Human Health Benefits through Daily Consumption of Jerusalem Artichoke (Helianthus tuberosus L.) Tubers
Abstract
1. Introduction
2. Main Uses of H. tuberosus
2.1. Bioethanol
2.2. Biological Control
2.3. Human Health
3. Nutritional Value of H. tuberosus
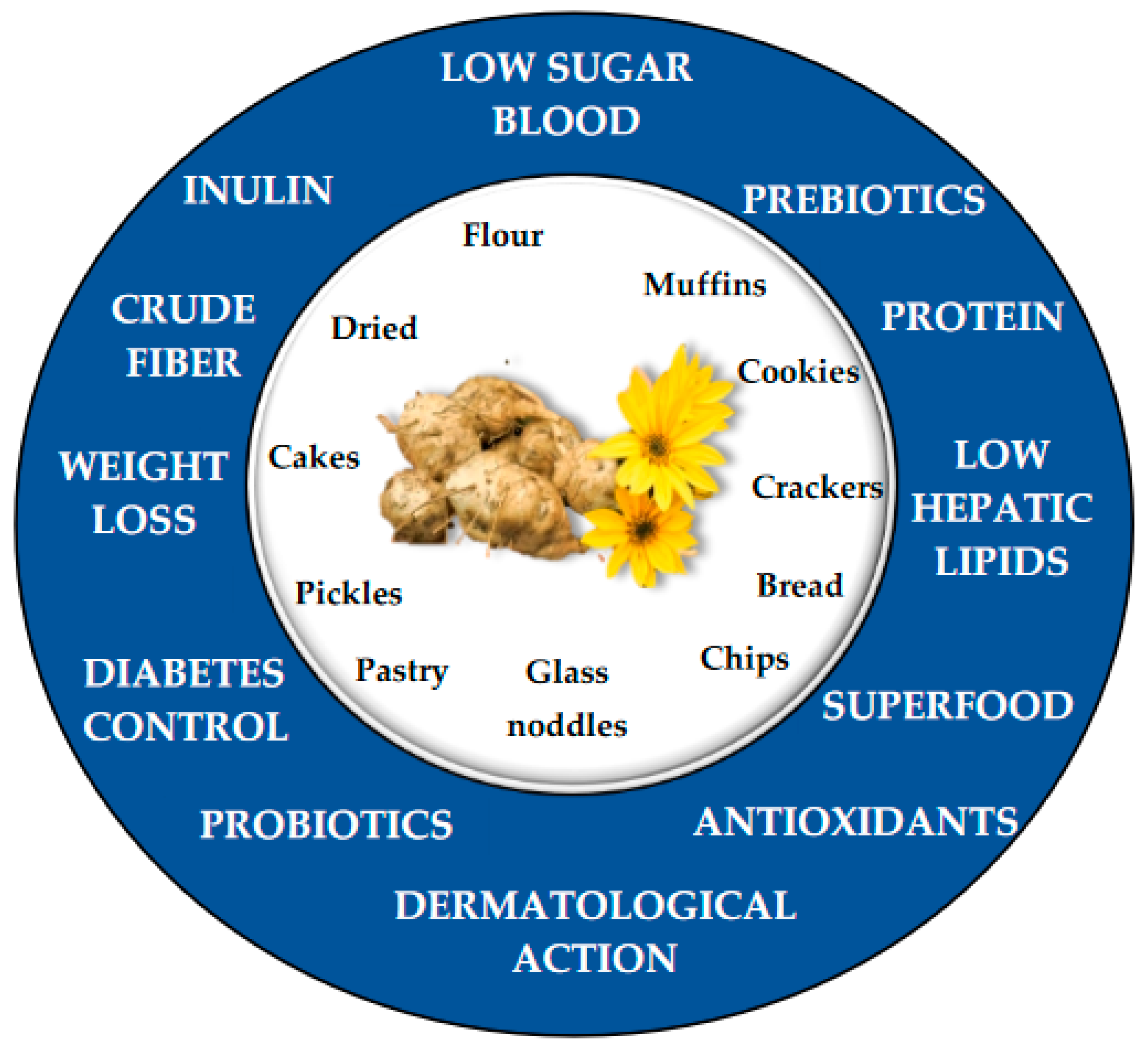
4. Applications of H. tuberosus in Human Health
4.1. Antioxidant Capacity
4.2. Dermatological Treatments
4.3. Digestive System
4.4. Improvement of Biochemical Parameters
4.5. Superfood
5. Helianthus tuberosus: Increasing Quality of Foods for Cooking
6. Challenges and Perspectives
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Bock, D.G.; Kane, N.C.; Ebert, D.P.; Rieseberg, L.H. Genome skimming reveals the origin of the Jerusalem Artichoke tuber crop species: Neither from Jerusalem nor an artichoke. New Phytol. 2014, 201, 1021–1030. [Google Scholar] [CrossRef]
- Rossini, F.; Provenzano, M.E.; Kuzmanovic, L.; Ruggeri, R. Jerusalem artichoke (Helianthus tuberosus L.): A versatile and sustainable crop for renewable energy production in Europe. Agronomy 2019, 9, 528. [Google Scholar] [CrossRef]
- Liava, V.; Karkanis, A.; Danalatos, N.; Tsiropoulos, N. Cultivation practices, adaptability and phytochemical composition of Jerusalem Artichoke (Helianthus tuberosus L.): A weed with economical value. Agronomy 2021, 11, 914. [Google Scholar] [CrossRef]
- Alla, N.A.; Domokos-Szabolcsy, E.; El-Ramady, H.; Hodossi, S.; Fári, M.; Ragab, M.; Taha, H. Jerusalem artichoke (Helianthus tuberosus L.): A review of in vivo and in vitro propagation. Int. J. Hortic. Sci. 2014, 20, 131–136. [Google Scholar] [CrossRef][Green Version]
- Smekalova, T.N.; Lebedeva, N.V.; Novikova, L.Y. Morphological analysis of Jerusalem Artichoke (Helianthus tuberosus L.) accessions of different origin from VIR collection. Proc. Latv. Acad. Sci. 2011, 73, 502–512. [Google Scholar]
- Serieys, H.; Souyris, I.; Gil, A.; Poinso, B.; Bervillé, A. Diversity of Jerusalem artichoke clones (Helianthus tuberosus L.) from the INRA-Montpellier collection. Genet. Resour. Crop Evol. 2010, 57, 1207–1215. [Google Scholar] [CrossRef]
- Kays, S.J.; Nottingham, S.F. Pollinators, pests, and diseases. In Biology and Chemistry of Jerusalem Artichoke: Helianthus tuberosus L.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Tokyo, Japan, 2008. [Google Scholar]
- Pacanoski, Z.; Mehmeti, A. The first report of the invasive alien weed Jerusalem Artichoke (Helianthus tuberosus L.) in the Republic of North Macedonia. Agric. For. 2020, 66, 115–127. [Google Scholar]
- Danilčenko, H.; Jariené, E.; Aleknavičiene, P.; Gajewski, M. Quality of Jerusalem Artichoke (Helianthus tuberosus L.) Tubers in relation to storage conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2008, 36, 23–27. [Google Scholar]
- Bach, V.; Kidmose, U.; Bjørn, G.; Edelenbos, M. Effects of harvest time and variety on sensory quality and chemical composition of Jerusalem artichoke (Helianthus tuberosus) tubers. Food Chem. 2012, 133, 82–89. [Google Scholar]
- Yuan, X.; Gao, M.; Xiao, H.; Tan, C.; Du, Y. Free radical scavenging activities and bioactive substances of Jerusalem artichoke (Helianthus tuberosus L.) leaves. Food Chem. 2012, 133, 10–14. [Google Scholar]
- Kapusta, L.; Krok, E.S.; Jamro, D.B.; Cebulak, T.; Kaszuba, J.; Salach, R.T. Identification and quantification of phenolic compounds from Jerusalem artichoke (Helianthus tuberosus L.) tubers. J. Food Agric. Environ. 2013, 11, 601–606. [Google Scholar]
- Barkhatova, T.V.; Nazarenko, M.N.; Kozhukhova, M.A.; Khripko, I.A. Obtaining and identification of inulin from Jerusalem artichoke (Helianthus tuberosus) tubers. Foods Raw Mater. 2015, 3, 13–22. [Google Scholar] [CrossRef]
- Ozgoren, E.; Isik, F.; Yapar, A. Effect of Jerusalem artichoke (Helianthus tuberosus L.) supplementation on chemical and nutritional properties of crackers. J. Food Meas. Charact. 2019, 13, 2812–2821. [Google Scholar] [CrossRef]
- Gunnarsson, I.B.; Svensson, S.E.; Johansson, E.; Karakashev, D.; Angelidaki, I. Potential of Jerusalem artichoke (Helianthus tuberosus L.) as biorefinery crop. Ind. Crops Prod. 2014, 56, 231–240. [Google Scholar] [CrossRef]
- Yang, L.; He, Q.S.; Corscadden, K.; Udenigwe, C.C. The prospects of Jerusalem artichoke in functional food ingredients and bioenergy production. Biotechnol. Rep. 2015, 5, 77–88. [Google Scholar] [CrossRef]
- Kim, S.; Kim, C.H. Evaluation of whole Jerusalem artichoke (Helianthus tuberosus L.) for consolidated bioprocessing ethanol production. Renew. Energy 2014, 65, 83–91. [Google Scholar] [CrossRef]
- Song, Y.; Wi, S.G.; Ki, H.M.; Bae, H. Cellulosic bioethanol production from Jerusalem artichoke (Helianthus tuberosus L.) using hydrogen peroxide-acetic acid (HPAC) pretreatment. Bioresour. Technol. 2016, 214, 30–36. [Google Scholar] [CrossRef]
- Bhagia, S.; Akinosho, H.; Ferreira, J.F.S.; Ragauskas, A.J. Biofuel production from Jerusalem artichoke tuber inulins: A review. Biofuel Res. J. 2017, 14, 587–599. [Google Scholar] [CrossRef]
- Tesio, F.; Weston, L.A.; Ferrero, A. Allelochemicals identified from Jerusalem artichoke (Helianthus tuberosus L.) residues and their potential inhibitory activity in the field and laboratory. Sci. Hortic. 2011, 129, 361–368. [Google Scholar] [CrossRef]
- Vidotto, F.; Tesio, F.; Ferrero, A. Allelopathic effects of Helianthus tuberosus L. on germination and seedling growth of several crops and weeds. Biol. Agric. Hortic. 2007, 26, 55–68. [Google Scholar] [CrossRef]
- Chen, F.; Long, X.; Liu, Z.; Shao, H.; Liu, L. Analysis of phenolics acids of Jerusalem artichoke (Helianthus tuberosus L.) responding to salt-stress by liquid chromatography/tandem mass spectrometry. Sci. World J. 2014, 568043. [Google Scholar] [CrossRef]
- Kaszás, L.; Kovács, Z.; Nagy, E.; Elhawat, N.; Abdalla, N.; Domokos-Szabolcsy, E. Jerusalem artichoke (Helianthus tuberosus L.) as a potential chlorophyll source for humans and animals nutrition. Environ. Biodivers. Soil Secur. 2018, 2, 1–9. [Google Scholar] [CrossRef][Green Version]
- Gupta, D.; Chaturvedi, N. Prebiotic potential of underutilized Jerusalem artichoke in human health: A comprehensive review. Int. J. Environ. Agric. Biotechnol. 2020, 5, 97–103. [Google Scholar] [CrossRef]
- Bakku, R.K.; Gupta, R.; Min, C.; Kim, S.; Takahashi, G.; Shibato, J.; Shioda, S.; Takenoya, F.; Agrawal, G.K.; Rakwal, R. Unravelling the Helianthus tuberosus L. (Jerusalem artichoke, Kiku-Imo) tuber proteome by label-free quantitative proteomics. Molecules 2022, 27, 1111. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, E.; Gębusia, A.; Florkiewicz, A.; Mickowska, B. The content of protein and of amino acids in Jerusalem artichoke tubers (Helianthus tuberosus L.) of red variety Rote Zonenkugel. Acta Sci. Pol. Technol. Aliment. 2011, 10, 433–441. [Google Scholar]
- Catana, L.; Catana, M.; Iorga, E.; Lazar, A.; Lazar, M.; Teodorescu, R.I.; Asanica, A.C.; Belc, N.; Iancu, A. Valorification of Jerusalem artichoke tubers (Helianthus tuberosus) for achieving of functional ingredient with high nutritional value. Conf. Proc. Agric. Life Life Agric. 2018, 1, 276–283. [Google Scholar] [CrossRef][Green Version]
- Sawicka, B.; Danilčencko, H.; Jariene, E.; Skiba, D.; Rachon, L.; Barbas, P.; Pszczólkowski, P. Nutritional value of Jerusalem artichoke tubers (Helianthus tuberosus L.) grown in organic system under Lithuanian and Polish conditions. Agriculture 2021, 11, 440. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Xue, F.; Nan, X.; Wang, H.; Hua, D.; Liu, J.; Yang, L.; Jiang, L.; Xiong, B. Nutritional value, bioactivity, and application potential of Jerusalem artichoke (Helianthus tuberosus L.) as a neotype feed resource. Anim. Nutr. 2020, 6, 429–437. [Google Scholar] [CrossRef]
- Kocsis, L.; Liebhard, P.; Praznik, W. Effect of seasonal changes on content and profile of soluble carbohydrates in tubers of different varieties of Jerusalem artichoke (Helianthus tuberosus L.). J. Agric. Food Chem. 2007, 55, 9401–9408. [Google Scholar] [CrossRef]
- Brkljača, J.; Bodroza-Solarov, M.; Krulj, J.; Terzić, S.; Mikić, A.; Marjanović Jeromela, A. Quantification of inulin content in selected accessions of Jerusalem artichoke (Helianthus tuberosus L.). Helia 2014, 37, 105–112. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. Plants of the Asteraceae family as agents in the protection of human health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Rubel, I.A.; Iraporda, C.; Manrique, G.D.; Genovese, D.B.; Abraham, A.G. Inulin from Jerusalem artichoke (H. tuberosus L.): From its biosynthesis to its application as bioactive ingredient. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100281. [Google Scholar] [CrossRef]
- Jantaharn, P.; Mongkoltharnaruk, W.; Senawong, T.; Jogloy, S.; McCloskey, S. Bioactive compounds from organic extracts of Helianthus tuberosus L. flowers. Ind. Crops. Prod. 2018, 119, 57–63. [Google Scholar] [CrossRef]
- Bedzo, O.K.K.; van Rensburg, E.; Görgens, J.F. Investigating the effect of different inulin-rich substrate preparations from Jerusalem artichoke (Helianthus tuberosus L.) tubers on efficient inulooligosaccharides production. Prep. Biochem. Biotechnol. 2020, 51, 440–449. [Google Scholar] [CrossRef]
- Srinameb, B.; Nuchadomrong, S.; Jogloy, S.; Patanothai, A.; Srijaranai, S. Preparation of inulin powder from Jerusalem artichoke (Helianthus tuberosus L.) tuber. Plant Foods Hum. Nutr. 2015, 70, 221–226. [Google Scholar] [CrossRef]
- Danilcenko, H.; Jariene, E.; Gajewski, M.; Sawicka, B.; Kulaitiene, J.; Cerniauskiene, J. Changes in amino acids content in tubers of Jerusalem artichoke (Helianthus tuberosus L.) cultivars during storage. Acta Sci. Pol.-Hortorum Cultus 2013, 12, 97–105. [Google Scholar]
- Pinar, H.; Kara, K.; Hanci, F.; Kaplan, M. Nutritional composition of herbage of different Jerusalem artichoke genotypes. J. Anim. Feed Sci. 2021, 30, 141–148. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Yang, J.; Idong, P.T.; Mei, L.; Tao, Y.; Shi, Y. Antioxidant and α-glucosidase inhibitory ingredients identified from Jerusalem artichoke flowers. Nat. Prod. Res. 2019, 33, 584–588. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.B.; Han, S.Y.; Kim, S.J.; Hwang, I.H.; Kim, D.K. Antioxidant activity of Helianthus tuberosus L. flower in Caenorhabditis elegans. Kor. J. Pharmacogn. 2019, 50, 96–101. [Google Scholar]
- Mariadoss, A.V.A.; Park, S.; Saravanakumar, K.; Sathiyaseelan, A.; Wang, M. Ethyl acetate fraction of Helianthus tuberosus L. induces anti-diabetic, and wound-healing activities in insulin-resistant human liver cancer and mouse fibroblast cells. Antioxidants 2021, 10, 99. [Google Scholar] [CrossRef]
- Zhou, H.; Li, B.; Wu, M.; Liu, Y. Evaluation of antioxidant capacity of polysaccharide in Jerusalem artichoke (Helianthus tuberosus L.) during overwintering. E3S Web Conf. 2019, 78, 02008. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Hwang, J.; Kim, E.; Park, P.; Jeon, B. Antioxidant activity and protective effects of extracts from Helianthus tuberosus L. leaves on t-BHP induced oxidative stress in Chang cells. J. Korean Soc. Food Sci. Nutr. 2011, 40, 1525–1531. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Z.; He, C.; Yuan, X. The antioxidant activities of flavonoids in Jerusalem artichoke (Helianthus tuberosus L.) leaves and their quantitative analysis. Nat. Prod. Res. 2020, 36, 1009–1013. [Google Scholar] [CrossRef]
- Showkat, M.M.; Falck-Ytter, A.B.; Straetkvern, O. Phenolic acids in Jerusalem Artichoke (Helianthus tuberosus L.): Plant organ dependent antioxidant activity and optimized extraction from leaves. Molecules 2019, 24, 3296. [Google Scholar] [CrossRef]
- Malm, A.; Grzegorczyk, A.; Biernasiuk, A.; Baj, T.; Rój, E.; Tyskiewicz, K.; Dębczak, A.; Stolarski, M.J.; Krzyzaniak, M.; Olba-Ziety, E. Could supercritical extracts from the aerial parts of Helianthus salicifolius A. Dietr and Helianthus tuberosus L. be regarded as potential raw materials for biocidal purposes? Agriculture 2020, 11, 10. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, K.; An, H. Inhibitory effects of Helianthus tuberosus ethanol extract on Dermatophagoides farina body-induced atopic dermatitis mouse model and human keratinocytes. Nutrients 2018, 10, 1657. [Google Scholar] [CrossRef]
- Niziol-Lukaszewska, Z.; Bujak, T.; Wasilewski, T.; Szmuc, E. Inulin as an effectiveness and safe ingredient in cosmetics. Pol. J. Chem. Technol. 2019, 21, 44–49. [Google Scholar] [CrossRef]
- Gupta, K.; Talwar, G.; Jain, V.; Dhawan, K.; Jain, S. Salad crops: Root, bulb and tuber crops. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 5060–5073. [Google Scholar]
- Elaheh, M.; Mohamadi, S.A.; Milani, E.; Ladan, N. Prebiotic effect of Jerusalem artichoke (Helianthus tuberosus) fructans on the growth performance of Bifiobacterium bifium and Escherichia coli. Asian Pac. J. Trop. Dis. 2016, 6, 385–389. [Google Scholar] [CrossRef]
- Iraporda, C.; Rubel, I.A.; Manrique, G.D.; Abraham, A.G. Influence of inulin rich carbohydrates from Jerusalem artichoke (Helianthus tuberosus L.) tubers on probiotic properties of Lactobacillus strains. LWT 2019, 101, 738–746. [Google Scholar] [CrossRef]
- El-Kholy, W.M.; Mahrous, H. Biological studies on Bio-Yoghurt fortified with prebiotic obtained from Jerusalem artichoke. Food Nutr. Sci. 2015, 6, 1552–1564. [Google Scholar] [CrossRef]
- Ramnani, P.; Gaudier, E.; Bingham, M.; van Bruggen, P.; Tuohy, K.M.; Gibson, G.R. Prebiotic effect of fruit and vegetable shots containing Jerusalem artichoke inulin: A human intervention study. Br. J. Nutr. 2010, 104, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chijiki, H.; Nanba, T.; Ozaki, M.; Sasaki, H.; Takahashi, M.; Shibata, S. Ingestion of Helianthus tuberosus at breakfast rather than at dinner is more effective for suppressing glucose levels and improving the intestinal microbiota in older adults. Nutrients 2020, 12, 3035. [Google Scholar] [CrossRef] [PubMed]
- Samal, L.; Chaturvedi, V.B.; Pattanaik, A.K. Effects of dietary supplementation with Jerusalem artichoke (Helianthus tuberosus L.) tubers on growth performance, nutrient digestibility as well as activity and composition of large intestinal microbiota in rats. J. Anim. Feed Sci. 2017, 26, 50–58. [Google Scholar] [CrossRef]
- Okrouhlá, M.; Cítek, J.; Svejstil, R.; Zadinová, K.; Pokorná, K.; Urbanová, D.; Stupka, R. Populations of pig faecal bacteria and the prevalence of skatole. Animals 2020, 10, 693. [Google Scholar] [CrossRef]
- Chang, W.; Jia, H.; Aw, W.; Saito, K.; Hasegawa, S.; Kato, H. Beneficial effects of soluble dietary Jerusalem artichoke (Helianthus tuberosus) in the prevention of the onset of type 2 diabetes and non-alcoholic fatty liver disease in high-fructose diet-fed rats. Br. J. Nutr. 2014, 112, 709–717. [Google Scholar] [CrossRef]
- Okada, N.; Kobayashi, S.; Moriyama, K.; Miyataka, K.; Abe, S.; Sato, C.; Kawazoe, K. Helianthus tuberosus (Jerusalem artichoke) tubers improve glucose tolerance ande hepatic lipid profile in rats fed a high-fat diet. Asian Pac. J. Trop. Med. 2017, 10, 439–443. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, B.K.; Park, B.Y.; Kim, J.M.; Lee, Y.J.; Lee, M.K.; Yee, S.; Kang, M.Y. Effects of Jerusalem artichoke extract and inulin on blood glucose levels and insulin secretion in streptozotocin induced diabetic mice. Korean Diabetes J. 2021, 22, 60–70. [Google Scholar] [CrossRef]
- Abd El-Mola, A.E.; Aboulfotoh, G. Effect of feeding Helianthus tuberosus (Jerusalem artichoke) on rations nutritive value and some blood parameters of Ossimi rams. Egypt. J. Nutr. Feed. 2018, 21, 3365–3371. [Google Scholar] [CrossRef][Green Version]
- Slapkauskaite, J.; Sekmokiene, D.; Kabasinskiene, A.; Bartkiene, E.; Juodeikiene, G.; Sarkinas, A. Influence of lactic acid bacteria-fermented Helianthus tuberosus L. and Lupinus luteus on quality of milk products. CyTA J. Food 2015, 14, 482–488. [Google Scholar]
- Singthong, J.; Thongkaew, C. Effect of Jerusalem artichoke (Helianthus tuberosus) powder on quality of glass noodles. Food Res. 2020, 4, 17–26. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Ji, J.; Deng, L.; Feng, Q.; Shi, W.; Gao, J. Inactivation of inulinase and marination of High-Quality Jerusalem Artichoke (Helianthus tuberosus L.) pickles with screened dominant strains. Front. Bioeng. Biotechnol. 2021, 8, 626861. [Google Scholar] [CrossRef] [PubMed]
- Rubel, I.A.; Pérez, E.E.; Manrique, G.D.; Genovese, D.B. Fiber enrichment of wheat bread with Jerusalem Artichoke inulin: Effect on dough rheology and bread quality. Food Struct. 2015, 3, 21–29. [Google Scholar] [CrossRef]
- Chirsanova, A.; Capcanari, T.; Gincu, E. Jerusalem artichoke (Helianthus tuberosus) flour impact on bread quality. J. Food Eng. 2021, 28, 131–143. [Google Scholar] [CrossRef]
- Gedrovica, I.; Karklina, D. Influence of Jerusalem Artichoke powder on the nutritional value of pastry products. Int. J. Food Sci. Nutr. 2012, 6, 524–527. [Google Scholar]
- Ibarguren, L.; Calderon, M.; Tessaro, S.; Bertona, A.; Rebora, C. Evaluación sensorial del topinambur (Helianthus tuberosus L.) como alimento. RIA Rev. Investig. Agropecu. 2019, 45, 204–210. [Google Scholar]
- Lee, Y.; Kim, D.; Lee, O.; Yoon, W.B. Characterizing texture, color and sensory attributes of cookies made with Jerusalem Artichoke (Helianthus tuberosus L.) flour using a mixture design and browning reaction kinetics. Int. J. Food Eng. 2016, 12, 107–126. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, O.; Yoon, W.B. Effect of inulin in Jerusalem Artichoke (Helianthus tuberosus L.) flour on the viscoelastic behavior of cookie dough and quality of cookies. FoodOPS 2017, 7, 35–44. [Google Scholar]
- Gedrovica, I.; Karklina, D.; Straumite, E. Sensory and qualitative indices (hardness and color) evaluation of cakes with Jerusalem artichoke (Helianthus tuberosus L.) powder. Res. Rural Dev. 2010, 1, 138–144. [Google Scholar]
- Celik, I.; Isik, F.; Gursoy, O.; Yilmaz, Y. Use of Jerusalem Artichoke (Helianthus tuberosus) tubers as a natural source of inulin in cakes. J. Food Process. Preserv. 2012, 37, 483–488. [Google Scholar] [CrossRef]
- Park, G. Optimization of muffin preparation upon addition of Jerusalem artichoke powder and oligosaccharide by response surface methodology. J. Korean Soc. Food Cult. 2014, 29, 101–110. [Google Scholar] [CrossRef]
- Baltacioglu, C.; Esin, A. Chips production from Jerusalem artichoke (Helianthus tuberosus L.). Food Nutr. Sci. 2012, 3, 1321–1328. [Google Scholar] [CrossRef]
- Khuenpet, K.; Jittanit, W.; Sirisansaneeyakul, S.; Srichamnong, W. Inulin powder production from Jerusalem artichoke (Helianthus tuberosus L.) tuber powder and its application to commercial food products. J. Food Process. Preserv. 2016, 41, e13097. [Google Scholar] [CrossRef]
| Fungi | Annelids | Nematode | Insects |
|---|---|---|---|
| Sclerotinia sclerotiorum | Strauzia longipennis | Ditylenchus dipsaci | Macrosiphum euphorbiae |
| Sclerotium rolfsii | Meloidogyne spp. | Trama penecaeca | |
| Botrytis cinerea | Heterodera schachtii | Trama troglodytes | |
| Alternaria helianthi | Heterodera marioni | Uroleucon compositae | |
| Rhizopus nigricans | Caconema radicicola | Uroleucon gobonis | |
| Erysiphe cichoracearum | Aphelenchoides ritzemabosi | Uroleucon helianthicola | |
| Puccinia helianthi | Cochylichroa hospe | ||
| Bipolaris zeae | Homoeosoma electellum | ||
| Fusarium spp. | |||
| Penicillium spp. |
| H. tuberosus Variety | Composition | Total Protein (%) | Amino Acids | Vitamins | Minerals | Reference |
|---|---|---|---|---|---|---|
| Rote zonenkugel (red variety) | 6.36% | H, I, L, K, M + C, F + Y, T, W, V | - | - | [26] | |
| Red and white varieties | Water, ash, protein, fat, crude fiber, inulin-type fructans | - | - | Thiamin, niacin, pantothenic acid, pyridoxine, vitamin C | Fe, Ca, Mg, P, K | [27] |
| Albik and Rubik varieties | Soluble dry mass, inulin, crude fiber, crude fat, crude protein, true protein, total amino acids | - | - | Ascorbic acid | N, P, K, Mg, Ca, Na | [28] |
| Product | Organoleptic Qualities | Reference |
|---|---|---|
| Cookies | Cookies with antioxidant capacity. Hard cookies with more H. tuberosus flour. Darker cookies with H. tuberosus flour, in comparison with potato flour. Elastic dough with more H. tuberosus flour. | [68] [69] |
| Cake | 30% of H. tuberosus powder improves organoleptic qualities such as aroma, texture, softness, porosity, appearance, and color, among others. 5–10% of H. tuberosus powder change cake color, decreases softness, but evaluators qualified well as a source of inulin. | [70] [71] |
| Muffins | The addition of 10.99% of H. tuberosus powder and 71.40% of oligosaccharide is the optimum formulation to prepare muffins with good organoleptic qualities. | [72] |
| Pickles | Inactivation of inulinase enzyme and marination to produce a better taste. | [63] |
| Bread | A percentage of H. tuberosus flour in the bread preparation (5%), adds essential amino acids, micro, and macronutrients, extends its useful life, and improves its organoleptic qualities. | [65] |
| Chips | Chips with low or without calories and sugar. Probing the best preparation of H. tuberosus chips in a deep fat fryer or microwave oven. | [73] |
| Glass noodles | Define optimum concentrations for glass noodles with more fiber and sugar, with other good organoleptic qualities such as cohesiveness and gumminess. | [62] |
| Other cooking preparations | Inulin extracted from H. tuberosus is used in preparations such as ice porridge, instant cereal drinks, ready mixed soya power chocolate malt mixed beverage | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Yáñez, A.; Ramos, P.; Morales-Quintana, L. Human Health Benefits through Daily Consumption of Jerusalem Artichoke (Helianthus tuberosus L.) Tubers. Horticulturae 2022, 8, 620. https://doi.org/10.3390/horticulturae8070620
Méndez-Yáñez A, Ramos P, Morales-Quintana L. Human Health Benefits through Daily Consumption of Jerusalem Artichoke (Helianthus tuberosus L.) Tubers. Horticulturae. 2022; 8(7):620. https://doi.org/10.3390/horticulturae8070620
Chicago/Turabian StyleMéndez-Yáñez, Angela, Patricio Ramos, and Luis Morales-Quintana. 2022. "Human Health Benefits through Daily Consumption of Jerusalem Artichoke (Helianthus tuberosus L.) Tubers" Horticulturae 8, no. 7: 620. https://doi.org/10.3390/horticulturae8070620
APA StyleMéndez-Yáñez, A., Ramos, P., & Morales-Quintana, L. (2022). Human Health Benefits through Daily Consumption of Jerusalem Artichoke (Helianthus tuberosus L.) Tubers. Horticulturae, 8(7), 620. https://doi.org/10.3390/horticulturae8070620








