Fine Mapping and Candidate Gene Validation of Tomato Gene Carpelloid Stamen and Parthenocarpy (CSP)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. A Phenotypic Analysis of the csp Mutant
2.3. Molecular Marker Development and Genotyping
2.4. The Genetic Analysis and Fine Mapping of the csp Locus
2.5. Candidate Gene Analysis
2.6. RNA Extraction, cDNA Synthesis, and qPCR Analysis
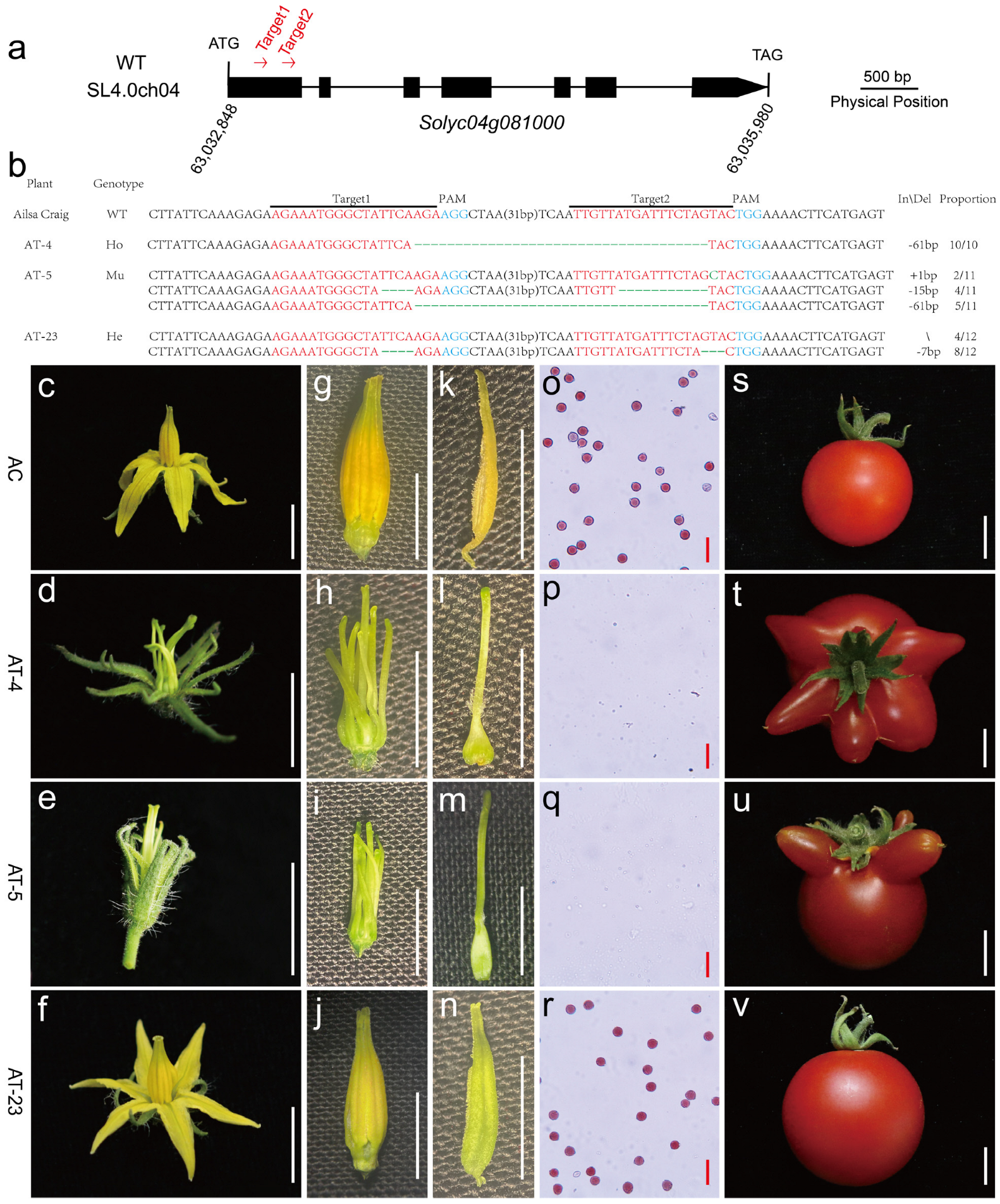
2.7. CRISPR/cas9 Gene Editing Vector Construction, Plant Transformation, and Mutant Phenotypic Analysis
3. Results
3.1. A Phenotypic Analysis of the csp Mutant
3.2. A Genetic Analysis of the csp Locus
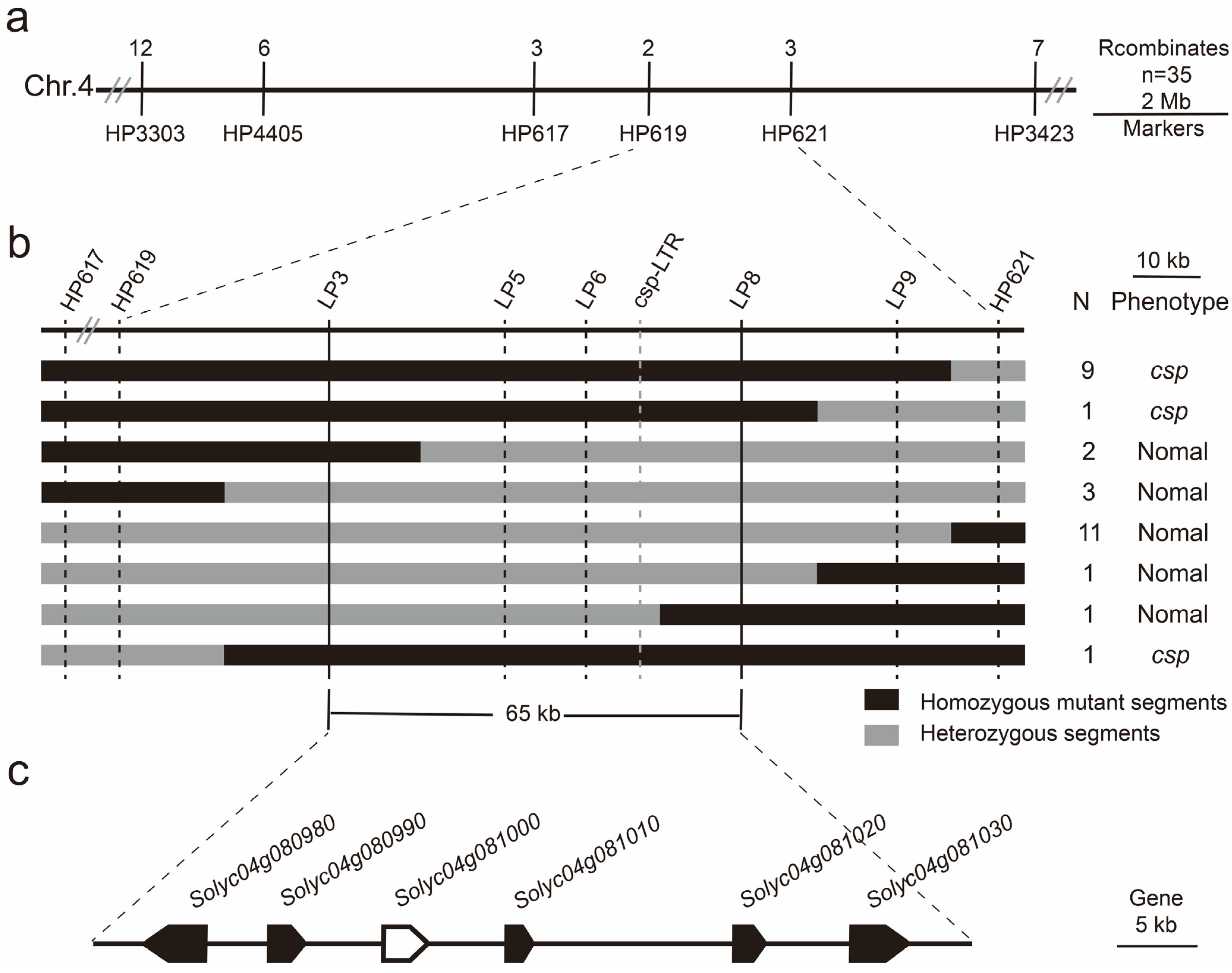
3.3. The Fine Mapping of the csp Locus
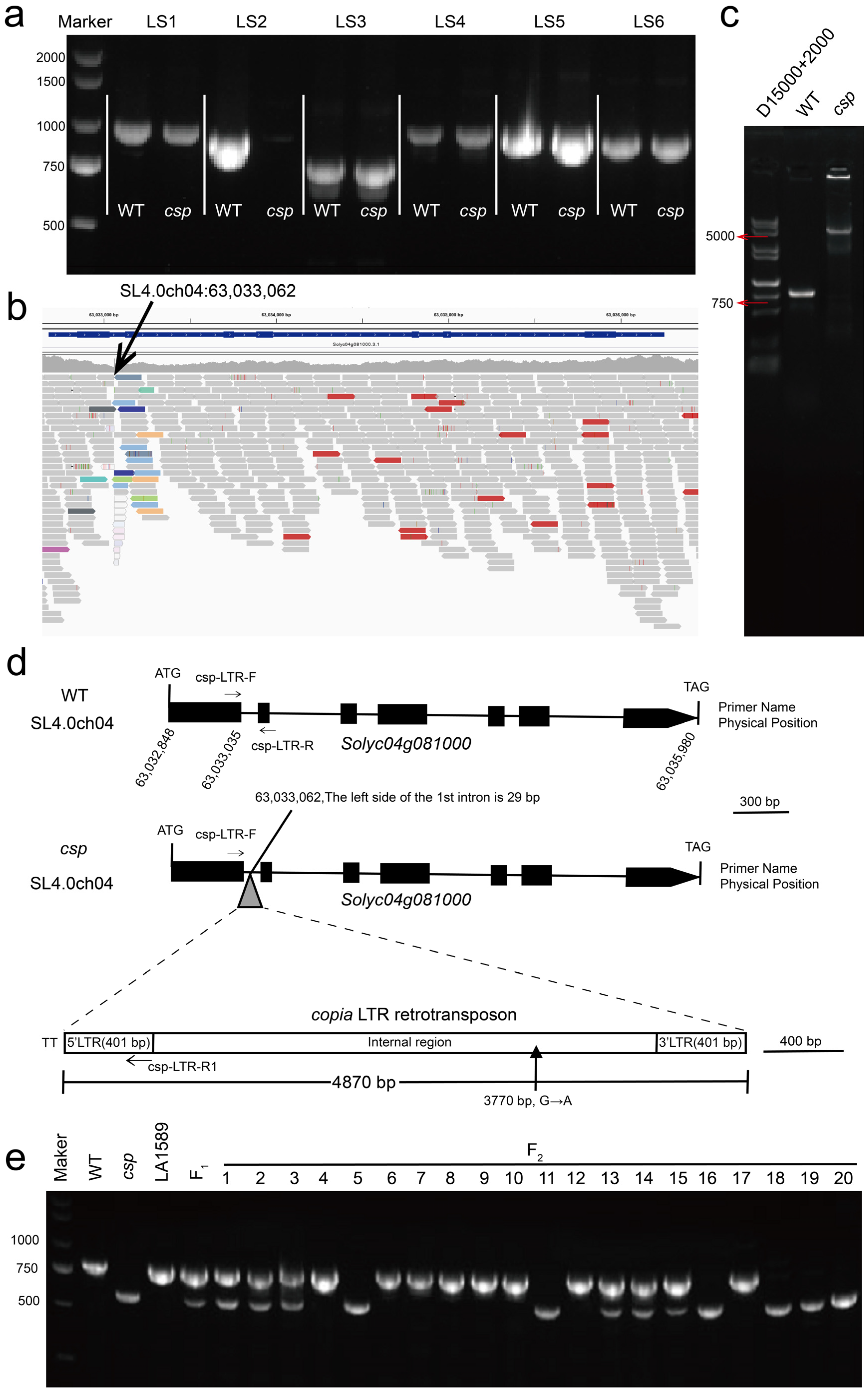
3.4. Candidate Gene Analysis
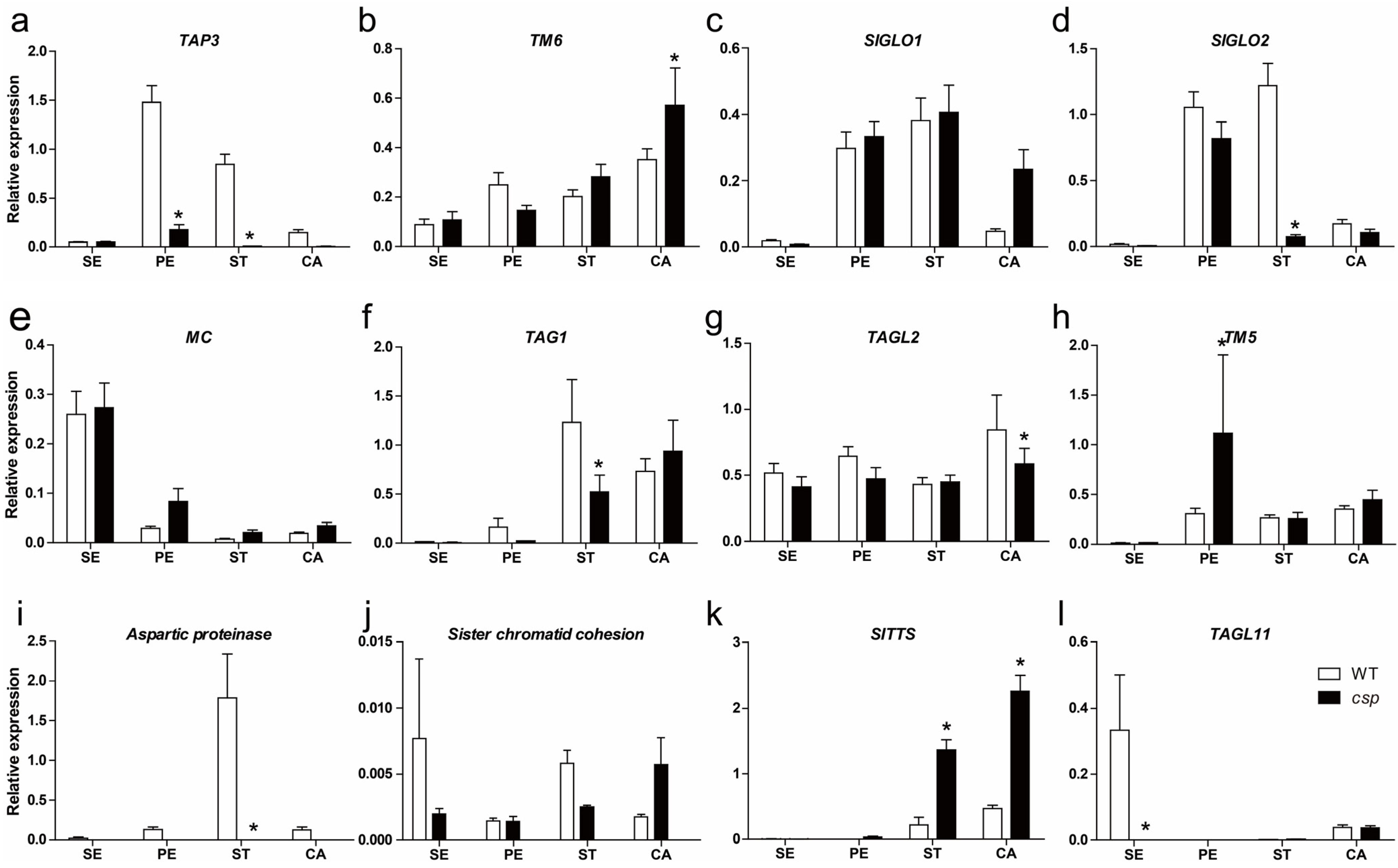
3.5. Expression Analysis of Floral Organ Identity Genes, Pollen Development Marker Genes, and Pistil-Specific Genes
3.6. Validation of Candidate Gene by Gene Editing and Allelism Test
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shu, J.; Liu, Y.; Li, Z.; Zhang, L.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H. Organelle simple sequence repeat markers help to distinguish carpelloid stamen and normal cytoplasmic male sterile sources in Broccoli. PLoS ONE 2015, 10, e0138750. [Google Scholar] [CrossRef] [PubMed]
- Kramer, E.M.; Dorit, R.L.; Irish, V.F. Molecular evolution of genes controlling petal and stamen development: Duplication and divergence within the APETALA3 and PISTILLATA MADS-box gene lineages. Genetics 1998, 149, 765–783. [Google Scholar] [CrossRef]
- Jack, T. Relearning our ABCs: New twists on an old model. Trends Plant Sci. 2001, 6, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yoo, M.J.; Albert, V.A.; Farris, J.S.; Soltis, P.S.; Soltis, D.E. Phylogeny and diversification of B-function MADS-box genes in angiosperms: Evolutionary and functional implications of a 260-million-year-old duplication. Am. J. Bot. 2004, 91, 2102–2118. [Google Scholar] [CrossRef]
- Murai, K.; Takumi, S.; Koga, H.; Ogihara, Y. Pistillody, homeotic transformation of stamens into pistil-like structures, caused by nuclear-cytoplasm interaction in wheat. Plant J. 2002, 29, 169–181. [Google Scholar] [CrossRef]
- Kang, L.; Li, P.; Wang, A.; Ge, X.; Li, Z. A novel cytoplasmic male sterility in Brassica napus (inap CMS) with carpelloid stamens via protoplast fusion with Chinese woad. Front. Plant Sci. 2017, 8, 529. [Google Scholar] [CrossRef]
- Wang, A.; Kang, L.; Yang, G.; Li, Z. Transcriptomic and iTRAQ-Based quantitative proteomic analyses of inap CMS in Brassica napus L. Plants 2022, 11, 2460. [Google Scholar] [CrossRef] [PubMed]
- Pucci, A. Characterization of Tomato (Solanum lycopersicum L.) Male Sterile Mutants Putatively Affected in Class B MADS-Box Transcription Factors; Università Degli Studi Della Tuscia—Viterbo: Viterbo, Italy, 2015; Available online: http://hdl.handle.net/2067/2946 (accessed on 10 March 2024).
- Weigel, D.; Meyerowitz, E.M. The ABCs of floral homeotic genes. Cell 1994, 78, 203–209. [Google Scholar] [CrossRef]
- Geuten, K.; Irish, V. Hidden variability of floral homeotic B Genes in solanaceae provides a molecular basis for the evolution of novel functions. Plant Cell 2010, 22, 2562–2578. [Google Scholar] [CrossRef]
- Cao, X.; Liu, X.; Wang, X.; Yang, M.; van Giang, T.; Wang, J.; Liu, X.; Sun, S.; Wei, K.; Wang, X.; et al. B-class MADS-box TM6 is a candidate gene for tomato male sterile-1526. Theor. Appl. Genet. 2019, 132, 2125–2135. [Google Scholar] [CrossRef]
- Fonseca, R.; Capel, C.; Lebrón, R.; Ortiz-Atienza, A.; Yuste-Lisbona, F.J.; Angosto, T.; Capel, J.; Lozano, R. Insights into the functional role of tomato TM6 as transcriptional regulator of flower development. Hortic. Res. 2024, 11, uhae019. [Google Scholar] [CrossRef] [PubMed]
- Quinet, M.; Bataille, G.; Dobrev, P.I.; Capel, C.; Gómez, P.; Capel, J.; Lutts, S.; Motyka, V.; Angosto, T.; Lozano, R. Transcriptional and hormonal regulation of petal and stamen development by STAMENLESS, the tomato (Solanum lycopersicum L.) orthologue to the B-class APETALA3 gene. J. Exp. Bot. 2014, 65, 2243–2256. [Google Scholar] [CrossRef]
- De Martino, G.; Pan, I.; Emmanuel, E.; Levy, A.; Irish, V.F. Functional analyses of two tomato APETALA3 genes demonstrate diversification in their roles in regulating floral development. Plant Cell 2006, 18, 1833–1845. [Google Scholar] [CrossRef]
- Okabe, Y.; Yamaoka, T.; Ariizumi, T.; Ushijima, K.; Kojima, M.; Takebayashi, Y.; Sakakibara, H.; Kusano, M.; Shinozaki, Y.; Pulungan, S.I.; et al. Aberrant stamen development is associated with parthenocarpic fruit set through up-regulation of gibberellin biosynthesis in tomato. Plant Cell Physiol. 2018, 60, 38–51. [Google Scholar] [CrossRef]
- Pucci, A.; Picarella, M.E.; Mazzucato, A. Phenotypic, genetic and molecular characterization of 7B-1, a conditional male-sterile mutant in tomato. Theor. Appl. Genet. 2017, 130, 2361–2374. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Li, X.; Cao, X.; Li, S.; Zhang, L.; Lu, F.; Liu, C.; Guo, Y.; Liu, L.; Zhu, C.; et al. Candidate gene identification and transcriptome analysis of tomato male sterile-30 and functional marker development for ms-30 and its alleles, ms-33, 7B-1, and stamenless-2. Int. J. Mol. Sci. 2024, 25, 3331. [Google Scholar] [CrossRef]
- Fayaz, Z.; Nazir, G.; Masoodi, U.; Afroza, B.; Asma, S.; Rashid, M. Parthenocarpy: “A potential trait to exploit in vegetable crops”. Environ. Ecol. 2021, 39, 1332–1346. [Google Scholar]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Hormonal interactions underlying parthenocarpic fruit formation in horticultural crops. Hortic. Res. 2022, 9, uhab024. [Google Scholar] [CrossRef]
- Beraldi, D.; Picarella, M.E.; Soressi, G.P.; Mazzucato, A. Fine mapping of the parthenocarpic fruit (pat) mutation in tomato. Theor. Appl. Genet. 2004, 108, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Selleri, L. Positional Cloning of the Parthenocarpic Fruit (Pat) Mutant Gene and Identification of New Parthenocarpic Sources in Tomato (Solanum lycopersicum, L.); Università degli studi della Tuscia—Viterbo: Viterbo, Italy, 2011. [Google Scholar]
- Nunome, T. Map-based cloning of tomato parthenocarpic pat-2 gene. J-STAGE 2016, 51, 37–40. [Google Scholar] [CrossRef]
- Takisawa, R.; Nakazaki, T.; Nunome, T.; Fukuoka, H.; Kataoka, K.; Saito, H.; Habu, T.; Kitajima, A. The parthenocarpic gene Pat-k is generated by a natural mutation of SlAGL6 affecting fruit development in tomato (Solanum lycopersicum L.). BMC Plant Biol. 2018, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Klap, C.; Yeshayahou, E.; Bolger, A.M.; Arazi, T.; Gupta, S.K.; Shabtai, S.; Usadel, B.; Salts, Y.; Barg, R. Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Plant Biotechnol. J. 2017, 15, 634–647. [Google Scholar] [CrossRef]
- Bassel, G.W.; Mullen, R.T.; Bewley, J.D. procera is a putative DELLA mutant in tomato (Solanum lycopersicum): Effects on the seed and vegetative plant. J. Exp. Bot. 2008, 59, 585–593. [Google Scholar] [CrossRef]
- Saito, T.; Ariizumi, T.; Okabe, Y.; Asamizu, E.; Hiwasa-Tanase, K.; Fukuda, N.; Mizoguchi, T.; Yamazaki, Y.; Aoki, K.; Ezura, H. Tomatoma: A novel tomato mutant database distributing micro-tom mutant collections. Plant Cell Physiol. 2011, 52, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Mazzucato, A.; Cellini, F.; Bouzayen, M.; Zouine, M.; Mila, I.; Minoia, S.; Petrozza, A.; Picarella, M.E.; Ruiu, F.; Carriero, F. A TILLING allele of the tomato Aux/IAA9 gene offers new insights into fruit set mechanisms and perspectives for breeding seedless tomatoes. Mol. Breed. 2015, 35, 22. [Google Scholar] [CrossRef]
- Mazzucato, A.; Taddei, A.R.; Soressi, G.P. The parthenocarpic fruit (pat) mutant of tomato (Lycopersicon esculentum Mill.) sets seedless fruits and has aberrant anther and ovule development. Development 1998, 125, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Rajam, M.V. RNAi silencing of three homologues of S-adenosylmethionine decarboxylase gene in tapetal tissue of tomato results in male sterility. Plant Mol. Biol. 2013, 82, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Brukhin, V.; Hernould, M.; Gonzalez, N.; Chevalier, C.; Mouras, A. Flower development schedule in tomato Lycopersicon esculentum cv. sweet cherry. Sex. Plant Reprod. 2003, 15, 311–320. [Google Scholar] [CrossRef]
- Cao, X.; Qiu, Z.; Wang, X.; Van Giang, T.; Liu, X.; Wang, J.; Wang, X.; Gao, J.; Guo, Y.; Du, Y.; et al. A putative R3 MYB repressor is the candidate gene underlying atroviolacium, a locus for anthocyanin pigmentation in tomato fruit. J. Exp. Bot. 2017, 68, 5745–5758. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.X.; Liu, X.L.; Cao, C.; Wei, K.; Ning, Y.; Yang, P.; Li, S.S.; Chen, Z.Y.; Wang, X.X.; Guo, Y.M.; et al. Construction and application of a CRISPR/Cas9 system for multiplex gene editing in tomato. Acta Hortic. Sin. 2023, 50, 1215–1229. (In Chinese) [Google Scholar]
- Van Eck, J.; Keen, P.; Tjahjadi, M. Agrobacterium tumefaciens-mediated transformation of tomato. Methods Mol. Biol. 2019, 1864, 225–234. [Google Scholar] [CrossRef]
- Vekemans, D.; Viaene, T.; Caris, P.; Geuten, K. Transference of function shapes organ identity in the dove tree inflorescence. New Phytol. 2012, 193, 216–228. [Google Scholar] [CrossRef]
- Paz, R.C.; Kozaczek, M.E.; Rosli, H.G.; Andino, N.P.; Sanchez-Puerta, M.V. Diversity, distribution and dynamics of full-length Copia and Gypsy LTR retroelements in Solanum lycopersicum. Genetica 2017, 145, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Roque, E.; Pineda, B.; Cañas, L.; Rodriguez-Concepción, M.; Beltrán, J.P.; Gómez-Mena, C. Early anther ablation triggers parthenocarpic fruit development in tomato. Plant Biotechnol. J. 2013, 11, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Roque, E.; Gómez-Mena, C.; Hamza, R.; Beltrán, J.P.; Cañas, L.A. Engineered male sterility by early anther ablation using the pea anther-specific promoter PsEND1. Front. Plant Sci. 2019, 10, 819. [Google Scholar] [CrossRef]
- Guo, X.; Hu, Z.; Yin, W.; Yu, X.; Zhu, Z.; Zhang, J.; Chen, G. The tomato floral homeotic protein FBP1-like gene, SlGLO1, plays key roles in petal and stamen development. Sci. Rep. 2016, 6, 20454. [Google Scholar] [CrossRef]
- Alseekh, S.; Scossa, F.; Fernie, A.R. Mobile transposable elements shape plant genome diversity. Trends Plant Sci. 2020, 25, 1062–1064. [Google Scholar] [CrossRef]
- Catlin, N.S.; Josephs, E.B. The important contribution of transposable elements to phenotypic variation and evolution. Curr. Opin. Plant Biol. 2022, 65, 102140. [Google Scholar] [CrossRef] [PubMed]
- Pulido, M.; Casacuberta, J.M. Transposable element evolution in plant genome ecosystems. Curr. Opin. Plant Biol. 2023, 75, 102418. [Google Scholar] [CrossRef] [PubMed]
- Jouffroy, O.; Saha, S.; Mueller, L.; Quesneville, H.; Maumus, F. Comprehensive repeatome annotation reveals strong potential impact of repetitive elements on tomato ripening. BMC Genom. 2016, 17, 624. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, Q.; Wang, J.; Wang, B.; Zhao, J.; Huang, S.; Yang, T.; Tang, Y.; Yang, S.; Aisimutuola, P.; et al. Super-pangenome analyses highlight genomic diversity and structural variation across wild and cultivated tomato species. Nat. Genet. 2023, 55, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.; Dugas, E.; Benchouaia, M.; Leduque, B.; Jiménez-Gómez, J.M.; Colot, V.; Quadrana, L. The impact of transposable elements on tomato diversity. Nat. Commun. 2020, 11, 4058. [Google Scholar] [CrossRef] [PubMed]
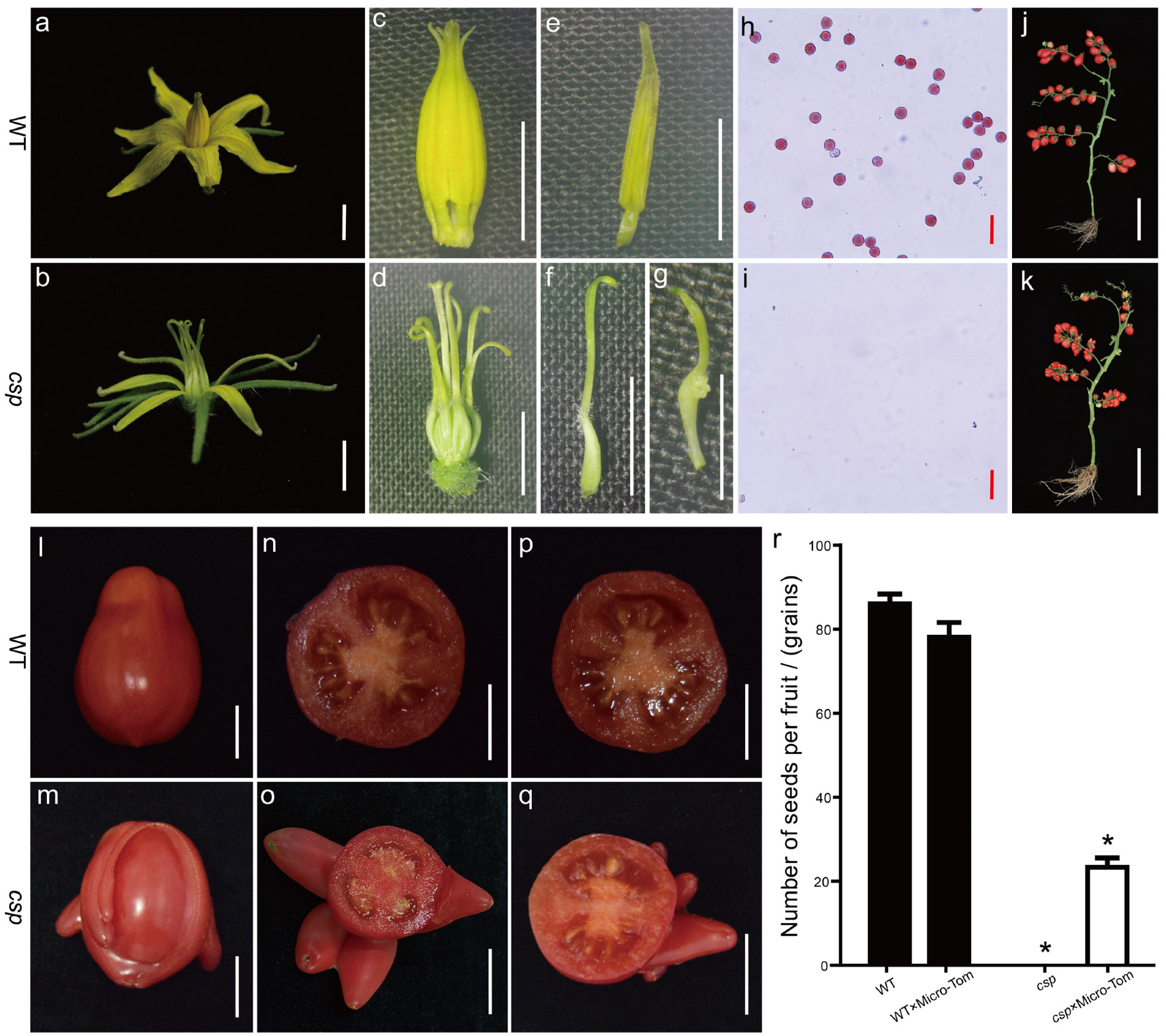
| Phenotypic Statistics | WT | csp |
|---|---|---|
| No. of observed stamens | 228 | 198 |
| Normal stamens (NOs) | 228 | 0 |
| Carpelloid structures (CSs) | 0 | 0 |
| Naked external ovules on the adaxial surface (EOs) | 0 | 12 (6%) |
| Complete transformation into carpels (TC) | 0 | 186 (94%) |
| Gene | Position | Function |
|---|---|---|
| Solyc04g080980.2 | SL4.0ch04: 63,014,916…63,020,499 (−) | Coatomer subunit alpha |
| Solyc04g080990.2 | SL4.0ch04: 63,025,202…63,027,315 (+) | S-type anion channel SLAH1 |
| Solyc04g081000.3 | SL4.0ch04: 63,032,681…63,036,255 (+) | TAP3, Tomato locus SlDEF (Deficiens), MADS box transcription factor |
| Solyc04g081010.2 | SL4.0ch04: 63,041,514…63,042,316 (+) | Unknown protein |
| Solyc04g081020.3 | SL4.0ch04: 63,056,424…63,057,867 (+) | B-box zinc finger protein 24 |
| Solyc04g081030.3 | SL4.0ch04: 63,064,187…63,069,499 (+) | Protein disulfide-isomerase 5-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wei, K.; Zhang, L.; Ning, Y.; Lu, F.; Wang, X.; Guo, Y.; Liu, L.; Li, X.; Zhu, C.; et al. Fine Mapping and Candidate Gene Validation of Tomato Gene Carpelloid Stamen and Parthenocarpy (CSP). Horticulturae 2024, 10, 403. https://doi.org/10.3390/horticulturae10040403
Li S, Wei K, Zhang L, Ning Y, Lu F, Wang X, Guo Y, Liu L, Li X, Zhu C, et al. Fine Mapping and Candidate Gene Validation of Tomato Gene Carpelloid Stamen and Parthenocarpy (CSP). Horticulturae. 2024; 10(4):403. https://doi.org/10.3390/horticulturae10040403
Chicago/Turabian StyleLi, Shanshan, Kai Wei, Li Zhang, Yu Ning, Feifei Lu, Xiaoxuan Wang, Yanmei Guo, Lei Liu, Xin Li, Can Zhu, and et al. 2024. "Fine Mapping and Candidate Gene Validation of Tomato Gene Carpelloid Stamen and Parthenocarpy (CSP)" Horticulturae 10, no. 4: 403. https://doi.org/10.3390/horticulturae10040403
APA StyleLi, S., Wei, K., Zhang, L., Ning, Y., Lu, F., Wang, X., Guo, Y., Liu, L., Li, X., Zhu, C., Du, Y., Li, J., & Huang, Z. (2024). Fine Mapping and Candidate Gene Validation of Tomato Gene Carpelloid Stamen and Parthenocarpy (CSP). Horticulturae, 10(4), 403. https://doi.org/10.3390/horticulturae10040403






