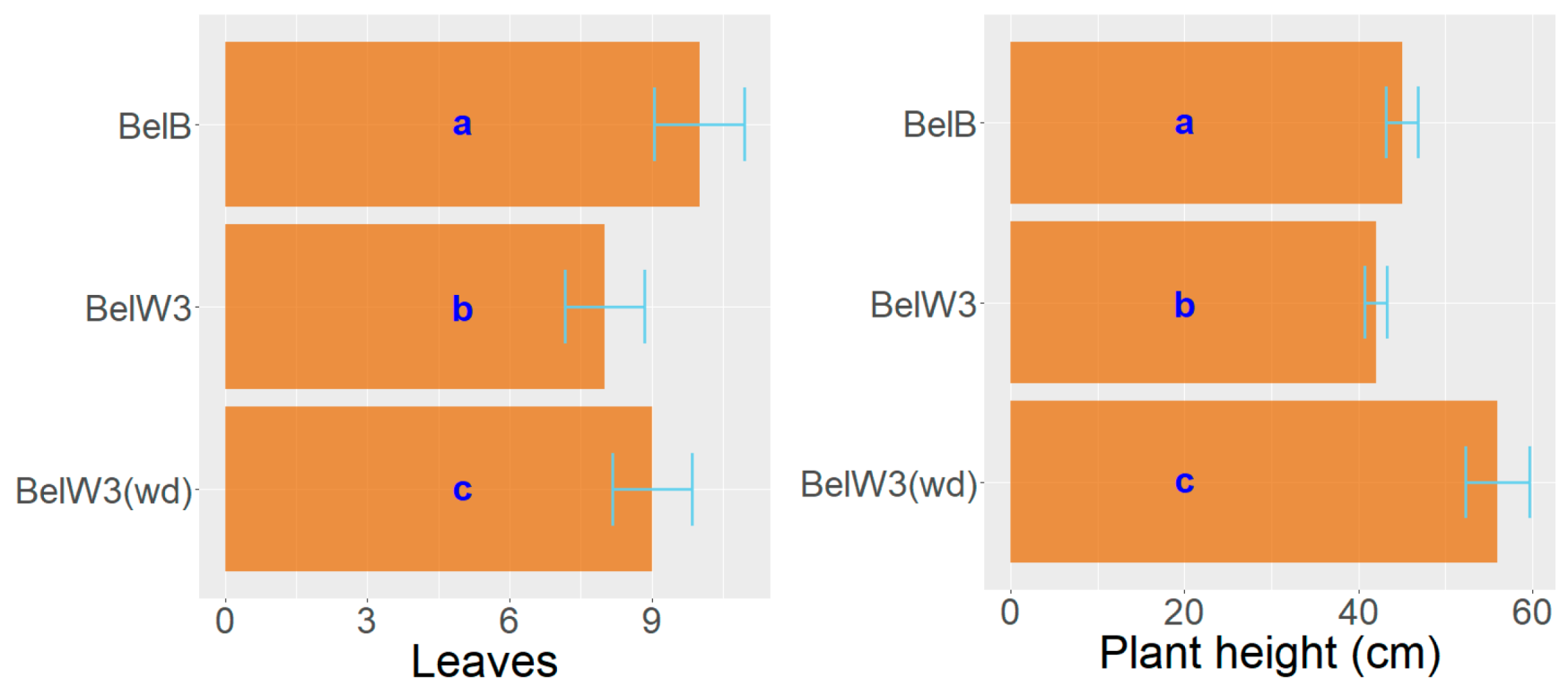
3. Results
3.1. Tobacco Growth and Vitality Six Weeks after Treatment with WD
3.2. Tobacco Growth and Vitality 11 Weeks after Treatment with WD
3.3. Tobacco Growth and Vitality Three Weeks after the End of the Treatments with WD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grulke, N.E.; Heath, R.L. Ozone Effects on Plants in Natural Ecosystems. Plant Biol. J. 2020, 22, 12–37. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Agrawal, S.B. The Role of Elevated Ozone on Growth, Yield and Seed Quality amongst Six Cultivars of Mung Bean. Ecotoxicol. Environ. Saf. 2015, 111, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Booker, F.; Muntifering, R.; McGrath, M.; Burkey, K.; Decoteau, D.; Fiscus, E.; Manning, W.; Krupa, S.; Chappelka, A.; Grantz, D. The Ozone Component of Global Change: Potential Effects on Agricultural and Horticultural Plant Yield, Product Quality and Interactions with Invasive Species. JIPB 2009, 51, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Agrawal, M. Tropospheric Ozone and Its Impacts on Crop Plants; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-71872-9. [Google Scholar]
- Feng, Z.; Kobayashi, K. Assessing the Impacts of Current and Future Concentrations of Surface Ozone on Crop Yield with Meta-Analysis. Atmos. Environ. 2009, 43, 1510–1519. [Google Scholar] [CrossRef]
- Pleijel, H.; Broberg, M.C.; Uddling, J.; Mills, G. Current Surface Ozone Concentrations Significantly Decrease Wheat Growth, Yield and Quality. Sci. Total Environ. 2018, 613–614, 687–692. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.M.; Betzelberger, A.M.; Wang, S.; Shook, E.; Zhu, X.-G.; Long, S.P.; Ainsworth, E.A. An Analysis of Ozone Damage to Historical Maize and Soybean Yields in the United States. Proc. Natl. Acad. Sci. USA 2015, 112, 14390–14395. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.; Buse, A.; Gimeno, B.; Bermejo, V.; Holland, M.; Emberson, L.; Pleijel, H. A Synthesis of AOT40-Based Response Functions and Critical Levels of Ozone for Agricultural and Horticultural Crops. Atmos. Environ. 2007, 41, 2630–2643. [Google Scholar] [CrossRef]
- Schucht, S.; Tognet, F.; Létinois, L.; Ineris, I.N. Wheat Yield Loss in 2019 in Europe Due to Ozone Exposure. Eionet Report-ETC/ATNI. 2021. Available online: https://www.eionet.europa.eu/etcs/etc-atni/products/etc-atni-report-17-2021-wheat-yield-loss-in-2019-in-europe-due-to-ozone-exposure (accessed on 4 April 2024).
- ANSA. La produzione di grano in Italia a 4 milioni di tonnellate, in crescita del 12%. 2023. Available online: https://www.ansa.it/canale_terraegusto/notizie/in_breve/2023/05/17/grano-produzione-italia-a-4-milioni-di-tonnellate-12_38565fbb-dc4b-4e14-9d79-1e7b2eea799a.html (accessed on 4 April 2024).
- ARPAE. Available online: https://apps.arpae.it/qualita-aria/bollettino-qa-provinciale/pr/20221020 (accessed on 4 April 2024).
- Mishra, A.K.; Rai, R.; Agrawal, S.B. Differential Response of Dwarf and Tall Tropical Wheat Cultivars to Elevated Ozone with and without Carbon Dioxide Enrichment: Growth, Yield and Grain Quality. Field Crops Res. 2013, 145, 21–32. [Google Scholar] [CrossRef]
- Emberson, L. Effects of Ozone on Agriculture, Forests and Grasslands. Phil. Trans. R. Soc. A. 2020, 378, 20190327. [Google Scholar] [CrossRef] [PubMed]
- Didyk, N.P.; Blum, O.B. Natural antioxidants of plant origin against ozone damage of sensitive crops. Acta Physiol. Plant 2011, 33, 25–34. [Google Scholar] [CrossRef]
- Manning, W.J.; Paoletti, E.; Sandermann, H.; Ernst, D. Ethylenediurea (EDU): A Research Tool for Assessment and Verification of the Effects of Ground Level Ozone on Plants under Natural Conditions. Environ. Pollut. 2011, 159, 3283–3293. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Zakaria, Z.A. Pyroligneous Acid—The Smoky Acidic Liquid from Plant Biomass. Appl. Microbiol. Biotechnol. 2015, 99, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Saitanis, C.J.; Lekkas, D.V.; Agathokleous, E.; Flouri, F. Screening Agrochemicals as Potential Protectants of Plants against Ozone Phytotoxicity. Environ. Pollut. 2015, 197, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; De Tullio, M.C. Ascorbic acid and ozone: Novel perspectives to explain an elusive relationship. Plants 2019, 8, 122. [Google Scholar] [CrossRef]
- Kittipornkul, P.; Treesubsuntorn, C.; Thiravetyan, P. Effect of exogenous catechin and salicylic acid on rice productivity under ozone stress: The role of chlorophyll contents, lipid peroxidation, and antioxidant enzymes. ESPR 2020, 27, 25774–25784. [Google Scholar] [CrossRef] [PubMed]
- Macias-Benitez, S.; Navarro-Torre, S.; Caballero, P.; Martín, L.; Revilla, E.; Castaño, A.; Parrado, J. Biostimulant capacity of an enzymatic extract from rice bran against ozone-induced damage in Capsicum annum. Front. Plant Sci. 2021, 12, 749422. [Google Scholar] [CrossRef] [PubMed]
- Saitanis, C.J.; Agathokleous, E. Exogenous application of chemicals for protecting plants against ambient ozone pollution: What should come next? Curr. Opin. Environ. Sci. Health 2021, 19, 100215. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.; Lord, A.; Gunupuru, L. R. Production, Prospects and Potential Application of Pyroligneous Acid in Agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Cândido, N.R.; Pasa, V.M.D.; Vilela, A.D.O.; Campos, Â.D.; De Fátima, Â.; Modolo, L.V. Understanding the Multifunctionality of Pyroligneous Acid from Waste Biomass and the Potential Applications in Agriculture. Sci. Total Environ. 2023, 881, 163519. [Google Scholar] [CrossRef]
- Ofoe, R.; Qin, D.; Gunupuru, L.R.; Thomas, R.H.; Lord, A. Effect of Pyroligneous Acid on the Productivity and Nutritional Quality of Greenhouse Tomato. Plants 2022, 11, 1650. [Google Scholar] [CrossRef]
- Vannini, A.; Fedeli, R.; Guarnieri, M.; Loppi, S. Foliar Application of Wood Distillate Alleviates Ozone-Induced Damage in Lettuce (Lactuca sativa L.). Toxics 2022, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Effects of wood distillate (pyroligneous acid) on sensitive bioindicators (lichen and moss). Ecotoxicol. Environ. Saf. 2020, 204, 111117. [Google Scholar] [CrossRef] [PubMed]
- Van Buuren, M.L.; Guidi, L.; Fornalè, S.; Ghetti, F.; Franceschetti, M.; Soldatini, G.F.; Bagni, N. Ozone-response mechanisms in tobacco: Implications of polyamine metabolism. New Phytol. 2002, 156, 389–398. [Google Scholar] [CrossRef]
- Städtler, S.; Ziegler, H. Illustration of the genetic differences in ozone sensitivity between the varieties Nicotiana tabacum Bel W3 and Bel B using various plant systems. Bot. Acta 1993, 106, 265–276. [Google Scholar] [CrossRef]
- Fernandez, M.G.S.; Becraft, P.W.; Yin, Y.; Lübberstedt, T. From dwarves to giants? Plant height manipulation for biomass yield. Trends Plant Sci. 2009, 14, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; McClure, M.A.; Jaja, N.; Tyler, D.D.; Hayes, R.M. In-season prediction of corn yield using plant height under major production systems. Agron. J. 2011, 103, 923–929. [Google Scholar] [CrossRef]
- Farjon, G.; Itzhaky, Y.; Khoroshevsky, F.; Bar-Hillel, A. Leaf counting: Fusing network components for improved accuracy. Front. Plant Sci. 2021, 12, 575751. [Google Scholar] [CrossRef] [PubMed]
- Novichonok, E.V.; Novichonok, A.O.; Kurbatova, J.A.; Markovskaya, E.F. Use of the atLEAF+ chlorophyll meter for a nondestructive estimate of chlorophyll content. Photosynthetica 2016, 54, 130–137. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis Mechanism, Regulation & Adaptation, 1st ed.; Mohammad, Y., Uday, P., Eds.; CRC Press: New York, NY, USA, 2000; pp. 445–483. [Google Scholar]
- Stefanov, M.A.; Rashkov, G.D.; Apostolova, E.L. Assessment of the Photosynthetic Apparatus Functions by Chlorophyll Fluorescence and P700 Absorbance in C3 and C4 Plants under Physiological Conditions and under Salt Stress. IJMS 2022, 23, 3768. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. In R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 7 December 2023).
- Saitanis, C.J.; Riga-Karandinos, A.N.; Karandinos, M.G. Effects of Ozone on Chlorophyll and Quantum Yield of Tobacco (Nicotiana tabacum L.) Varieties. Chemosphere 2001, 42, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Restivo, F.M. Indoor and Outdoor Genotoxic Load Detected by the Comet Assay in Leaves of Nicotiana Tabacum Cultivars Bel B and Bel W3. Mutagenesis 2002, 17, 127–134. [Google Scholar] [CrossRef][Green Version]
- Ahmad, S.; Su, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Javed, T.; Han, Q. Foliar Application of Melatonin Delay Leaf Senescence in Maize by Improving the Antioxidant Defense System and Enhancing Photosynthetic Capacity under Semi-Arid Regions. Protoplasma 2020, 257, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous Melatonin Application Delays Senescence of Kiwifruit Leaves by Regulating the Antioxidant Capacity and Biosynthesis of Flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Numan, M.; Khan, A.L.; Lee, I.-J.; Imran, M.; Asaf, S.; Al-Harrasi, A. Melatonin: Awakening the Defense Mechanisms during Plant Oxidative Stress. Plants 2020, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Saitanis, C.J.; Karandinos, M.G. Effects of Ozone on Tobacco (Nicotiana tabacum L.) Varieties. J. Agron. Crop. Sci. 2002, 188, 51–58. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. Does Enhanced Photosynthesis Enhance Growth? Lessons Learned from CO2 Enrichment Studies. Plant Physiol. 2011, 155, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Moratelli, F.; Monaci, F.; Loppi, S. Effects of Wood Distillate and Soy Lecithin on the Photosynthetic Performance and Growth of Lettuce (Lactuca sativa L.). SN Appl. Sci. 2021, 3, 113. [Google Scholar] [CrossRef]
- Masum, S.M.; Malek, M.; Mandal, M.S.H.; Haque, M.N.; Akther, Z. Influence of plant extracted pyroligneous acid on transplanted aman rice. J. Expt. Biosci. 2013, 4, 31–34. [Google Scholar]
- Zulkarami, B.; Ashrafuzzaman, M.; Husni, M.O.; Ismail, M.R. Effect of Pyroligneous Acid on Growth, Yield and Quality Improvement of Rockmelon in Soilless Culture. AJCS 2011, 5, 1508–1514. [Google Scholar]
- Travero, J.T.; Mihara, M. Erd Effects of Pyroligneous Acid to Growth and Yield of Soybeans. IJERD 2016, 7-1, 50–54. [Google Scholar]
- Bussotti, F.; Strasser, R.J.; Schaub, M. Photosynthetic Behavior of Woody Species under High Ozone Exposure Probed with the JIP-Test: A Review. Environ. Pollut. 2007, 147, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Chang, Y.M.; Wang, T.K.; Lin, S.H.; Knee, J.L. Ultrafast Spectroscopy and its Applications. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 217–226. ISBN 9780122274107. [Google Scholar] [CrossRef]
- Björn, L.O.; Papageorgiou, G.C.; Blankenship, R.E.; Govindjee. A Viewpoint: Why Chlorophyll a? Photosynth. Res. 2009, 99, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H. Reactive Oxygen Species, Oxidative Signaling and the Regulation of Photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Cong, Y.; Zhu, P.; Xing, J.; Cui, J.; Xu, W.; Shi, Q.; Diao, M.; Liu, H.Y. Ascorbic acid-induced photosynthetic adaptability of processing tomatoes to salt stress probed by fast OJIP fluorescence rise. Front. Plant Sci. 2021, 12, 594400. [Google Scholar] [CrossRef]
- Allan, A.C.; Fluhr, R. Ozone and Reactive Oxygen Species. eLS 2001, 11, 41–53. [Google Scholar] [CrossRef]
- Maliba, B.G.; Inbaraj, P.M.; Berner, J.M. The Use of OJIP Fluorescence Transients to Monitor the Effect of Elevated Ozone on Biomass of Canola Plants. Water Air Soil Pollut 2019, 230, 75. [Google Scholar] [CrossRef]
- Gupta, A.; Yadav, D.S.; Agrawal, S.B.; Agrawal, M. Sensitivity of Agricultural Crops to Tropospheric Ozone: A Review of Indian Researches. Environ. Monit Assess 2022, 194, 894. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A. Understanding and Improving Global Crop Response to Ozone Pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Values |
|---|---|
| Peat | 50% |
| Organic carbon (dw) | 25% |
| Humic and fulvic carbon (dw) | 7% |
| Organic nitrogen (dw) | 80% of total nitrogen |
| Salinity | 1.20 dS/m |
| Parameters | Description |
|---|---|
| FV/FM | Maximum quantum efficiency of Photosystem II |
| PITOT | Performance Index for energy conservation from excitation to the reduction of PSII and PSI |
| RC/CS0 | Density of reaction centers (QA reducing PSII RC) |
| TR0/CS0 | Maximum trapped exciton flux per excited cross section |
| ET0/CS0 | Electron transport flux from QA to QB per excited cross section |
| RE0/CS0 | Electron transport flux until PSI acceptors per excited cross section |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannini, A.; Petraglia, A. Wood Distillate Mitigates Ozone-Induced Visible and Photosynthetic Plant Damage: Evidence from Ozone-Sensitive Tobacco (Nicotiana tabacum L.) BelW3. Horticulturae 2024, 10, 480. https://doi.org/10.3390/horticulturae10050480
Vannini A, Petraglia A. Wood Distillate Mitigates Ozone-Induced Visible and Photosynthetic Plant Damage: Evidence from Ozone-Sensitive Tobacco (Nicotiana tabacum L.) BelW3. Horticulturae. 2024; 10(5):480. https://doi.org/10.3390/horticulturae10050480
Chicago/Turabian StyleVannini, Andrea, and Alessandro Petraglia. 2024. "Wood Distillate Mitigates Ozone-Induced Visible and Photosynthetic Plant Damage: Evidence from Ozone-Sensitive Tobacco (Nicotiana tabacum L.) BelW3" Horticulturae 10, no. 5: 480. https://doi.org/10.3390/horticulturae10050480
APA StyleVannini, A., & Petraglia, A. (2024). Wood Distillate Mitigates Ozone-Induced Visible and Photosynthetic Plant Damage: Evidence from Ozone-Sensitive Tobacco (Nicotiana tabacum L.) BelW3. Horticulturae, 10(5), 480. https://doi.org/10.3390/horticulturae10050480









