The First Study on Cultivating Roman Chamomile (Chamaemelum nobile (L.) All.) for Its Flower and Essential Oil in Southeast Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Localities with Experimental Cultivations of Roman Chamomile
2.2. Soil Analyses
2.3. Morphological and Productive Traits
2.4. Plant Material
2.5. Essential oil (EO) Extraction Procedure
2.6. Essential Oil Sample Preparation and Chemical Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Meteorological Conditions during the Research Period
3.2. Agrochemical Properties of Soil
3.3. Morphological and Productive Traits of Roman Chamomile
3.3.1. Influence of Year
| Year | Soil Type | Locality | Plant Height (cm) | Number | Fresh Flower Heads | Yield (g/m2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Branches | Leaves | Flowers | Diameter (cm) | Weight (g) | Fresh | Dry | ||||
| 2022 | Alluvial | Gadžin Han | 38.0 | 1.5 | 9.0 | 2.8 | 1.3 | 0.05 | 1578.7 | 890.1 |
| Rendzina | Svrljig | 49.8 | 2.8 | 10.5 | 2.3 | 1.6 | 0.05 | 1736.8 | 1314.1 | |
| Calcomelanosol | Bela Palanka | 46.3 | 3.0 | 12.0 | 3.3 | 1.5 | 0.07 | 485.5 | 319.3 | |
| 2023 | Alluvial | Gadžin Han | 41.3 | 2.8 | 12.8 | 3.8 | 1.5 | 0.11 | 1624.5 | 791.9 |
| Rendzina | Svrljig | 48.8 | 5.5 | 12.8 | 5.0 | 1.7 | 0.12 | 1963.5 | 1289.9 | |
| Calcomelanosol | Bela Palanka | 46.3 | 3.0 | 12.0 | 3.3 | 1.5 | 0.07 | 1014.7 | 447.7 | |
| Average | Alluvial | Gadžin Han | 39.6 c | 2.1 b | 10.9 a | 3.3 a | 1.4 | 0.08 a | 1601.6 | 841.0 b |
| Rendzina | Svrljig | 49.3 a | 4.1 a | 11.6 a | 3.6 a | 1.6 | 0.08 a | 1850.2 | 1302.0 a | |
| Calcomelanosol | Bela Palanka | 46.3 b | 3.0 ab | 12.0 a | 3.3 a | 1.5 | 0.07 a | 750.1 | 383.5 c | |
| Years | v.r. | 0.08 | 6.15 | 11.40 | 6.04 | 0.85 | 45.85 | 29.22 | ||
| F pr. | 0.779 | 0.025 | 0.004 | 0.027 | 0.372 | <0.001 | <0.001 | |||
| LSD 0.05 | 5.60 | 1.14 | 1.42 | 1.08 | 0.17 | 0.01 | 105.4 | 82.4 | ||
| Soil type | v.r. | 4.68 | 4.64 | 7.62 | 0.24 | 3.52 | 3.33 | 181.63 | ||
| F pr. | 0.026 | 0.027 | 0.005 | 0.788 | 0.056 | 0.064 | <0.001 | <0.001 | ||
| LSD 0.05 | 6.86 | 1.40 | 1.74 | 1.33 | 0.21 | 0.01 | 129.0 | 100.9 | ||
| Interaction | v.r. | 0.24 | 2.19 | 3.80 | 2.50 | 0.28 | 12.11 | 8.14 | ||
| F pr. | 0.791 | 0.147 | 0.046 | 0.116 | 0.758 | <0.001 | 0.004 | 0.081 | ||
| LSD 0.05 | 9.70 | 1.98 | 2.46 | 1.88 | 0.30 | 0.02 | 182.5 | 142.7 | ||
3.3.2. Influence of Soil Type
3.3.3. Influence of Interaction Year x Soil Type
3.4. Content and Composition of Roman Chamomile Essential Oil (EO)
| Essential Oil Content (%) | CV | ||||
|---|---|---|---|---|---|
| Locality | Soil Type | 2022 | 2023 | Average | (%) |
| Gadžin Han | Alluvial soil | 0.72 b | 0.89 b | 0.80 b | 21.01 |
| Svrljig | Rendzina | 1.50 a | 1.74 a | 1.62 a | 17.45 |
| Bela Palanka | Calcomelanosol | 0.68 b | 0.80 b | 0.74 b | 10.17 |
| Average | 0.97 | 1.14 | 1.05 | 16.21 | |
| LSD 0.05 | 0.22 | 0.31 | 0.27 | ||
| F-test | ** | ** | ** | ||
| Contribution to Essential Oil (%. w/w) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Alluvial Soil Gadžin Han | Rendzina Svrljig | Calcomenasol Bela Palanka | ||||||
| RIlit | RIexp | Components | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 |
| 869 | 873 | Isopentyl acetate | 0.5 | 0.4 | 0.3 | 0.4 | ||
| 875 | 875 | 2-Methyl butyl acetate | 0.2 | 0.5 | 0.2 | 0.3 | 0.3 | |
| 908 | 912 | Isobutyl isobutyrate | 3.2 | 3.2 | 2.0 | 1.9 | 2.2 | 2.2 |
| 914 | 919 | 3-methyl-2-Buten-1-ol acetate | 5.1 | 5.0 | 4.4 | 4.5 | 0.5 | 0.5 |
| 932 | 936 | α–Pinene | 10.5 | 10.9 | 4.7 | 4.7 | 6.0 | 6.1 |
| 946 | 952 | Camphene | 12.1 | 12.3 | 3.2 | 3.4 | 1.4 | 1.4 |
| 974 | 979 | β–Pinene | 1.0 | 0.7 | 1.5 | 1.2 | 0.7 | 0.9 |
| 988 | 991 | Myrcene | 1.1 | 1.0 | 1.3 | 1.3 | 0.8 | 0.8 |
| 1007 | 1009 | Isoamyl isobutyrate | 2.3 | 0.7 | 3.1 | 0.6 | 3.2 | 0.4 |
| 1045 | 1049 | Isobutyl angelate | 12.3 | 13.6 | 4.3 | 4.4 | 10.6 | 10.7 |
| 1048 | 1054 | Prenyl isobutyrate | 1.7 | 2.0 | 3.0 | 3.1 | 1.4 | 1.6 |
| 1056 | 1064 | Artemisia ketone | 15.9 | 4.1 | 10.7 | 11.4 | 8.8 | 9.0 |
| 1063 | 1070 | n–Octanol | 0.6 | 0.5 | 0.2 | |||
| 1085 | 1086 | Butyl angelate | 0.6 | 0.6 | 0.4 | 0.4 | 0.4 | 0.4 |
| 1088 | 1089 | Isobutyl tiglate | 0.2 | 0.1 | ||||
| 1100 | 1101 | 2–Methyl butyl-2-methyl butyrate | 0.5 | 0.4 | 0.9 | 0.9 | 1.1 | 1.1 |
| 1127 | 1133 | Octyl formate | 6.0 | 5.4 | 7.9 | 7.9 | 3.5 | 3.6 |
| 1143 | 1147 | Isoamyl angelate | 5.6 | 5.5 | 8.6 | 8.7 | 13.8 | 13.7 |
| 1148 | 1151 | Isoamyl tiglate | 5.5 | 5.6 | 6.3 | 6.4 | 11.4 | 11.2 |
| 1145 | 1156 | Camphene hydrate | 0.4 | 0.8 | 0.4 | |||
| 1145 | 1158 | Myrcenone | 1.3 | 0.8 | 0.7 | 0.8 | 0.5 | |
| 1160 | 1169 | Pinocarvone | 1.4 | 1.1 | 4.0 | 3.9 | 8.7 | 8.5 |
| 1165 | 1173 | Borneol | 0.5 | 0.3 | 0.4 | 0.5 | ||
| 1189 | 1190 | Prenyl angelate | 3.4 | 3.0 | 4.7 | 4.7 | 3.2 | 3.2 |
| 1197 | 1201 | Butanoic acid. 2-methyl-4-methylpentyl ester | 0.5 | 2.0 | 2.4 | |||
| 1195 | 1202 | Myrtenal | 2.6 | 3.1 | ||||
| 1249 | 1251 | 3–Methyl pentyl angelate | 4.9 | 19.1 | 20.7 | 20.9 | 15.2 | 15.1 |
| 1275 | 1276 | 3Z–Hexenyl angelate | 0.3 | 0.1 | ||||
| 1417 | 1435 | Caryophyllene(E-) | 0.1 | |||||
| 1493 | 1497 | trans–Muurola-4(14).5-diene | 0.2 | 0.2 | 1.0 | 1.0 | ||
| 1627 | 1627 | 1-epi-Cubenol | 0.4 | 0.2 | ||||
| Monoterpene hydrocarbons | 23.7 | 23.9 | 9.4 | 9.4 | 8.2 | 8.4 | ||
| Oxygenated monoterpenes | 72.7 | 72.3 | 86.8 | 85.1 | 88.9 | 86.7 | ||
| Sesquiterpene hydrocarbons | 0.2 | 0.0 | 0.2 | 0.0 | 1.1 | 1.0 | ||
| Oxygenated sesquiterpenes | 0.4 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | ||
| Total identified | 97.0 | 96.2 | 96.4 | 94.5 | 98.4 | 96.1 | ||
3.5. Correlations among Variables
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicine, 3rd ed.; Pharmaceutical Press: London, UK, 2007. [Google Scholar]
- Anonymous. Roman Chamomile EO (CAS N° 8015-92-7). Available online: https://www.scentree.co/en/Roman_Chamomile_EO.html (accessed on 20 December 2022).
- EMEA; European Medicines Agency; Committee on Herbal Medicinal Products (HMPC). Assessment Report on Chamaemelum nobile (L.) All. Flos; EMA/HMPC/560906/2010; European Medicines Agency: London, UK, 2011; Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-chamaemelum-nobile-l-all-flos_en.pdf (accessed on 20 October 2023).
- Marković, T. Essential Oils and Their Safe Use; Institute for Medicinal Plant Research “Dr. Josif Pančić”: Belgrade, Serbia, 2011. (In Serbian) [Google Scholar]
- Ph Eur 7.0. 2011 European Pharmacopoeia, 7th ed.; Council of Europe: Strasbourg, France, 2011.
- Sharafzadeh, S.; Alizadeh, O. German and Roman chamomile. J. Appl. Pharm. Sci. 2011, 1, 1–5. [Google Scholar]
- Chamomillae romanae flos (Roman Chamomile Flower). ESCOP Monograph. 2019. Available online: https://www.escop.com/downloads/chamomillae-romanae-flos-roman-chamomile-flover/ (accessed on 20 October 2023).
- Moss, M.; Howarth, R.; Wilkinson, L.; Wesnes, K. Expectancy and the aroma of Roman chamomile influence mood and cognition in healthy volunteers. Int. J. Aromather. 2006, 16, 63–73. [Google Scholar] [CrossRef]
- Buckle, J. Basic plant taxonomy. basic essential oil chemistry. extraction. biosynthesis. and analysis. In Clinical Aromatherapy; Barlow, J., Ed.; Churchill Livingstone: St. Louis, MI, USA, 2015. [Google Scholar]
- Magro, A.; Carolino, M.; Bastos, M.; Mexia, A. Efficacy of plant extracts against stored products fungi. Rev. Iberoam. Micol. 2006, 23, 176–178. [Google Scholar] [CrossRef]
- Arctander, S. Perfume and Flavor Materials of Natural Origin; Lulu Press: Morrisville, NC, USA, 1960. [Google Scholar]
- Guenther, E. The Essential Oils; RE Krieger: Huntington, NY, USA, 1975; pp. 433–437. [Google Scholar]
- FAO. Anthemis nobilis. Ecocrop; FAO: Rome, Italy, 2023; Available online: https://ecocrop.review.fao.org/ecocrop/srv/en/cropView?id=3254 (accessed on 7 November 2023).
- Franke, R.; Schilcher, H. Chamomile: Industrial Profiles; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Liuc, J.; Pank, B. Effect of vermicompost and fertility levels on growth and oil yield of Roman chamomile. Sci. Pharm. 2005, 46, 63–69. [Google Scholar]
- ISO-11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: London, UK, 1995.
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor- und Kaliumbestimmung. K. Lantbrukshögskolans Ann. 1960, 26, 199–215. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Compounds by Gas Chromatography and Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2009. [Google Scholar]
- Radmanović, S.; Djordjević, A.; Nikolić, N. Humus composition of rendzina soils in different environmental conditions of Serbia. Arch. Tech. Sci. 2018, 19, 57–64. [Google Scholar] [CrossRef]
- Raičević, P.R. Karakteristike Zemljišta Pivske Planine. Ph.D. Thesis, Agricultural Faculty of University of Belgrade, Belgrade, Serbia, 2018. (In Serbian). [Google Scholar]
- Naghani, S.S. Irrigation and salinity effects on some morpho-physiological characteristics in German and Roman chamomile populations. Rom. J. Biol. Plant Biol. 2019, 64, 73–81. [Google Scholar]
- Asai, I.; Yoshihara, K.; Omoto, T.; Shimomura, K. Growth and essential oil production in shoot culture and regenerates of Anthemis nobilis L. Plant Tissue Cult. Lett. 1995, 12, 303–311. [Google Scholar] [CrossRef]
- Wagner, S.; Pfleger, A.; Mandl, M.; Böchzelt, H. Changes in the qualitative and quantitative composition of essential oils of clary sage and roman chamomile during steam distillation in pilot plant scale. In Distillation—Advances from Modelling to Application; Zereshki, S., Ed.; InTech: Melbourne, VC, Australia, 2012; pp. 141–158. ISBN 9789535104285. [Google Scholar]
- Carnat, A.; Carnat, A.P.; Fraisse, D.; Ricoux, L.; Lamaison, J.L. The aromatic and polyphenolic composition of Roman camomile tea. Fitoterapia 2004, 75, 32–38. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Sefidkon, F.; Kazemi, F. Influence of drying methods on the essential oil content and composition of Roman chamomile. Flavour Frag. J. 2004, 19, 196–198. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with a bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [PubMed]
- Fauconnier, M.L.; Jaziri, M.; Homes, J.; Shimomura, K.; Marlier, M. Anthemis nobilis L. (Roman Chamomile): In vitro culture. micropropagation. and the production of essential oils. In Medicinal and Aromatic Plants IX; Springer: Berlin/Heidelberg, Germany, 1996; pp. 16–37. [Google Scholar]
- Silveira, V.; Rubio, K.T.S.; Martucci, M.E.P. Anxiolytic effect of Anthemis nobilis L. (roman chamomile) and Citrus reticulata Blanco (tangerine) essential oils using the light-dark test in zebrafish (Danio rerio). J. Ethnopharmacol. 2022, 298, 115580. [Google Scholar] [CrossRef] [PubMed]
- Drobac, M.; Lakušić, D.; Kovačević, N.; Tzakou, O. Comparative Analysis of Essential Oils of Six Anthemis Taxa from Serbia and Montenegro. Chem. Biodivers. 2010, 7, 1231–1244. [Google Scholar]
- Baranauskienė, R.; Venskutonis, P.R.; Ragažinskienė, O. Valorisation of Roman chamomile (Chamaemelum nobile L.) herb by comprehensive evaluation of hydrodistilled aroma and residual non-volatile fractions. Food Res. Int. 2022, 160, 111715. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.C.; Hará, M.; Uemura, M. Proline synthesis. physiological responses and biomass yield of eggplants during and after repetitive soil moisture stress. Sci. Hortic. 2005, 103, 387–402. [Google Scholar] [CrossRef]
- Sándor, Z.; Mottaghipisheh, J.; Veres, K.; Hohmann, J.; Bencsik, T.; Horváth, A.; Kelemen, D.; Papp, R.; Barthó, L.; Csupor, D. Evidence supports tradition: The in vitro effects of roman chamomile on smooth muscles. Front. Pharmacol. 2018, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Farkas, P.; Hollá, M.; Vaverková, S.; Stahlova, B.; Tekel, J.; Havránek, E. Composition of the essential oil from the flowerheads of Chamaemelum nobile (L.) All. (Asteraceae) cultivated in Slovak Republic. J. Esset. Oil Res. 2003, 15, 83–85. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Raman, V.; Avonto, C.; Yang, X.; Demirci, B.; Chittiboyina, A.; Khan, I. Chemical Compositions of Essential Oils from German. Roman. and Chinese Chamomile Flowers and Their Biological Activities against Three Economically Important Insect Pests. Rec. Nat. Prod. 2023, 17, 595–614. [Google Scholar] [CrossRef]
- Antonelli, A.; Fabbri, C. Study on Roman chamomile (Chamaemelum nobile L. All.) oil. J. Essent. Oil Res. 1998, 10, 571–574. [Google Scholar] [CrossRef]
- Bail, S.; Buchbauer, G.; Jirovetz, L.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Schmidt, E.; Geissler, M. Antimicrobial activities of Roman chamomile oil from France and its main compounds. J. Essent. Oil Res. 2009, 21, 283–286. [Google Scholar] [CrossRef]
- Umezu, T.; Sano, T.; Hayashi, J.; Yoshikawa, Y.; Shibata, Y. Identification of isobutyl angelate. isoamyl angelate and 2-methylbutyl isobutyrate as active constituents in Roman chamomile essential oil that promotes mouse ambulation. Flavour Frag. J. 2017, 32, 433–439. [Google Scholar] [CrossRef]
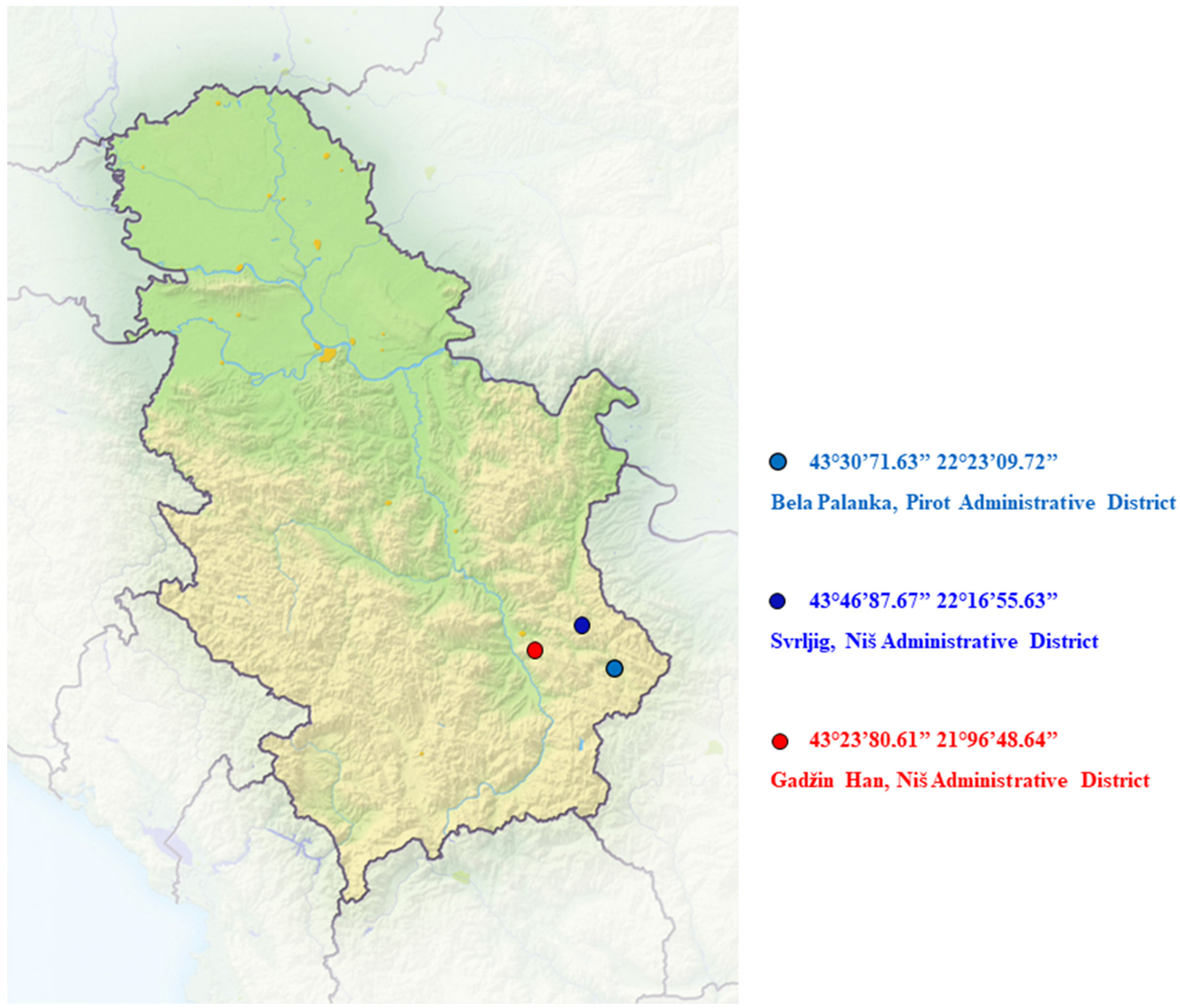
| Locality | Soil Type | Altitude (m) | Latitude (°N) | Longitude (°E) |
|---|---|---|---|---|
| Gadžin Han | Alluvial soil | 230 | 43°23′80.61″ | 21°96′48.64″ |
| Svrljig | Rendzina | 400 | 43°46′87.67″ | 22°16′55.63″ |
| Bela Palanka | Calcomelanosol | 270 | 43°30′71.63″ | 22°23′09.72″ |
| Administrative District Niš Localities Gadžin Han and Svrljig | Administrative District Pirot Locality Bela Palanka | Republic of Serbia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Month | Precipitations (mm) | T (°C) | Precipitations (mm) | T (°C) | Precipitations (mm) | T (°C) | ||||
| 2021/22 | 2022/23 | 2021/22 | 2022/23 | 2021/22 | 2022/23 | 2021/22 | 2022/23 | 1981–2010 | ||
| XI | 45.6 | 87.1 | 9.3 | 9.8 | 26.2 | 79.5 | 7.2 | 8.2 | 58.3 | 5.2 |
| XII | 86.6 | 64.8 | 4.2 | 6.5 | 93.9 | 58.0 | 0.4 | 4.1 | 55.3 | 0.8 |
| I | 22.0 | 76.0 | 1.5 | 5.6 | 43.3 | 26.0 | −1.3 | 3.1 | 43.4 | −0.4 |
| II | 15.6 | 19.6 | 6.1 | 4.7 | 38.8 | 12.5 | 3.1 | 1.2 | 41.1 | 1.0 |
| III | 6.7 | 58.6 | 5.7 | 9.4 | 28.6 | 13.9 | 2.2 | 6.3 | 46.8 | 5.5 |
| Sum Average | 176.5 | 306.1 | 5.4 | 7.2 | 230.8 | 189.9 | 2.3 | 4.6 | 244.9 | 2.4 |
| Month | Precipitations (mm) | T (°C) | Precipitations (mm) | T (°C) | Precipitations (mm) | T (°C) | ||||
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 1981–2010 | ||
| IV | 58.1 | 40.9 | 11.9 | 10.0 | 24.4 | 29.8 | 10.4 | 8.64 | 56.7 | 10.7 |
| V | 23.0 | 41.9 | 19.0 | 16.0 | 72.4 | 52.6 | 16.9 | 14.5 | 64.9 | 15.9 |
| VI | 43.5 | 99.1 | 22.9 | 20.0 | 79.2 | 102.0 | 20.2 | 18.4 | 82.8 | 19.0 |
| VII | 58.6 | 59.6 | 24.4 | 24.0 | 61.8 | 15.2 | 21.8 | 23.2 | 63.5 | 21.0 |
| Sum Average | 183.2 | 241.5 | 19.6 | 17.5 | 237.8 | 199.6 | 17.3 | 16.2 | 267.9 | 14.9 |
| Soil Type (Locality) | pH | CaCO3 (%) | Humus (%) | Total N (%) | Available (mg/100 g Soil) | ||
|---|---|---|---|---|---|---|---|
| in H2O | in KCl | P2O5 | K2O | ||||
| Alluvial soil (Gadžin Han) | 7.88 | 7.15 | 6.36 | 2.32 | 0.117 | 7.6 | 12.9 |
| Rendzina (Svrljig) | 7.58 | 6.73 | 1.74 | 4.78 | 0.302 | 4.7 | 18.3 |
| Calcomelanosol (Bela Palanka) | 6.35 | 5.74 | 5.87 | 7.46 | 0.469 | 1.6 | 12.8 |
| Parameters | Dry Flower Weight (g/m2) | Plant Height (cm) | Number of Branches | Number of Leaves | Number of Heads | Diameter of the Head (cm) | Head Mass (g) | Essential Oil Content (%) | 3–Methyl Pentyl Angelate | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dry flower weight (g/m2) | Pearson Corr. | 1 | 0.10324 | 0.19063 | 0.39543 | 0.06092 | 0.13824 | 0.13949 | 0.77382 * | 0.34533 |
| Sig. | -- | 0.63118 | 0.37225 | 0.0558 | 0.77734 | 0.51946 | 0.51564 | 9.18 × 10−6 | 0.09838 | |
| Plant height (cm) | Pearson Corr. | 0.10324 | 1 | 0.60262 * | 0.38235 | 0.19124 | 0.55899 * | 0.06729 | 0.42262 * | 0.38038 |
| Sig. | 0.63118 | -- | 0.00183 | 0.0652 | 0.3707 | 0.00452 | 0.75472 | 0.03965 | 0.06671 | |
| Number of branches | Pearson Corr. | 0.19063 | 0.60262 * | 1 | 0.59464 * | 0.40059 | 0.59318 * | 0.43237 * | 0.53974 * | 0.48398 * |
| Sig. | 0.37225 | 0.00183 | -- | 0.00218 | 0.0524 | 0.00225 | 0.03485 | 0.00649 | 0.01656 | |
| Number of leaves | Pearson Corr. | 0.39543 | 0.38235 | 0.59464 * | 1 | 0.49592 * | 0.50794 * | 0.51538 * | 0.57743 * | 0.75219 * |
| Sig. | 0.0558 | 0.0652 | 0.00218 | -- | 0.01372 | 0.01128 | 0.00995 | 0.00313 | 2.24 × 10−5 | |
| Number of heads | Pearson Corr. | 0.06092 | 0.19124 | 0.40059 | 0.49592 * | 1 | 0.24333 | 0.57502 * | 0.24743 | 0.27496 |
| Sig. | 0.77734 | 0.3707 | 0.0524 | 0.01372 | -- | 0.25189 | 0.00329 | 0.24374 | 0.19348 | |
| Diameter of the head (cm) | Pearson Corr. | 0.13824 | 0.55899 * | 0.59318 * | 0.50794 * | 0.24333 | 1 | 0.37451 | 0.3579 | 0.45056 * |
| Sig. | 0.51946 | 0.00452 | 0.00225 | 0.01128 | 0.25189 | -- | 0.07138 | 0.08595 | 0.02714 | |
| Head mass (g) | Pearson Corr. | 0.13949 | 0.06729 | 0.43237 * | 0.51538 * | 0.57502 * | 0.37451 | 1 | 0.33309 | 0.44416 * |
| Sig. | 0.51564 | 0.75472 | 0.03485 | 0.00995 | 0.00329 | 0.07138 | -- | 0.11173 | 0.02968 | |
| Essential oil content (%) | Pearson Corr. | 0.77382 * | 0.42262 * | 0.53974 * | 0.57743 * | 0.24743 | 0.3579 | 0.33309 | 1 | 0.64151 * |
| Sig. | 9.18 × 10−6 | 0.03965 | 0.00649 | 0.00313 | 0.24374 | 0.08595 | 0.11173 | -- | 7.29 × 10−4 | |
| 3–Methyl pentyl angelate | Pearson Corr. | 0.34533 | 0.38038 | 0.48398 * | 0.75219 * | 0.27496 | 0.45056 * | 0.44416 * | 0.64151 * | 1 |
| Sig. | 0.09838 | 0.06671 | 0.01656 | 2.24 × 10−5 | 0.19348 | 0.02714 | 0.02968 | 7.29 × 10−4 | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipović, V.; Marković, T.; Dimitrijević, S.; Song, A.; Prijić, Ž.; Mikić, S.; Čutović, N.; Ugrenović, V. The First Study on Cultivating Roman Chamomile (Chamaemelum nobile (L.) All.) for Its Flower and Essential Oil in Southeast Serbia. Horticulturae 2024, 10, 396. https://doi.org/10.3390/horticulturae10040396
Filipović V, Marković T, Dimitrijević S, Song A, Prijić Ž, Mikić S, Čutović N, Ugrenović V. The First Study on Cultivating Roman Chamomile (Chamaemelum nobile (L.) All.) for Its Flower and Essential Oil in Southeast Serbia. Horticulturae. 2024; 10(4):396. https://doi.org/10.3390/horticulturae10040396
Chicago/Turabian StyleFilipović, Vladimir, Tatjana Marković, Snežana Dimitrijević, Aiping Song, Željana Prijić, Sara Mikić, Natalija Čutović, and Vladan Ugrenović. 2024. "The First Study on Cultivating Roman Chamomile (Chamaemelum nobile (L.) All.) for Its Flower and Essential Oil in Southeast Serbia" Horticulturae 10, no. 4: 396. https://doi.org/10.3390/horticulturae10040396
APA StyleFilipović, V., Marković, T., Dimitrijević, S., Song, A., Prijić, Ž., Mikić, S., Čutović, N., & Ugrenović, V. (2024). The First Study on Cultivating Roman Chamomile (Chamaemelum nobile (L.) All.) for Its Flower and Essential Oil in Southeast Serbia. Horticulturae, 10(4), 396. https://doi.org/10.3390/horticulturae10040396









