Abstract
Allium vegetables attract attention for their flavor and aroma in Asia, especially in China and Japan. The aim of this experiment was to uncover the differences in the unique flavor compounds of two Welsh onions that are typical cultivars in China and Japan (‘Zhangqiu’ and ‘Tenko’). Chemical methods and solid-phase microextraction–gas chromatography-mass spectrometry were performed to determine the nutritional quality and quantity of volatile compounds of various organs of Welsh onions. The results show that a total of 30, 37, and 28 compounds were detected in the roots, pseudostem, and leaves of ‘Zhangqiu’, respectively, while 21, 27, and 20 compounds were detected in the corresponding organs of ‘Tenko’. The distribution of sulfur compounds in the roots, pseudostem, and leaves of ‘Zhangqiu’ accounted for 72%, 83%, and 26% of the total content, while those of ‘Tenko’ accounted for 55%, 84%, and 57%, respectively. Aldehydes are the second largest class of volatiles in Welsh onions. The distribution of aldehydes in the leaves was notably different: 52% and 27% in ‘Zhangqiu’ and ‘Tenko’, respectively. The contribution of S to the volatile substances was outstanding, and through forward selection, it was found that P, Ca, and Mg contribute to the volatile substances of Welsh onions. The above results indicate that the different genotypes of Welsh onions have various flavors, and mineral elements contribute variously to these flavors. Calcium could be a new topic of interest for our subsequent research on elements and volatiles.
1. Introduction
Plants can synthesize hundreds of thousands of metabolites with different taste and odor characteristics. The primary metabolites include sugars, acids, salts, bitterness-related metabolites, and volatiles [1,2]. Among them, volatile aromatic compounds receive increased attention because they are essential to unique flavors [1]. The nature and origin of volatile compounds have been studied in Allium species. A series of volatile sulfides produce the typical flavor characteristics of alliums, primarily including S-1-propenyl cysteine sulfoxide (PECSO), S-propyl cysteine sulfoxide (PCSO), and S-methyl cysteine sulfoxide (MCSO) [3]. Intact allium cells have no odor, but when the cells are disrupted, 5-alk (en) yl cysteine sulfoxides are hydrolyzed to produce the many volatile sulfur compounds related to flavor and odor. In recent years, various studies have used different analytical techniques to describe the differences in flavor quality among alliums [4,5].
Flavor and odor, the core concerns of consumers, are restricted by the genetic factors of vegetable crops and environmental conditions and cultivation techniques [6]. Therefore, in detecting and improving the flavor quality of vegetables, the effects of growth and cultivation conditions on their chemical composition must be considered [7]. Previous studies have shown that different genotypes, developmental stages, and growth conditions result in distinct capsaicin content in pepper fruits [8], and mushrooms have various flavor substances based on their quality [9]. Sulfur is essential for the synthesis of sulfides in allium plants. The supply of sulfur distinctively affects the flavor and odor of onions. Sulfur metabolism is closely related to nitrogen metabolism through its production of the amino acid cysteine, which is the first organosulfur compound in sulfur assimilation [10]. Therefore, the nitrogen supply may affect sulfur absorption and CSO formation [10]. Plants of different genotypes also exhibit dissimilar elemental absorption. Plant species vary under the same environmental conditions, which may be one reason for the difference in their flavors.
Due to its flavor and aroma, Welsh onion (Allium fistulosum L.) is a popular vegetable and spice in Asia, especially in China and Japan [11]. There are many varieties of Welsh onion in China, with the ‘Zhangqiu’ Welsh onion being the most popular vegetable eaten fresh. Meanwhile, in Japan, Welsh onions have been cultivated for a long time and are characterized by their shorter and more slender pseudostems. The leaves and pseudostems of Japanese Welsh onions are used in cooking, and the ‘Tenko’, introduced to China from Japan in 2003, is an excellent example of this. ‘Tenko’ is known for its early maturity and robust disease resistance. The two cultivars have different flavor characteristics, thanks to which ‘Zhangqiu’ is mainly eaten fresh, while ‘Tenko’ is more suitable for cooking. However, few studies have focused on the difference in the flavor qualities between Chinese and Japanese Welsh onions. Given this, the flavor characteristics of the Chinese Welsh onion (‘Zhangqiu’) and Japanese Welsh onion (‘Tenko’) were analyzed using solid-phase microextraction (SPME) combined with GC-MS. The analyses aimed to determine the difference in volatile components between the cultivars, analyze the elements and flavors of the Welsh onions, and provide a reference for improving Welsh onion cultivars.
2. Materials and Methods
2.1. Plant Material and Cultivation
This research was conducted in 2022 at Shandong Agricultural University’s horticultural pilot station. All experiments used the two cultivars of Welsh onion, ‘Zhangqiu’ and ‘Tenko’. Seeds of the two cultivars were obtained from Zhangqiu, China and Musashino Seed Co., Ltd., Tokyo, Japan. The seeds were rinsed with sterilized distilled water, sown in plugs containing substrate composed of a sandy loam soil–peat mixture (1:1, v:v), and grown in a greenhouse with standard irrigation and fertilization. Full-strength Hoagland nutrient solution, which contained 0.5 mM NH4H2PO4, 2.0 mM Ca(NO3)2·4H2O, 3.2 mM KNO3, and 1.0 mM MgSO4·7H2O, was used. The nutrient solutions’ electrical conductivity (EC) and pH were maintained at 2.2–2.5 ms/cm and 6.8–7.0, respectively. The nutrient solutions were aerated every 2 h by air pumps. The original volumes were supplemented daily, and the solution was refreshed every 2–3 days. Finally, the plastic containers were transferred to a growth room at 24 °C under a 12 h photoperiod.
Each cultivar as a treatment was replicated three times and arranged into a completely randomized block design. Each replicate contained 20 plants. Representative seedlings with four true leaves were used for all experiments, which were transferred and cultured hydroponically in darkened plastic containers (area 0.036 m2, twenty plants per container). After the seedlings showed five true leaves, the measurements were performed.
2.2. Determination of Nutritional Quality
Three individual plants of each cultivar were randomly selected. Before the measurement, the fresh pseudostems and leaves were separated, washed with distilled water, chopped, and ground to determine the nutritional quality immediately. The same fresh weight was used to determine all nutritional qualities.
After the sample was extracted by boiling water, an anthrone colorimetric method was used to determine the soluble sugar content [12]. The crude fiber was collected by heating under acidic conditions and determined by an anthrone colorimetric method [12]. The soluble protein was measured using Coomassie brilliant blue [12]. The free amino acid in the sample was extracted with 10% acetic acid, and the concentration was determined by colorimetry after adding ninhydrin and heating in boiling water for 15 min [12]. After removing the sample protein with trichloroacetic acid, the pyruvic acid content was determined by 2,4-dinitrophenylhydrazine colorimetry [13].
2.3. Determination of Volatile Compounds
The samples, various organs of fresh individual Welsh onions, were used to determine the volatile compounds immediately.
Headspace solid-phase microextraction (HS-SPME) was performed to determine the volatile production of Welsh onion, as described previously [14], with minor modifications. Briefly, 2 g was accurately, quickly weighed, placed into a 40 mL headspace bottle, sealed, and put in a 45 °C water bath. The volatile materials were then captured using SPME fibers (DVB/CAR/PDMS, 50/30 μm, Millipore-Sigma, Burlington, MA, USA). The volatile compounds of samples were extracted at 45 °C for 30 min. The fibers were then removed from the vial and inserted into the injection port of a gas chromatography-mass spectrometer (GC-MS QP-2010, Shimadzu, Japan) to identify the volatile compounds.
We used helium as a carrier gas and separated it on a Rtx-5 ms capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness; maximum temperature, 350 °C) at a constant flow rate of 0.89 mL/min. The sample was desorbed with a 230 °C GC inlet for 3 min. The temperature setting procedure was as follows: the initial column temperature was 40 °C for 2 min, then ramped up to 70 °C at 4 °C/min for 1 min, then ramped up to 230 °C at 10 °C/min for 5 min. The mass spectrometer’s operating parameters were as follows: ion source temperature of 200 °C, full-spectrum scanning, and mass scanning range of 45–500 m/z. Volatile materials were identified by GC-MS analysis and compared to mass spectrometry data contained in the National Institute of Standards and Technology (2017). Available RetIndex values were assayed to describe the volatile components. The relative amounts given herein are based on the percentage of peak areas, expressed as a percentage of the total peak area of all identified compounds.
2.4. Determination of Mineral Elements
Samples of three organs of five individual plants of two cultivars were randomly selected, and the green parts (105 °C for 20 min) were removed and dried at 75 °C to a constant weight. The dried sample was ground and sieved. These powders were used to measure mineral content.
The dried sample (0.1 g) was weighed into a digestive tract and diluted with H2SO4-H2O2 until clarified. The total nitrogen (N) and total phosphorus (P) contents were measured using the Kjeldahl method [15] and the molybdenum blue colorimetry method [16], respectively.
A 0.1 g sample was digested with 8 mL of HNO3 in a pickled digestion tube using a CEM high-throughput closed microwave digestion system (CEM MARS6, CEM Corporation, Matthews, NC, USA). After digestion, the solution was diluted to 50 mL with deionized water. Potassium (K), sodium (Na), calcium (Ca), magnesium (Mg), sulfur (S), and iron (Fe) in the samples were determined by inductively coupled plasma mass spectrometry (ICP; iCAP 7000; Thermo Fisher Scientific, Waltham, MA, USA).
2.5. Statistical Analysis
Experimental data were collected randomly from three organs of two cultivars using three independent replicates. The data are expressed as three independent experiments’ mean ± standard deviation. Principal component analysis (PCA) and redundancy analysis (RDA) were performed using the R language. PCA was performed to determine the differences in volatile compounds from the samples. RDA was used to analyze the effects of different mineral elements on the volatile substances in various organs of the Welsh onions. All statistical analyses were performed using the DPS software [17] (Data Processing System, v9.01). Calculations were performed using one-way ANOVA and Duncan’s multiple range test. p < 0.05 was considered to be statistically significant.
3. Results
3.1. Nutritional Quality of Edible Parts of Two Welsh Onion Cultivars
Table 1 shows the quality changes in the edible parts of the two Welsh onion cultivars. The pseudostem soluble sugar content of ‘Zhangqiu’ was significantly higher than that of ‘Tenko’, being 85.6%. The difference between soluble proteins was not significant. The contents of free amino acids and crude fiber in the pseudostem and leaves of ‘Tenko’ were significantly higher than those in ‘Zhangqiu’. The soluble protein content in the leaves of ‘Tenko’ was higher. Generally, the pseudostem’s quality was superior to that of the leaves in the two Welsh onion cultivars. Compared with ‘Tenko’, the pyruvic acid content of the pseudostems of ‘Zhangqiu’ was higher by 0.13-fold. In leaves, the pyruvic acid content of ‘Tenko’ was higher by 0.49-fold when compared to ‘Zhangqiu’.

Table 1.
Nutritional qualities of ‘Zhangqiu’ and ‘Tenko’ edible parts.
3.2. Volatile Compounds
Using GC-MS, we detected 57 volatile substances in the two cultivars’ various organs (Table S1). Accurately, 30, 37, and 18 volatile substances were detected in the roots, pseudostem, and leaves of ‘Zhangqiu’, respectively, while 21, 27, and 20 substances were detected in corresponding organs of ‘Tenko’ (Figure 1 and Figure S1). The volatile components of the roots and pseudostem of ‘Zhangqiu’ were significantly higher than those of ‘Tenko’. The Welsh onion contained the most distinct compounds, with three (X09, (E)-2-Hexenal; X20, 2-Ethyl-1-Hexanol; X51, 1-Hexanol) root-specific compounds, followed by two (X30, (E, Z)-Di-1-propenyl disulfide; X33, (E)-Propenyl propanedithioate) pseudostem-specific compounds.
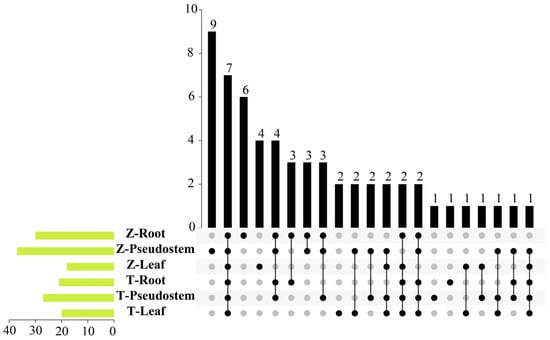
Figure 1.
UpSet plot of ‘Zhangqiu’ and ‘Tenko’ organs. The vertical bars’ height indicates the number of volatile compounds in this set overlap, as indicated by the dots and connecting lines in the plot’s bottom part. The height of the horizontal bars indicates the total number of volatile compounds per set. The letter ‘Z’ represents ‘Zhangqiu’, and ‘T’ represents ‘Tenko’.
Figure 2 shows the differentiation between the two cultivars using PCA based on the volatile compound profiles. We observed apparent differences in the ‘Zhangqiu’ and ‘Tenko’ profiles. The PCA analysis extracted two main components. The samples of the three organs were scattered in different quadrants, and there was an obvious distinction. The leaves and roots of ‘Tenko’ had a negative response value toward PC1. In the PC2 direction, the leaves and pseudostem were positive, while the roots were in the negative direction.
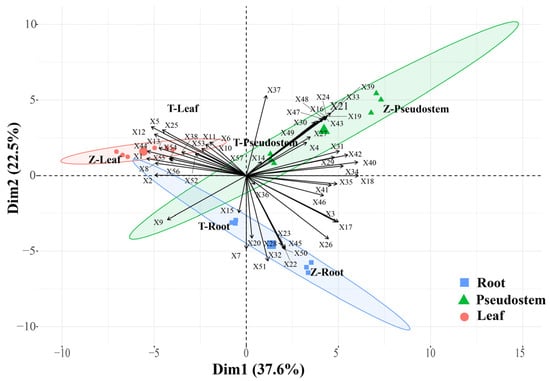
Figure 2.
Principal component analysis (PCA) graphs of volatiles extracted from Welsh onions. Numbers on the loading plot represent compounds characteristic of the analyzed Welsh onions. Points of different types represent the three organs of ‘Zhangqiu’ and ‘Tenko’. The letter ‘Z’ means ‘Zhangqiu’, and ‘T’ represents ‘Tenko’. The percentage indicates the proportion of variance explained by each axis. Compound numbering is the same as the data provided in Table S1.
The compounds were classified according to their functional groups, and they principally comprised alcohols, aldehydes, ketones, hydrocarbons, pyrazines, and sulfur compounds (Table S2). The predominant volatile compounds in the roots and pseudostem of the Welsh onions were sulfur compounds (Figure 3). The sulfur compounds in the roots, pseudostem, and leaves of ‘Zhangqiu’ accounted for 72%, 83%, and 26% of the total content. In comparison, those of ‘Tenko’ accounted for 55%, 84%, and 57%, respectively. Aldehydes comprised no less than 11% of all the organs, and the highest content was in the ‘Zhangqiu’ leaves, accounting for 52% of the total peak area. In addition, the pyrazine, ketone, and alcohol contents were tiny in the three organs of the two cultivars. Hydrocarbons and ketones were not detected in the root, and pyrazines and alcohols comprised less than 3%.
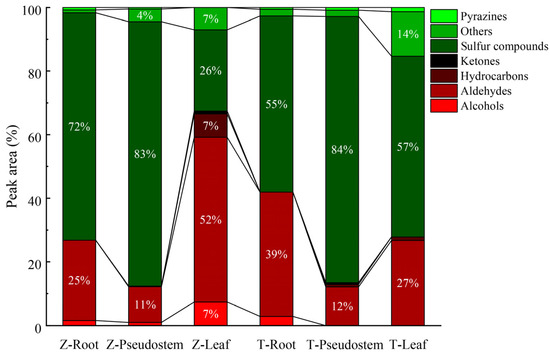
Figure 3.
Percentage contents of different compounds in all analyzed samples. The letters ‘Z’ represent ‘Zhangqiu’, and ‘T’ represent ‘Tenko’.
Twenty and eighteen sulfur compounds were detected in the edible pseudostem portions of ‘Zhangqiu’ and ‘Tenko’. There was no significant difference in the peak area of sulfur compounds between the two cultivars’ pseudostems. In the pseudostem, the predominant sulfur compounds were (E)-propenyl-2-propyl disulfane, accounting for 28.77% and 33.24%, respectively, in ‘Zhangqiu’ and ‘Tenko’. The relative content of the sulfur compounds in the ‘Zhangqiu’ roots was distinctly higher than that of ‘Tenko’, 29.1%. The main sulfur compounds in the roots of the two cultivars were disulfides (dipropyl disulfide). The sulfur content of the ‘Tenko’ leaves was 1.22-fold higher than that of ‘Zhangqiu’. 2-methyl-2-pentenal was detected in all the Welsh onion cultivars’ organs, contributing to the total aldehyde content. The aldehyde content in the leaves of ‘Zhangqiu’ was more abundant than that of ‘Tenko’, 0.94-fold. The leaves’ aldehyde content was significantly more extensive than that of the pseudostem and roots, and the aldehydes in the leaves were abundant.
3.3. Mineral Elements
The element contents differed distinctly from the two cultivars’ three organs (Table 2). The content of each element in the roots and leaves was higher than that of the pseudostem. The sulfur content was the highest in the leaves, followed by the roots, and lowest in the pseudostem. The sulfur content of the pseudostem of ‘Zhangqiu’ was distinctly high, but the difference between the two cultivars’ root sulfur content was insignificant. Through the forward selection of the measured elements, we obtained a higher correlation between S, P, Ca, and Mg and various organs of the Welsh onion (Figure 4). The ordination diagram shows three distinct groups corresponding to different cultivars of volatiles. The first and second canonical axes show 45.56% and 23.74% of the data variance, respectively. The first axis separates the leaves from the pseudostem and roots, while the second axis separates the pseudostem from the roots. The RDA indicated that sulfur and calcium greatly affected the elements in the cultivars and organs.

Table 2.
Mineral element uptake by three organs of ‘Zhangqiu’ and ‘Tenko’.
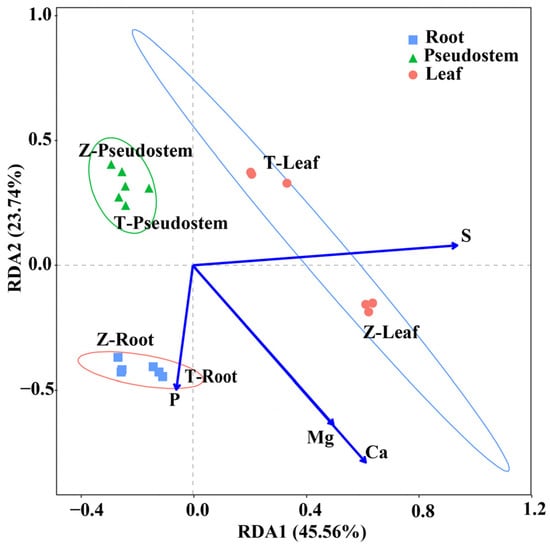
Figure 4.
Redundancy analysis (RDA) of the relationship between mineral elements and volatile compounds of three organs of ‘Zhangqiu’ and ‘Tenko’. Points of different types represent the three organs of ‘Zhangqiu’ and ‘Tenko’. The percentage indicates the proportion of variance explained by each axis. The letter ‘Z’ represents ‘Zhangqiu’, and ‘T’ represents ‘Tenko’.
4. Discussion
Welsh onions are a significant economic crop; their pseudostems and leaves can be eaten fresh or dried. Generally, the complex interaction of taste and aroma produces flavor [6,18]. The aroma of plants is a complex feature because it is affected by different factors, such as phytohormonal changes, microbial effects, or environmental conditions [2,4,19]. To minimize these differences, we planted two cultivars of green onions in the same growth chamber, using the same nutrient solution concentration, and harvested the onions on the same day. Therefore, we can roughly determine that the quality of the edible portion of the Welsh onion and the difference in the volatile matter measured in this study were mainly attributable to the cultivar and not to environmental factors.
The sugars and acids determined the taste. The sugar and acid levels are the main factors affecting the acceptability of fruit flavor, and a high sugar content indicates more sweetness. The accumulation of sugar can also determine the intensity of aromas. In tomatoes, cultivars with increased sugar levels were found to exhibit enhanced aroma intensity, making the overall flavor more acceptable. Sugar is positively correlated with overall flavor acceptability [20]. The results showed that the sugar accumulation in the pseudostem and leaves of ‘Zhangqiu’ was significantly higher than that in ‘Tenko’. Pyruvic acid is a product of the hydrolysis of all flavor precursors. Because pyruvate is easy to measure, it is estimated that enzymatically produced pyruvate has been the most widely used analytical method. In the range of 1.2–9.3 μmol pyruvic acid·g−1 fresh weight, the sensory intensity has a significant linear relationship with pyruvic acid [21]. The pseudostem pyruvate content of ‘Zhangqiu’ was higher than that of ‘Tenko’, while the pyruvate content of the leaves of ‘Tenko’ was significantly higher than that of ‘Zhangqiu’. The coarse fiber content of the stem and leaves of ‘Tenko’ was considerably higher than that of ‘Zhangqiu’, which led to the poor taste of ‘Tenko’.
HS-SPME, combined with GC-MS, provides a simple, fast, and reliable technique for investigating volatiles in Welsh onions. The volatile compounds found in every organ of the two cultivars are shown in Supplemental Table S1. These 57 compounds correspond to the volatile headspace fraction from the Welsh onions. The production of volatile aromatic compounds is essential for the onions’ flavor and is a defining feature of different cultivars. The characteristic aroma comes from one or several volatile compounds and a complex mixture of volatile compounds [22]. Through the PCA analysis, the samples of both cultivars and individual organs were distinguished according to their content of volatile components. The leaves’ volatile substances were unique compared to the pseudostem and roots, and the sulfide content was diminished while the aldehyde levels expanded, particularly in the ‘Zhangqiu’ leaves. Among these chemical classes, we should also pay attention to aldehydes. 2-methyl-2-pentenal is a self-condensation product formed from propionaldehyde, which provided a fruity taste in onion samples [23]. In this study, the primary aldehyde detected was 2-methyl-2-pentenal. The ratio of the volatile compounds in ‘Zhangqiu’ leaves differed from that in the ‘Tenko’ leaves. The sulfide ratio in the ‘Tenko’ leaves was significantly higher than that in ‘Zhangqiu’.
Several volatile sulfur compounds produce the typical allium features [24]. In general, sulfur compounds have a minimum threshold for all kinds of aromatic activity [25]. These compounds are generally present in low concentrations but have a great deal of commonality with aromas; therefore, they play an essential role in overall food flavor characteristics [26]. High concentrations of sulfur compounds frequently have an unpleasant smell. However, when diluted to ppb or ppm concentrations, their aroma changes substantially, producing one similar to that of fresh onion and garlic [25]. Allicin is the most abundant and vital dialkyl sulfosuccinate present in garlic; dipropyl disulfide and propyl sulfide-S-oxide contribute to the flavor of onion, but in fresh amaranth [27], the critical aromatic compounds are dipropyl disulfide, methacryl diamide, and propyl propenyl diacid diene. Similarly, the Welsh onions’ compounds were mainly disulfide and trisulfide, and the prominent components of these sulfur compounds were diverse in the three organs. These main sulfur compounds were responsible for the flavor of the onion. Early studies demonstrated that the intensity of the flavor was related to enzymatically produced pyruvate levels in the onion, primarily due to the linear relationship between the ACSO content and pyruvate [21]. We obtained similar results. The pyruvic acid content in the Welsh onion’s pseudostem and leaves was directly proportional to the sulfur-containing compounds.
Previous studies have shown that the chemical variation caused by environmental factors is much more considerable than that caused by genetic divergence [19]. Environmental influences on chemical components’ growth and culture conditions must be considered to improve vegetable flavor. Different cultivation measures, such as defoliation, foliar application of N, and S fertilizer addition to N fertilizer, can improve the aromatic content of Allium cultivars [28,29]. Sulfur metabolism is closely related to nitrogen metabolism, and flavor compound biosynthesis is thought to rely on primary and secondary metabolites derived from photosynthesis [30]. Cysteine and glutathione metabolism were associated with the biosynthesis of Allium flavor precursors, which are secondary metabolites in alliums. This process is essential for plants’ absorption of sulfur. Sulfur and nitrogen can be produced as the first organosulfur compounds in sulfur assimilation by producing the amino acid cysteine so that the nitrogen supply may affect sulfur absorption and CSO formation [31]. Welsh onions of various genotypes exhibit the absorption of diverse mineral elements from the soil. The distribution of mineral elements in the three organs was extraordinary. The RDA found that sulfur significantly affected the Welsh onions’ volatile content, and the response of different cultivars to sulfur was unique. This is similar to previous research results [5]. However, based on forward selection, we did not discover contrasts in nitrogen relative to the volatiles. Strikingly, P, Ca, and Mg in various organs demonstrated a critical impact on the volatile substances’ distribution.
5. Conclusions
The soluble sugar content of the pseudostem and leaves in ‘Zhangqiu’ was higher than that of ‘Tenko’, and in the pseudostem was 85.6% higher. The characteristic flavors of the different organs of Welsh onions are different. The pseudostem mainly contains sulfur compounds. The distribution of volatile substances in the roots was similar to that in the pseudostems, but the types of substances decreased. The volatile substances in the leaves were more prominent in terms of aldehydes, especially in the ‘Zhangqiu’ leaves, where they made up 52%. There was no significant difference in the sulfur compounds in the pseudostems of the two cultivars. The relative content of sulfur compounds in the roots of ‘Zhangqiu’ was significantly higher than that of ‘Tenko’, which was 29.1%. In ‘Zhangqiu’, the edible leaves acted synergistically with aldehydes, sulfur compounds, hydrocarbons, and alcohols to form their unique flavor, and sulfides, aldehydes, pyrazines, and aromatic hydrocarbons contributed to the flavor of the ‘Tenko’ leaves. In addition, according to the results of the RDA analysis, we determined that S, P, Ca, and Mg significantly contribute to the volatile matter in the Welsh onion, and this result provides a new perspective on the relationship between elements and volatile substances. Focusing on a characteristic volatile substance will provide a point of view for future studies. Integrating volatile compounds, nutritional qualities, and mineral contents provides a perspective for further research on the quality of Welsh onions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10050446/s1, Table S1: Relative content of flavor compounds (%) in different ‘Zhangqiu’ and ‘Tenko’ organs; Table S2: The classes of volatile compounds in three organs of ‘Zhangqiu’ and ‘Tenko’; Figure S1: Volatile compound contents extracted from three organs of ‘Zhangqiu’ and ‘Tenko’.
Author Contributions
Conceptualization, X.L. and K.X. (Kang Xu); methodology, X.L.; software, X.L. and Z.C.; formal analysis, X.L. and K.X. (Kun Xu); investigation, X.L.; data curation, K.X. (Kun Xu); writing—original draft preparation, X.L. and J.G.; writing—review and editing, K.X. (Kang Xu); visualization, X.L. and J.G.; supervision, Z.C. and K.X. (Kun Xu); project administration, K.X. (Kun Xu) and K.X. (Kang Xu) All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shandong Province key research and development plan (Action plan to boost science, technology and innovation for rural revitalization), grant number 2023TZXD029.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, G.; Gou, J.; Klee, H.; Huang, S. Next-Gen Approaches to Flavor-Related Metabolism. Annu. Rev. Plant Biol. 2019, 70, 187–212. [Google Scholar] [CrossRef] [PubMed]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived from the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 454686. [Google Scholar] [CrossRef] [PubMed]
- Khandagale, K.; Krishna, R.; Roylawar, P.; Ade, A.B.; Benke, A.; Shinde, B.; Singh, M.; Gawande, S.J.; Rai, A. Omics approaches inAlliumresearch: Progress and way ahead. PeerJ 2020, 8, e9824. [Google Scholar] [CrossRef]
- Gao, S.; Kong, Y.; Lv, Y.; Cao, B.; Chen, Z.; Xu, K. Effect of different LED light quality combination on the content of vitamin C, soluble sugar, organic acids, amino acids, antioxidant capacity and mineral elements in green onion (Allium fistulosum L.). Food Res. Int. 2022, 156, 111329. [Google Scholar] [CrossRef] [PubMed]
- Biancolillo, A.; Aloia, R.; Rossi, L.; D’Archivio, A.A. Organosulfur volatile profiles in Italian red garlic (Allium sativum L.) varieties investigated by HS-SPME/GC-MS and chemometrics. Food Control 2022, 131, 108477. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Walczak, M.; Skrzypczak-Zielinska, M.; Jelen, H.H. Bitter taste of Brassica vegetables: The role of genetic factors, receptors, isothiocyanates, glucosinolates, and flavor context. Crit. Rev. Food Sci. Nutr. 2018, 58, 3130–3140. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; da Silva, A.L.B.R.; Valdez-Aguilar, L.A. Seasonal plant growth, leaf and bulb mineral nutrients, and bulb yield and quality under chemical, mixed, and organic fertilization in sweet onion (Allium cepa L.). J. Plant Nutr. 2021, 45, 153–167. [Google Scholar] [CrossRef]
- Gil-Pérez, I.; Rebollar, R.; Lidón, I.; Martín, J.; van Trijp, H.C.M.; Piqueras-Fiszman, B. Hot or not? Conveying sensory information on food packaging through the spiciness-shape correspondence. Food Qual. Prefer. 2019, 71, 197–208. [Google Scholar] [CrossRef]
- Yin, C.; Fan, X.; Fan, Z.; Shi, D.; Yao, F.; Gao, H. Comparison of non-volatile and volatile flavor compounds in six Pleurotus mushrooms. J. Sci. Food Agric. 2019, 99, 1691–1699. [Google Scholar] [CrossRef]
- Khokhar, K.M. Mineral nutrient management for onion bulb crops—A review. J. Hortic. Sci. Biotechnol. 2019, 94, 703–717. [Google Scholar] [CrossRef]
- Vuković, S.; Popović-Djordjević, J.B.; Kostić, A.Ž.; Pantelić, N.D.; Srećković, N.; Akram, M.; Laila, U.; Katanić Stanković, J.S. Allium Species in the Balkan Region—Major Metabolites, Antioxidant and Antimicrobial Properties. Horticulturae 2023, 9, 408. [Google Scholar] [CrossRef]
- Li, H. Principle and Technology of Plant Physiological and Biochemical Experiments; SHigher Education Press: Beijing, China, 2002. [Google Scholar]
- Randle, W.M.; Bussard, M.; Warnock, D. Ontogeny and sulfur fertility affect leaf sulfur in short-day onions. J. Am. Soc. Hortic. Sci. 1993, 118, 762–765. [Google Scholar] [CrossRef]
- Kremr, D.; Bajerová, P.; Bajer, T.; Eisner, A.; Adam, M.; Ventura, K. Using headspace solid-phase microextraction for comparison of volatile sulphur compounds of fresh plants belonging to families Alliaceae and Brassicaceae. J. Food Sci. Tech. 2015, 52, 5727–5735. [Google Scholar] [CrossRef]
- Ishida, H.; Suzuno, H.; Sugiyama, N.; Innami, S.; Tadokoro, T.; Maekawa, A. Nutritive evaluation on chemical components of leaves, stalks and stems of sweet potatoes (Ipomoea batatas poir). Food Chem. 2000, 68, 359–367. [Google Scholar] [CrossRef]
- Pai, S.; Yang, C.; Riley, J.P. Effects of acidity and molybdate concentration on the kinetics of the formation of the phosphoantimonylmolybdenum blue complex. Anal. Chim. Acta 1990, 229, 115–120. [Google Scholar] [CrossRef]
- Tang, Q.-Y.; Zhang, C.-X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Inaoka, T.; Nakamura, T.; Kimura, K.; Sekiyama, Y.; Tomita, S. Nuclear magnetic resonance- and gas chromatography/mass spectrometry-based metabolomic characterization of water-soluble and volatile compound profiles in cabbage vinegar. J. Biosci. Bioeng. 2018, 126, 53–62. [Google Scholar] [CrossRef]
- Kim, S.-H.; Yoon, J.; Han, J.; Seo, Y.; Kang, B.-H.; Lee, J.; Ochar, K. Green Onion (Allium fistulosum): An Aromatic Vegetable Crop Esteemed for Food, Nutritional and Therapeutic Significance. Foods 2023, 12, 4503. [Google Scholar] [CrossRef]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Crowther, T.; Collin, H.A.; Smith, B.; Tomsett, A.B.; O’Connor, D.; Jones, M.G. Assessment of the flavour of fresh uncooked onions by taste-panels and analysis of flavour precursors, pyruvate and sugars. J. Sci. Food Agric. 2005, 85, 112–120. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Jelen, H.H. Volatile compounds of selected raw and cooked brassica vegetables. Molecules 2019, 24, 391. [Google Scholar] [CrossRef]
- Villière, A.; Le Roy, S.; Fillonneau, C.; Guillet, F.; Falquerho, H.; Boussely, S.; Prost, C. Evaluation of aroma profile differences between sué, sautéed, and pan-fried onions using an innovative olfactometric approach. Flavour 2015, 4, 24. [Google Scholar] [CrossRef]
- Kusano, M.; Kobayashi, M.; Iizuka, Y.; Fukushima, A.; Saito, K. Unbiased profiling of volatile organic compounds in the headspace of Allium plants using an in-tube extraction device. BMC Res. Notes 2016, 9, 133. [Google Scholar] [CrossRef]
- McGorrin, R.J. The significance of volatile sulfur compounds in food flavors. In Volatile Sulfur Compounds in Food; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1068, pp. 3–31. [Google Scholar]
- Zhang, N.; Sun, B.; Mao, X.; Chen, H.; Zhang, Y. Flavor formation in frying process of green onion (Allium fistulosum L.) deep-fried oil. Food Res. Int. 2019, 121, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.S.; Poll, L. Determination of odor active aroma compounds in freshly cut leek (Allium ampeloprasum Var. Bulga) and in long-term stored frozen unblanched and blanched leek slices by gas chromatography olfactometry analysis. J. Agr. Food Chem. 2004, 52, 1642–1646. [Google Scholar] [CrossRef]
- Vyavahare, G.D.; Lee, Y.; Seok, Y.J.; Kim, H.N.; Sung, J.; Park, J.H. Monitoring of Soil Nutrient Levels by an EC Sensor during Spring Onion (Allium fistulosum) Cultivation under Different Fertilizer Treatments. Agronomy 2023, 13, 2156. [Google Scholar] [CrossRef]
- Kong, L.; Xu, K.; Wang, L.; He, P.; Zhang, Y. Influence of nitrogen and sulfur interaction on growth and quality of Chinese spring onion. J. Plant Nutr. Fertil. 2013, 19, 1272–1278. [Google Scholar]
- McCallum, J.; Porter, N.; Searle, B.; Shaw, M.; Bettjeman, B.; McManus, M. Sulfur and nitrogen fertility affects flavour of field-grown onions. Plant Soil 2005, 269, 151–158. [Google Scholar] [CrossRef]
- Liu, P.; Weng, R.; Sheng, X.; Wang, X.; Zhang, W.; Qian, Y.; Qiu, J. Profiling of organosulfur compounds and amino acids in garlic from different regions of China. Food Chem. 2020, 305, 125499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).