Abstract
In this study, the physiological indicators, reactive oxygen species (ROS) levels, and activities and expressions of key enzymes related to ROS metabolism were monitored to explore the mechanism of ozone treatment on the shelf life of postharvest Korla fragrant pears. The results show that postharvest fragrant pears treated with ozone had a higher firmness and lower weight loss rate and decay rate during their shelf life, especially in the late stage. Ozone treatment could also delay the occurrence of the respiratory peak and reduce the peak value. The generation rate of superoxide anion (O2−), the hydrogen peroxide (H2O2) content, and the malondialdehyde (MDA) level were reduced in the ozone-treated group, while the activities of key enzymes, superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), related to ROS metabolism in postharvest fragrant pears were stimulated by ozone treatment, especially in the middle and late stages of shelf life. Analysis of the proteomics results indicates that the POD family enzymes were the main target protein in postharvest fragrant pears treated by ozone during the middle and late stages of shelf life. The activity and expression of antioxidant-related enzymes in postharvest fragrant pears were stimulated by ozone to accelerate the metabolism of ROS and maintain high quality, especially in the middle and late shelf lives.
1. Introduction
Korla fragrant pear (Pyrus sinkiangensis) is an ancient pear variety originating from Korla, Xinjiang province, China [1,2]. It was a kind of favorite fruit among consumers in autumn and winter due to its thin skin, high water content, crispy texture, sweet flesh, and richness in vitamin C and other minerals [2]. In addition, the year-round market supply of Korla fragrant pears has been made possible with the continuous development of low-temperature storage and preservation technology [3,4]. For instance, ‘Korla’ fragrant pears can be stored at 0 °C for 120 days without a significant impact on quality. However, the quality of pears declines rapidly when the fruit is transferred from a low-temperature environment to shelf-life temperature (e.g., usually 0 °C). This includes a decrease in firmness, decay, and water loss [5]. It is difficult for retailers to maintain the low temperatures required to preserve pears because of the high cost of cold storage, especially in China [6]. A simple and convenient way to extend the shelf life of ‘Korla’ pears is needed urgently.
Ozone is an active gas with the advantages of no residue and broad-spectrum sterilization compared with ordinary chemical preservatives [7]. Studies have shown that the shelf life of raspberries [8], mulberries [9], bananas [10], and kiwis [11] was extended by increasing antioxidant enzyme activity and gene expression, with produce quality being maintained using ozone treatment. Ernandes et al. have found that ozone fumigation significantly slowed down the ripening of pears (Pyrus communis L.) at room temperature, but the mechanisms of how the treatment affected the ripening process were unclear [12]. A large number of studies have shown that an accumulation of active oxygen is a sign of maturity and quality decline in harvested fruits and vegetables [13,14]. The antioxidant enzymatic activity system, phenylalanine metabolism, and ascorbic acid pathway of melon [15], strawberry [16], winter jujube [17], and ‘Qiushui’ pear [18] were activated by ozone to increase antioxidant capacity and extend shelf life. However, there is almost no research on the antioxidant mechanism of ozone treatment in maintaining the quality of fragrant pears during shelf life.
The application of proteomics can be used to provide evidence that the antioxidant capacity of postharvest fresh produce was induced by ozone treatment. Specifically, label-free proteomics was found to be an effective way to provide information and unveil the latent mechanism [17]. Ioannis et al. have found that the expression of proteins related to antioxidant defense during the ripening process of ozone-treated kiwifruit was identified using one-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (1D-SDS-PAGE) and mass spectrometry [19], which was a starting point for the practical development of ozone-based treatment strategies to control fruit ripening and quality [19]. Luo et al. have found that the level of protein-associated antioxidants and the redox system as well as phenylpropanoid metabolism are directly linked to the soft scald development of ‘Ambrosia’ apple fruit at low temperatures using label-free proteomics [20]. Zhang et al. have also concluded that the abundance of the protein associated with the ascorbic acid–glutathione (ASA-GSH) pathway detected by label-free proteomics of postharvest strawberries had accumulated in the ozone-treated group compared with the control group [16]. The combined analysis of label-free quantification proteomics and a weighted gene co-expression network (WGCNA) unveiled that the metabolites of ribosome and glutathione were stimulated in ozone-treated winter jujube [17].
In this study, Korla fragrant pears (Pyrus sinkiangensis) planted in Xinjiang, China, and stored in cold storage for 60 days were used as research materials to investigate the impact of ozone treatment on shelf-life quality. The physical traits, ROS levels, and antioxidant enzyme (SOD, CAT, and POD) activities of fragrant pears were detected throughout the shelf life period. Label-free proteomics was used to analyze the expression profiles of related antioxidant proteins, and correlation models of physiological traits, antioxidant indicators, and the level of protein were constructed to further reveal the mechanism of the ozone preservation effect on postharvest fragrant pears during shelf life.
2. Material and Method
2.1. Fruit Samples and Ozone Treatment
Korla fragrant pears (Pyrus sinkiangensis) from the Aksu region of Xinjiang province were sourced, individually packaged in foam mesh bags, and transported to the National Engineering Technology Research Center for Preservation of Agriculture Product, Tianjin, China. Pears with uniform size (≈110 g), no diseases, and free of mechanical damage were selected and stored in cold storage at 0 ± 0.5 °C for 60 days. Then, about of the 100 pears were transferred to an ozone storage device developed by our research team, with the temperature set to 20 ± 0.5 °C [16]. The ozone treatment group (OT) was separated and treated with 1 ppm ozone for 1 h every 4 days (the final concentration of 1 ppm of ozone was previously tasted in this study), and the control group (CK) was treated with air. The test samples of fragrant pears were not treated with ozone on the first day of shelf life.
2.2. Rotting Rate
The incidence of rotten fruit during storage was calculated using the following formula:
2.3. Respiration Rate
About 500 g of the pears were sealed in a 3 L seal tank for three hours, and the respiration rate of postharvest pears was detected by a gas analyzer (CheckPoint O2/CO2, Dongguan spectrum label experimental equipment technology Co., Ltd., Dongguan, China), according to the method of Trina; the results were represented in mg CO2·kg−1·h−1 [15].
2.4. Weight Loss
The weight loss rate was determined by the following formula:
where m0 is the mass of pears before storage, and m1 is the mass of the sampling point.
2.5. Firmness
The firmness of the pears (2 cm × 2 cm) was measured after storage using a texture analyzer (TA.XT plus, Stable Micro Systems, Godalming, UK) with a 2 mm diameter probe. A tissue sample was compressed at a speed of 2 mm·s−1 on selected points in the equatorial region, with a final penetration depth of 20 mm. The firmness was defined as the recorded maximum compression force and was expressed in kg·cm−2.
2.6. Malondialdehyde (MDA) Content
The MDA content was detected using the Thiobarbituric acid colorimetric method according to the method of Li et al. [21], and the results were represented by nmol·g−1.
2.7. Superoxide Anion (O−−) Generation Rate and Hydrogen Peroxide (H2O2) Content
The O2− generation rate and H2O2 content were determined using the Plant ELISA Kit (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China) and the H2O2 kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively, as we described in our previous study [16]. The results of the O2− generation rate were described as nmol min−1·g−1, and H2O2 content was represented by μmol·L−1.
2.8. Superoxide Dismutase (SOD), Catalase (CAT), and Peroxidase (POD) Activity
The activities of pear catalase (CAT) and superoxide dismutase (SOD) were detected using commercial detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions provided by the manufacturer [16]. CAT and SOD activity units are expressed in U·g−1. The POD activity of postharvest pear was detected according to the method of Li et al. [22]. Hydrogen peroxide (0.24 mL, 250 mmol·L−1) and guaiacol (3.0 mL, 25 mmol·L−1) were mixed with crude enzyme (240 μL) to determine POD activity. All three enzyme activities were expressed by U·g−1.
2.9. Proteomics Detection and Analysis
The protein extraction of pear was conducted using the methods described in our previous study [16,17]. Approximately 1 g of tissue was separately ground and digested with trypsin (substrate to enzyme mass to mass ratio of 50:1) at 37 °C for 12 h. The digested peptides were analyzed on a Q-Extractive HF mass spectrometer (Thermo Scientific, Waltham, MA, USA) coupled with an Ultimate 3000 system (Thermo Scientific). The tryptic samples were resuspended with buffer A (water, 0.1% formic acid) and then separated with a C18 column (120 Å, 2 μm, 75 μm × 15 mm) at a flow rate of 0.6 μL·min−1.
The gradient elution profile was as follows: 4% buffer B (80% ACN, 0.1% formic acid) for 100 min, 34% buffer B for 15 min, 45% buffer B for 4 min, 99% buffer B for 4 min, 4% buffer B for 5 min. Sequest and Proteome Discoverer2.2.0.388 (Thermo Scientific) were used for library identification and quantitative analysis. Database search was performed through the UniProtKB pear database, where a total of 87,242 protein sequences were included.
2.10. Statistic Analysis
All measurements were performed in triplicate. The statistics were calculated using SPSS (version 22, IBM, Armonk, NY, USA) and Origin software (version 9.1, OriginLab, Northampton, MA, USA). The data were expressed as mean ± standard deviation and a difference was considered to be significant at the 95% level of significance (p < 0.05) using one-way ANOVA with Tukey’s adjustment.
3. Results and Discussion
3.1. Physical Quality Traits
The rot incidence and weight loss of both the control and treatment groups gradually increased with the progression of shelf time (Figure 1). However, the rot incidence of fragrant pears was lower in the ozone-treated group after four days of shelf life, with a more pronounced difference observed after the 8 days (Figure 1a). The increase in weight loss rate was also slowed down by ozone treatment, especially after 12 days (Figure 1c).
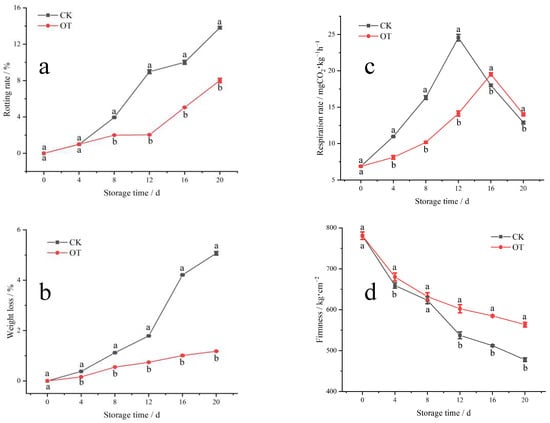
Figure 1.
The effect of ozone treatment on rot incidence (a), weight loss (b), respiration rate (c), and firmness (d) of fragrant pears during shelf life. Different letters indicate a significant difference between the ozone-treated (OT) group and the control group (CK) at the same time (p < 0.05), and the same letter indicates no significant difference.
Respiration rate is closely related to the metabolism and senescence of fruit, and the acceleration of respiration rate is considered to be a sign of senescence [23]. As shown in Figure 1b, the respiration rate of postharvest fragrant pears increased continuously until it peaked at approx. 12–16 days after storage, and then decreased during the later stage of shelf life. This corresponded to an increase in metabolic activities when the pears were transferred from cold to room temperatures in the early stage of shelf life, followed by senescence at room temperature [5,24], and subsequently the development of decay in the later stage. The respiration rate and peak value of the fragrant pears in the ozone-treated group were reduced, and the occurrence of the peak respiratory rate was delayed from the 12th day to the 16th day compared with the control group (Figure 1b).
The firmness of the fragrant pears gradually decreased during shelf-life storage, and the firmness of the ozone-treated group was better retained compared to that of the control group, especially after a shelf life of 8 days (Figure 1d).
3.2. Antioxidant Index
The removal of active oxygen from harvested fruits and vegetables is a crucial issue to maintaining quality and extending shelf life. A large number of studies have found that the effective removal of reactive oxygen species (ROS) can extend the shelf life of harvested fruits and vegetables [14]. Superoxide anion and hydrogen peroxide are the two most important active oxygen species in harvested fruits and vegetables [13,16].
As shown in Figure 2a,b, the production rate of superoxide anions and the accumulation of hydrogen peroxide content in fragrant pears increased rapidly during early shelf-life storage and then decreased after reaching a peak on the 12th day. The accumulation amount and production rate of reactive oxygen species in the ozone-treated group were inhibited, and the appearance of the peak values was delayed from day 12 to day 15 compared with the control group. Zhang et al. have also found that the generation rates of O2− and H2O2 in postharvest strawberries were inhibited by ozone treatment [16]. Membrane lipid peroxidation was found to be related to the level of lipid oxidation in cell membranes, reflecting the degree of oxidative damage in harvested fruits and vegetables [25]. The degree of membrane lipid peroxidation in postharvest fragrant pears continued to intensify as the shelf life extended, and it rose rapidly after 12 days (Figure 2c). Ozone treatment did not change the trend of membrane lipid peroxidation in postharvest fragrant pears but did reduce the degree of peroxidation. The activities of key antioxidant enzymes, SOD, CAT, and POD, in the ozone-treated and the control groups all showed a trend of gradual increase followed by a rapid decrease (Figure 2d–f). The activities of SOD and CAT in the fragrant pears treated with ozone were higher than those of the control group, and the activity of POD was also higher than that of the control group, except on the 12th day. Additionally, the ozone treatment extended the peak period of SOD, CAT, and POD enzyme activities of from the 12th day to 16th day. In general, the superoxide anion generation rate of postharvest fragrant pears gradually increased with storage time, which stimulated the increase in SOD enzyme activity, and the hydrogen peroxide produced by the metabolism of superoxide anion induced the activities of the POD and CAT enzymes. The antioxidant enzyme activity of fragrant pears was promoted by ozone to improve the antioxidant capacity of the tissue and maintain fruit quality. This finding is in agreement with previous work on Acibenzolar-S-methyl treated pears [25,26], and Zhao et al. have also concluded that the antioxidant enzyme activities of postharvest pears were stimulated by ozone at appropriate concentrations, especially the POD enzyme [18]. In addition, the effect of ozone treatment on postharvest fragrant pears was more pronounced in the later period of shelf life.
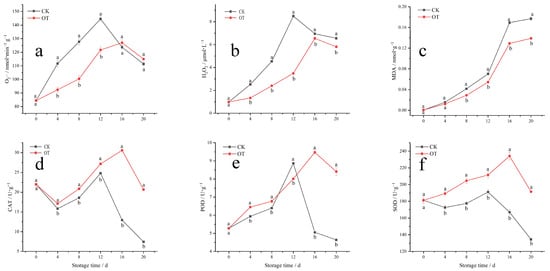
Figure 2.
The effect of ozone treatment on the O2− (a), H2O2 (b), MDA (c), CAT (d), POD (e), and SOD (f) in fragrant pears during shelf life. Different letters indicate a significant difference between the OT group and the control group at the same time (p < 0.05), and the same letter indicates no significant difference.
3.3. Identification and Expression Analysis of Antioxidant Proteins
Label-free proteomics was used to further analyze the effect of ozone treatment on postharvest fragrant pear antioxidant enzymes. A total of 19 SOD, CAT, and POD enzymes were identified, including 10 POD enzymes, 7 SOD enzymes, and 2 CAT enzymes (Table 1).

Table 1.
Differentially expressed proteins of the Korla fragrant pear proteome.
According to the method of Chen et al. [27], a fold change (FC) greater than 1.5 and less than 0.67 represent up-regulation and down-regulation of protein expression, respectively. Figure 3 shows that the expressions of POD2, POD3, POD5, and POD9 were increased in the ozone-treated group compared to the control group at the early stage of shelf life (Figure 3a), while no significant difference in the expression of CAT and SOD proteins was found between the two groups (Figure 3b,c). The POD7, POD7, and SOD6 proteins in the ozone treatment group accumulated on the 12th day of shelf life, and the high expression levels of POD2, POD5, SOD2, and SOD6 were detected in the ozone treatment group on the 16th day of shelf life (Figure 3a). These results suggest that the POD proteins of postharvest pears may be the key proteins induced by ozone during early shelf life, and that the rapid metabolism of reactive oxygen species in the ozone-treated group at the end of the shelf life was achieved through the antioxidant network of SOD and POD enzymes [28]. On the 16th day of shelf life, the respiration rate, superoxide anion production rate, and hydrogen peroxide content of the postharvest fragrant pears in the ozone treatment group reached their peaks, which were consistent with the peaks of SOD, POD, and CAT enzyme activities, while the difference in the protein expression levels of SOD2, SOD6, CAT2, and POD2 also reached the maximum fold. Li et al. have also concluded that the decrease in postharvest ‘Yali’ pear quality was related to the decrease in the enzyme activities of SOD, CAT, and POD [29]. The gene expression of POD was stimulated in postharvest ‘Zaosu’ and ‘Nanguo’ pears to maintain high quality during storage time [26,30].
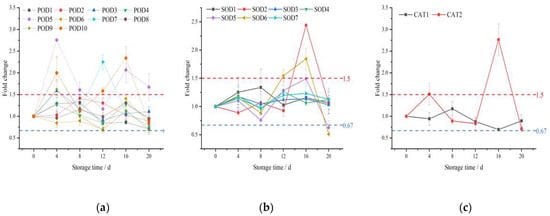
Figure 3.
The fold change (FC, ratio between OT and CK) of the SOD, CAT, and POD proteins in fragrant pears during shelf life.
3.4. Correlation between Physicochemical Quality and Proteins
Linear models and Pearson coefficients were used to analyze the correlation between the dynamic changes in physiological indicators, enzyme activity, and protein expression in postharvest fragrant pears of the OT group/CK group throughout the shelf period. Correlation coefficients of R > 0.75 and <−0.75 represent positive and negative correlations, respectively. As shown in Figure 4, there was a significant positive correlation between the activities of POD, SOD, and CAT enzymes (p < 0.05), which indicates that the regulatory pattern of antioxidant enzymes is unified. The changes in CAT and SOD enzyme activities were consistent with the changes in firmness during the shelf life of postharvest fragrant pears. The generation rate of O2−, H2O2 content, and changes in respiration rate showed a clear positive correlation (p < 0.05), and some studies have found that the increase in respiration rate and the accumulation of ROS were signs of aging in harvested fruits and vegetables [31,32]. The MDA content showed a clear positive correlation with the weight loss rate and a clear negative correlation with firmness (p < 0.05), and the permeability of harvested fruit and vegetable cells was induced by the peroxidation of cell membranes, resulting in a decrease in hardness and an increase in weight loss rate [33].
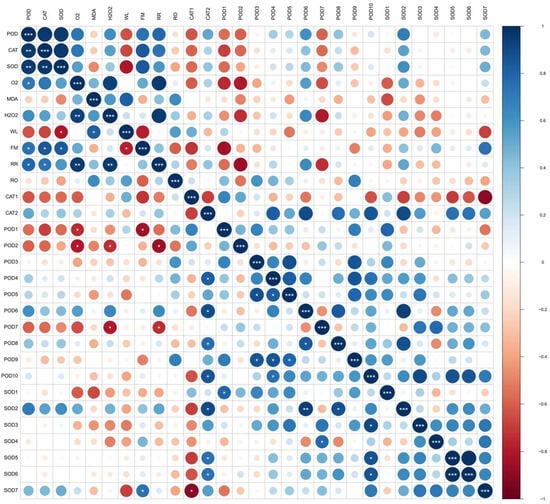
Figure 4.
The linear correlation analysis between the dynamic changes in physiological indicators, enzyme activity, and protein expression in postharvest fragrant pears of the treatment group/control group throughout the shelf period. Where WL is weight loss, RO is the rotting rate, RR is the respiration rate, and FM is the firmness. Additionally, *** indicates extremely significant differences at p < 0.001, ** indicates significant differences at p < 0.01, and * indicates significant differences at p < 0.05.
These statistical results indicate that the accumulation of ROS induced a deterioration in the quality of the fragrant pears, whilst an enhancement of antioxidant capacity was promoted by enzymatic activities and improved the physiological quality of the pears. The same results were found in 1-MCP-treated ‘Gem’ pears [34] and Nanguo pears [35]. There were obvious aggregations and correlations between the expression patterns of different families of proteins, but no significant linear correlation was found between protein expression patterns and physiological indicators in fragrant pears. POD5 and POD9 were positively correlated with multiple POD family proteins. Similarly, expression of the SOD2 protein was closely related to the expression of proteins in the POD family (Figure 4), indicating that POD family proteins, especially POD5 and POD9, may be key proteins implicated in the shelf life of postharvest pears.
4. Conclusions
Ozone treatment was an effective way to extend the shelf life of ‘Korla’ fragrant pears. Compared with the control group, ozone treatment could delay the occurrence of the respiration rate peak and reduce the peak value. The weight loss rate and decay rate of the ozone-treated fragrant pears were lower, and the firmness retention was better, especially in the middle-to-late stages of the shelf life. Ozone treatment reduced the accumulation of ROS and the degree of membrane lipid peroxidation in fragrant pears during shelf life, and it increased the enzymatic activities and protein abundance of SOD, CAT, and POD, especially of POD2, POD5, POD9, and SOD2. The correlation analysis suggests that the superior quality of fragrant pears treated by ozone may be due to the combined effect of the activity and expression of antioxidant system enzymes.
Author Contributions
Conceptualization, C.D. and M.L.; methodology, P.Z., H.J. and X.M.; data curation, C.G., N.Z. and C.C.; writing—original draft preparation, X.Z.; writing—review and editing, S.L. and Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [Cunkun Chen, grant number 32172268]; Natural Science Foundation of Tianjin [Cunkun Chen, grant number 23JCYBJC00840]; Research, development and application of key technologies for freshness preservation in the supply chain of several bulk vegetables [Cunkun Chen, grant number XLYC2204024]; Research, development and demonstration of key new technologies for preservation and damage reduction of Northwest specialty fruits [Na Zhang, grant number 23ZYCGSN00920]; Demonstration and promotion of green storage, preservation and quality control technology of winter pear [Na Zhang, grant number 23CXNA0026]; Laboratory of Storage of Agricultural Products, Ministry of Agriculture and Rural Affairs [Xiaojun Zhang, grant number kf2024002].
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to application for intellectual property rights.
Conflicts of Interest
Author Changyu Gu was employed by the company Sinolight Inspection & Certification Co., Ltd., Beijing, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhang, Y.; Liu, H.; Gong, P.; He, X.; Wang, J.; Wang, Z.; Zhang, J. Irrigation method and volume for korla fragrant pear: Impact on soil water and salinity, yield, and fruit quality. Agronomy 2022, 12, 1980. [Google Scholar] [CrossRef]
- Han, S.; Zhao, J.; Liu, Y.; Xi, L.; Liao, J.; Liu, X.; Su, G. Effects of green manure planting mode on the quality of korla fragrant pears (Pyrus sinkiangensis yu). Front. Plant Sci. 2022, 13, 1027595. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Pan, L.; Sun, K.; Tu, S.; Sun, Y.; Wei, Y.; Tu, K. Differentiation of deciduous-calyx and persistent-calyx pears using hyperspectral reflectance imaging and multivariate analysis. Comput. Electron. Agric. 2017, 137, 150–156. [Google Scholar] [CrossRef]
- Jia, X.; Wang, W.; Du, Y.; Tong, W.; Wang, Z.; Gul, H. Optimal storage temperature and 1-mcp treatment combinations for different marketing times of korla xiang pears. J. Integr. Agric. 2018, 17, 693–703. [Google Scholar] [CrossRef]
- Wang, J.; Lv, M.; He, H.; Jiang, Y.; Yang, J.; Ji, S. Glycine betaine alleviated peel browning in cold-stored ‘nanguo’ pears during shelf life by regulating phenylpropanoid and soluble sugar metabolisms. Sci. Hortic. 2020, 262, 109100. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Xu, X. Application and research progress of cold storage technology in cold chain transportation and distribution. J. Therm. Anal. Calorim. 2019, 139, 1419–1434. [Google Scholar] [CrossRef]
- Meena, N.K.; Vinod, B.R.; Menaka, M. Ethylene control in postharvest handling of fruits and vegetables. In Postharvest Physiology and Handling of Horticultural Crops; CRC Press: Boca Raton, FL, USA, 2024; Volume 167–195. [Google Scholar]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Sójka, M.; Balawejder, M. Changes in phenolic compounds profile and glutathione status in raspberry fruit during storage in ozone-enriched atmosphere. Postharvest Biol. Technol. 2020, 168, 111277. [Google Scholar] [CrossRef]
- Tabakoğlu, N.; Karaca, H. Effects of ozone-enriched storage atmosphere on postharvest quality of black mulberry fruits (Morus nigra L.). LWT 2018, 92, 276–281. [Google Scholar] [CrossRef]
- Siriprom, W.; Teanchai, K.; Chamchoi, N. Quality assessment of different ozone treatments to extend shelf-life of banana (Musa acuminata). Mater. Today Proc. 2022, 65, 2452–2455. [Google Scholar] [CrossRef]
- Allai, F.M.; Azad, Z.R.A.A.; Mir, N.A.; Gul, K. Recent advances in non-thermal processing technologies for enhancing shelf life and improving food safety. Appl. Food Res. 2022, 3, 100258. [Google Scholar] [CrossRef]
- R Alencar, E. Effectiveness of ozone on postharvest conservation of pear (Pyrus communis L.). J. Food Process. Technol. 2014, 5, 317. [Google Scholar] [CrossRef]
- Min, D.; Li, F.; Ali, M.; Zhang, X.; Liu, Y. Application of methyl jasmonate to control disease of postharvest fruit and vegetables: A meta-analysis. Postharvest Biol. Technol. 2024, 208, 112667. [Google Scholar] [CrossRef]
- Li, N.; Zhai, K.; Yin, Q.; Gu, Q.; Zhang, X.; Melencion, M.G.; Chen, Z. Crosstalk between melatonin and reactive oxygen species in fruits and vegetables post-harvest preservation: An update. Front. Nutr. 2023, 10, 1143511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, N.; Zhang, H.; Chen, C.; Li, L.; Dong, C.; Cheng, Y. Comparative transcriptomic analysis of cantaloupe melon under cold storage with ozone treatment. Food Res. Int. 2021, 140, 109993. [Google Scholar] [CrossRef]
- Zhang, H.; Li, K.; Zhang, X.; Dong, C.; Ji, H.; Ke, R.; Ban, Z.; Hu, Y.; Lin, S.; Chen, C. Effects of ozone treatment on the antioxidant capacity of postharvest strawberry. RSC Adv. 2020, 10, 38142–38157. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tian, Y.; Xing, J.; Chong, Y.; Chen, C.; Hou, Z. Coexpression modules constructed identifies regulation pathways of winter jujube (Ziziphus jujuba mill. ‘dongzao’) following postharvest treatment with ozone. Postharvest Biol. Technol. 2023, 197, 112183. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, G.; Han, Z.; Li, Q.; Chen, Y.; Li, D. Effect of ozone on the antioxidant capacity of “qiushui” pear (Pyrus pyrifolia nakai cv. Qiushui) during postharvest storage. J. Food Qual. 2013, 36, 190–197. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Belghazi, M.; Job, D.; Manganaris, G.A.; Molassiotis, A.; Vasilakakis, M. Physiological and proteomic approaches to address the active role of ozone in kiwifruit post-harvest ripening. J. Exp. Bot. 2012, 63, 2449–2464. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Song, J.; Toivonen, P.; Gong, Y.; Forney, C.; Palmer, L.C.; Fillmore, S.; Pang, X.; Zhang, Z. Proteomic changes in ‘ambrosia’ apple fruit during cold storage and in response to delayed cooling treatment. Postharvest Biol. Technol. 2018, 137, 66–76. [Google Scholar] [CrossRef]
- Li, H.; James, A.; He, X.; Zhang, M.; Cai, Q.; Wang, Y. Effect of hypobaric treatment on the quality and reactive oxygen species metabolism of blueberry fruit at storage. CYTA J. Food 2019, 17, 937–948. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Guo, Y.; Zhang, S.; Xu, H.; Ge, Y. Activation of the calcium signaling, mitogen-activated protein kinase cascade and phenylpropane metabolism contributes to the induction of disease resistance in pear fruit upon phenylalanine treatment. Postharvest Biol. Technol. 2024, 210, 112782. [Google Scholar] [CrossRef]
- Salehi, F. Recent advances in the modeling and predicting quality parameters of fruits and vegetables during postharvest storage: A review. Int. J. Fruit Sci. 2020, 20, 506–520. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Liu, Y.; Wang, S. Effect of mechanical vibration on postharvest quality and volatile compounds of blueberry fruit. Food Chem. 2021, 349, 129216. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Cheng, Y.; Hou, J.; Zhang, J.; Ge, Y. Postharvest application of acibenzolar-s-methyl delays the senescence of pear fruit by regulating reactive oxygen species and fatty acid metabolism. J. Agric. Food. Chem. 2020, 68, 4991–4999. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Ji, S. Influence of melatonin treatment on peel browning of cold-stored “nanguo” pears. Food Bioprocess Technol. 2020, 13, 1478–1490. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Zhang, H.; Ban, Z.; Li, L.; Dong, C.; Ji, H.; Xue, W. Label-free quantitative proteomics to investigate the response of strawberry fruit after controlled ozone treatment. RSC Adv. 2019, 9, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Wang, Y.; Li, B.; Gu, X.; Zhang, X.; Boateng, N.A.S.; Zhang, H. Effect of β-glucan on the biocontrol efficacy of cryptococcus podzolicus against postharvest decay of pears and the possible mechanisms involved. Postharvest Biol. Technol. 2020, 160, 111057. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Fan, X.; Wang, J.; Liang, L.; Yan, S.; Xiao, L. Relationship between activated oxygen metabolism and browning of “yali” pears during storage. J. Food Process. Preserv. 2020, 44, e14392. [Google Scholar] [CrossRef]
- Guo, Y.; Li, C.; Wang, M.; Xu, H.; Zhang, S.; Liu, J.; Jin, Y.; Ge, Y. Postharvest caffeic acid dipping enhances disease resistance and storage capacity of ‘zaosu’ pear fruit via regulating phenylpropane metabolism. Postharvest Biol. Technol. 2024, 209, 112716. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Chen, Y.; Wang, H.; Lin, M.; Ritenour, M.A.; Lin, Y. The role of ros-induced change of respiratory metabolism in pulp breakdown development of longan fruit during storage. Food Chem. 2020, 305, 125439. [Google Scholar] [CrossRef] [PubMed]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yang, H.; Cheng, S.; Zhang, Z.; Zhang, L.; Su, R.; Li, Y.; Zhan, X.; Yang, B.; Lin, L.; et al. Combination effects of ultrasound and citral nanoemulsion against shigella flexneri and the preservation effect on fresh-cut carrots. Food Control 2023, 155, 110069. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.; Einhorn, T.C. Postharvest physiology, storage quality and physiological disorders of ‘gem’ pear (Pyrus communis L.) Treated with 1-methylcyclopropene. Sci. Hortic. 2018, 240, 631–637. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Wei, B.; Cheng, S.; Zhou, Q.; Ji, S. Gaba application improves the mitochondrial antioxidant system and reduces peel browning in ‘nanguo’ pears after removal from cold storage. Food Chem. 2019, 297, 124903. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).