Abstract
Penicillium digitatum and Penicillium italicum are responsible for citrus green and blue moulds (GM and BM), respectively, which are major citrus postharvest diseases. The aim of this study was to develop an optimal dipping mixture of an aqueous solution of different food additives: sodium bicarbonate (SB), sodium benzoate (SBen), and potassium sorbate (PS), in combination with heat, to control GM and BM using response surface methodology. The ranges of SB (0.0%, 3.0%, 6.0%), SBen (0.0%, 0.5%, 1.0%), PS (0.0%, 0.5%, 1.0%) and temperature (20 °C, 35 °C, 50 °C) with a dipping time of 60s were tested for their impact on GM and BM on artificially inoculated oranges. Within these tested ranges, SB reduced GM severity and incidences of both GM and BM. PS affected BM severity and incidence, but not GM. SBen and temperature did not have impact on GM and BM. The most suitable food additive concentrations were identified to be 4.7% SB, 1.0% SBen and 0.7% PS, with a dipping solution temperature of 50 °C. This treatment was shown to reduce GM and BM incidence from 85 and 86% on control fruit dipped in tap water at 20 °C to 3 and 10%, respectively. Additionally, the severity of GM and BM was reduced from 64 and 26 mm on control fruit to <1 and 2.8 mm, respectively.
1. Introduction
Oranges are a widely grown and traded commodity which often require significant shipping and storage times. The optimum storage temperature for citrus is 2 to 3 °C at 85 to 90% relative humidity (RH) allowing for storage times of between 4 and 8 weeks []. Postharvest decay is a major problem for the storage of citrus fruit. Penicillium digitatum (Pers.: Fr.) Sacc. and Penicillium italicum Wehmer are responsible for green and blue mould (GM and BM) in citrus, respectively, and are the major postharvest pathogens causing significant commercial costs []. These pathogens are currently controlled with commercial synthetic fungicides such as imazalil. However, consumer and regulatory concerns are leading to renewed interest in environmentally friendly and safe alternatives to the use of postharvest fungicides [,]. Some food additives or salts classified as generally recognised as safe (GRAS), such as sodium bicarbonate (SB), potassium sorbate (PS) and sodium benzoate (SBen), are useful in controlling postharvest decay [,,] and some commercial products based on GRAS compounds have been registered to be used within the European Union and other markets [].
PS is a broad-spectrum food preservative that has been considered safe for human consumption as a food additive []. Extensive research has shown synergies between heat and fungicides when used as a dipping treatment or incorporated into waxes or edible coatings [,,]. In both in vitro studies and in vivo trials with citrus, PS has been shown to inhibit disease-causing fungi and control postharvest decay caused by diseases such as GM, BM, and stem-end rot caused by Lasiodiplodia theobromae [,] and some PS-based antifungal commercial products for postharvest application are currently available in the market. Furthermore, PS has also been found to provide additional benefits by inhibiting Escherichia coli when incorporated into an edible film [].
The postharvest application of SB has been shown to reduce postharvest diseases such as GM and BM in citrus and SB dips are commercially used in some citrus packing houses [,]. Short hot water dips can also significantly reduce BM in oranges, although the treatment was not very persistent and the range between effective and phytotoxic temperatures was very narrow (45–50 °C) []. Hot water dips can also reduce postharvest decay caused by GM and BM in citrus [], indicating a direct fungistatic effect of hot water. SBen is another widely used food additive which has been shown to reduce the incidence of both GM and BM in citrus []. Furthermore, increasing treatment temperatures resulted in increased effectiveness and synergistic activity in controlling postharvest decay. For example, dip treatments for 60 s with 3% (w/v) SBen solutions heated to 50 °C resulted in about 90% reduction in the incidence of GM and BM on Valencia []. In addition, higher solution temperatures were phytotoxic and longer immersion times did not generally provide additional effectiveness []. Nevertheless, in contrast to PS, SBen has not been registered in the European Union (EU) as a plant protection product for postharvest usage in citrus, so it cannot be currently used commercially. SB is commercially used in European citrus packinghouses because it has been included in the list of basic substances allowed for citrus postharvest applications [].
These research studies have clearly shown the positive effects of food additives (PS, SB and SBen) and elevated treatment temperatures on reducing postharvest decay in citrus. However, each salt solution, heated or not, was applied as a stand-alone treatment and little information is available on the effectiveness of mixtures of these different GRAS salts to control postharvest decay in citrus. Response surface methodology (RSM) is a useful methodology to show the interactive effects between different independent and response variables [], which can reduce time and cost by simultaneously assessing numerous experimental parameters at once. Therefore, RSM can be a valuable resource to systematically investigate the interactions and additive effects of these food additives and high treatment temperatures. This study seeks to fill this knowledge gap and determine the optimal dipping solutions of the food additives SB, SBen and PS (at concentrations of 0.5 to 6.0%) combined with elevated but non-phytotoxic treatment temperatures (20–50 °C) using RSM for the control of GM and BM in artificially inoculated ‘Valencia’ oranges.
2. Materials and Methods
2.1. Fruit and Fungal Inoculation
Fungicide-free oranges (Citrus sinensis L. Osbeck) cv. Valencia from a commercial orchard (Hanwood, NSW Australia), were used for decay assessments at NSW Department of Primary Industries at Ourimbah, NSW Australia. Blemish-free fruit were harvested at commercial maturity with total soluble solids (TSS) 13.7% °Brix, titratable acidity (TA) 0.85% citric acid and TSS/TA ratio of 16.1. After harvest, fruit were washed with 50 µL/L free chlorine (HyChlor, Glendenning NSW Australia) water at room temperature (20 °C) and allowed to air dry. Fruit were then sorted, graded, and randomised into treatment units.
Fruit were inoculated with GM and BM spores at opposite points on each orange. Wild type GM and BM spores (3.4 × 106 spores per mL) obtained from NSW Department of Primary Industries citrus pathology laboratory were inoculated into a wound in each orange. Infection was conducted by dipping a stainless-steel rod into the inoculum solution, and then puncturing the rod tip 2 mm deep into the fruit []. Inoculated fruits were then incubated for 24 h at 20 °C and 95% RH before treatment [].
2.2. Food Additives
Chemicals used in this study were all food grade and purchased from different sources. SB (NaHCO3) was purchased from ETi SODA Co., Ltd. (Beypazarı Ankara, Turkey). SBen (C7H5NaO2) was purchased from Wuhan Youji Industries Co., Ltd. (Wuhan, China). PS (C6H7KO2) was sourced from Nantong Acetic Acid Chemical Co., Ltd. (Nantong, China). All these products were stored for a maximum period of 12 weeks.
2.3. Experimental Design for Effectiveness of Food Additive Solutions Using RSM
Individual food additives were weighed and mixed with 10 L of tap water at the required temperature in a 20 L food safe bucket. Hot tap water (60 °C) was mixed with cold tap water (~17 °C) to create the treatment temperature which was measured to be within 0.5 °C immediately before start of 60 s treatment. The water’s temperature was sustained solely by its thermal mass throughout the 60 s treatment period, experiencing a decrease of less than 2 °C. The treatment suspension was thoroughly mixed for at least 2 min to ensure the food additives were completely dissolved. The oranges were then dipped in each treatment combination for 60 s. Each treatment combination was applied to 20 oranges and replicated 3 times, except the centre point which was repeated 3 times, meaning it was replicated a total of 9 times. Each solution was independently prepared and applied.
The infection rates (disease incidence, %) and the lesion diameters (disease severity, mm) of decay caused by Penicillium spp. on ‘Valencia’ oranges were assessed 7 days after treatment and incubation at 20 °C and 95% RH. For each disease, GM and BM, the incidence of decay was determined for each treatment unit of 20 fruit, while disease severity was determined in each orange by measuring the rind decayed soft tissue around the inoculation point with a flexible ruler. The average of the two directions of lesion radial growth was used for statistical analysis.
RSM with the Box–Behnken design was employed for starting the experiment and analysing the impact of four independent factors: different concentrations of SB (0.0–6.0%), SBen (0.0–1.0%), and PS (0.0–1.0%), and different treatment temperatures (20, 35 or 50 °C). Immersion time was considered as a constant factor and fixed at 60 s. Four different response variables (GM severity, GM incidence, BM severity, and BM incidence) were observed from each trial assessment. All the different treatment combinations (4 variables x 3 levels) are presented in Table 1.

Table 1.
Box–Behnken treatment design using a 60 s dipping duration.
The Box–Behnken design (Table 1) was utilised to determine the treatment combinations. Each of these combinations was then tested using 3 replicates with a sample size of 20 inoculated fruit, which helped in predicting the optimal concentrations and temperatures. These predictions were subsequently validated through a confirmation test. This test was conducted using the optimal concentrations, with 4 replicates and a sample size of 20 inoculated fruit for each replicate, and each replicate was independently prepared and treated.
2.4. Statistical Analysis
RSM experimental design and analysis was performed using JMP®, (Version <14>, SAS Institute Inc., Cary, NC, USA, 1989–2023). A model was created using a Box–Behnken design at three levels for each independent variable with three centre points replicates to create the equations, to graph the 3D plots, 2D contours of the responses and to calculate the optimum values for the three concentrations of food additives and dipping temperatures from the response variables. The Student’s t-test was conducted using the JMP® (Version <14>, SAS Institute Inc., Cary, NC, USA, 1989–2023) statistical software to compare the means of each replicate obtained from the experiment samples. Differences between the means of GM and BM incidence (%) and severity were taken to be statistically significant at p < 0.05.
3. Results and Discussion
3.1. Variable Responses from Box–Behnken Design Experiment
The results of the Box–Behnken design with the 25 different dipping treatments with combinations of concentrations of SB (0.0–6.0%), SBen (0.0–1.0%) and PS (0.0–1.0%) and solution temperature (20–50 °C) are presented in Table 2. The untreated control treatment, which was only dipped in tap water at 20 °C, resulted in GM severity and incidence of 103 mm and 87%, respectively, while BM severity and incidence were 71 mm and 96%. The fungal inoculation and experimental procedures used in these experiments proved appropriate, as they achieved good infection rates on the control fruit. All treatments were significantly different from the control with tested concentrations of SB 3.0%, SBen 1.0%, and PS 0.5% at 50 °C (run number 24) being most effective at reducing GM severity (3.8 mm) and disease incidence (4%), compared with the treatment of SB 3.0%, SBen 0.5%, and PS 1.0% at 50 °C (run number 25) being most effective at reducing BM severity (7.3 mm) and disease incidence (8%) (Table 2).

Table 2.
Box–Behnken design and observed responses of GM and BM severity (mm) and incidence (%) using 60 s dipping duration.
3.2. Fitting of the Models for Prediction of Impact of Food Additives and Temperature on the In Vivo Development of GM and BM
To assess the reliability of the RSM models for prediction of the impact of food additives and temperature on the development of GM and BM in oranges, ANOVA was conducted, and the results are presented in Figure 1. The results show that the models for prediction of the impact of food additives and temperature on severity and incidence of both GM and BM were reliable.
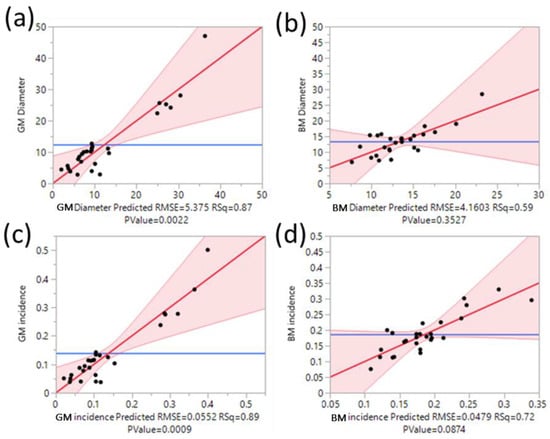
Figure 1.
Correlations between predicted and experimental values and lack of fit and analysis of variance for (a) green mould (GM) severity, p > F 0.494, F ratio 1.373; (b) blue mould (BM) severity, p > F 0.0190, F ratio 52.625; (c) GM incidence (%) p > F 0.624, F ratio 0.924; and (d) BM incidence (%) p > F 0.791, F ratio 12.031. Each point represents the average from 20 fruit. The light red zone is the 95% confidence region. Horizontal blue line represents the null hypothesis, with the red line indicating the alternative hypothesis.
For GM severity, the values predicted by the model matched the actual values from the experimental data approximately 87% of the time (Figure 1a). While the p value of lack of fit was not significant (p > 0.05), the p value of the model was significant (p < 0.05), further confirming the fitting of the model for prediction of the impact of the tested food additives and temperatures on this response (Figure 1). For BM severity, approximately 59% of the predicted values using the model matched the actual values (Figure 1b). For the GM and BM incidence, 89% and 72% of the values predicted by the models matched the actual values (Figure 1c,d). The p value for the lack of fit was not significant while the p value of the models was significant, showing that the models are reliable for prediction of the impact of the tested food additives and temperatures.
The models show the relationships between the variables, including SB, SBen, and PS concentrations and different temperatures, and responses (severity (mm) and incidence (%) of GM and BM) are as follows (Equations (1)–(4)):
where SB = sodium bicarbonate concentration, SBen = sodium benzoate concentration, PS = potassium sorbate concentration, GM = green mould, and BM = blue mould.
3.3. Effects of Food Additives and Temperature on Development of GM and BM
The incidence of GM and its severity were significantly affected by SB while SBen, PS, and temperature did not have an impact on GM incidence and severity (Table 3). This was unexpected as many other reports have shown the beneficial effects of SBen and PS on reducing GM and also their synergistic effects with heat [,,]. This observation may be due to the low concentrations of these salts used in this trial (up to 1%), since higher concentrations (2–3%) were usually used in most of the cited works. The severity of BM was impacted by PS, while BM incidence was impacted by both SB and PS. There was no interaction between SB and SBen; SB and PS; SB and temperature; SBen and PS; SBen and temperature; or PS and temperature. SBen at a maximum concentration of 1.0% showed no impact on the disease severity and incidence. For instance, Montesinos-Herrero et al. [] reported that SBen effectively reduced GM and BM incidence at concentrations of 3.0%, but this was not found in this experiment due to the lower concentrations which were used (<1.0%).

Table 3.
Regression coefficients for the quadratic polynomial and analysis of variance from RSM.
The results presented in Table 3 show that PS at concentrations of 1.0% or lower did not have a significant impact on both GM severity and incidence, which is supported by [], which also found that 1.0% PS at 20 °C had no effect on the in vivo development of GM on lemons infected by a thiabendazole (TBZ)-sensitive strain of GM. In contrast, brief dips (30–60 s duration) in PS aqueous solutions at 3% effectively reduced GM and BM on different citrus species and cultivars, including ‘Valencia’ oranges, previously wound-inoculated with P. digitatum and P. italicum, respectively [].
The response surface plots presented in Figure 2 show the effect of SB vs. Sben, and treatment temperature vs. PS, on both GM and BM. It can be seen that all treatment components influence the development of decay. Figure 2a shows that an increase in SB predicts that the incidence of GM declines whilst the results in Figure 2b show that an increase in both temperature and PS results in a decrease in GM incidence. These observations are consistent with other studies working with mandarin fruit dipped for 60 or 150 s in SB solutions at 20 °C [].
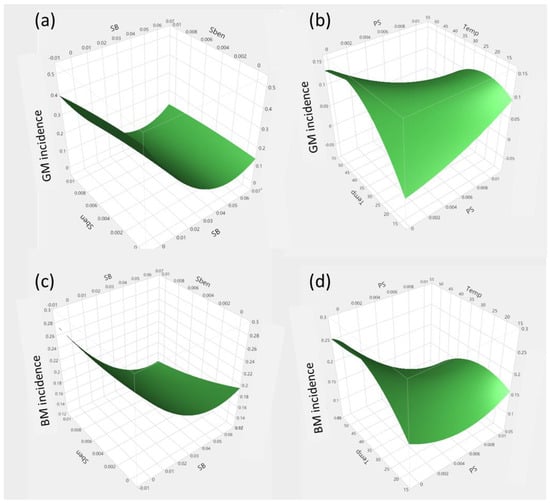
Figure 2.
Response surface plots showing the interaction impact of independent variables. (a) SB vs. SBen on incidence of GM, (b) treatment temperature vs. PS on incidence of GM, (c) SB vs. Sben on incidence of BM, and (d) treatment temperature vs. PS on incidence of BM.
3.4. Prediction and Validation of Optimal Mixture and Concentration of Food Additives and Temperature to Control GM and BM
The response surface map from the RSM predicted that the ideal concentrations for the three food additives were SB 4.7%, SBen 1.0%, PS 0.7% and a treatment temperature of 50 °C. It was predicted that this optimum combination would result in the greatest reductions in incidence and severity for both GM and BM (Figure 3). A further confirmatory trial was conducted with these experimental conditions and the results were within the expected value tolerances permitted (>0.05), thereby validating the model.
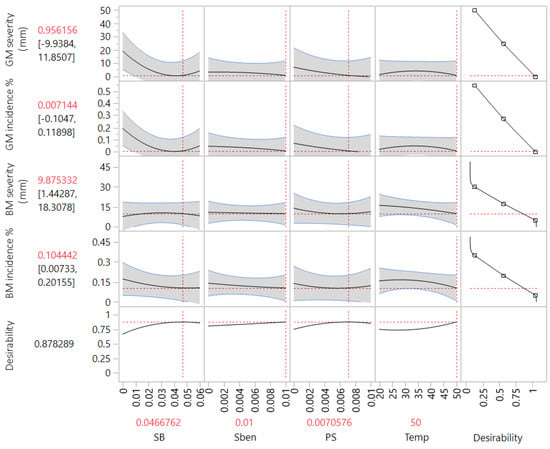
Figure 3.
Response surface methodology model predictions for the dependent variables’ responses for the different independent variables. Sodium bicarbonate concentration (SB, 0.0–6.0%); sodium benzoate concentration (SBen, 0.0–1.0%); potassium sorbate concentration (PS, 0.0–1.0%); temperature (Temp, 20–50 °C).
The results from the optimum combination experiment showed that the experimental value was within the range of the predicted value, suggesting that this model can be accepted. The optimal solution was able to greatly reduce both GM and BM incidence and severity (Figure 4).
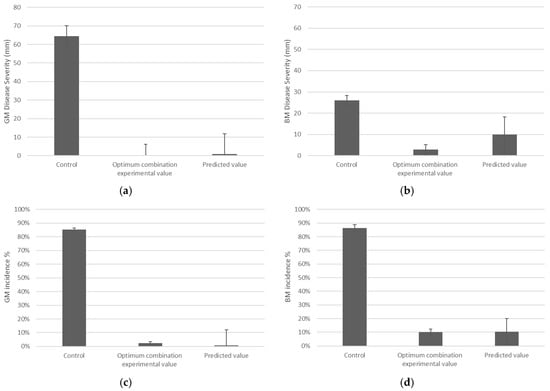
Figure 4.
Severity (mm) of green mould (GM) (a), severity (mm) of blue mould (BM) (b), incidence (%) of GM (c), and incidence (%) of BM (d). Control and optimum combination experimental value error bar representing SE and predicted value error bar representing the predicted range. Each graph was analysed independently with an alpha 0.05 GM severity (mm) LSD 26.163; BM severity (mm) LSD 10.582; GM incidence (%) LSD 0.05012; and BM incidence (%) LSD 0.10676.
4. Conclusions
This study successfully applied RSM to identify effective combinations of SB, SBen, PS, and temperature for controlling GM and BM, caused by P. digitatum and P. italicum, respectively, in ‘Valencia’ oranges. SB notably reduced both GM severity and incidence, along with BM incidence, while it did not significantly affect BM severity. In contrast, SBen and treatment temperature showed no substantial influence on GM and BM at the tested concentrations, likely due to the low concentrations of SBen used in these trials (<1%). By comparison, other research showed significant effectiveness of SBen at higher concentrations, suggesting a threshold-dependent action. PS, although ineffective against GM at the tested concentrations, significantly impacted BM severity and incidence.
Utilising RSM models, this study identified an optimal postharvest treatment using a mixture of SB at 4.7%, SBen at 1.0%, and PS at 0.7%, at a dip temperature of 50 °C, which decreased the incidence and severity of both GM and BM. The reduction in GM incidence was from 85% in the control group treated with water at 20 °C to less than 5% in oranges treated with this combination. In the case of BM incidence, this reduction was from 87% to 10%.
Future experimentation should scale up these experimental results for broader commercial application with the aim to control GM and BM on ‘Valencia’ oranges. Furthermore, similar research could reduce postharvest decay of other commercially important orange cultivars, such as Navels, mandarins, and lemons. The broader implications of this study are significant where the recommended treatment could potentially improve postharvest disease control in ‘Valencia’ oranges, reducing food wastage and increasing shipping tolerances, all achieved without the use of synthetic fungicides. Therefore, this approach offers an environmentally friendly and efficient way of preserving the quality and extending the shelf life of citrus fruit.
Author Contributions
Conceptualization, J.A., P.P., Q.V.V., L.P. and J.B.G.; methodology, J.A., P.P., Q.V.V., L.P. and J.B.G.; software, J.A., P.P. and Q.V.V.; validation, J.A., P.P., Q.V.V., L.P. and J.B.G.; formal analysis, J.A., P.P., Q.V.V., L.P. and J.B.G.; investigation, J.A., P.P., Q.V.V., L.P. and J.B.G.; resources, J.A. and J.B.G.; data curation, J.A., P.P., Q.V.V., L.P. and J.B.G.; writing—original draft preparation, J.A.; writing—review and editing, J.A., P.P., Q.V.V., L.P. and J.B.G.; visualization, J.A., P.P., Q.V.V., L.P. and J.B.G.; supervision, J.B.G.; project administration, J.B.G.; funding acquisition, J.B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by NSW Department of Primary Industries and the University of Newcastle. This project was co-funded by Horticulture Innovation Australia—‘Citrus Postharvest Program’ (CT19003). This is also a contribution of the Euphresco Project—‘Basic substances as an environmentally friendly alternative to synthetic pesticides for plant protection (BasicS)’.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Golding, J.; Archer, J. Advances in postharvest handling of citrus fruit. In Achieving Sustainable Cultivation of Tropical Fruits; Yahia, E.M., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 65–90. [Google Scholar]
- Palou, L. Chapter 2—Penicillium digitatum, Penicillium italicum (Green Mold, Blue Mold). In Postharvest Decay; Bautista-Baños, S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 45–102. [Google Scholar]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, plant- and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Gutiérrez Martínez, P.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Cerioni, L.; Rapisarda, V.; Dtor, J.; Fikkert, S.; Ruiz, T.; Fassel, R.; Smilanick, J. Use of phosphite salts in laboratory and semicommercial tests to control citrus postharvest decay. Plant Dis. 2013, 97, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Deliopoulos, T.; Kettlewell, P.; Hare, M. Fungal disease suppression by inorganic salts: A review. Crop Prot. 2010, 29, 1059–1075. [Google Scholar] [CrossRef]
- Palou, L.; Pérez-Gago, M.B. GRAS salts as alternative low-toxicity chemicals for postharvest preservation of fresh horticultural products. In Postharvest Pathology: Next Generation Solutions to Reducing Losses and Enhancing Safety. Plant Pathology in the 21st Century, Vol. 11; Spadaro, D., Droby, S., Gullino, M.L., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 163–179. ISBN 9783030565299. [Google Scholar]
- D’Aquino, S.; Palma, A. Reducing or replacing conventional postharvest fungicides with low toxicity acids and salts. In Postharvest Pathology of Fresh Horticultural Produce; Palou, L., Smilanick, J.L., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2020; pp. 595–632. ISBN 9781138630833. [Google Scholar]
- Chevalier, E.; Chaabani, A.; Assezat, G.; Prochazka, F.; Oulahal, N. Casein/wax blend extrusion for production of edible films as carriers of potassium sorbate—A comparative study of waxes and potassium sorbate effect. Food Packag. Shelf Life 2018, 16, 41–50. [Google Scholar] [CrossRef]
- Kitagawa, H.; Tani, T. Effect of potassium sorbate and thiabendazole mixture on the control of green and blue molds of citrus fruit. J. Jpn. Soc. Hortic. Sci. 1984, 52, 464–468. [Google Scholar] [CrossRef]
- Palou, L.; Montesinos-Herrero, C.; Pastor, C.; del Rio, M. Evaluation of heated potassium sorbate solutions to control postharvest green and blue molds on commercially important citrus cultivars. Phytopathology 2008, 98, 119. [Google Scholar]
- Parra, J.; Ripoll, G.; Orihuel-Iranzo, B. Potassium sorbate effects on citrus weight loss and decay control. Postharvest Biol. Technol. 2014, 96, 7–13. [Google Scholar] [CrossRef]
- Guimarães, J.E.R.; de la Fuente, B.; Pérez-Gago, M.B.; Andradas, C.; Carbó, R.; Mattiuz, B.-H.; Palou, L. Antifungal activity of GRAS salts against Lasiodiplodia theobromae in vitro and as ingredients of hydroxypropyl methylcellulose-lipid composite edible coatings to control Diplodia stem-end rot and maintain postharvest quality of citrus fruit. Int. J. Food Microbiol. 2019, 301, 9–18. [Google Scholar] [CrossRef]
- Youssef, K.; Ligorio, A.M.; Franco, N.; Ippolito, A. Activity of salts incorporated in wax in controlling postharvest diseases of citrus fruit. Postharvest Biol. Technol. 2012, 65, 39–43. [Google Scholar] [CrossRef]
- Palou, L.; Usall, J.; Muñoz, J.A.; Smilanick, J.L.; Viñas, I. Hot water, sodium carbonate, and sodium bicarbonate for the control of postharvest green and blue molds of clementine mandarins. Postharvest Biol. Technol. 2002, 24, 93–96. [Google Scholar] [CrossRef]
- Palou, L.; Smilanick, J.L.; Usall, J.; Viñas, I. Control of postharvest blue and green molds of oranges by hot water, sodium carbonate, and sodium bicarbonate. Plant Dis. 2001, 85, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Deng, L.; Zeng, K. Enhancement of biocontrol efficacy of Pichia membranaefaciens by hot water treatment in postharvest diseases of citrus fruit. Crop Prot. 2014, 63, 89–96. [Google Scholar] [CrossRef]
- Montesinos-Herrero, C.; Moscoso-Ramírez, P.A.; Palou, L. Evaluation of sodium benzoate and other food additives for the control of citrus postharvest green and blue molds. Postharvest Biol. Technol. 2016, 115, 72–80. [Google Scholar] [CrossRef]
- Romanazzi, G.; Orçonneau, Y.; Moumni, M.; Davillerd, Y.; Marchand, P.A. Basic substances, a sustainable tool to complement and eventually replace synthetic pesticides in the management of pre and postharvest diseases: Reviewed instructions for Users. Molecules 2022, 27, 3484. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Saberi, B.; Pristijono, P.; Stathopoulos, C.E.; Golding, J.B.; Scarlett, C.J.; Bowyer, M.; Vuong, Q.V. Use of response surface methodology (RSM) to optimize pea starch–chitosan novel edible film formulation. J. Food Sci. Technol. 2017, 54, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Montesinos-Herrero, C.; Smilanick, J.L.; Tebbets, J.S.; Walse, S.; Palou, L. Control of citrus postharvest decay by ammonia gas fumigation and its influence on the efficacy of the fungicide imazalil. Postharvest Biol. Technol. 2011, 59, 85–93. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Mansour, M.F.; Mlikota-Gabler, F.; Sorenson, D. Control of citrus postharvest green mold and sour rot by potassium sorbate combined with heat and fungicides. Postharvest Biol. Technol. 2008, 47, 226–238. [Google Scholar] [CrossRef]
- Montesinos-Herrero, C.; del Río, M.A.; Pastor, C.; Brunetti, O.; Palou, L. Evaluation of brief potassium sorbate dips to control postharvest Penicillium decay on major citrus species and cultivars. Postharvest Biol. Technol. 2009, 52, 117–125. [Google Scholar] [CrossRef]
- D’Aquino, S.; Fadda, A.; Barberis, A.; Palma, A.; Angioni, A.; Schirra, M. Combined effects of potassium sorbate, hot water and thiabendazole against green mould of citrus fruit and residue levels. Food Chem. 2013, 141, 858–864. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).