Appropriate Nitrogen Application for Alleviation of Soil Moisture-Driven Growth Inhibition of Okra (Abelmoschus esculentus L. (Moench))
Abstract
1. Introduction
2. Material and Methods
2.1. Soil Conditions of the Pot Experiments
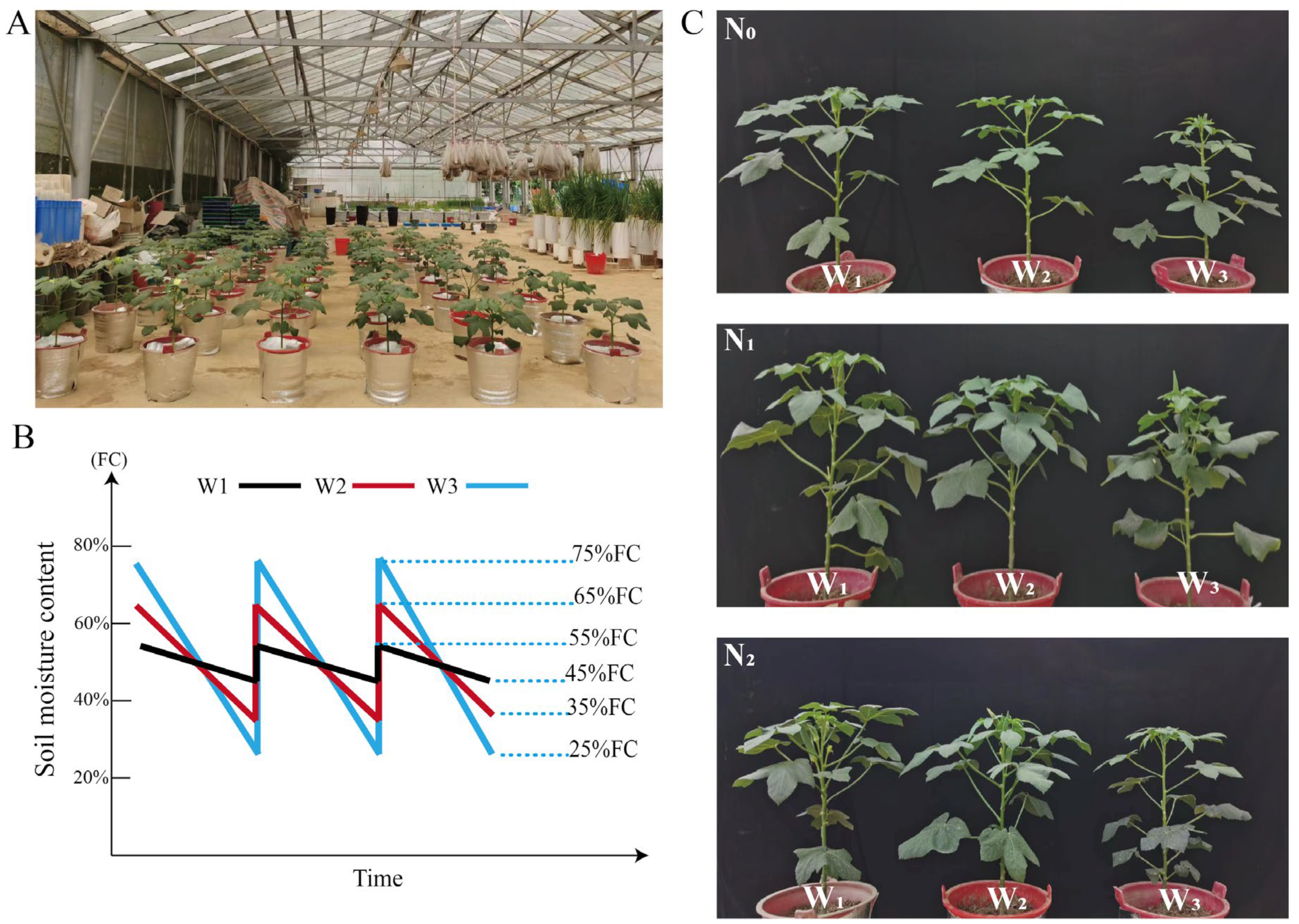
2.2. Experimental Design and Crop Management
2.3. Soil Moisture Content
2.3.1. Soil Volumetric Moisture Content
2.3.2. Soil Mass Moisture Content
2.3.3. Field Water Holding Capacity
2.3.4. Relative Soil Water Content
2.4. Agronomic Traits
2.5. Gas Exchange Measurements
2.6. Stomatal Morphology
2.7. Antioxidant Enzymes, Malondialdehyde and Proline
2.8. Fruit Yield
2.9. Statistical Analysis
3. Results
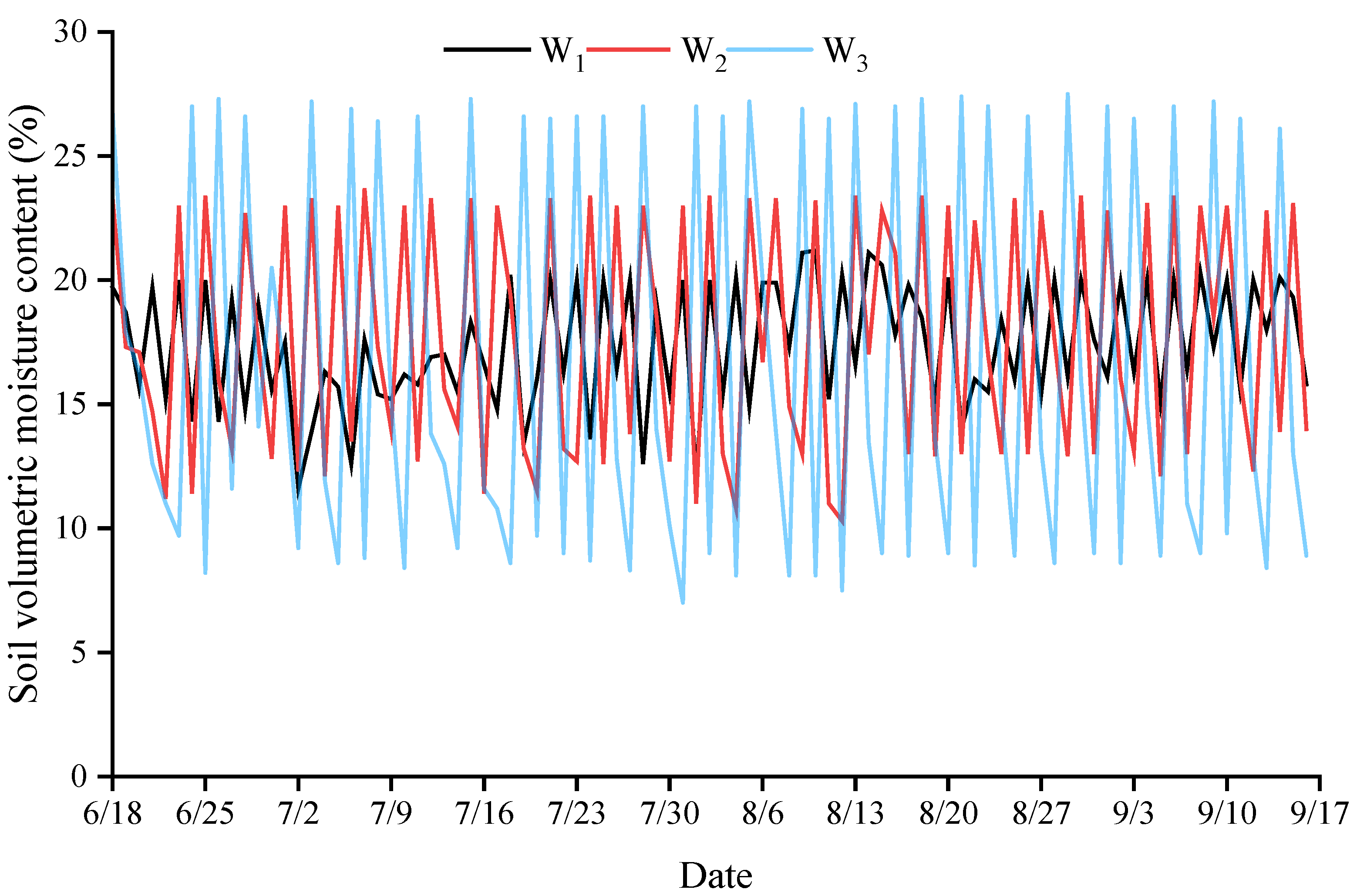
3.1. Dynamics of Soil Volumetric Water Content under Different Irrigation Conditions
3.2. Agronomic Traits
3.3. Fruit Yield
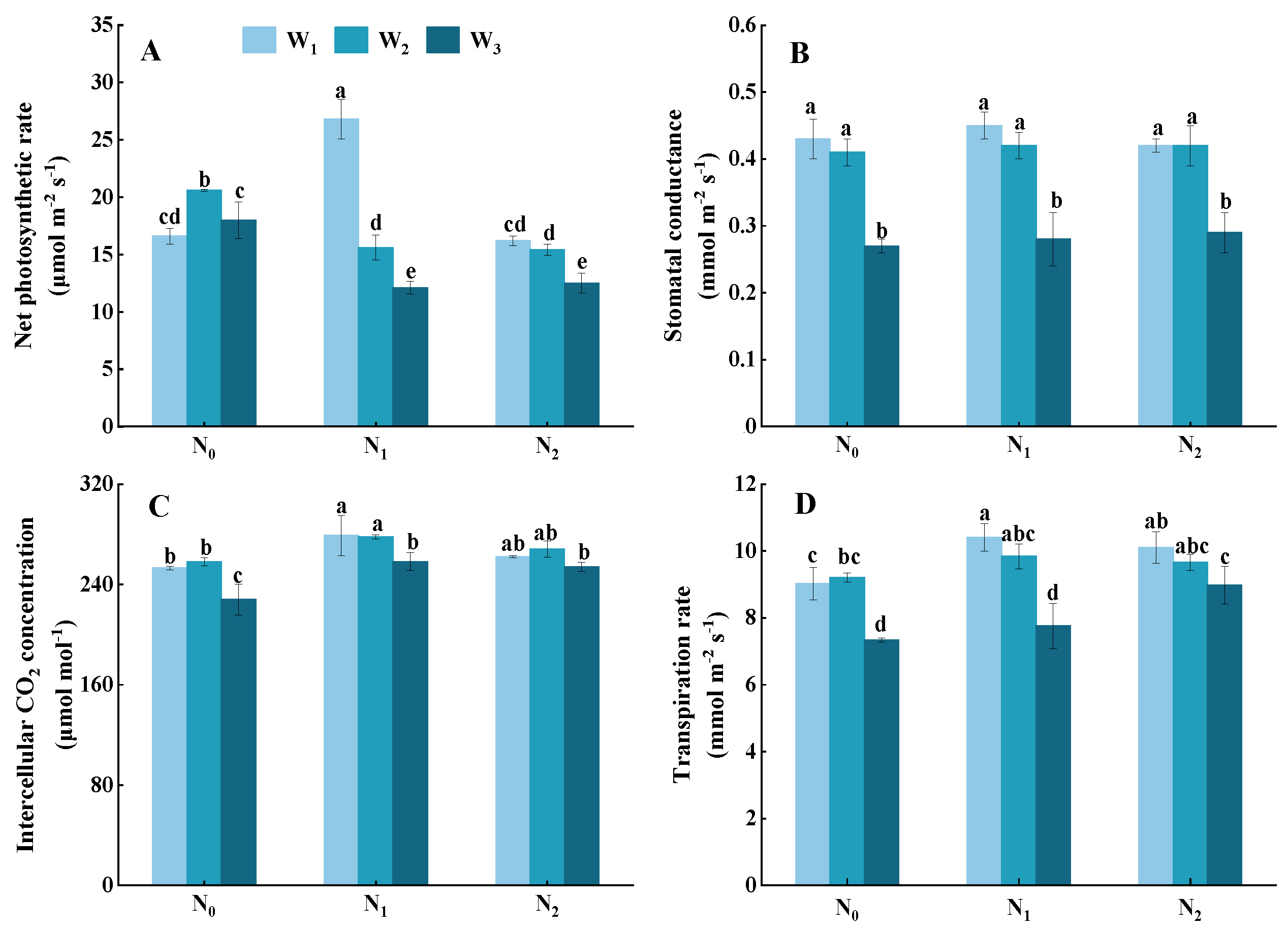
3.4. Photosynthesis
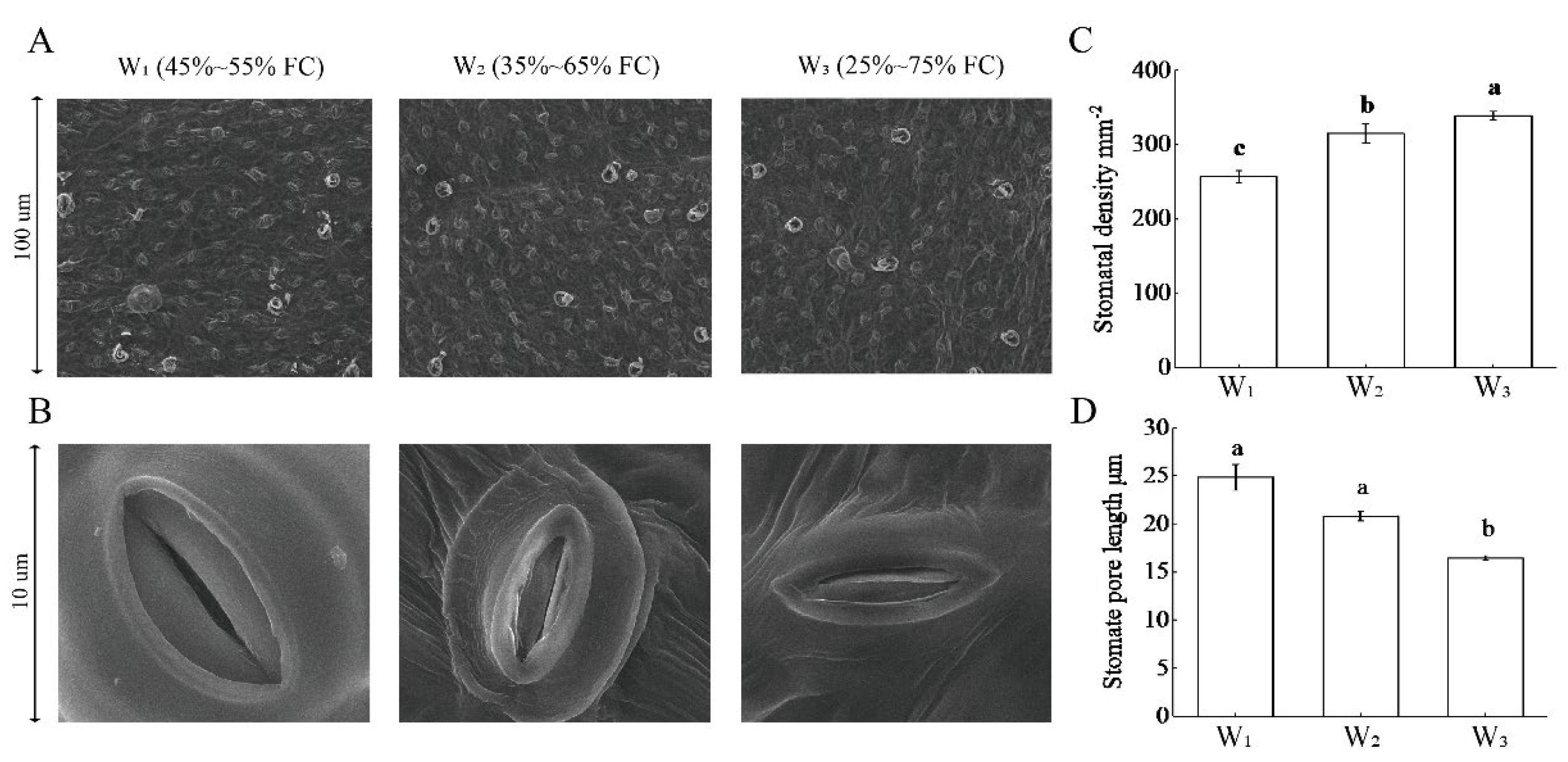
3.5. Stomatal Morphology
3.6. Antioxidant Enzymes
3.7. Malondialdehyde and Proline
3.8. Correlation Analysis
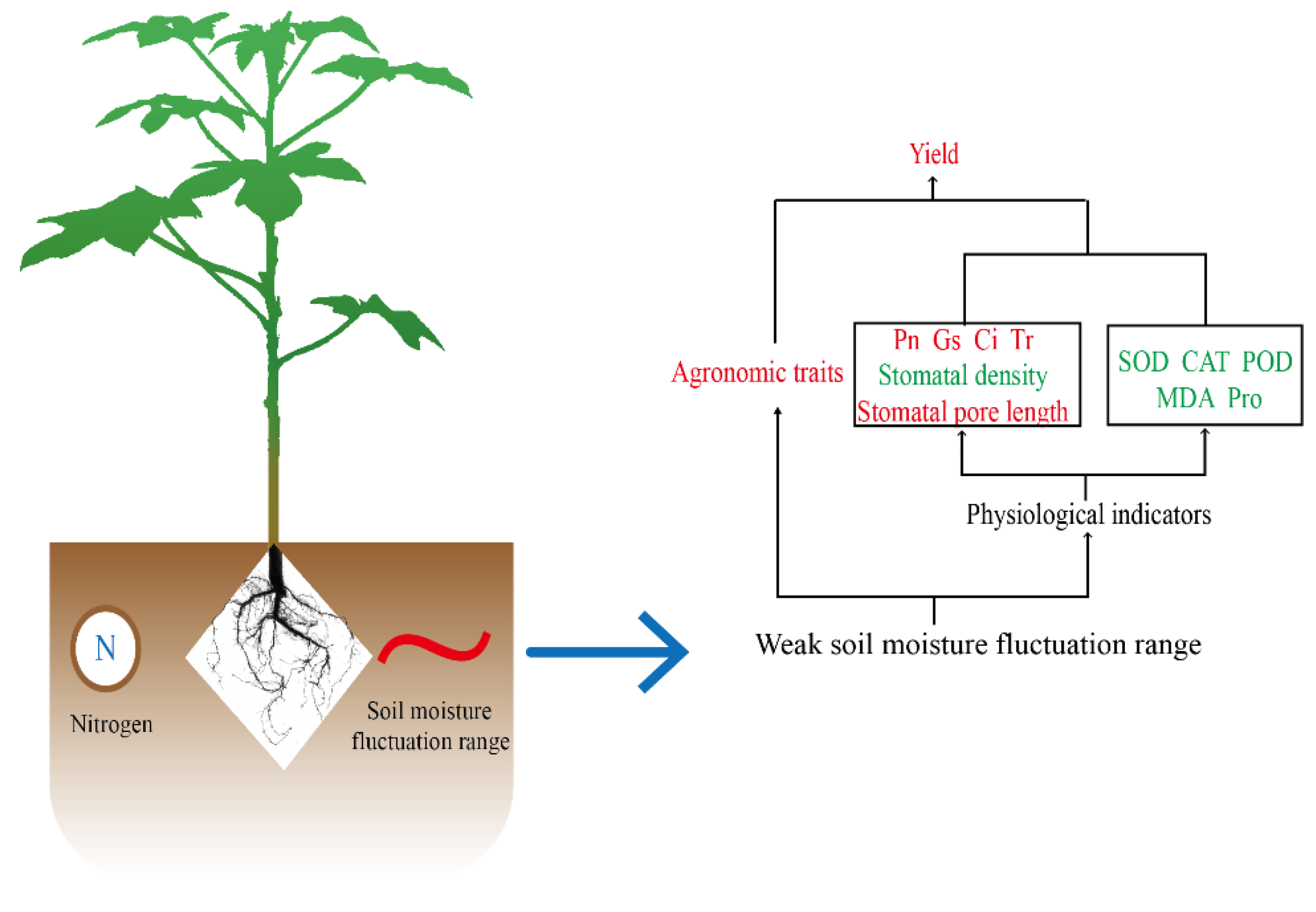
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El-Fattah, B.E.S.A.; Haridy, A.G.; Abbas, H.S. Response to Planting Date, Stress Tolerance and Genetic Diversity Analysis among Okra (Abelmoschus esculentus (L.) Moench.) Varieties. Genet. Resour. Crop Evol. Int. J. 2020, 67, 831–851. [Google Scholar] [CrossRef]
- Nawaz, A.; Ali, H.; Gogi, M.D. Resistance Assessment of Different Cultivars of Okra (Abelmoschus esculentus) Against Whitefly (Bemisia tabaci). Gesunde Pflanz. 2020, 72, 361–369. [Google Scholar] [CrossRef]
- Food Agriculture Organization of the United Nations. FAOSTAT; FAO: Rome, Italy, 2020. [Google Scholar]
- Kaur, J.; Pathak, M.; Pathak, D. Inheritance of Resistance to Yellow Vein Mosaic Virus Disease in Interspecific Crosses of Abelmoschus. Indian J. Agric. Sci. 2021, 90, 2302–2306. [Google Scholar] [CrossRef]
- Tian, Z.-H.; Miao, F.-T.; Zhang, X.; Wang, Q.-H.; Lei, N.; Guo, L.-C. Therapeutic Effect of Okra Extract on Gestational Diabetes Mellitus Rats Induced by Streptozotocin. Asian Pac. J. Trop. Med. 2015, 8, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; He, Y.; Xiang, P.Y. Influences of different drying methods on the structural characteristics and multiple bioactivities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2020, 147, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Nana, R.; Sawadogo, M.; Tamini, Z. Response of Okra [Abelmoschus esculentus (L.) Moench] to Water Stress in the Soil. Afr. J. Biotechnol. 2012, 13, 3591–3596. [Google Scholar]
- O’Grady, C. Time Grows Short to Curb Warming, Report Warns. Science 2021, 373, 723. [Google Scholar] [CrossRef] [PubMed]
- Oki, T.; Kanae, S. Global Hydrological Cycles and World Water Resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef]
- Wu, X.C.; Liu, H.Y.; Li, X.Y.; Ciais, P.; Babst, F.; Guo, W.C.; Zhang, C.C.; Magliulo, V.; Pavelka, M.; Liu, S.M.; et al. Differentiating Drought Legacy Effects on Vegetation Growth over the Temperate Northern Hemisphere. Glob. Chang. Biol. 2018, 2, 504–516. [Google Scholar] [CrossRef]
- Touma, D.; Ashfaq, M.; Nayak, M.A.; Kao, S.-C.; Diffenbaugh, N.S. A Multi-Model and Multi-Index Evaluation of Drought Characteristics in the 21st Century. J. Hydrol. 2015, 526, 196–207. [Google Scholar] [CrossRef]
- Wu, J.; Chen, X.; Yao, H.; Liu, Z.; Zhang, D. Hydrological Drought Instantaneous Propagation Speed Based on the Variable Motion Relationship of Speed-Time Process. Water Resour. Res. 2018, 54, 9549–9565. [Google Scholar] [CrossRef]
- Ponce, C.G.E.; Moran, M.S.; Huete, A.; Zhang, Y.; Bresloff, C.; Huxman, T.E.; Eamus, D.; Bosch, D.D.; Buda, A.R.; Gunter, S.A.; et al. Ecosystem Resilience Despite Large-Scale Altered Hydroclimatic Conditions. Nature 2013, 494, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Poulter, B.; Frank, D.; Ciais, P.; Myneni, R.B.; Andela, N.; Bi, J.; Broquet, G.; Canadell, J.G.; Chevallier, F.; Liu, Y.Y.; et al. Contribution of Semi-Arid Ecosystems to Interannual Variability of the Global Carbon Cycle. Nature 2014, 509, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, P.; Gao, Y.H.; Ma, G. Effects of soil moisture fluctuations on CO2 and N2O emission in alpine meadow ecosystem. Pratacultural Sci. 2018, 35, 266–275. [Google Scholar]
- Suralta, R.R.; Inukai, Y.; Yamauchi, A. Dry Matter Production in Relation to Root Plastic Development, Oxygen Transport, and Water Uptake of Rice under Transient Soil Moisture Stresses. Plant Soil 2010, 332, 87–104. [Google Scholar] [CrossRef]
- Niones, J.M.; Suralta, R.R.; Inukai, Y.; Yamauchi, A. Field Evaluation on Functional Roles of Root Plastic Responses on Dry Matter Production and Grain Yield of Rice under Cycles of Transient Soil Moisture Stresses Using Chromosome Segment Substitution Lines. Plant Soil 2012, 359, 107–120. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Surendran, U.; Gopinath, G.; Chandran, K.M.; NK, A.; CT, M.F. Elucidation of Stage Specific Physiological Sensitivity of Okra to Drought Stress through Leaf Gas Exchange, Spectral Indices, Growth and Yield Parameters. Agric. Water Manag. 2019, 222, 92–104. [Google Scholar] [CrossRef]
- Barzegar, T.; Moradi, P.; Nikbakht, J.; Ghahremani, Z. Physiological Response of Okra Cv. Kano to Foliar Application of Putrescine and Humic Acid under Water Deficit Stress. Int. J. Hortic. Sci. Technol. 2016, 3, 187–197. [Google Scholar]
- Bahadur, A.; Singh, A.K.; Chaurasia, S.N.S. Physiological and Yield Response of Okra (Abelmoschus esculentus Moench.) to Drought Stress and Organic Mulching. J. Appl. Hortic. 2013, 15, 187–190. [Google Scholar] [CrossRef]
- Abid, M.; Tian, Z.; Ata-Ul-Karim, S.T.; Cui, Y.; Liu, Y.; Zahoor, R.; Jiang, D.; Dai, T. Nitrogen Nutrition Improves the Potential of Wheat (Triticum aestivum L.) to Alleviate the Effects of Drought Stress during Vegetative Growth Periods. Front. Plant Sci. 2016, 7, 981. [Google Scholar] [CrossRef]
- Saud, S.; Fahad, S.; Yajun, C.; Ihsan, M.Z.; Hammad, H.M.; Nasim, W.; Amanullah, Jr.; Arif, M.; Alharby, H. Effects of Nitrogen Supply on Water Stress and Recovery Mechanisms in Kentucky Bluegrass Plants. Front. Plant Sci. 2017, 8, 983. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A.; Alamri, S.A.M.; El-Mageed, T.A.A.; Abousekken, M.S.M.; Hashem, M. Role of Exogenous Nitrogen Supply in Alleviating the Deficit Irrigation Stress in Wheat Plants. Agric. Water Manag. 2018, 210, 261–270. [Google Scholar] [CrossRef]
- Wei, G.; Pufan, Z.; Li, T.; Mei, G.; Lixin, Z.; Aisha, A.N.; Muhammad, A. Exogenous Application of Urea and a Urease Inhibitor Improves Drought Stress Tolerance in Maize (Zea mays L.). J. Plant Res. 2017, 130, 599–609. [Google Scholar]
- Sparks, D.L. Soil Chemistry, Soil Fertility, and Nutrient Management; John Wiley and Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Brady, N.C.; Weil, R.R. Elements of the Nature and Properties of Soils, 2nd ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2004. [Google Scholar]
- Dow, G.J.; Bergmann, D.C.; Berry, J.A. An integrated model of stomatal development and leaf physiology. New Phytol. 2014, 201, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Young, T.E.; Gallie, D.R.; Demason, D.A. Ethylene-mediated programmed cell death during maize endosperm development of wild-type and shrunken2 genotypes. Plant Physiol. 1997, 115, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Limones, C.; HervÃs, A.; Navas-Cortés, J.A. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f.sp.ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol. Plant. 1996, 97, 104–110. [Google Scholar] [CrossRef]
- Planchet, E.; Verdu, I.; Delahaie, J. Abscisic acid-induced nitric oxide and proline accumulation in independent pathways under water-deficit stress duri ng seedling establishment in Medicago truncatula. J. Exp. Bot. 2014, 65, 2161–2170. [Google Scholar] [CrossRef]
- Xu, J.P.; Yu, Y.C.; Zhang, T.; Ma, Q.; Yang, H.B. Effects of ozone water irrigation and spraying on physiological characteristics and gene expression of tomato seedlings. Hortic. Res. 2021, 8, 180. [Google Scholar] [CrossRef]
- Adejumo, S.A.; Ezeh, O.S.; Mur, L.A.J. Okra Growth and Drought Tolerance When Exposed to Water Regimes at Different Growth Stages. Int. J. Veg. Sci. 2019, 25, 226–258. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, M.N.; Luo, T.; Zhang, K.K. Compensation of high nitrogen toxicity and nitrogen deficiency with biochar amendment through enhancement of soil fertility and nitrogen use efficiency promoted rice growth and yield. Glob. Chang. Biol. Bioenergy 2021, 13, 1765–1784. [Google Scholar] [CrossRef]
- Fatima, S.; Khan, M.S.; Nadeem, M.; Khan, I.; Waseem, K.; Nisar, M.; Iqbal, M. Interactive Effects of Genotype and Nitrogen on the Phenology and Yield Determination of Okra [Abelmoschus esculentus (L.)]. Int. J. Plant Prod. 2019, 13, 73–90. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Basra, N.K.D.F.S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Teixeira, E.I.; George, M.; Herreman, T.; Brown, H.; Fletcher, A.; Chakwizira, E.; de Ruiter, J.; Maley, S.; Noble, A. The Impact of Water and Nitrogen Limitation on Maize Biomass and Resource-Use Efficiencies for Radiation, Water and Nitrogen. Field Crops Res. 2014, 168, 109–118. [Google Scholar] [CrossRef]
- Xiong, B.L.; Wang, S.W.; Wang, X.Y.; Ma, Q.; Chen, D.Q.; Yin, L.N.; Deng, X.P.; Chen, D.Q. Effects of nitrogenous fertilizer on leaf senescence of maize and the associate with carbon/nitrogen balance under drought stress. J. Maize Sci. 2016, 24, 138–146. [Google Scholar]
- Li, W.; Wang, Y.; Ren, H.; Guo, Z.; Li, N.; Zhao, C.; Xie, Z. Transcriptomic and physiological analyses identifying Lanzhou lily(Lilium davidii var. unicolor) drought adaptation strategies. Hortic. Plant J. 2023, 9, 145–157. [Google Scholar] [CrossRef]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the World: Improving Photosynthetic Efficiency for Sustainable Crop Production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef]
- Verma, K.K.; Liu, X.-H.; Wu, K.-C.; Singh, R.K.; Song, Q.-Q.; Malviya, M.K.; Song, X.-P.; Singh, P.; Verma, C.L.; Li, Y.-R. The Impact of Silicon on Photosynthetic and Biochemical Responses of Sugarcane under Different Soil Moisture Levels. Silicon 2019, 12, 1355–1367. [Google Scholar] [CrossRef]
- Massai, R.; Remorini, D.; Tattini, M. Gas Exchange, Water Relations and Osmotic Adjustment in Two Scion/Rootstock Combinations of Prunus under Various Salinity Concentrations. Plant Soil 2004, 259, 153–162. [Google Scholar] [CrossRef]
- Taheri, A.; Abad, H.H.S.; Nourmohammadi, G.; Ardabili, M.S. Investigating Quantitative and Qualitative Performance of Bread Wheat Genotypes under Different Climatic Conditions. Gesunde Pflanz. 2021, 73, 229–238. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop Yield, Plant Nutrient Uptake and Soil Physicochemical Properties under Organic Soil Amendments and Nitrogen Fertilization on Nitisols. Soil Tillage Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Bresta, P.; Nikolopoulos, D.; Economou, G.; Vahamidis, P.; Lyra, D.; Karamanos, A.; Karabourniotis, G. Modification of Water Entry (Xylem Vessels) and Water Exit (Stomata) Orchestrates Long Term Drought Acclimation of Wheat Leaves. Plant Soil 2011, 347, 179–193. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of Leaf Stomatal Density to Water Status and Its Relationship with Photosynthesis in a Grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- Keshavarz Mirzamohammadi, H.; Modarres-Sanavy, S.A.M.; Sefidkon, F.; Mokhtassi-Bidgoli, A. Irrigation and fertilizer treatments affecting rosmarinic acid accumulation, total phenolic content, antioxidant potential and correlation between them in peppermint (Mentha piperita L.). Irrig. Sci. 2021, 39, 671–683. [Google Scholar] [CrossRef]
- Mardomi, S.; Najafi, N.; Reyhanitabar, A.; Dehgan, G. Antioxidant Enzyme Activities and Dry Matter of Rice Plant as Affected by Interactions of Lead, Phosphorus and Zinc. Philipp. Agric. Sci. 2019, 102, 310–321. [Google Scholar]
- Davoudi, M.; Song, M.F.; Zhang, M.R.; Chen, J.F.; Lou, Q.F. Long-distance control of the scion by the rootstock under drought stress as revealed by transcriptome sequencing and mobile mRNA identification. Hortic. Res. 2022, 9, uhab033. [Google Scholar] [CrossRef]
- Hu, H.M.; Li, W. Effects of coupling water and fertilizer on physio-morphological indices of foxtail millet at seedling stage. Chin. J. Ecol. 2015, 34, 1917–1923. [Google Scholar]
- Zhang, P.; Zhang, Y.L.; Chi, D.C.; Zou, H.T.; Gao, N.; Qu, J.; Yu, N. The combined effect of irrigation and potassium fertilization on the physiological characteristics and yield of peanut. Chin. J. Eco-Agric. 2016, 24, 1473–1481. [Google Scholar]
- Li, L.; Zhang, Z.; Tian, H.; Mo, Z.; Ashraf, U.; Duan, M.; Wang, Z.; Wang, S.; Tang, X.; Pan, S. Roles of Nitrogen Deep Placement on Grain Yield, Nitrogen Use Efficiency, and Antioxidant Enzyme Activities in Mechanical Pot-Seedling Transplanting Rice. Agronomy 2020, 10, 1252. [Google Scholar] [CrossRef]
- Li, B.; Feng, Y.; Zong, Y.; Zhang, D.; Hao, X.; Li, P. Elevated Co(2)-Induced Changes in Photosynthesis, Antioxidant Enzymes and Signal Transduction Enzyme of Soybean under Drought Stress. Plant Physiol. Biochem. 2020, 154, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, R.; Zhang, G.; Guo, J.; Dong, Z. Effects of Soil Drought on Photosynthetic Traits and Antioxidant Enzyme Activities in Hippophae Rhamnoides Seedlings. J. For. Res. 2016, 28, 255–263. [Google Scholar] [CrossRef]
- Edreva, A. Generation and scavenging of reactive oxygen species in chloroplasts: A submolecular approach. Agric. Ecosyst. Environ. 2005, 106, 119–133. [Google Scholar] [CrossRef]
- Guo, Z.W.; Hu, J.J.; Chen, S.L.; Li, Y.C.; Yang, Q.P.; Cai, H.J. Nitrogen Addition and Clonal Integration Alleviate Water Stress of Dependent Ramets of Indocalamus Decorus under Heterogeneous Soil Water Environment. Sci. Rep. 2017, 7, 44524. [Google Scholar] [CrossRef]
- Filippou, P.; Antoniou, C.; Fotopoulos, V. The Nitric Oxide Donor Sodium Nitroprusside Regulates Polyamine and Proline Metabolism in Leaves of Medicago Truncatula Plants. Free Radic. Biol. Med. 2013, 56, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Miyazaki, S.; Veronese, P.; Fujita, T.; Ibeas, J.I.; Damsz, B.; Narasimhan, M.L.; Hasegawa, P.M.; Joly, R.J.; Bressan, R.A. Does Proline Accumulation Play an Active Role in Stress-Induced Growth Reduction? Plant J. 2002, 31, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Wang, Z.; Zhu, G.; Yu, K.; Li, G.; Long, H. Stable Soil Moisture Improves the Water Use Efficiency of Maize by Alleviating Short-Term Soil Water Stress. Front. Plant Sci. 2022, 13, 833041. [Google Scholar] [CrossRef] [PubMed]
- Tayyab, N.; Naz, R.; Yasmin, H.; Nosheen, A.; Keyani, R.; Sajjad, M.; Hassan, M.N.; Roberts, T.H. Combined Seed and Foliar Pre-Treatments with Exogenous Methyl Jasmonate and Salicylic Acid Mitigate Drought-Induced Stress in Maize. PLoS ONE 2020, 15, e0232269. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jamsheed, S.; Hameed, A.; Rasool, S.; Sharma, I.; Azooz, M.; Hasanuzzaman, M. Drought Stress Induced Oxidative Damage and Antioxidants in Plants. Oxidative Damage Plants 2014, 11, 345–367. [Google Scholar]
- Silber, A.; Xu, G.; Levkovitch, I. High fertigation frequency: The effects on uptake of nutrients, water and plant growth. Plant Soil 2003, 253, 467–477. [Google Scholar] [CrossRef]

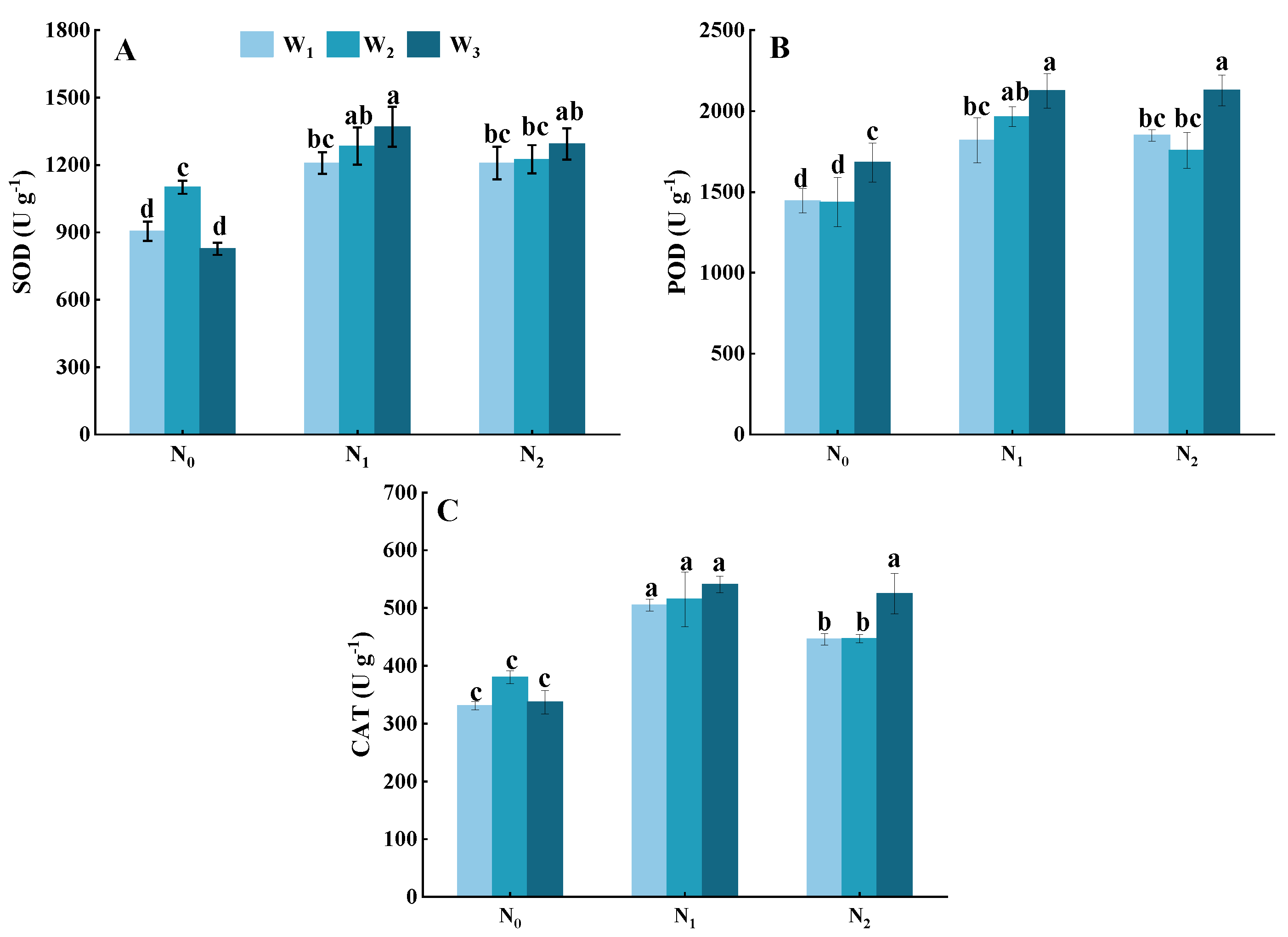
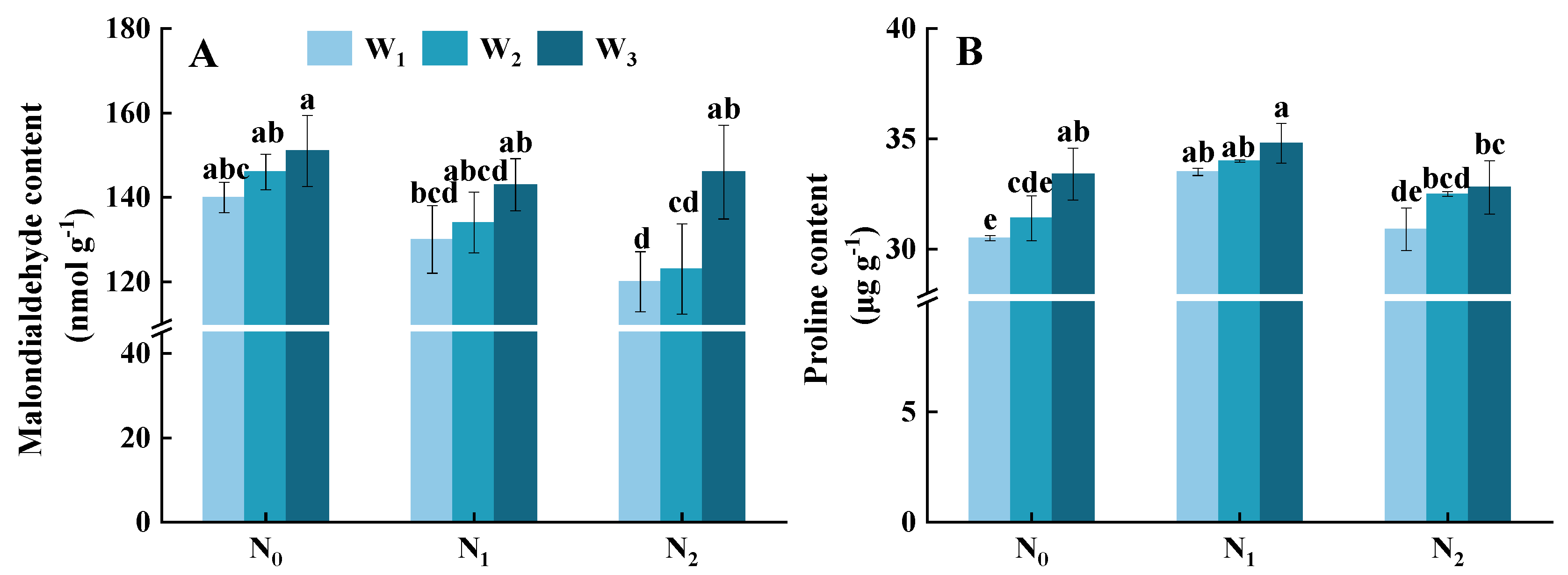

| Soil Texture(%) | Field Capacity (v/v%) | Soil Bulk Density (g cm−3) | Total Nitrogen (g kg−1) | Total Phosphorus (g kg−1) | Alkaline Hydrolysis Nitrogen (mg kg−1) | Available Phosphorus (mg kg−1) | Available Potassium (mg kg−1) | Organic Matter (g kg−1) | pH Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | |||||||||
| 43.2 | 25.2 | 31.5 | 27.6 | 1.1 | 1.28 | 0.84 | 164.5 | 10.3 | 200.0 | 19.63 | 5.53 |
| Treatment | Plant Height (cm) | Stem Diameter (mm) | Maximum Leaf Length (cm) | Maximum Leaf Width (cm) | Number of Leaves | |
|---|---|---|---|---|---|---|
| N0 | W1 | 38.7 ± 6.13 abc | 12.1 ± 0.17 b | 15.8 ± 0.47 ab | 22.3 ± 0.94 abc | 15.7 ± 0.94 bc |
| W2 | 34.3 ± 4.92 bc | 11.9 ± 0.23 b | 15.5 ± 1.47 ab | 21.0 ± 1.63 bcd | 15.7 ± 0.47 bc | |
| W3 | 34.3 ± 2.05 bc | 10.9 ± 0.45 c | 14.3 ± 0.94 b | 20.3 ± 0.94 cd | 14.3 ± 0.47 c | |
| N1 | W1 | 40.0 ± 1.63 ab | 13.5 ± 0.56 a | 16.0 ± 0.82 ab | 24.7 ± 0.47 a | 20.0 ± 2.16 a |
| W2 | 36.0 ± 0.82 abc | 13.0 ± 0.45 a | 15.7 ± 0.47 ab | 23.3 ± 0.47 ab | 17.0 ± 0.82 abc | |
| W3 | 34.7 ± 2.49 bc | 11.5 ± 0.61 bc | 15.3 ± 0.47 ab | 21.5 ± 1.47 bcd | 15.3 ± 2.62 bc | |
| N2 | W1 | 42.2 ± 0.62 a | 12.0 ± 0.65 b | 17.2 ± 1.03 a | 24.7 ± 1.70 a | 17.7 ± 1.25 abc |
| W2 | 36.0 ± 1.41 abc | 12.1 ± 0.23 b | 14.3 ± 0.24 b | 20.3 ± 0.47 cd | 16.7 ± 0.47 bc | |
| W3 | 32.7 ± 1.70 c | 9.2 ± 0.33 d | 12.3 ± 0.24 c | 19.3 ± 0.47 d | 14.3 ± 0.47 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Huang, Y.; Zhang, R.; Niu, L.; Long, H. Appropriate Nitrogen Application for Alleviation of Soil Moisture-Driven Growth Inhibition of Okra (Abelmoschus esculentus L. (Moench)). Horticulturae 2024, 10, 425. https://doi.org/10.3390/horticulturae10050425
Xu S, Huang Y, Zhang R, Niu L, Long H. Appropriate Nitrogen Application for Alleviation of Soil Moisture-Driven Growth Inhibition of Okra (Abelmoschus esculentus L. (Moench)). Horticulturae. 2024; 10(5):425. https://doi.org/10.3390/horticulturae10050425
Chicago/Turabian StyleXu, Shenghui, Yunxiang Huang, Renlian Zhang, Li Niu, and Huaiyu Long. 2024. "Appropriate Nitrogen Application for Alleviation of Soil Moisture-Driven Growth Inhibition of Okra (Abelmoschus esculentus L. (Moench))" Horticulturae 10, no. 5: 425. https://doi.org/10.3390/horticulturae10050425
APA StyleXu, S., Huang, Y., Zhang, R., Niu, L., & Long, H. (2024). Appropriate Nitrogen Application for Alleviation of Soil Moisture-Driven Growth Inhibition of Okra (Abelmoschus esculentus L. (Moench)). Horticulturae, 10(5), 425. https://doi.org/10.3390/horticulturae10050425






