Lasiodiplodia iraniensis and Diaporthe spp. Are Associated with Twig Dieback and Fruit Stem-End Rot of Sweet Orange, Citrus sinensis, in Florida
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling and Fungal Isolation
2.2. Molecular Characterization
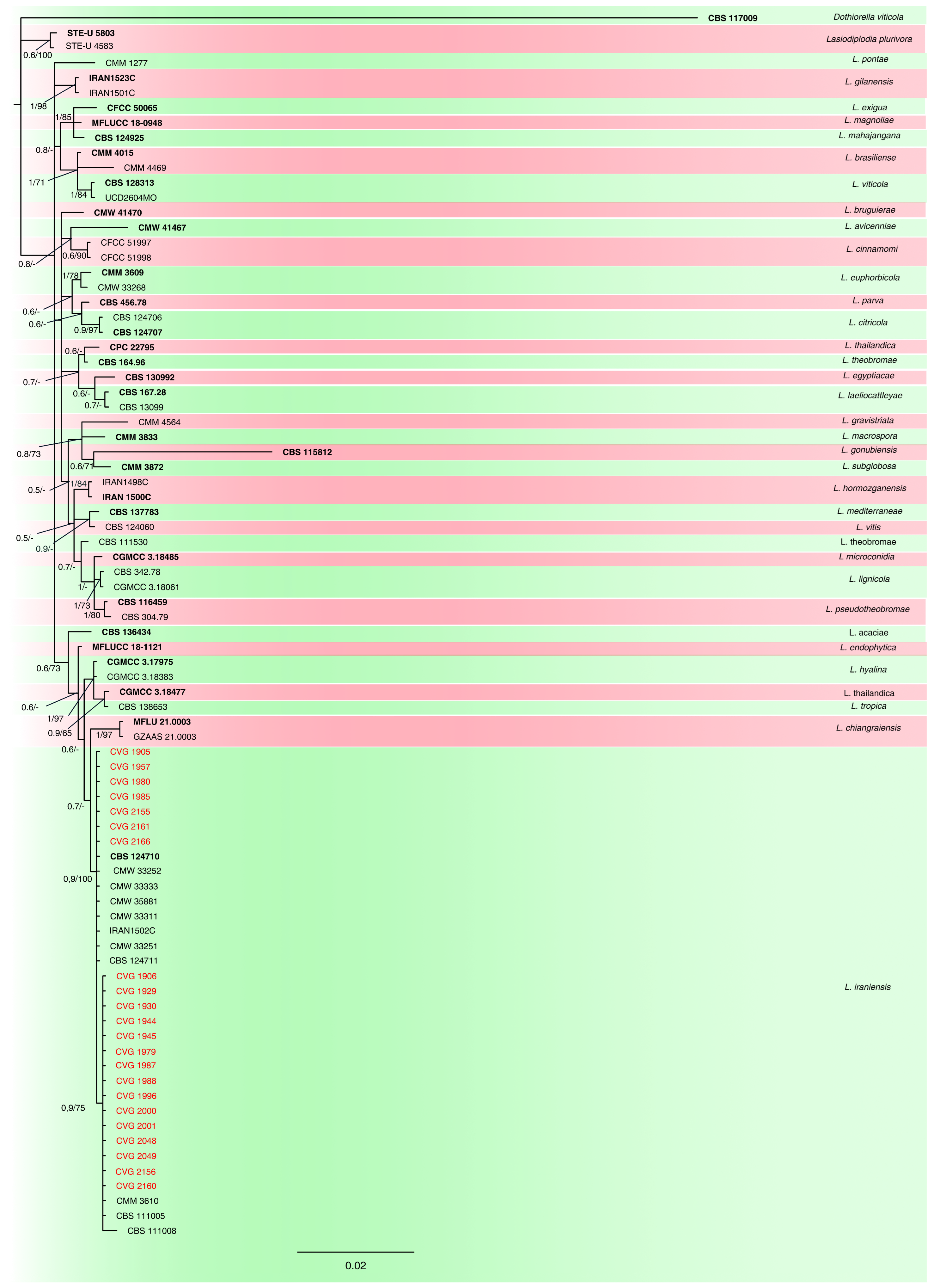
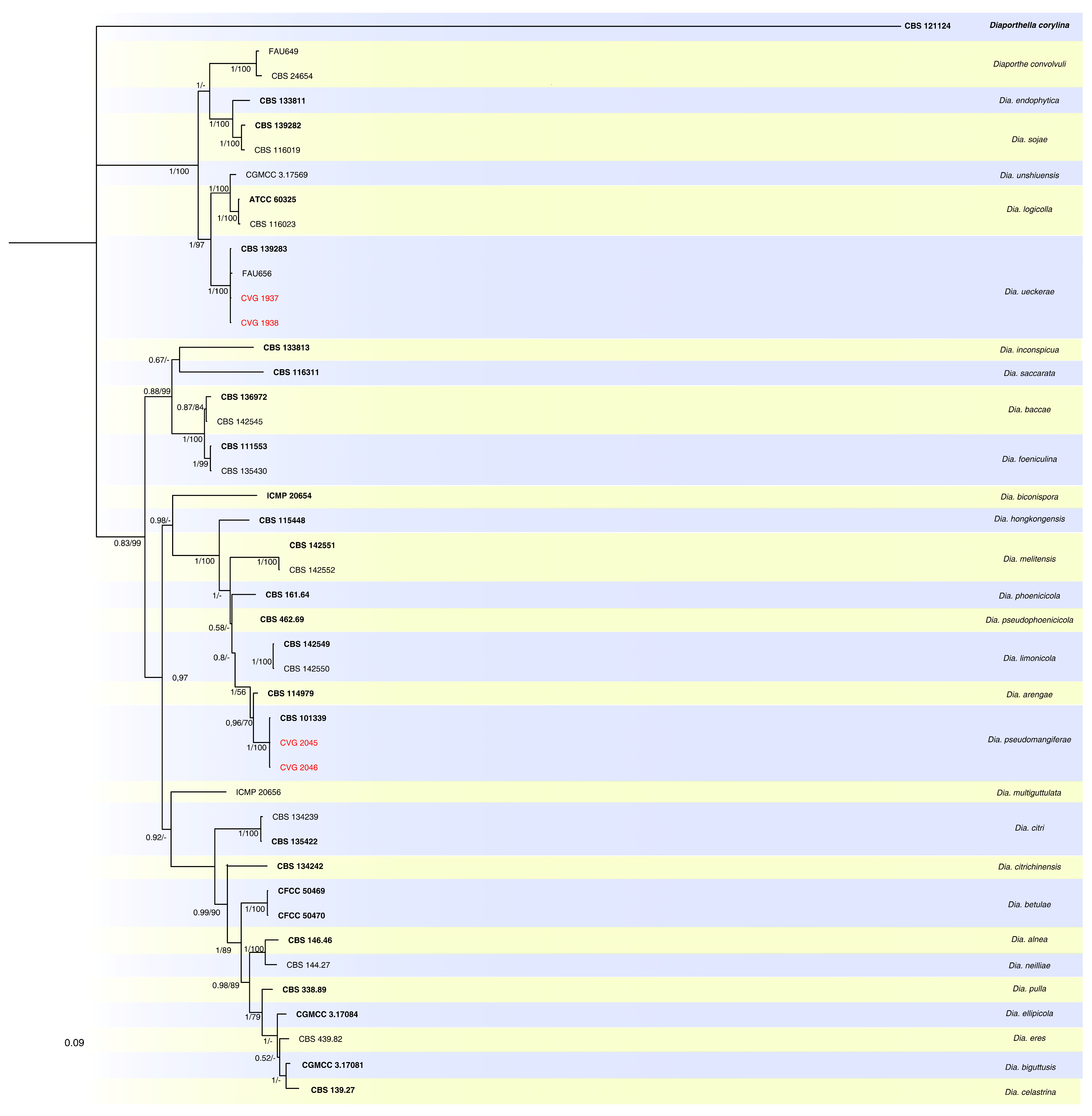
2.3. Phylogenetic Analyses
2.4. Morphological Characterization
2.5. Effect of Temperature on Mycelial Growth
2.6. Pathogenicity Tests
2.6.1. Pathogenicity on Fruit
2.6.2. Pathogenicity on Plants
2.7. Statistical Analysis
3. Results
3.1. Field Sampling and Isolation
3.2. Phylogenetic Analyses
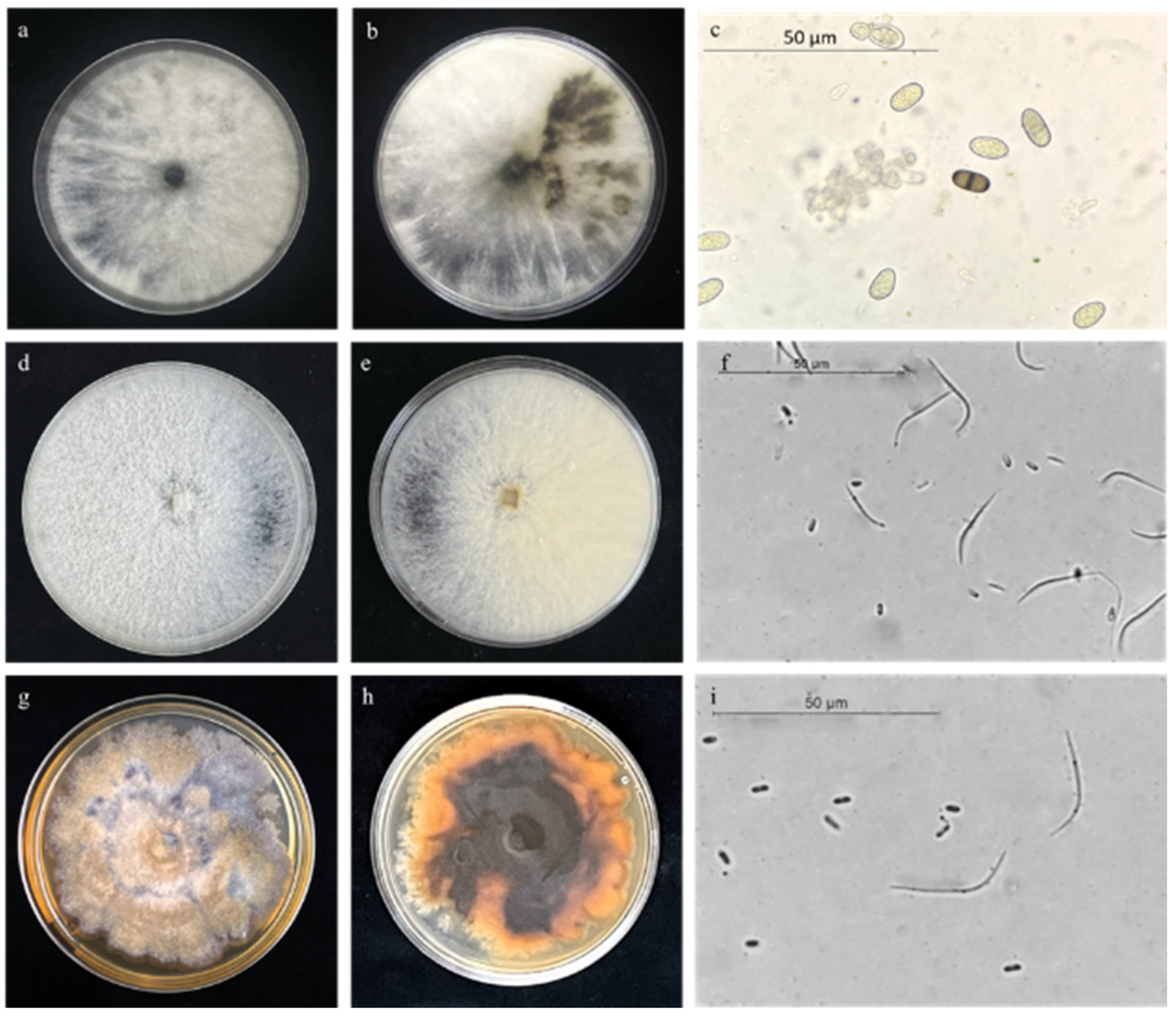
3.3. Morphology
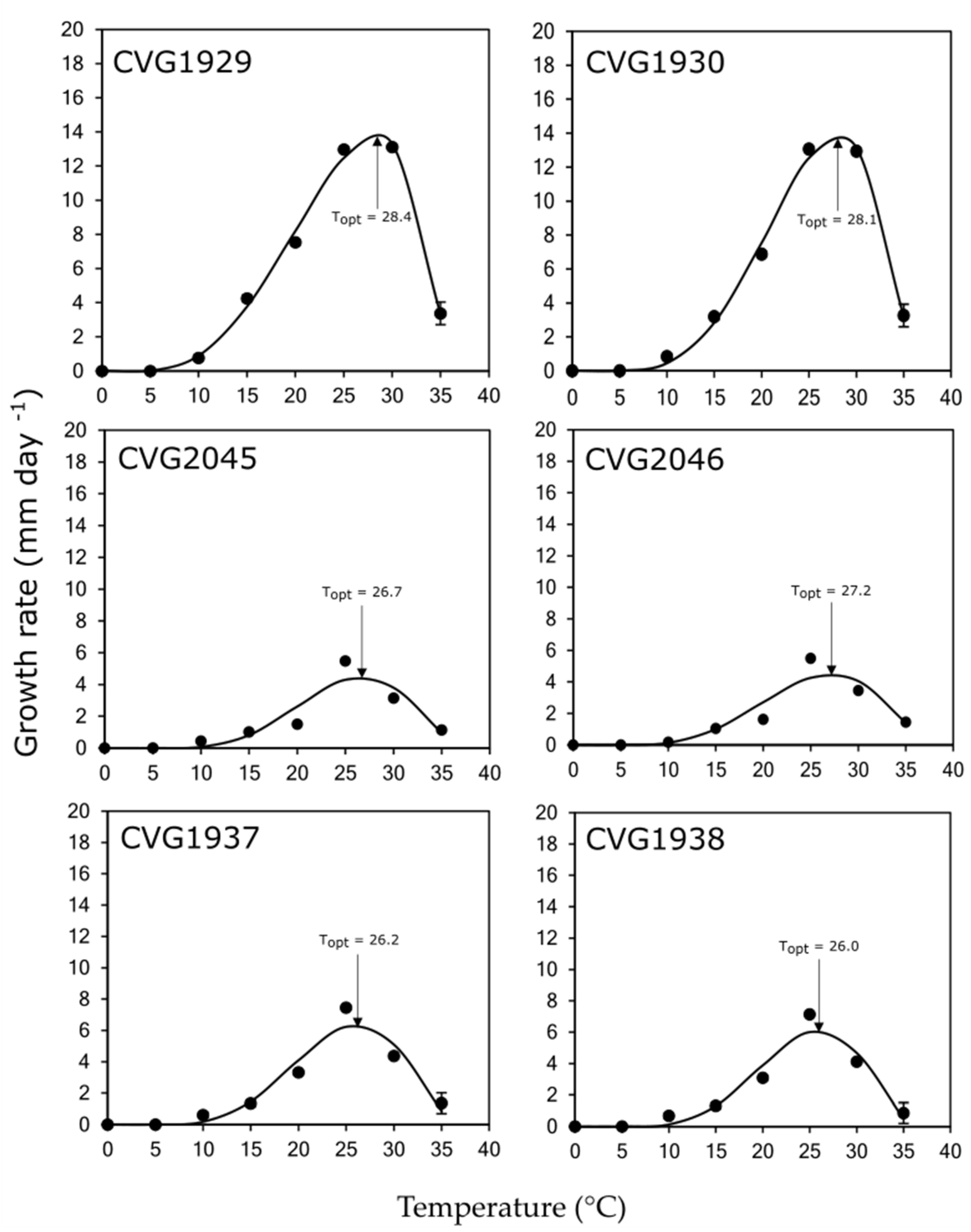
3.4. Effect of Temperature on Mycelial Growth
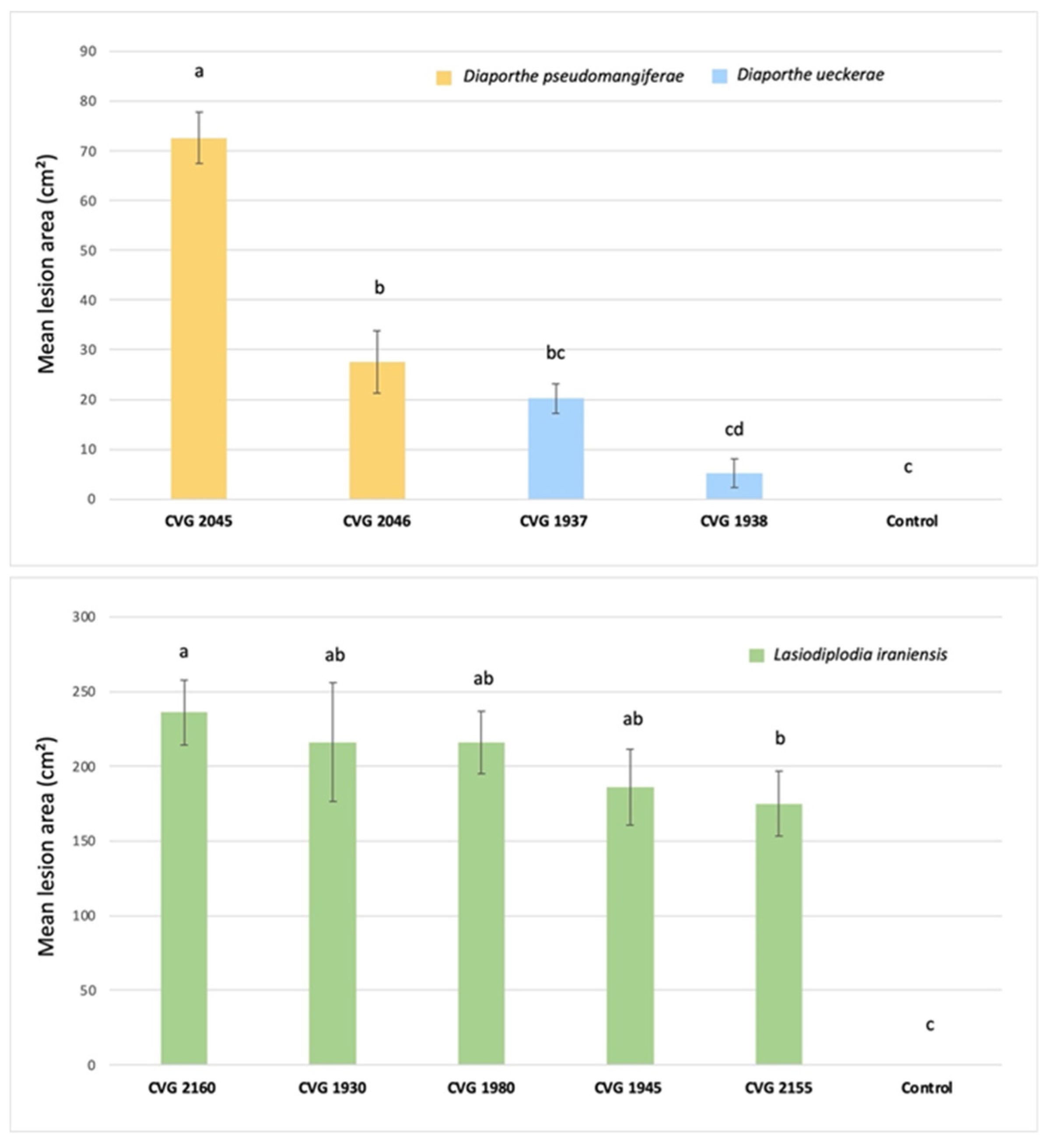
3.5. Pathogenicity on Fruit
3.6. Pathogenicity on Plants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rehman, S.U.; Abbasi, K.S.; Qayyum, A.; Jahangir, M.; Sohail, A.; Nisa, S.; Tareen, M.N.; Tareen, M.J.; Sopade, P. Comparative analysis of citrus fruits for Nutraceutical properties. Food Sci. Technol. 2020, 40, 153–157. [Google Scholar] [CrossRef]
- ISTAT. Istituto Nazionale di Statistica. 2023. Available online: https://www.istat.it/ (accessed on 1 November 2023).
- FAOSTAT. Food and Agriculture Organization Corporate Statistical Database. 2023. Available online: https://www.fao.org (accessed on 20 December 2023).
- USDA. United States Department of Agriculture. 2023. Available online: https://www.usda.gov (accessed on 20 December 2023).
- Timmer, L.W.; Garnsey, S.M.; Graham, J.H. Compendium of Citrus Diseases; American Phytopathology Society: Saint Paul, MN, USA, 2000. [Google Scholar]
- Bezerra, J.D.P.; Crous, P.W.; Aiello, D.; Gullino, M.L.; Polizzi, G.; Guarnaccia, V. Genetic Diversity and Pathogenicity of. Plants 2021, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Chaisiri, C.; Liu, X.Y.; Lin, Y.; Luo, C.X. Diaporthe citri: A Fungal Pathogen Causing Melanose Disease. Plants 2022, 11, 1600. [Google Scholar] [CrossRef] [PubMed]
- Galsurker, O.; Diskin, S.; Maurer, D.; Feygenberg, O.; Alkan, N. Fruit Stem-End Rot. Horticulturae 2018, 4, 50. [Google Scholar] [CrossRef]
- Mayorquin, J.S.; Wang, D.H.; Twizeyimana, M.; Eskalen, A. Identification, Distribution, and Pathogenicity of Diatrypaceae and Botryosphaeriaceae Associated with Citrus Branch Canker in the Southern California Desert. Plant Dis. 2016, 100, 2402–2413. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Aiello, D.; Azzaro, A.; Guarnaccia, V.; Polizzi, G. An Eleven-Year Survey on Field Disease Susceptibility of Citrus Accessions to Colletotrichum and Alternaria Species. Agriculture 2021, 11, 536. [Google Scholar] [CrossRef]
- Fawcett, H.S.; Burger, O.F. A gum-inducing Diplodia of peach and orange. Mycologia 1911, 3, 151–153. [Google Scholar] [CrossRef]
- Punithalingam, E. Plant Diseases Attributed to Botryodiplodia Theobromae Pat; J. Cramer.: Englewood Cliffs, NJ, USA, 1980. [Google Scholar]
- Adesemoye, A.O.; Mayorquin, J.S.; Wang, D.H.; Twizeyimana, M.; Lynch, S.C.; Eskalen, A. Identification of Species of Botryosphaeriaceae Causing Bot Gummosis in Citrus in California. Plant Dis. 2014, 98, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Espargham, N.; Mohammadi, H.; Gramaje, D. A Survey of Trunk Disease Pathogens within Citrus Trees in Iran. Plants 2020, 9, 754. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J. Fungi infecting woody plants: Emerging frontiers. Persoonia 2018, 40, I–III. [Google Scholar] [CrossRef]
- Berraf-Tebbal, A.; Mahamedi, A.E.; Aigoun-Mouhous, W.; Špetík, M.; Čechová, J.; Pokluda, R.; Baránek, M.; Eichmeier, A.; Alves, A. Lasiodiplodia mitidjana sp. nov. and other Botryosphaeriaceae species causing branch canker and dieback of Citrus sinensis in Algeria. PLoS ONE 2020, 15, e0232448. [Google Scholar] [CrossRef]
- Camiletti, B.X.; Lichtemberg, P.S.F.; Paredes, J.A.; Carraro, T.A.; Velascos, J.; Michailides, T.J. Characterization of. Phytopathology 2022, 112, 1454–1466. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Groenewald, J.Z.; Polizzi, G.; Crous, P.W. High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia 2017, 39, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, V.; Crous, P.W. Emerging citrus diseases in Europe caused by species of. IMA Fungus 2017, 8, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Riolo, M.; Aloi, F.; Pane, A.; Cara, M.; Cacciola, S. Twig and Shoot Dieback of Citrus, a New Disease Caused by Colletotrichum Species. Cells 2021, 10, 449. [Google Scholar] [CrossRef]
- Leonardi, G.R.; Aiello, D.; Camilleri, G.; Piattino, V.; Polizzi, G.; Guarnaccia, V. A new disease of kumquat (Fortunella margarita) caused by Colletotrichum karsti: Twig and branch dieback. Phytopathol. Mediterr. 2023, 62, 333–348. [Google Scholar] [CrossRef]
- Ismail, M.; Zhang, J. Post-harvest Citrus Diseases and their control. Outlooks Pest Manag. 2004, 15, 29–35. [Google Scholar] [CrossRef]
- Brown, G.E.; Eckert, J.W. Diplodia stem-end rot. Compend. Citrus Dis. 2000, 2, 43–44. [Google Scholar]
- Zhang, J. Lasiodiplodia theobromae in Citrus Fruit (Diplodia Stem-End Rot). In Postharvest Decay: Control Strategies; Academic Press: Cambridge, MA, USA, 2014; pp. 309–335. [Google Scholar]
- Lim, L.; Mohd, M.H.; Zakaria, L. Identification and pathogenicity of Diaporthe species associated with stem-end rot of mango (Mangifera indica L.). Eur. J. Plant Pathol. 2019, 155, 687–696. [Google Scholar] [CrossRef]
- Guimarães, J.E.R.; de la Fuente, B.; Pérez-Gago, M.B.; Andradas, C.; Carbó, R.; Mattiuz, B.H.; Palou, L. Antifungal activity of GRAS salts against Lasiodiplodia theobromae in vitro and as ingredients of hydroxypropyl methylcellulose-lipid composite edible coatings to control Diplodia stem-end rot and maintain postharvest quality of citrus fruit. Int. J. Food Microbiol. 2019, 301, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Awan, Q.N.; Akgül, D.S.; Unal, G. First Report of Lasiodiplodia pseudotheobromae Causing Postharvest Fruit Rot of Lemon in Turkey. Plant Dis. 2016, 100, 2327. [Google Scholar] [CrossRef]
- Huang, F.; Hou, X.; Dewdney, M.M.; Fu, Y.; Chen, G.Q.; Hyde, K.D.; Li, H.Y. Diaporthe species occurring on citrus in China. Fungal Divers. 2013, 61, 237–250. [Google Scholar] [CrossRef]
- Kucharek, T.; Whiteside, J.O.; Brown, E. Melanose and Phomopsis Stem-End Rot of Citrus. In Plant Pathology Fact Sheet; Florida Cooperative Extension Service; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2000; pp. 26–30. [Google Scholar]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef]
- Slippers, B.; Boissin, E.; Phillips, A.J.L.; Groenewald, J.Z.; Lombard, L.; Wingfield, M.J.; Postma, A.; Burgess, T.; Crous, P.W. Phylogenetic lineages in the Botryosphaeriales: A systematic and evolutionary framework. Stud. Mycol. 2013, 76, 31–49. [Google Scholar] [CrossRef]
- Batista, E.; Lopes, A.; Alves, A. What do we know about Botryosphaeriaceae? An overview of a worldwide cured dataset. Forests 2021, 12, 313. [Google Scholar] [CrossRef]
- Bautista-Cruz, M.A.; Almaguer-Vargas, G.; Leyva-Mir, S.G.; Colinas-León, M.T.; Correia, K.C.; Camacho-Tapia, M.; Robles-Yerena, L.; Michereff, S.J.; Tovar-Pedraza, J.M. Phylogeny, Distribution, and Pathogenicity of Lasiodiplodia Species Associated with Cankers and Dieback Symptoms of Persian Lime in Mexico. Plant Dis. 2019, 103, 1156–1165. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; Ismail, A.M.; Iqbal, Z.; Elshewy, E.S.; Alhudaib, K.A.; Almaghasla, M.I.; Magistà, D. Diversity among Lasiodiplodia Species Causing Dieback, Root Rot and Leaf Spot on Fruit Trees in Egypt, and a Description of Lasiodiplodia newvalleyensis sp. nov. J. Fungi 2022, 8, 1203. [Google Scholar] [CrossRef]
- Polizzi, G.; Aiello, D.; Vitale, A.; Giuffrida, F.; Groenewald, J.Z.; Crous, P.W. First Report of Shoot Blight, Canker, and Gummosis Caused by Neoscytalidium dimidiatum on Citrus in Italy. Plant Dis. 2009, 93, 1215. [Google Scholar] [CrossRef]
- Xiao, X.E.; Wang, W.; Crous, P.W.; Wang, H.K.; Jiao, C.; Huang, F.; Pu, Z.X.; Zhu, Z.R.; Li, H.Y. Species of Botryosphaeriaceae associated with citrus branch diseases in China. Persoonia 2021, 47, 106–135. [Google Scholar] [CrossRef]
- Sultana, R.; Islam, M.S.; Rahman, H.; Alam, M.S.; Islam, M.A.; Sikdar, B. Characterization of Lasiodiplodia pseudotheobromae associated with citrus stem-end rot disease in Bangladesh. Int. J. Biosci. 2018, 13, 252–262. [Google Scholar]
- Guarnaccia, V.; Groenewald, J.Z.; Woodhall, J.; Armengol, J.; Cinelli, T.; Eichmeier, A.; Ezra, D.; Fontaine, F.; Gramaje, D.; Gutierrez-Aguirregabiria, A.; et al. diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe. Persoonia 2018, 40, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.R.; Glienke, C.; Videira, S.I.; Lombard, L.; Groenewald, J.Z.; Crous, P.W. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 2013, 31, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Aiello, D.; Guarnaccia, V.; Costanzo, M.B.; Leonardi, G.R.; Epifani, F.; Perrone, G.; Polizzi, G. Woody Canker and Shoot Blight Caused by Botryosphaeriaceae and Diaporthaceae on Mango and Litchi in Italy. Horticulturae 2022, 8, 330. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Vitale, A.; Cirvilleri, G.; Aiello, D.; Susca, A.; Epifani, F.; Perrone, G.; Polizzi, G. Characterisation and pathogenicity of fungal species associated with branch cankers and stem-end rot of avocado in Italy. Eur. J. Plant Pathol. 2016, 146, 963–976. [Google Scholar] [CrossRef]
- Hilario, S.; Santos, L.; Alves, A. Diversity and Pathogenicity of Diaporthe Species Revealed from a Survey of Blueberry Orchards in Portugal. Agriculture 2021, 11, 1271. [Google Scholar] [CrossRef]
- Lombard, L.; van Leeuwen, G.C.M.; Guarnaccia, V.; Polizzi, G.; van Rijswick, P.C.J.; Rosendahl, K.; Gabler, J.; Crous, P.W. Diaporthe species associated with Vaccinium, with specific reference to Europe. Phytopathol. Mediterr. 2014, 53, 287–299. [Google Scholar]
- Manawasinghe, I.S.; Dissanayake, A.J.; Li, X.H.; Liu, M.; Wanasinghe, D.N.; Xu, J.P.; Zhao, W.S.; Zhang, W.; Zhou, Y.Y.; Hyde, K.D.; et al. High Genetic Diversity and Species Complexity of Diaporthe Associated with Grapevine Dieback in China. Front. Microbiol. 2019, 10, 473533. [Google Scholar] [CrossRef] [PubMed]
- Martino, I.; Tabone, G.; Giordano, R.; Gullino, M.L.; Guarnaccia, V. First Report of Diaporthe eres Causing Stem Blight and Dieback on Highbush Blueberry (Vaccinium corymbosum) in Italy. Plant Dis. 2023, 107, 1236. [Google Scholar] [CrossRef] [PubMed]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Chukeatirote, E.; Hyde, K.D. The Diaporthe sojae species complex: Phylogenetic re-assessment of pathogens associated with soybean, cucurbits and other field crops. Fungal Biol. 2015, 119, 383–407. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Hyde, K.D. Species limits in Diaporthe: Molecular re-assessment of D-citri, D-cytosporella, D-foeniculina and D-rudis. Persoonia 2014, 32, 83–101. [Google Scholar] [CrossRef]
- Chaisiri, C.; Liu, X.Y.; Lin, Y.; Li, J.B.; Xiong, B.; Luo, C.X. Phylogenetic Analysis and Development of Molecular Tool for Detection of Diaporthe citri Causing Melanose Disease of Citrus. Plants 2020, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.N.; Vicent, A.; Reis, R.F.; Timmer, L.W. Saprophytic colonization of citrus twigs by Diaporthe citri and factors affecting pycnidial production and conidial survival. Plant Dis. 2007, 91, 387–392. [Google Scholar] [CrossRef]
- Chen, G.Q.; Jiang, L.Y.; Xu, F.S.; Xu, F.; Li, H.Y. In vitro and in vivo screening of fungicides for controlling citrus melanose caused by Diaporthe citri. J. Zhejiang Univ. Agric. Life Sci. 2010, 36, 440–444. [Google Scholar]
- Guarnaccia, V.; Crous, P.W. Species of Diaporthe on Camellia and Citrus in the Azores Islands. Phytopathol. Mediterr. 2018, 57, 307–319. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, F.; Huang, Z.; Huang, F.; Chen, G.; Li, H. Occurrence and control of citrus melanose caused by Diaporthe citri. Acta Agric. Zhejiangensis 2012, 24, 647–653. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungusfusariumare nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Pavlic, D.; Slippers, B.; Coutinho, T.A.; Wingfield, M.J. Multiple gene genealogies and phenotypic data reveal cryptic species of the Botryosphaeriaceae: A case study on the Neofusicoccum parvum/N. ribis complex. Mol. Phylogenet. Evol. 2009, 51, 259–268. [Google Scholar] [CrossRef]
- Boratyn, G.M.; Camacho, C.; Cooper, P.S.; Coulouris, G.; Fong, A.; Ma, N.; Madden, T.L.; Matten, W.T.; McGinnis, S.D.; Merezhuk, Y.; et al. BLAST: A more efficient report with usability improvements. Nucleic Acids Res. 2013, 41, W29–W33. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kumar, V.; Reinartz, W. Creating Enduring Customer Value. J. Mark. 2016, 80, 36–68. [Google Scholar] [CrossRef]
- Zhang, W.; Groenewald, J.Z.; Lombard, L.; Schumacher, R.K.; Phillips, A.J.L.; Crous, P.W. Evaluating species in Botryosphaeriales. Persoonia 2021, 46, 63–115. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Fan, X.L.; Hyde, K.D.; Yang, Q.; Liang, Y.M.; Tian, C.M. Phylogeny and morphology reveal two new species of Diaporthe from Betula spp. in China. Phytotaxa 2016, 269, 90–102. [Google Scholar] [CrossRef][Green Version]
- Nylander, J. MrModeltest v. 2 Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Cummings, M.P. PAUP* Phylogenetic Analysis Using Parsimony (* and Other Methods). In Dictionary of Bioinformatics Computational Biology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Page, R.D.M. Tree View: An application to display phylogenetic trees on personal computers. Bioinformatics 1996, 12, 357–358. [Google Scholar] [CrossRef]
- Smith, H.; Wingfield, M.J.; Crous, P.W.; Coutinho, T.A. Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp and Eucalyptus spp in South Africa. S. Afr. J. Bot. 1996, 62, 86–88. [Google Scholar] [CrossRef]
- Rayner, R.W. A Mycological Colour Chart; Commonwealth Mycological Institute: Kew, Australia, 1970. [Google Scholar]
- Hau, B.; Kranz, J. Mathematics and statistics for analyses in epidemiology. In Epidemics of Plant Diseases: Mathematical Analysis and Modeling; Springer: Berlin/Heidelberg, Germany, 1990; pp. 12–52. [Google Scholar]
- López-Moral, A.; Raya-Ortega, M.C.; Agustí-Brisach, C.; Roca, L.F.; Lovera, M.; Luque, F.; Arquero, O.; Trapero, A. Morphological, pathogenic, and molecular characterization of Colletotrichum acutatum isolates causing almond anthracnose in Spain. Plant Dis. 2017, 101, 2034–2045. [Google Scholar] [CrossRef]
- Aiello, D.; Carrieri, R.; Guarnaccia, V.; Vitale, A.; Lahoz, E.; Polizzi, G. Characterization and pathogenicity of colletotrichum gloeosporioides and C. karstii causing preharvest disease on citrus sinensis in Italy. J. Phytopathol. 2015, 163, 168–177. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H. Bioestadística: Principios y Procedimientos; McGraw-Hill: New York, NY, USA, 1985. [Google Scholar]
- Software, A. Statistix 10 User’s Manual; Analytical Software: Tallahassee, FL, USA, 2013. [Google Scholar]
- Karadžić, D.; Stanivuković, Z.; Milanović, S.; Sikora, K.; Radulović, Z.; Račko, V.; Kardošová, M.; Ďurkovič, J.; Milenković, I. Development of Neonectria punicea pathogenic symptoms in juvenile Fraxinus excelsior trees. Front. Plant Sci. 2020, 11, 592260. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Richardson, D.M.; Le Roux, J.J.; Strasberg, D.; Edwards, J.; Roets, F.; Hubka, V.; Taylor, P.W.; Heykoop, M.; et al. Fungal Planet description sheets: 400–468. Persoonia 2016, 36, 316–458. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, J.; Javadi, A.; Mohammadi Goltapeh, E.; Zare, R.; Phillips, A.J. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 2010, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Netto, M.S.B.; Lima, W.G.; Correia, K.C.; Da Silva, C.F.B.; Thon, M.; Martins, R.B.; Miller, R.N.G.; Michereff, S.J.; Câmara, M.P.S. Analysis of phylogeny, distribution, and pathogenicity of Botryosphaeriaceae species associated with gummosis of Anacardium in Brazil, with a new species of Lasiodiplodia. Fungal Biol. 2017, 121, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Li GuoQing Li, G.; Arnold, R.J.; Liu FeiFei Liu, F.; Li JieQiong Li, J.; Chen ShuaiFei Chen, S. Identification and pathogenicity of Lasiodiplodia species from Eucalyptus urophylla × grandis, Polyscias balfouriana and Bougainvillea spectabilis in southern China. J. Phytopathol. 2015, 163, 956–967. [Google Scholar]
- Negi, N.; Krishna, R.; Meena, R.K.; Pandey, A.; Bhandari, M.S.; Pandey, S. First report of Lasiodiplodia iraniensis causing leaf spot disease of Eucalyptus in India. Physiol. Mol. Plant Pathol. 2023, 127, 102113. [Google Scholar] [CrossRef]
- Al-Sadi, A.M.; Al-Wehaibi, A.N.; Al-Shariqi, R.M.; Al-Hammadi, M.S.; Al-Hosni, I.A.; Al-Mahmooli, I.H.; Al-Ghaithi, A.G. Population Genetic Analysis Reveals Diversity in Lasiodiplodia Species Infecting Date Palm, Citrus, and Mango in Oman and the UAE. Plant Dis. 2013, 97, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.W.; Lima, N.B.; de Morais, M.A.; Barbosa, M.A.G.; Souza, B.O.; Michereff, S.J.; Phillips, A.J.L.; Câmara, M.P.S. Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers. 2013, 61, 181–193. [Google Scholar] [CrossRef]
- Fayyaz, A.; Bonello, P.; Tufail, M.R.; Amrao, L.; Habib, A.; Gai, Y.; Sahi, S.T. First report of citrus withertip (tip dieback), a disease complex caused by Colletotrichum siamense and Lasiodiplodia iraniensis, on Citrus reticulata cv. Kinnow in Punjab, Pakistan. Plant Dis. 2018, 102, 2659. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.; Fu, Y.; Cheng, J.; Xie, J.; Lin, Y. Identification of Lasiodiplodia pseudotheobromae Causing Fruit Rot of Citrus in China. Plants 2021, 10, 202. [Google Scholar] [CrossRef]
- Thompson, S.M.; Grams, R.A.; Neate, S.M.; Shivas, R.G.; Ryley, M.J.; Tan, Y.P.; Aitken, E.A.B.; Wright, G.C.; O’Connor, D.J. First Reports of Diaporthe kongii, D. masirevicii, and D. ueckerae Associated with Stem and Peg Dieback on Peanut in Australia. Plant Dis. 2018, 102, 1459. [Google Scholar] [CrossRef]
- Gao, Y.H.; Liu, F.; Cai, L. Unravelling Diaporthe species associated with Camellia. Syst. Biodivers. 2016, 14, 102–117. [Google Scholar] [CrossRef]
- Liao, W.J.; Luo, L.F.; Zou, D.X.; Wu, Y.J.; Chen, S.Y.; Luo, J. First Report of Branch Blight Caused by Diaporthe ueckerae on Eucalyptus citriodora in China. Plant Dis. 2023, 107, 563. [Google Scholar] [CrossRef]
- López-Cardona, N.; Guevara-Castro, A.; Gañán-Betancur, L.; Amaya-Gomez, C.V. First Report of Diaporthe ueckerae Causing Stem Canker on Soybean (Glycine max) in Colombia. Plant Dis. 2021, 105, 4162. [Google Scholar] [CrossRef]
- Wang, Y.C.; Liu, J.H.; Huang, C.C.; Hong, C.F. First Report of Dragon Fruit (Hylocereus undatus) Stem Rot Caused by Diaporthe ueckerae in Taiwan. Plant Dis. 2022, 106, 1527. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Rivera-Vargas, L.I.; French-Monar, R.D. First Report of Diaporthe pseudomangiferae Causing Inflorescence Rot, Rachis Canker, and Flower Abortion of Mango. Plant Dis. 2014, 98, 1004–1005. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Zhang, W. First Report of Postharvest Stem-End Rot of Mango Fruit (Mangifera indica) Caused by Diaporthe pseudomangiferae in China. Plant Dis. 2023, 107, 582. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Ayala-Silva, T.; Goenaga, R. First Report of Diaporthe tulliensis and D. pseudomangiferae Causing Cacao Pod Rot in Puerto Rico. Plant Dis. 2022, 106, 2530. [Google Scholar] [CrossRef]
- Seungeun, G.; Wonyong, K.; Kwang-Yeol, Y. Emergence of multiple Diaporthe species causing kiwifruit rot and occurrence of resistance to a methyl benzimidazole carbamate fungicide in South Korea. Crop Prot. 2022, 158, 106016. [Google Scholar] [CrossRef]
- Yi, R.; Chen, Y.; Han, J.; Hu, Q.; Li, H.; Wu, H. Identification and biological characteristics of Diaporthe ueckerae causing dieback disease on Michelia shiluensis. Sci. Silvae Sin. 2018, 54, 80–88. [Google Scholar]
- PRISM Climate Group. PRISM Spatial Climate Datasets for the Coterminous United States. Oregon State University. 2022. Available online: https://prism.oregonstate.edu/ (accessed on 1 November 2023).
- Batista, E.; Lopes, A.; Miranda, P.; Alves, A. Can species distribution models be used for risk assessment analyses of fungal plant pathogens? A case study with three Botryosphaeriaceae species. Eur. J. Plant Pathol. 2023, 165, 41–56. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Li, L.; Mohd, M.; Mohamed Nor, N.; Subramaniam, S.; Latiffah, Z. Identification of Botryosphaeriaceae associated with stem-end rot of mango (Mangifera indica L.) in Malaysia. J. Appl. Microbiol. 2021, 130, 1273–1284. [Google Scholar] [CrossRef]
- Sutton, T.B.; Aldwinckle, H.S.; Agnello, A.M.; Walgenbach, J.F. Compendium of Apple and Pear Diseases and Pests, 2nd ed.; American Phytopathological Society: St. Paul, MN, USA, 2014. [Google Scholar]
- Aćimović, S.G.; Rooney-Latham, S.; Albu, S.; Grosman, D.M.; Doccola, J.J. Characterization and pathogenicity of Botryosphaeriaceae fungi associated with declining urban stands of coast redwood in California. Plant Dis. 2018, 102, 1950–1957. [Google Scholar] [CrossRef]
- Schoeneweiss, D.F. Role of environmental stress in diseases of woody plants. Plant Dis. 1981, 65, 308–314. [Google Scholar] [CrossRef]
- Wene, E.; Schoeneweiss, D. Localized freezing predisposition to Botryosphaeria canker in differentially frozen woody stems. Can. J. Bot. 1980, 58, 1455–1458. [Google Scholar] [CrossRef]
- Ganesan, S.; Kumari, N.; Sahu, S.; Pattanaik, M.; Kishore, K. Identification of Lasiodiplodia species inciting stem rot of dragon fruit in India through polyphasic approach. 3 Biotech 2023, 13, 333. [Google Scholar]
- Mohankumar, V.; Dann, E.K.; Akinsanmi, O.A. Diversity and Pathogenicity of Botryosphaeriaceae Associated with Macadamia Branch Dieback in Australia. Plant Dis. 2022, 106, 2576–2582. [Google Scholar] [CrossRef]
- Dutta, S.K.; Gurung, G.; Yadav, A.; Laha, R.; Mishra, V.K. Factors associated with citrus fruit abscission and management strategies developed so far: A review. N. Z. J. Crop Hortic. Sci. 2022, 51, 467–488. [Google Scholar] [CrossRef]
- Moreira, R.R.; Machado, F.J.; Lanza, F.E.; Trombin, V.G.; Bassanezi, R.B.; De Miranda, M.P.; Barbosa, J.C.; Da Silva, G.J., Jr.; Behlau, F. Impact of diseases and pests on premature fruit drop in sweet orange orchards in Sao Paulo state citrus belt, Brazil. Pest Manag. Sci. 2022, 78, 2643–2656. [Google Scholar] [CrossRef]
- Tang, L.; Chhajed, S.; Vashisth, T. Preharvest Fruit Drop in Huanglongbing-affected ‘Valencia’ Sweet Orange. J. Am. Soc. Hortic. Sci. 2019, 144, 107–117. [Google Scholar] [CrossRef]
- Zhao, W.; Gottwald, T.; Bai, J.H.; McCollum, G.; Irey, M.; Plotto, A.; Baldwin, E. Correlation of Diplodia (Lasiodiplodia theobromae) infection, huanglongbing, ethylene production, fruit removal force and pre-harvest fruit drop. Sci. Hortic. 2016, 212, 162–170. [Google Scholar] [CrossRef]
- Moral, J.; Morgan, D.; Trapero, A.; Michailides, T.J. Ecology and epidemiology of diseases of nut crops and olives caused by Botryosphaeriaceae fungi in California and Spain. Plant Dis. 2019, 103, 1809–1827. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Travadon, R.; Baumgartner, K. Diversity of Diaporthe species associated with wood cankers of fruit and nut crops in northern California. Mycologia 2015, 107, 926–940. [Google Scholar] [CrossRef]
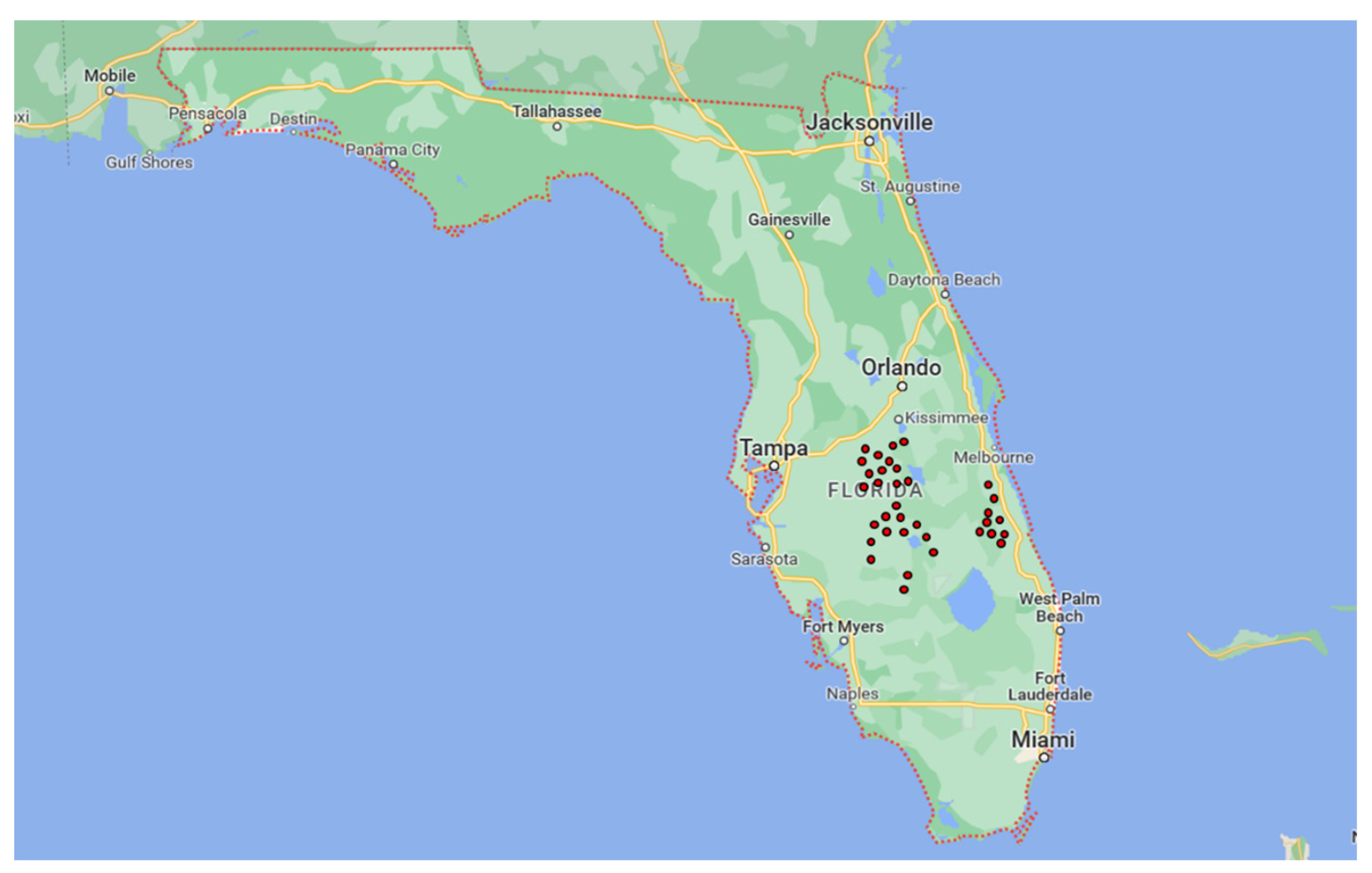



| Locality | Orchard | GPS Coordinates |
|---|---|---|
| Groves | 1 | 27.5471351 N–81.6733289 W |
| Sebring | 2 | 27.1606129 N–81.3105547 W |
| 3 | 27.1597714 N–81.3176948 W | |
| 4 | 27.50406 N–81.33028 W | |
| 5 | 27.50373 N–81.34373 W | |
| 6 | 27.50437 N–81.22116 W | |
| 7 | 27.28401 N–81.13406 W | |
| 8 | 27.28227 N–81.17358 W | |
| DeSoto County | 9 | 27.3130647 N–81.6936935 W |
| 10 | 27.2813967 N–81.7091179 W | |
| Polk County | 11 | 27.976646 N–81.48899 W |
| 12 | 27.95506 N–81.471910 W | |
| St. Lucie County School District | 13 | 27.4730 N–80.62818 W |
| 14 | 27.48017 N–80.62691 W | |
| 15 | 27.46471 N–80.62985 W | |
| 16 | 27.49084 N–80.62699 W | |
| 17 | 27.45678 N–80.49815 W | |
| 18 | 27.45860 N–80.51303 W | |
| 19 | 27.45855 N–80.50092 W | |
| Lakeland | 20 | 27.84340 N–81.57374 W |
| 21 | 27.88448 N–81.54064 W | |
| 22 | 27.55239 N–81.34291 W | |
| 23 | 27.92471 N–81.47095 W | |
| 24 | 27.92141 N–81.6386 W | |
| 25 | 27.87577 N–81.55236 W | |
| Lake Wales | 26 | 27.55475 N–81.33492 W |
| 27 | 27.55481 N–81.33383 W | |
| 28 | 27.55232 N–81.34283 W | |
| 29 | 27.56271 N–81.33534 W | |
| 30 | 27.553237 N–81.34307 W | |
| Osceola County | 31 | 28.15235 N–81.41578 W |
| 32 | 28.09085 N–81.24568 W | |
| Hillcrest Heights | 33 | 27.82674 N–81.53213 W |
| Indian River County School District | 34 | 27.81489 N–80.62050 W |
| 35 | 27.62212 N–80.6298 W |
| Species | Isolate Code (1) | Host | Country | GenBank Accession Number (2) | ||
|---|---|---|---|---|---|---|
| ITS | tef1 | tub2 | ||||
| Diaporthe alnea | CBS 146.46 | Betulaceae | Netherlands | KC343008 | KC343734 | KC343976 |
| Diaporthe arengae | CBS 114979 | Arenga engleri | Hong Kong | KC343034 | KC343760 | KC344002 |
| Diaporthe baccae | CBS 136972 | Vaccinium corymbosum | Italy | KJ160565 | KJ160597 | MF418509 |
| CBS 142545 | Citrus sinensis cv. Tarocco | Italy | MF418351 | MF418430 | MF418519 | |
| Diaporthe betulae | CFCC 50469 | Betula platyphylla | China | KT732950 | KT733016 | KT733020 |
| CFCC 50470 | Betula platyphylla | China | KT732951 | KT733017 | KT733021 | |
| Diaporthe biconispora | ICMP20654 | Citrus grandis | China | KJ490597 | KJ490476 | KJ490418 |
| Diaporthe biguttusis | CGMCC 3.17081 | Lithocarpus glabra | China | KF576282 | KF576257 | KF576306 |
| Diaporthe celastrina | CBS 139.27 | Celastrus sp. | USA | KC343047 | KC343773 | KC344015 |
| Diaporthe citri | CBS 134239 | Citrus sinensis | USA, Florida | KC357553 | KC357522 | KC357456 |
| CBS 135422 | Citrus sp. | USA | KC843311 | KC843071 | KC843187 | |
| Diaporthe citrichinensis | CBS 134242 | Citrus sp. | China | JQ954648 | JQ954666 | MF418524 |
| Diaporthe convolvuli | FAU649 | Convolvulus arvensis | Canada | KJ590721 | KJ590765 | - |
| CBS 124654 | Convolvulus arvensis | Turkey | KC343054 | KC343780 | KC344022 | |
| Diaporthe ellipicola | CGMCC 3.17084 | Lithocarpus glabra | China | KF576270 | KF576245 | KF576294 |
| Diaporthe endophytica | CBS 133811 | Schinus terebinthifolius | Brazil | KC343065 | KC343791 | KC344033 |
| Diaporthe eres | CBS 439.82 | Cotoneaster sp. | Scotland | KC343090 | KC343816 | KC344058 |
| Diaporthe foeniculina | CBS 111553 | Foeniculum vulgare | Spain | KC343101 | KC343827 | KC344069 |
| CBS 135430 | Citrus limon | USA | KC843301 | KC843110 | KC843215 | |
| Diaporthe hongkongensis | CBS 115448 | Dichroa febrifuga | China | KC343119 | kc343845 | KC344087 |
| Diaporthe inconspicua | CBS 133813 | Maytenus ilicifolia | Brazil | KC343123 | KC343849 | KC344091 |
| Diaporthe limonicola | CBS 142549 | Citrus limon | Malta, Gozo | MF418422 | MF418501 | MF418582 |
| CBS 142550 | Citrus limon | Malta, Zurrieq | MF418423 | MF418502 | MF418583 | |
| Diaporthe logicolla | ATCC 60325 | Glycine max | USA | KJ590728 | KJ590767 | KJ610883 |
| CBS 116023 | Glycine max | USA | KC343198 | KC343924 | KC344166 | |
| Diaporthe melitensis | CBS 142551 | Citrus limon | Malta, Gozo | MF418424 | MF418503 | MF418584 |
| CBS 142552 | Citrus limon | Malta, Gozo | MF418425 | MF418504 | MF418585 | |
| Diaporthe multiguttulata | ICMP20656 | Citrus grandis | China | KJ490633 | KJ490512 | KJ490454 |
| Diaporthe neilliae | CBS 144. 27 | Spiraea sp. | USA | KC343144 | KC343870 | KC344112 |
| Diaporthe phoenicicola | CBS 161.64 | Areca catechu | India | KC343032.1 | KC343758.1 | KC344000.1 |
| Diaporthe pseudomangiferae | CBS 101339 | Mangifera indica | Dominican Republic | KC343181 | KC343907 | KC344149 |
| CVG 2045 | Citrus sinensis cv. Valencia | USA, Florida | PP312931 | PP329599 | PP329603 | |
| CVG 2046 | Citrus sinensis cv. Valencia | USA, Florida | PP312932 | PP329600 | PP329604 | |
| Diaporthe pseudophoenicicola | CBS 462.69 | Phoenix dactylifera | Spain | KC343184 | KC343910 | KC344152 |
| Diaporthe pulla | CBS 338.89 | Hedera helix | Yugoslavia | KC343152 | KC343878 | KC344120 |
| Diaporthe saccarata | CBS 116311 | Protea repens | South Africa | KC343190 | KC343916 | KC344158 |
| Diaporthe sojae | CBS 139282 | Glycine max | USA | KJ590719 | KJ590762 | KJ610875 |
| CBS 116019 | Caperonia palustris | USA | KC343175 | KC343901 | KC344143 | |
| Diaporthe uekeri | CBS 139283 | Cucumis melo | USA, Oklahoma | NR 147543 | OM370952 | OM370953 |
| FAU656 | Cucumis melo | USA | KJ590726 | KJ590747 | KJ610881 | |
| CVG 1937 | Citrus sinensis cv. Valencia | USA, Florida | PP312929 | PP329597 | PP329601 | |
| CVG 1938 | Citrus sinensis cv. Valencia | USA, Florida | PP312930 | PP329598 | PP329602 | |
| Diaporthe unshiuensis | CGMCC 3.17569 | Citrus unshiu | China | KJ490587 | KJ490466 | KJ490408 |
| Diaporthella corylina | CBS 121124 | Corylus sp. | China | KC343004 | KC343488 | KC343972 |
| Dothiorella viticola | CBS 117009 | Citrus sinensis, twig | Italy | AY905554 | AY905559 | EU673104 |
| Lasiodiplodia acaciae | CBS 136434 | Acacia sp., leaf spot | Indonesia | MT587421 | MT592133 | MT592613 |
| Lasiodiplodia avicenniae | CMW 41467 | Avicennia marina | South Africa | KP860835 | KP860680 | KP860758 |
| Lasiodiplodia brasiliensis | CMM4015 | Mangifera indica | Brazil | JX464063 | JX464049 | - |
| CMM4469 | Anacardium occidentale | Brazil | KT325574.1 | KT325580.1 | - | |
| Lasiodiplodia bruguierae | CMW 41470 | Bruguiera gymnorrhiza | South Africa | NR_147358 | KP860678 | KP860756 |
| Lasiodiplodia chiangraiensis | MFLUCC 21- 0003 | - | Thailand | MW760854 | MW815630 | MW815628 |
| GZCC 21- 0003 | - | Thailand | MW760853 | MW815629 | MW815627 | |
| Lasiodiplodia cinnamomi | CFCC 51997 | Cinnamomum camphora | China | MG866028 | MH236799 | MH236797 |
| CFCC 51998 | Cinnamomum camphora | China | MG866029 | MH236800 | MH236798 | |
| Lasiodiplodia citricola | CBS 124707 | Citrus sp. | Iran | GU945354 | GU945340 | KP872405 |
| CBS 124706 | Citrus sp. | Iran | GU945353 | GU945339 | KU887504 | |
| Lasiodiplodia endophytica | MFLUCC 18-1121 | Magnolia candolii | China | MK501838 | MK584572 | MK550606 |
| Lasiodiplodia egyptiacae | CBS 130992 | Mangifera indica | Egypt | JN814397 | JN814424 | – |
| Lasiodiplodia euphorbiaceicola | CMM 3609 | Jatropha curcas | Brazil | KF234543 | KF226689 | KF254926 |
| CMW 33268 | Adansonia sp. | Senegal | KU887131 | KU887008 | KU887430 | |
| Lasiodiplodia exigua | CERC 1961 | Pistacia vera cv. Kerman, twigs | USA, Arizona | KP217059 | KP217067 | KP217075 |
| Lasiodiplodia gravistriata | CMM 4564 | Anacardium humile | Brazil | KT250949.1 | KT250950 | – |
| Lasiodiplodia gilanensis | IRAN 1523C | Citrus sp., fallen twigs | Iran | GU945351 | GU945342 | KP872411 |
| IRAN1501C | Citrus sp. | Iran | GU945352 | GU945341 | KU887510 | |
| Lasiodiplodia gonubiensis | CBS 115812 | Syzygium cordatum | South Africa | AY639595 | DQ103566 | DQ458860 |
| Lasiodiplodia hyalina | CGMCC 3.17975 | Acacia confusa | China | KX499879 | KX499917 | KX499992 |
| CGMCC 3.18383 | woody plant | China | KY767661 | KY751302 | KY751299 | |
| Lasiodiplodia hormozganensis | IRAN 1500C | Olea sp. | Iran | GU945355 | GU945343 | KP872413 |
| IRAN1498C | Mangifera indica | Iran | GU945356 | GU945344 | KU887514 | |
| Lasiodiplodia iraniensis | CBS 124710; IRAN 1520C | Salvadora persica, twigs | Iran | GU945346 | GU945334 | KP872415 |
| CMW 33252 | Adansonia sp. | - | KU887065 | KU886947 | KU887422 | |
| CMW 33333 | Adansonia sp. | - | KU887085 | KU886963 | KU887448 | |
| CMW 35881 | Adansonia sp. | - | KU887092 | KU886970 | KU887464 | |
| CMW 33311 | Adansonia sp. | - | KU887084 | KU886962 | KU887442 | |
| IRAN1502C | Juglans sp. | Iran | GU945347 | GU945335 | KU887517 | |
| CMM 3610 | Jatropha curcas | Brazil | KF234544 | KF226690 | KF254927 | |
| CBS 111005; STE-U 1136; CPC 1136 | - | - | MT587430 | MT592142 | MT592624 | |
| CBS 111008; STE-U 1135; CPC 1135 | - | - | MT587431 | MT592143 | MT592625 | |
| CBS 124711; IRAN 1502C | Juglans sp., twigs | Iran | GU945347 | GU945335 | KU887517 | |
| CVG 1905 | Citrus sinensis cv. Valencia | USA, Florida | PP309948 | PP389256 | PP319979 | |
| CVG 1906 | Citrus sinensis cv. Valencia | USA, Florida | PP309949 | PP389257 | PP319980 | |
| CVG 1929 | Citrus sinensis cv. Valencia | USA, Florida | PP309950 | PP389258 | PP319981 | |
| CVG 1930 | Citrus sinensis cv. Valencia | USA, Florida | PP309951 | PP389259 | PP319982 | |
| CVG 1944 | Citrus sinensis cv. Valencia | USA, Florida | PP309952 | PP389260 | PP319983 | |
| CVG 1945 | Citrus sinensis cv. Valencia | USA, Florida | PP309953 | PP389261 | PP319984 | |
| CVG 1957 | Citrus sinensis cv. Valencia | USA, Florida | PP309954 | PP389262 | PP319985 | |
| CVG 1979 | Citrus sinensis cv. Valencia | USA, Florida | PP309955 | PP389263 | PP319986 | |
| CVG 1980 | Citrus sinensis cv. Valencia | USA, Florida | PP309956 | PP389264 | PP319987 | |
| CVG 1985 | Citrus sinensis cv. Valencia | USA, Florida | PP309957 | PP389265 | PP319988 | |
| CVG 1987 | Citrus sinensis cv. Valencia | USA, Florida | PP309958 | PP389266 | PP319989 | |
| CVG 1988 | Citrus sinensis cv. Valencia | USA, Florida | PP309959 | PP389267 | PP319990 | |
| CVG 1996 | Citrus sinensis cv. Valencia | USA, Florida | PP309960 | PP389268 | PP319991 | |
| CVG 2000 | Citrus sinensis cv. Valencia | USA, Florida | PP309961 | PP389269 | PP319992 | |
| CVG 2001 | Citrus sinensis cv. Valencia | USA, Florida | PP309962 | PP389270 | PP319993 | |
| CVG 2048 | Citrus sinensis cv. Valencia | USA, Florida | PP309963 | PP389271 | PP319994 | |
| CVG 2049 | Citrus sinensis cv. Valencia | USA, Florida | PP309964 | PP389272 | PP319995 | |
| CVG 2155 | Citrus sinensis cv. Valencia | USA, Florida | PP309965 | PP389273 | PP319996 | |
| CVG 2156 | Citrus sinensis cv. Valencia | USA, Florida | PP309966 | PP389274 | PP319997 | |
| CVG 2160 | Citrus sinensis cv. Valencia | USA, Florida | PP309967 | PP389275 | PP319998 | |
| CVG 2161 | Citrus sinensis cv. Valencia | USA, Florida | PP309968 | PP389276 | PP319999 | |
| CVG 2166 | Citrus sinensis cv. Valencia | USA, Florida | PP309969 | PP389277 | PP320000 | |
| Lasiodiplodia lignicola | CBS 342.78 | Sterculia oblonga | Germany | KX464140 | KX464634 | KX464908 |
| CGMCC 3.18061 | Woody branch | China | KX499889 | KX499927 | KX500002 | |
| Lasiodiplodia laeliocattleyae | CBS 167.28 | Laeliocattleya | Italy | KU507487 | KU507454 | - |
| CBS 130992 | Mangifera indica | Egypt | NR_120002 | KU507454 | KU887508 | |
| Lasiodiplodia macrospora | CMM 3833 | Jatropha curcas | Brazil | KF234557 | KF226718 | KF254941 |
| Lasiodiplodia magnoliae | MFLUCC 18-0948 | Magnolia candolii, dead leaves | China | MK499387 | MK568537 | MK521587 |
| Lasiodiplodia mahajangana | CBS 124925; CMW 27801 | Terminalia catappa | Madagascar | FJ900595 | FJ900641 | FJ900630 |
| Lasiodiplodia mediterranea | CBS 137783 | Quercus ilex, branch canker | Italy | KJ638312 | KJ638331 | - |
| Lasiodiplodia microconidia | CGMCC 3.18485 | Aquilaria crassna | Laos | KY783441 | KY848614 | - |
| Lasiodiplodia parva | CBS 456.78 | Cassava field | Colombia | EF622083 | EF622063 | KP872419 |
| Lasiodiplodia plurivora | STE-U 5803 | Prunus salicina | South Africa | EF445362 | EF445395 | KP872421 |
| STE-U 4583 | Vitis vinifera | South Africa | AY343482 | EF445396 | KU887525 | |
| Lasiodiplodia pontae | CMM 1277 | Spondias purpure | Brazil | KT151794 | KT151791 | KT151797 |
| Lasiodiplodia pseudotheobromae | CBS 116459 | Gmelina arborea | Costa Rica | EF622077 | EF622057 | EU673111 |
| CBS 304.79 | Rosa cv. Ilona, branches | Netherlands | EF622079 | EF622061 | MT592630 | |
| Lasiodiplodia subglobosa | CMM 3872 | Jatropha curcas | Brazil | KF234558 | KF226721 | KF254942 |
| Lasiodiplodia thailandica | CPC 22795 | Mangifera indica | Thailand | KJ193637 | KJ193681 | - |
| Lasiodiplodia theobromae | CBS 164.96 | Fruit along coral reef coast | Papua New Guinea | AY640255 | AY640258 | EU673110 |
| Lasiodiplodia tropica | CGMCC 3.18477 | Aquilaria crassna | Laos | KY783454 | KY848616 | KY848540 |
| Lasiodiplodia viticola | CBS 128313 | Vitis vinifera | USA | HQ288227 | HQ288269 | HQ288306 |
| UCD 2604MO | Vitis vinifera | USA | HQ288228 | HQ288270 | HQ288307 | |
| Lasiodiplodia vitis | CBS 124060 | Vitis vinifera | Italy | KX464148 | KX464642 | KX464917 |
| Family/Fungal Species | Isolate | Analytis Beta Model (2) | Temperature (°C) (3,5) | MGR (mm day−1) (4,5) | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | a | b | Optimum | Minimum | Maximum | |||
| Lasiodiplodia iraniensis | CVG 1929 | 0.9927 | 2.65 | 0.78 | 28.4 a | 4.0 | 35.5 | 13.7 a |
| CVG 1930 | 0.9924 | 3.42 | 1.13 | 28.1 a | 4.0 | 36.0 | 13.8 a | |
| Diaporthe pseudomangiferae | CVG 2045 | 0.8474 | 4.20 | 2.19 | 26.7 b | 5.0 | 38.0 | 4.5 c |
| CVG 2046 | 0.8675 | 3.64 | 1.74 | 27.2 bc | 4.5 | 38.0 | 4.5 c | |
| Diaporthe ueckerae | CVG 1937 | 0.9257 | 4.11 | 2.00 | 26.2 cd | 4.0 | 37.0 | 6.3 b |
| CVG 1938 | 0.9774 | 4.20 | 2.05 | 26.0 d | 4.5 | 36.5 | 6.1 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piattino, V.; Aiello, D.; Dardani, G.; Martino, I.; Flores, M.; Aćimović, S.G.; Spadaro, D.; Polizzi, G.; Guarnaccia, V. Lasiodiplodia iraniensis and Diaporthe spp. Are Associated with Twig Dieback and Fruit Stem-End Rot of Sweet Orange, Citrus sinensis, in Florida. Horticulturae 2024, 10, 406. https://doi.org/10.3390/horticulturae10040406
Piattino V, Aiello D, Dardani G, Martino I, Flores M, Aćimović SG, Spadaro D, Polizzi G, Guarnaccia V. Lasiodiplodia iraniensis and Diaporthe spp. Are Associated with Twig Dieback and Fruit Stem-End Rot of Sweet Orange, Citrus sinensis, in Florida. Horticulturae. 2024; 10(4):406. https://doi.org/10.3390/horticulturae10040406
Chicago/Turabian StylePiattino, Valeria, Dalia Aiello, Greta Dardani, Ilaria Martino, Mauricio Flores, Srđan G. Aćimović, Davide Spadaro, Giancarlo Polizzi, and Vladimiro Guarnaccia. 2024. "Lasiodiplodia iraniensis and Diaporthe spp. Are Associated with Twig Dieback and Fruit Stem-End Rot of Sweet Orange, Citrus sinensis, in Florida" Horticulturae 10, no. 4: 406. https://doi.org/10.3390/horticulturae10040406
APA StylePiattino, V., Aiello, D., Dardani, G., Martino, I., Flores, M., Aćimović, S. G., Spadaro, D., Polizzi, G., & Guarnaccia, V. (2024). Lasiodiplodia iraniensis and Diaporthe spp. Are Associated with Twig Dieback and Fruit Stem-End Rot of Sweet Orange, Citrus sinensis, in Florida. Horticulturae, 10(4), 406. https://doi.org/10.3390/horticulturae10040406








