Optimizing Apricot Yield and Quality with Biostimulant Interventions: A Comprehensive Analysis
Abstract
1. Introduction
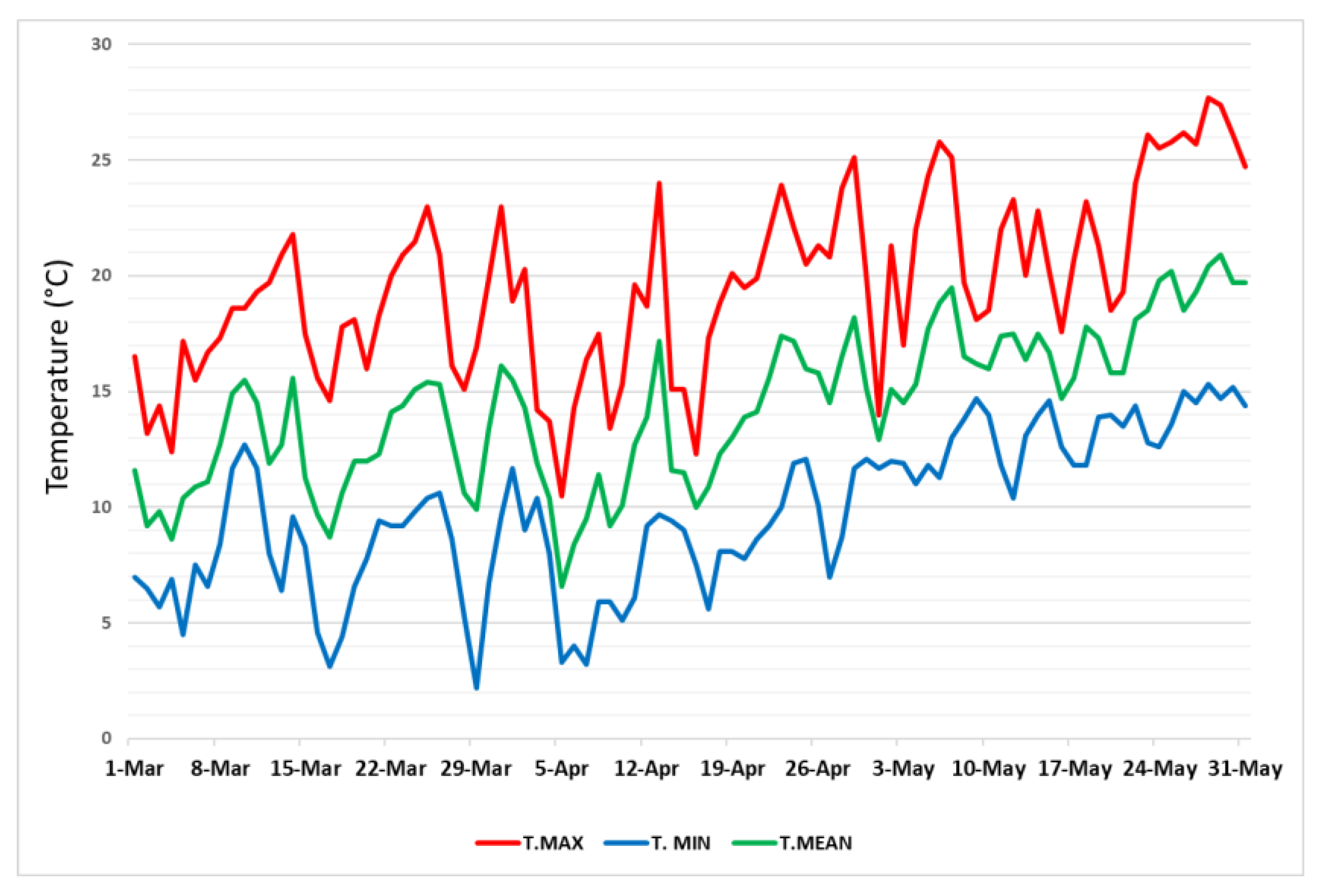
2. Materials and Methods
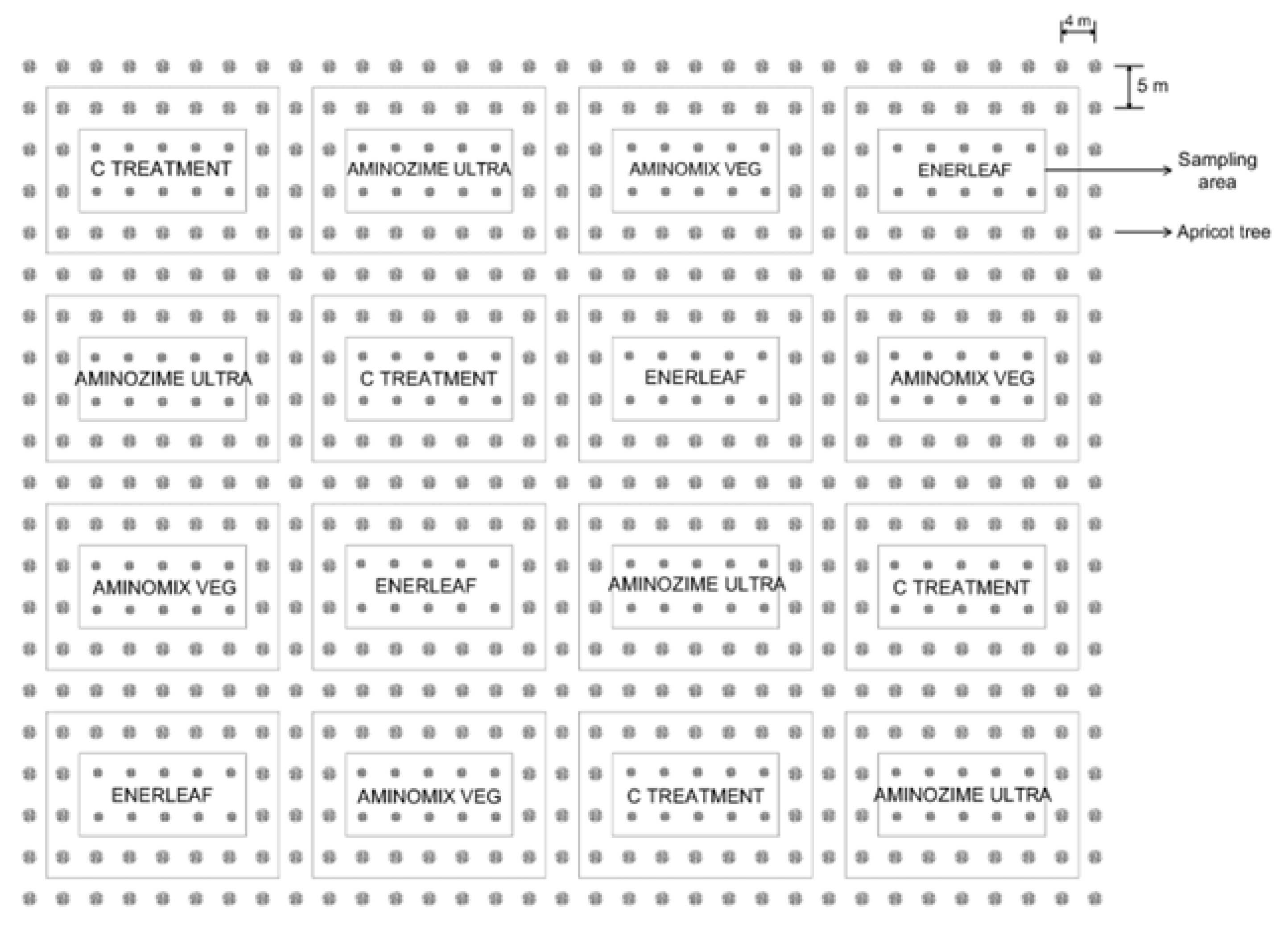
2.1. Site and Biostimulants
- (1)
- A biostimulant based on an extract derived from the seaweed Ascophyllum nodosum collected in the Atlantic Ocean (Nova Scotia, Canada), commercially known as Enerleaf. It was applied by foliar application at a dose of 400 mL/100 L of water.
- (2)
- A biostimulant based on an organic nitrogen fertilizer obtained through controlled enzymatic hydrolysis of selected plants with a high content of free amino acids, oligopeptides, enzymes, vitamins, elicitors, and substances with hormone-like functions, commercially known as Aminomix Vegetal. It was applied by foliar application at a dose of 400 mL/100 L of water.
- (3)
- A biostimulant based on a nitrogen-rich organic fertilizer and fluid suspension of meat by-products, commercially known as Aminozime Ultra. It was applied by foliar application at a dose of 200 mL/100 L of water.
- (4)
- The three selected commercial products were compared with a control group, where only water was administered.
2.2. Biometric Analysis and Physico-Chemical Analysis of Fruits
2.3. Polyphenol Extraction
2.4. Chemical Characterization of Polyphenols through UHPLC Q-Exactive Analysis
2.5. Total Phenolic Content Analysis
2.6. Antioxidant Activity
2.7. Statistical Analysis
3. Results and Discussion
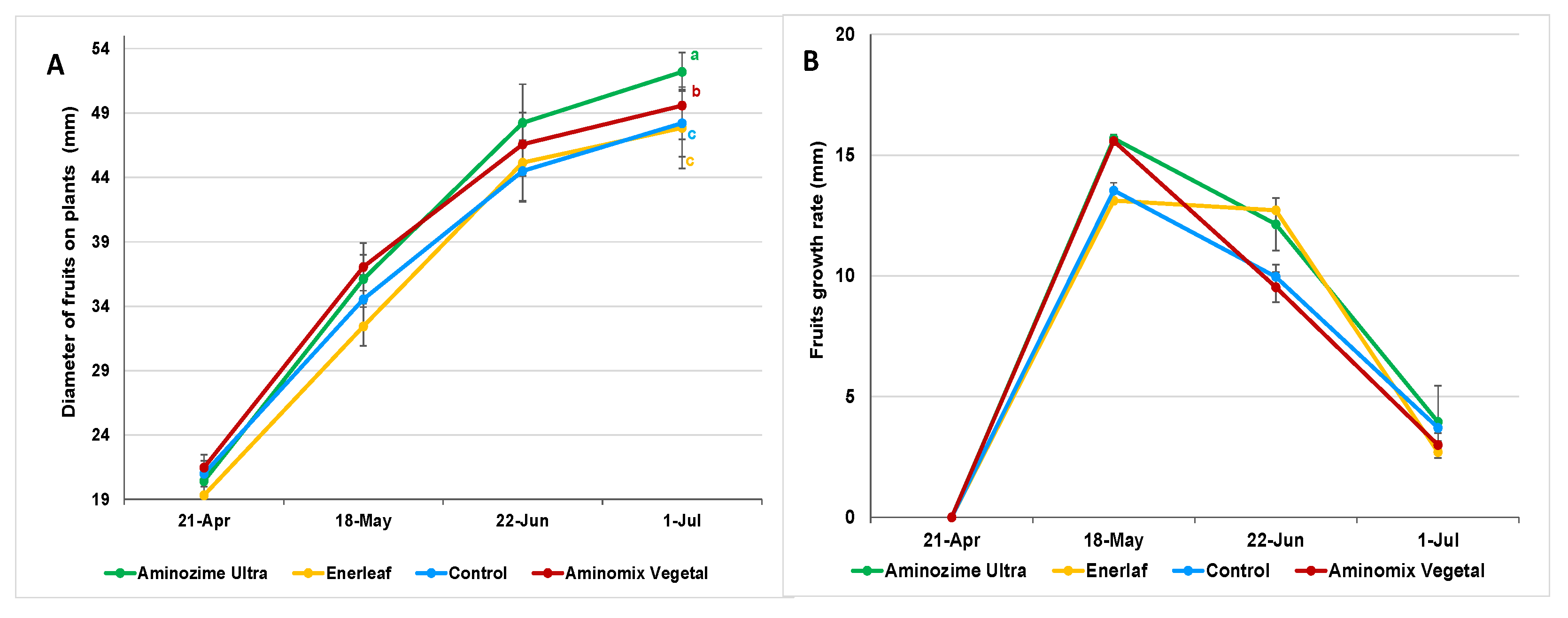
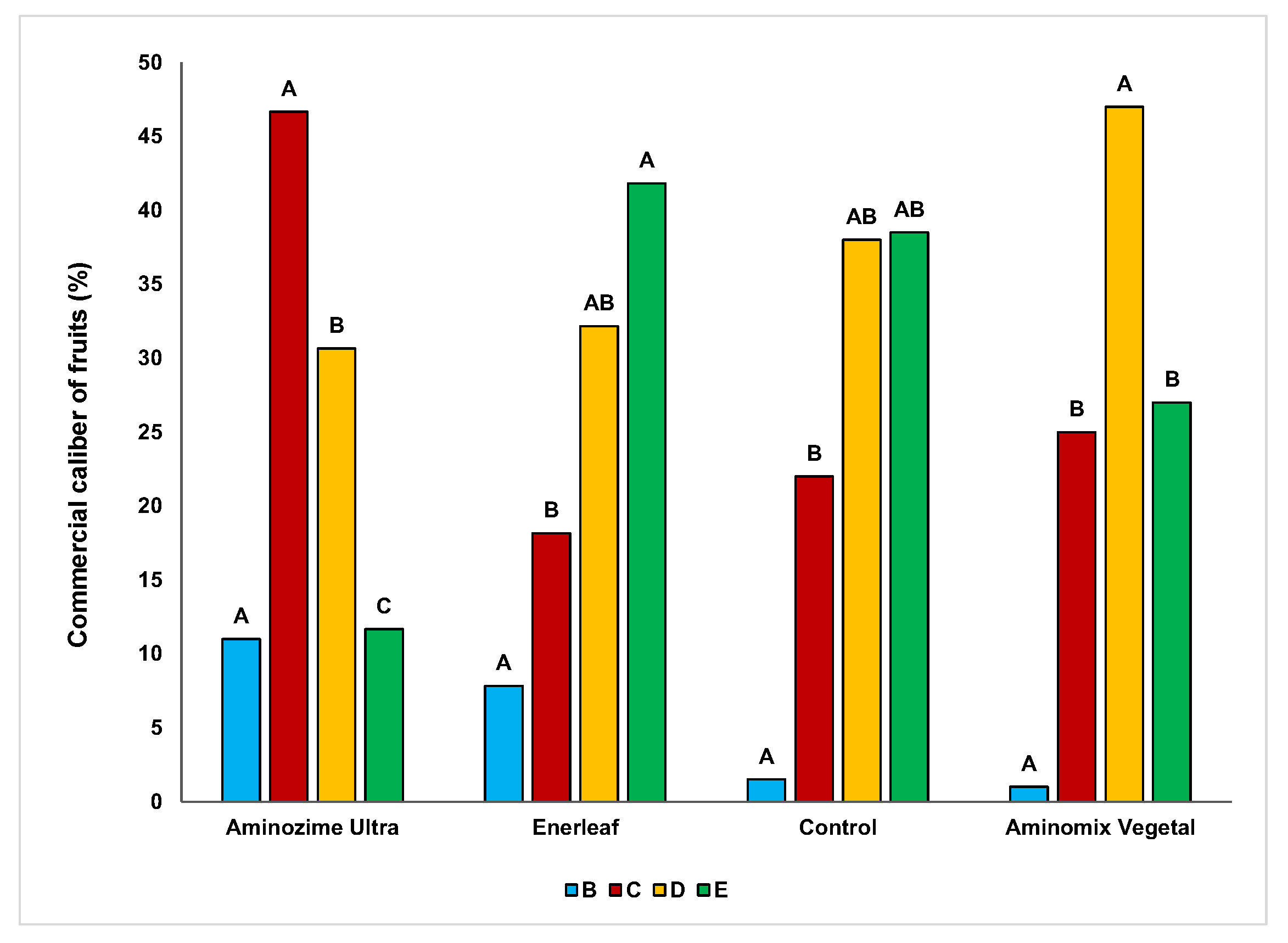
3.1. Effect of Biostimulants on Biometric and Physico-Chemical Traits of Fruits
3.2. Effects of Biostimulants on Color Fruits and Nutraceutical Parameters
3.3. Antioxidant Activity and Identification and Quantification of Apricot Bioactive Compounds
3.4. Heatmap and Cluster Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarantino, A.; Lops, F.; Disciglio, G.; Lopriore, G. Effects of Plant Biostimulants on Fruit Set, Growth, Yield and Fruit Quality Attributes of ‘Orange Rubis®’ Apricot (Prunus armeniaca L.) Cultivar in Two Consecutive Years. Sci. Hortic. 2018, 239, 26–34. [Google Scholar] [CrossRef]
- Kunicki, E.; Grabowska, A.; Sękara, A.; Wojciechowska, R. The Effect of Cultivar Type, Time of Cultivation, and Biostimulant Treatment on the Yield of Spinach ( Spinacia oleracea L.). Folia Hortic. 2010, 22, 9–13. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and Crop Responses: A Review. Biol. Agric. Hortic. 2015, 31, 964649. [Google Scholar] [CrossRef]
- Di-Vaio, C.; Cirillo, A.; Cice, D.; El-Nakhel, C.; Rouphael, Y. Biostimulant Application Improves Yield Parameters and Accentuates Fruit Color of Annurca Apples. Agronomy 2021, 11, 715. [Google Scholar] [CrossRef]
- Graziani, G.; Cirillo, A.; Giannini, P.; Conti, S.; El-Nakhel, C.; Rouphael, Y.; Ritieni, A.; Di Vaio, C. Biostimulants Improve Plant Growth and Bioactive Compounds of Young Olive Trees under Abiotic Stress Conditions. Agriculture 2022, 12, 227. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptistella, J.L.C.; Horz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic Plant Biostimulants and Fruit Quality—A Review. Agronomy 2020, 10, 988. [Google Scholar] [CrossRef]
- Graziani, G.; Ritieni, A.; Cirillo, A.; Cice, D.; Di Vaio, C. Effects of Biostimulants on Annurca Fruit Quality and Potential Nutraceutical Compounds at Harvest and during Storage. Plants 2020, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Egea, J. Phenotypic Diversity and Relationships of Fruit Quality Traits in Apricot (Prunus armeniaca L.) Germplasm. Euphytica 2008, 163, 143–158. [Google Scholar] [CrossRef]
- Velíšek, J.; Cejpek, K. Biosynthesis of Food Constituents: Vitamins. 1. Fat-Soluble Vitamins—A Review. Czech J. Food Sci. 2007, 25, 1–16. [Google Scholar] [CrossRef]
- Martín, C.; Herrero, M.; Hormaza, J.I. Molecular Characterization of Apricot Germplasm from an Old Stone Collection. PLoS ONE 2011, 6, e23979. [Google Scholar] [CrossRef]
- Cirillo, A.; De Luca, L.; Izzo, L.; Cepparulo, M.; Graziani, G.; Ritieni, A.; Romano, R.; Di Vaio, C. Biochemical and Nutraceutical Characterization of Different Accessions of the Apricot (Prunus armeniaca L.). Horticulturae 2023, 9, 546. [Google Scholar] [CrossRef]
- Garcia-Viguera, C.; Bridle, P.; Ferreres, F.; Tomas-Barberan, F.A. Influence of Variety, Maturity and Processing on Phenolic Compounds of Apricot Juices and Jams. Z. Für Leb. Und Forsch. A 1994, 199, 433–436. [Google Scholar] [CrossRef]
- La Vecchia, C.; Altieri, A.; Tavani, A. Vegetables, Fruit, Antioxidants and Cancer: A Review of Italian Studies. Eur. J. Nutr. 2001, 40, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, A.; Lops, F.; Disciglio, G.; Tarantino, E. Effect of Plant Biostimulants on Fruit Set, Yield, and Quality Attributes of “Farbaly” Apricot Cultivar. World Acad. Sci. Eng. Technol. Int. J. Agric. Biosyst. Eng. 2017, 11, 5. [Google Scholar]
- Eissa, F.; Fathi, M.; El-Shall, S. Response of Peach and Apricot Seedlings to Humic Acid Treatments under Salinity Condition. J. Plant Prod. 2007, 32, 3605–3620. [Google Scholar] [CrossRef]
- Hsiang, T.-F.; Lin, Y.-J.; Yamane, H.; Tao, R. Characterization of Japanese Apricot (Prunus mume) Floral Bud Development Using a Modified BBCH Scale and Analysis of the Relationship between BBCH Stages and Floral Primordium Development and the Dormancy Phase Transition. Horticulturae 2021, 7, 142. [Google Scholar] [CrossRef]
- Izzo, L.; Rodríguez-Carrasco, Y.; Pacifico, S.; Castaldo, L.; Narváez, A.; Ritieni, A. Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of a Red Cabbage Extract Chemically Profiled through UHPLC-Q-Orbitrap HRMS. Antioxidants 2020, 9, 955. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Toriello, M.; Sessa, R.; Izzo, L.; Lombardi, S.; Narváez, A.; Ritieni, A.; Grosso, M. Antioxidant and Anti-Inflammatory Activity of Coffee Brew Evaluated after Simulated Gastrointestinal Digestion. Nutrients 2021, 13, 4368. [Google Scholar] [CrossRef]
- Hsu, J. Multiple Comparisons: Theory and Methods; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2015; Volume 130, pp. 141–174. ISBN 978-0-12-802137-8. [Google Scholar]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Effect of Ascophyllum Extract Application on Plant Growth, Fruit Yield and Soil Microbial Communities of Strawberry. Can. J. Plant Sci. 2013, 93, 23–36. [Google Scholar] [CrossRef]
- Gentile, C.; Di Gregorio, E.; Di Stefano, V.; Mannino, G.; Perrone, A.; Avellone, G.; Sortino, G.; Inglese, P.; Farina, V. Food Quality and Nutraceutical Value of Nine Cultivars of Mango (Mangifera indica L.) Fruits Grown in Mediterranean Subtropical Environment. Food Chem. 2019, 277, 471–479. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar Applications of Biostimulants Promote Growth, Yield and Fruit Quality of Strawberry Plants Grown under Nutrient Limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Sas-Paszt, L.; Głuszek, S.; Górnik, K.; Anjum, M.A.; Saleh, A.A.; Abada, H.S.; Awad, R.M. Effect of Some Biostimulants on the Vegetative Growth, Yield, Fruit Quality Attributes and Nutritional Status of Apple. Horticulturae 2022, 9, 32. [Google Scholar] [CrossRef]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First Fruit in Season: Seaweed Extract and Silicon Advance Organic Strawberry (Fragaria×ananassa Duch.) Fruit Formation and Yield. Sci. Hortic. 2018, 242, 103–109. [Google Scholar] [CrossRef]
- Chouliaras, V.; Tasioula, M.; Chatzissavvidis, C.; Therios, I.; Tsabolatidou, E. The Effects of a Seaweed Extract in Addition to Nitrogen and Boron Fertilization on Productivity, Fruit Maturation, Leaf Nutritional Status and Oil Quality of the Olive (Olea europaea L.) Cultivar Koroneiki: Effect of Seaweed Extract on Olive Tree Nutritional Status and Productivity. J. Sci. Food Agric. 2009, 89, 984–988. [Google Scholar] [CrossRef]
- Fornes, F.; Almela, V.; Abad, M.; Agustí, M. Low Concentrations of Chitosan Coating Reduce Water Spot Incidence and Delay Peel Pigmentation of Clementine Mandarin Fruit. J. Sci. Food Agric. 2005, 85, 1105–1112. [Google Scholar] [CrossRef]
- Stern, R.A.; Flaishman, M.; Applebaum, S.; Ben-Arie, R. Effect of Synthetic Auxins on Fruit Development of ‘Bing’ Cherry (Prunus avium L.). Sci. Hortic. 2007, 114, 275–280. [Google Scholar] [CrossRef]
- Ayour, J.; Sagar, M.; Alfeddy, M.N.; Taourirte, M.; Benichou, M. Evolution of Pigments and Their Relationship with Skin Color Based on Ripening in Fruits of Different Moroccan Genotypes of Apricots (Prunus armeniaca L.). Sci. Hortic. 2016, 207, 168–175. [Google Scholar] [CrossRef]
- Carbone, K.; Ciccoritti, R.; Paliotta, M.; Rosato, T.; Terlizzi, M.; Cipriani, G. Chemometric Classification of Early-Ripening Apricot ( Prunus armeniaca L.) Germplasm Based on Quality Traits, Biochemical Profiling and in Vitro Biological Activity. Sci. Hortic. 2018, 227, 187–195. [Google Scholar] [CrossRef]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Sharma, R.R.; Kaushik, R.; Verma, M.K.; Tomar, M.; Kumar, M.; Mekhemar, M. Nutritional and Phytochemical Traits of Apricots (Prunus armeniaca L.) for Application in Nutraceutical and Health Industry. Foods 2021, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Xia, H.; Lin, L.; Wang, J.; Yuan, L.; Li, K.; Zhang, J.; Lv, X.; Liang, D. SUNRED, a Natural Extract-Based Biostimulant, Application Stimulates Anthocyanin Production in the Skins of Grapes. Sci. Rep. 2019, 9, 2590. [Google Scholar] [CrossRef]
- Jaafar, H.J. Effects of Apricot and Apricot Kernels on Human Health and Nutrition: A Review of Recent Human Research. Tech. Biochem. 2021, 2, 139–162. [Google Scholar] [CrossRef]
- Lo Bianco, R.; Farina, V.; Indelicato, S.G.; Filizzola, F.; Agozzino, P. Fruit Physical, Chemical and Aromatic Attributes of Early, Intermediate and Late Apricot Cultivars: Fruit Quality and Ripening Season in Apricot. J. Sci. Food Agric. 2010, 90, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Drogoudi, P.D.; Vemmos, S.; Pantelidis, G.; Petri, E.; Tzoutzoukou, C.; Karayiannis, I. Physical Characters and Antioxidant, Sugar, and Mineral Nutrient Contents in Fruit from 29 Apricot ( Prunus armeniaca L.) Cultivars and Hybrids. J. Agric. Food Chem. 2008, 56, 10754–10760. [Google Scholar] [CrossRef] [PubMed]
- Akhone, M.A.; Bains, A.; Tosif, M.M.; Chawla, P.; Fogarasi, M.; Fogarasi, S. Apricot Kernel: Bioactivity, Characterization, Applications, and Health Attributes. Foods 2022, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, B.E.; Ruiz, D.; Salazar, J.A.; Rubio, M.; Martínez-García, P.J.; Martínez-Gómez, P. Analysis of Metabolites and Gene Expression Changes Relative to Apricot (Prunus armeniaca L.) Fruit Quality During Development and Ripening. Front. Plant Sci. 2020, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The Application of a Plant Biostimulant Based on Seaweed and Yeast Extract Improved Tomato Fruit Development and Quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Basile, B.; Rao, R.; Corrado, G. Genotypic Diversity and Population Structure of the Apricot Landraces of the Campania Region (Southern Italy) Based on Fluorescent SSRs. Genet. Resour. Crop Evol. 2023, 70, 125–134. [Google Scholar] [CrossRef]
- Parrado, J.; Escudero-Gilete, M.L.; Friaza, V.; García-Martínez, A.; González-Miret, M.L.; Bautista, J.D.; Heredia, F.J. Enzymatic Vegetable Extract with Bio- Active Components: Influence of Fertiliser on the Colour and Anthocyanins of Red Grapes. J. Sci. Food Agric. 2007, 87, 2310–2318. [Google Scholar] [CrossRef]
- Cirillo, A.; De Luca, L.; Graziani, G.; Cepparulo, M.; El-Nakhel, C.; Giordano, M.; Rouphael, Y.; Ritieni, A.; Romano, R.; Di Vaio, C. Biostimulants Application on Olea europaea L. in Mediterranean Conditions Increase the Production and Bioactive Compounds of Drupes and Oil. Agriculture 2022, 12, 2173. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]

| Treatments | Production Plant (kg) | Yield Efficiency (kg/cm2) | First Harvast (kg/pt) |
|---|---|---|---|
| Control | 31.65 ± 3.17 b | 0.94 ± 0.10 b | 8.59 ± 1.62 |
| Enerleaf | 34.41 ± 3.17 b | 1.07 ± 0.10 b | 11.20 ± 1.62 |
| Aminomix Vegetal | 36.67 ± 3.17 b | 1.21 ± 0.10 b | 14.62 ± 1.62 |
| Aminozime Ultra | 48.68 ± 3.17 a | 1.47 ± 0.10 a | 10.15 ± 1.62 |
| Significance | * | * | ns |
| Treatments | Fruit Weight (g) | Polar Diameter (mm) | Equatorial Diameter (mm) | Transversal Diameter (mm) | Firmness (kg × 0.5 cm2) | |||
| Control | 61.00 ± 1.20 c | 48.92 ± 0.35 c | 46.03 ± 0.40 c | 50.50 ± 0.40 c | 2.33 ± 0.12 | |||
| Enerleaf | 59.20 ± 1.20 c | 49.17 ± 0.35 c | 45.88 ± 0.40 c | 50.33 ± 0.40 c | 2.37 ± 0.12 | |||
| Aminomix Vegetal | 65.62 ± 1.20 b | 50.55 ± 0.35 b | 48.07 ± 0.40 b | 51.93 ± 0.40 b | 2.09 ± 0.12 | |||
| Aminozime Ultra | 78.62 ± 1.20 a | 53.52 ± 0.35 a | 51.51 ± 0.40 a | 55.54 ± 0.40 a | 2.11 ± 0.12 | |||
| Significance | *** | *** | *** | *** | ns | |||
| Treatments | TSS | TA | TSS/TA | pH | ||||
| (°brix) | (g/L citric acid) | |||||||
| Control | 12.67 ± 0.31 | 18.44 ± 0.72 a | 0.72 ± 0.34 | 3.89 ± 0.09 | ||||
| Enerleaf | 12.44 ± 0.31 | 15.78 ± 0.72 b | 0.78 ± 0.34 | 3.89 ± 0.09 | ||||
| Aminomix Vegetal | 12.89 ± 0.31 | 17.33 ± 0.72 ab | 0.75 ± 0.34 | 4.00 ± 0.09 | ||||
| Aminozime Ultra | 13.22 ± 0.31 | 15.89 ± 0.72 b | 0.82 ± 0.34 | 3.89 ± 0.09 | ||||
| Significance | ns | * | ns | ns | ||||
| Treatments | *L | *a | *b | Chroma |
|---|---|---|---|---|
| Control | 46.23 ± 0.62 b | 54.71 ± 1.87 a | 29.87 ± 4.88 | 65.34 ± 47.89 |
| Enerleaf | 47.63 ± 0.62 ab | 51.03 ± 1.87 ab | 27.65 ± 4.88 | 157.87 ± 47.89 |
| Aminomix Vegetal | 48.85 ± 0.62 a | 46.78 ± 1.87 b | 33.25 ± 4.88 | 59.13 ± 47.89 |
| Aminozime Ultra | 48.87 ± 0.62 a | 51.18 ± 1.87 ab | 33.51 ± 4.88 | 63.11 ± 47.89 |
| Significance | ** | * | ns | ns |
| Polyphneols (µg/g) | Control | Aminozime Ultra | Aminomix Vegetal | Enerleaf | Significance |
|---|---|---|---|---|---|
| Quinic acid | 346.42 ± 20.77 b | 415.74 ± 20.77 a | 381.08 ± 20.77 b | 389.75 ± 20.77 a | *** |
| Protocatechuic acid | 1.51 ± 0.15 b | 2.53 ± 0.15 a | 2.01 ± 0.15 b | 2.66 ± 0.15 a | * |
| Caffeic acid | 16.60 ± 0.86 | 18.34 ± 0.86 | 16.72 ± 0.86 | 19.15 ± 0.86 | ns |
| Epicatechin | 30.34 ± 1.54 | 26.06 ± 1.54 | 24.68 ± 1.54 | 27.24 ± 1.54 | ns |
| Chlorogenic acid | 641.14 ± 29.97 | 663.93 ± 29.97 | 539.74 ± 29.97 | 649.41 ± 29.97 | ns |
| Catechin | 76.84 ± 4.23 | 82.42 ± 4.23 | 85.65 ± 4.23 | 102.55 ± 4.23 | ns |
| p-coumaric acid | 10.10 ± 0.67 b | 12.39 ± 0.67 ab | 13.92 ± 0.67 ab | 16.45 ± 0.67 a | * |
| acid Syringic acid | 24.38 ± 3.18 c | 65.53 ± 3.18 b | 68.63 ± 3.18 b | 104.92 ± 3.18 a | *** |
| Ferulic acid | 58.64 ± 2.52 b | 56.61 ± 2.52 b | 62.79 ± 2.52 b | 79.93 ± 2.52 a | * |
| Naringin | 0.99 ± 0.05 | 0.90 ± 0.05 | 0.94 ± 0.05 | 1.23 ± 0.05 | ns |
| Rutin hydrate | 508.06 ± 28.69 | 544.14 ± 28.69 | 383.58 ± 28.69 | 557.87 ± 28.69 | ns |
| Quercetin 3β-glucoside | 4.82 ± 0.33 | 4.58 ± 0.33 | 3.21 ± 0.33 | 4.52 ± 0.33 | ns |
| Kaempferolo 3-O-glucoside | 0.56 ± 0.04 | 0.67 ± 0.04 | 0.41 ± 0.04 | 0.64 ± 0.04 | ns |
| isorhamnetin-3-rutinoside | 1.89 ± 0.08 | 1.57 ± 0.08 | 1.59 ± 0.08 | 2.00 ± 0.08 | ns |
| Luteolin 7-glucoside | 0.85 ± 0.06 | 0.91 ± 0.06 | 0.56 ± 0.06 | 0.84 ± 0.06 | ns |
| Myricitrin | 4.64 ± 0.31 | 4.09 ± 0.31 | 3.19 ± 0.31 | 4.52 ± 0.31 | ns |
| Sum of Total (poly) phenols | 1727.75 ± 60.30 b | 1900.40 ± 60.30 a | 1588.7 ± 60.30 c | 1963.67 ± 60.30 a | * |
| Treatments | DPPH | ABTS | FRAP | FOLIN |
|---|---|---|---|---|
| mmol trolox/kg | mg/g | |||
| Control | 9.47 ± 1.06 | 19.15 ± 1.63 | 15.73 ± 2.25 | 5.32 ± 0.64 |
| Aminozime Ultra | 9.95 ± 1.06 | 18.72 ± 1.63 | 16.57 ± 2.25 | 5.64 ± 0.64 |
| Enerleaf | 9.55 ± 1.06 | 17.72 ± 1.63 | 15.33 ± 2.25 | 5.48 ± 0.64 |
| Aminomix Vegetal | 8.15 ± 1.06 | 14.73 ± 1.63 | 11.50 ± 2.25 | 4.84 ± 0.64 |
| Significance | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, A.; Izzo, L.; Ciervo, A.; Ledenko, I.; Cepparulo, M.; Piscitelli, A.; Di Vaio, C. Optimizing Apricot Yield and Quality with Biostimulant Interventions: A Comprehensive Analysis. Horticulturae 2024, 10, 447. https://doi.org/10.3390/horticulturae10050447
Cirillo A, Izzo L, Ciervo A, Ledenko I, Cepparulo M, Piscitelli A, Di Vaio C. Optimizing Apricot Yield and Quality with Biostimulant Interventions: A Comprehensive Analysis. Horticulturae. 2024; 10(5):447. https://doi.org/10.3390/horticulturae10050447
Chicago/Turabian StyleCirillo, Aurora, Luana Izzo, Andrea Ciervo, Ivana Ledenko, Marco Cepparulo, Alfonso Piscitelli, and Claudio Di Vaio. 2024. "Optimizing Apricot Yield and Quality with Biostimulant Interventions: A Comprehensive Analysis" Horticulturae 10, no. 5: 447. https://doi.org/10.3390/horticulturae10050447
APA StyleCirillo, A., Izzo, L., Ciervo, A., Ledenko, I., Cepparulo, M., Piscitelli, A., & Di Vaio, C. (2024). Optimizing Apricot Yield and Quality with Biostimulant Interventions: A Comprehensive Analysis. Horticulturae, 10(5), 447. https://doi.org/10.3390/horticulturae10050447








