1. Introduction
As the major revenue creators of fresh commodities, cut flowers have significantly boosted the production of floricultural crops worldwide, particularly in many developing countries [
1]. The value of fresh cut flowers lies in their quality and longevity, with the promotion of flower opening and delayed petal senescence being topics of great interest in research on optimizing postharvest flower quality [
2]. The improved performance of a cut flower directly correlates with its extended lifespan, fostering increased consumer approval and consequently elevating its economic value. Whether in an open or bud state after separation from the parent, cut flowers typically continue their metabolic activity, consuming available nutrients and water in the tissues [
3]. Petal senescence is a programmed and irreversible process [
4], characterized by ultrastructural modifications, increased lipid peroxidation and membrane leakage, heightened respiration rate, enhanced hydrolase activity, alterations in various organelles, and the degradation of macromolecules [
5,
6]. The metabolic disorders induced by factors such as microbial growth, water deficit, and ethylene release [
7] further promote this senescence process.
Although chemical treatments are currently the predominant approach for promoting flowering and delaying senescence by improving the metabolic activity of cut flowers, the increasing availability of synthetic nanomaterials further suggests an alternative solution with promising applications [
8,
9]. Those specially designed nanoparticles, like carbon-based nanoparticles, are defined as materials with dimensions less than 100 nm [
10]. These nanoparticles exhibit distinctive physicochemical properties including small surface area, atypical surface structure, and increased reactivity due to their small size [
9]. Those nanoparticles are believed to be low–medium- or even non-toxic, easy to use, highly efficient and durable, with excellent electrical conductivity, thermal conductivity, and antibacterial activity against pathogens [
11]. Recently, environmentally friendly carbon nanoparticles, such as graphene oxide (GO), fullerenes (C60), and graphene quantum dots (GQDs), have also emerged as common substances for the postharvest treatment of cut flowers. During the senescence of fresh cut flowers, elevated levels of reactive oxygen species (ROS) destabilize proteins and membrane integrity, leading to cell death and accelerated flower senescence [
12]. He et al. [
13] indicated that cut roses (
Rosa ‘Carola’) treated with 0.1 mg L
−1 GO reached full bloom faster, bloomed for longer, and maintained their color better. Zhang et al. [
14] also demonstrated that the vase life of cut carnations was prolonged by about 10% or more with 1 mg L
−1 C60 and 25 mg L
−1 GQD treatments. The potential physiological mechanisms in these studies suggest that carbon nanomaterials, similar to common preservatives, can enhance the ornamental performance of cut flowers by acting as effective germicides to improve water balance, and as antioxidant agents to moderate ROS-derived metabolic disorders [
15].
However, the use of carbon nanoparticles does not appear to be a foolproof solution for improving the quality of cut flowers, and further refinements are necessary. In studies employing metal-based nanoparticles, researchers found that appropriate concentrations of nano-silver, when in combined with sucrose, can be more effective in improving the ornamental performance of a wide range of cut flowers, including
Dianthus caryophyllus,
Gerbera jamesonii, and
Antirrhinum majus [
16,
17,
18,
19]. This aligns with the observation made by He et al. [
13] in their study, where GO treatment applied to cut roses was conducted with deionized water, an atypical medium for cut flowers. Consequently, this also suggests that the upgraded solution established by the addition of carbon nanomaterials to common preservatives (including carbohydrate and 8-hydroxyquinoline) may be more effective in improving the performance of cut flowers. Wu et al. showed that the combination of 0.15 mg L
−1 graphene oxide and preservative exhibited excellent antioxidant and moisture balancing ability, which could effectively prolong the vase life of fresh-cut roses [
20]. Nevertheless, further validation in this direction needs to be pursued.
Multi-walled carbon nanotubes (MWCNTs) are carbon nanomaterials consisting of more than one layer of graphene rolled on top of another layer [
21]. Positive effects of MWCNTs on plants include the promotion of callus proliferation, seed germination, seedling growth, root formation, stress tolerance, and flower and fruit production [
22]. However, the application of MWCNTs in cut flowers has been little reported so far, not to mention the combined treatment with commonly used preservatives. A study on carnations by Ahmadi-Majd et al. showed that MWCNTs in the vial solution could extend vase life by maintaining water balance and stimulating antioxidant defenses, compared to leaf spray, which had little effect on water relationship, flowering, and preservation quality [
23]. Thus, MWCNTs were chosen for application in cut chrysanthemums in this study.
Chrysanthemum is among the top ten traditional flowers in China, and one of the four major cut flowers in the world, constituting approximately 30% of the total fresh-cut flower production [
24]. As a well-established non-climacteric species, its floral senescence is virtually independent of the presence of ethylene, but sensitive to changes in both moisture and carbohydrate content to decrease the damage of excessive ROS [
25]. Here, we compared the postharvest quality improvement of cut chrysanthemums ‘Jinba’ under the combined treatment of different concentrations of MWCNTs with common preservatives (including sugar and 8-hydroxyquinoline).
2. Materials and Methods
2.1. Plant Material and Treatments
Chrysanthemum × morifolium (Ramat.) Hemsl. ‘Jinba’, a Japanese-bred autumn cultivar, was chosen for MWCNT treatment in this study. Renowned for its erect, firm stems and large, white inflorescences, it stands out as a highly sought-after cut flower in the Chinese market. The cut chrysanthemums with unopened buds were sourced from a commercial farm in mid-to-late May 2022. The multi-walled carbon nanotubes (MWCNTs) (30–50 nm OD, >95 wt % purity, <1.5 wt % ash content, 0.5–2.0 mm length) were obtained from Nanjing XFNANO Materials Tech Co., Ltd., Nanjing, China. Prior to applying the MWCNTs, a combination of nitric acid (HNO3) and sonication was employed to enhance their biocompatibility with deionized water.
Healthy chrysanthemums with single-headed, uniformed-size bud were carefully selected for the experiment. After harvesting, they were rapidly transported to the experimental room in buckets covered with plastic film to minimize water loss and physical damage during transit. Stems were pre-cut underwater in accordance with the same retained stem length of 45 cm, which would help the cut flowers to avoid air bolting, reduce water loss, and maintain water balance. Ten leaves were retained on each stem.
A preserved solution containing 30 g L−1 sucrose and 0.2 g L−1 8-hydroxyquinoline was used as the basic solvent, supplemented with MWCNTs at six different concentrations. The MWCNT concentrations used were 0, 1.0, 2.5, 5.0, and 10.0 mg L−1, denoted as CK2, T1, T2, T3, and T4, respectively. An additional control treatment involved using deionized water (CK1) only. All treated cut chrysanthemums were placed in a laboratory chamber maintained at 25 ± 1 °C, 60% ± 5% relative humidity, and 15 µmol m−2 s−1 photon irradiance. The number of cut chrysanthemums in each treatment was 20.
2.2. Definition of Flowering Index of Cut Chrysanthemums ‘Jinba’
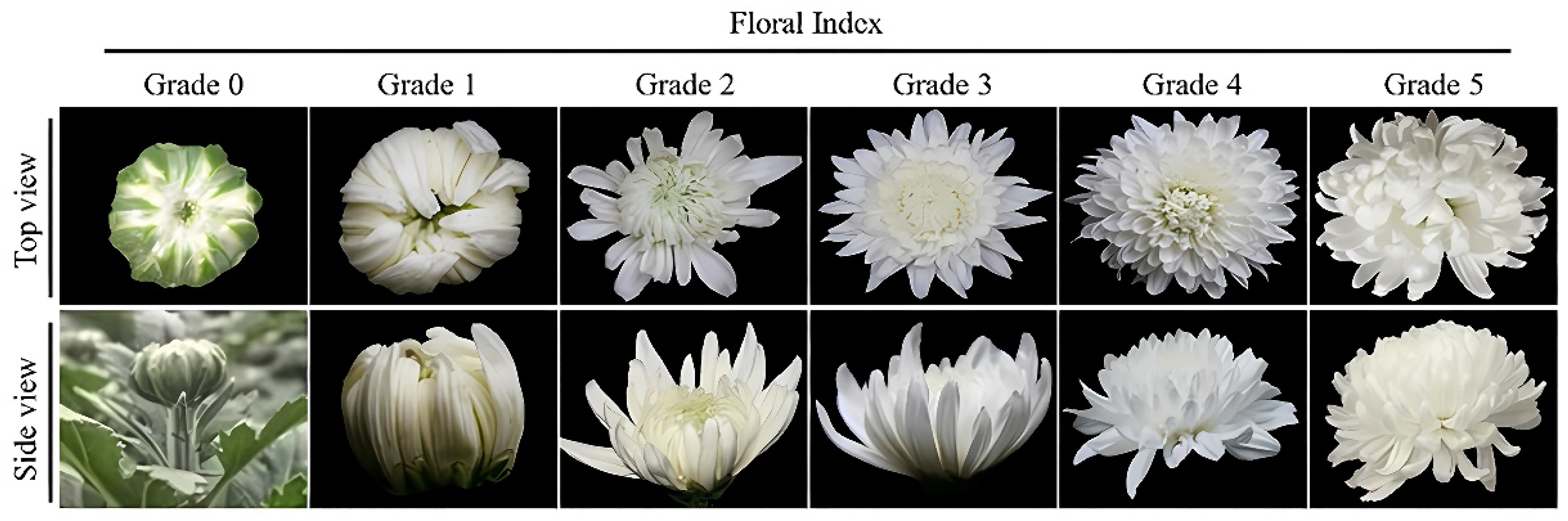
The floral index (FI) of cut chrysanthemums was characterized as six grades with specific morphological characteristics illustrated in
Figure 1. As depicted, the inflorescence bud at grade 0 is embraced by green bracts, while at grade 1, the floral color emerges and the outermost flowers commence to loosen. Flowers at grade 2 open only 1–2 layers, while those at grade 3 open 5 or more layers, and those at grade 4 opened all but the innermost 5 layers. The entire inflorescence at grade 5 become progressively flabby, with waterlogged drooping flowers. It can be observed that cut chrysanthemums at grade 4 exhibit the highest openness with the most visual ornamental value.
Subsequently, we meticulously monitored the transition of the floral index in cut chrysanthemums treated with different MWCNT concentrations every 2 d until 34 d. The diameter and longitudinal diameter of cut flowers in each treatment were measured with vernier calipers at 10:00 a.m. following the schedule outlined above. Moreover, the spherical index, defined as the ratio of inflorescence diameter to longitudinal diameter, was also calculated.
2.3. Physiological Changes in Cut Chrysanthemums Treated with MWCNTs
While observing the morphological characteristics, the moisture changes in the typical inflorescences at grade 0 to 5 were also determined. Three independent representative samples were randomly selected for each MWCNT treatment condition within every FI grade.
The fresh weight of entire cut chrysanthemums was weighed from 0 to 15 d and defined as the percentage of the initial original fresh weight (grade 0). Similarly, flowers in each FI stage were collected and weighed, and then dehydrated at 105 °C for 30 s, followed by an additional dehydration at 60 °C for 48 h before reweighing. The water content referred to the weight difference prior to and after flower dehydration.
The dehydrated flowers were further ground and processed for the determination of betaine and soluble sugar content. The acetone colorimetric method and the anthrone colorimetric method were employed to determine the contents of betaine and total soluble sugar, respectively. The acetone colorimetric method was employed to determine the contents of betaine according to Focht et al. [
26]. The anthrone colorimetric method was employed to determine the contents of total soluble sugar according to Yemm and Willis [
27]. The contents of proline were determined using the sulfosali-cylic acid method described by Cisse et al. [
28]. Three replications of the above experiments were performed for each sample.
2.4. Key Gene Expression in Cut Chrysanthemums Treated with MWCNTs
Fresh flowers at FI grade 1 to 5 were also harvested and rapidly frozen in liquid nitrogen for RNA extraction. Total RNA extraction and cDNA synthesis were performed according to the Instructions for the RNeasy Plant Mini Kit and RNA Transcription Synthesis Kit (Vazyme, Nanjing, China).
Table 1 displays three gene-specific PCR primers designed by Primer 3.0, with the Actin gene serving as the internal reference gene. SYBR green-based qRT-PCR was performed for all genes using the ABI StepOne Plus system (Applied Biosystems, San Diego, CA, USA). Each sample underwent three biological replicates. Relative gene expression levels for each sample were calculated using the ΔΔCT method.
2.5. Microscopic Observation of the MWCNT Distribution in Cut Inflorescence Stems
To confirm the absorbance of MWCNTs, we traced their uptake in the stems of cut chrysanthemums exposed to a preservative solution T5 containing 10 mg L−1 MWCNTs + 30 g L−1 sucrose + 0.2 g L−1 8-hydroxyquinoline. After 3 d, the stem sections were photographed with a camera under a stereomicroscope to visualize the distribution of MWCNTs in the cross-section of the cut chrysanthemum stems.
2.6. Statistical Analysis
In this study, all of the above experiments consisted of a minimum of three biological replicates. All quantitative data were summarized using means and standard deviations through SPSS Statistics 20.0 software (SPSS Inc., Chicago, IL, USA). Significance and multiple comparisons in each treatment group were analyzed using Analysis of Variance (ANOVA) and Tukey’s test with a significance level of 0.05. Statistically significant differences (* p < 0.05) were determined by Student’s t-test.
3. Results
3.1. The Treatment of MWCNTs Combined with Sucrose and 8-Hydroxyquinoline on Floral Index of Cut Chrysanthemums
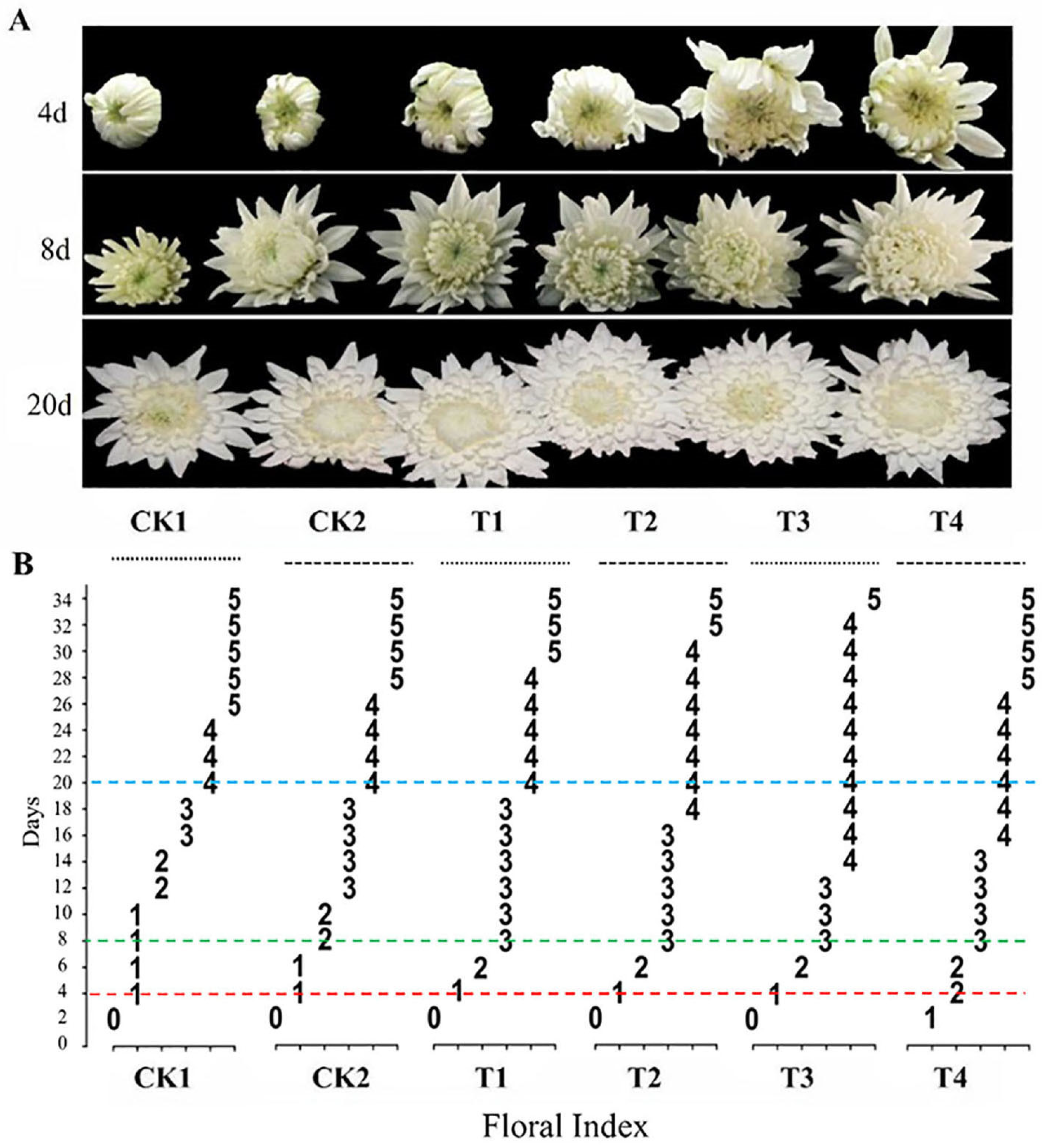
The morphological variations in cut chrysanthemum flowers subjected to distinct treatments are displayed in
Figure 2A, particularly focusing on three key days: 4, 8, and 20. Noticeable morphological differences are observed among the treatments with and without the addition of MWCNTs.
Figure 2B provides a more detailed representation of the initiation days of each stage of FI under each treatment of cut chrysanthemums and the duration of each stage. Under CK1 treatment (deionized water only), the development of cut chrysanthemums progressed to FI grade 1, grade 2, grade 3, grade 4, and grade 5 on day 4, 12, 16, 20, and 26, respectively. The durations in each FI were 8 d, 4 d, 4 d, 6 d, and 10 d, correspondingly. The flowering process of cut chrysanthemums was accelerated under CK2 treatment (30 g L
−1 sucrose and 0.2 g L
−1 8-hydroxyquinoline), reaching grade 2 and grade 3 on day 8 and 12, respectively. Additionally, the treatment delayed the senescence process, with the inflorescences at grade 5 on the 28th day. Typically, most ornamental chrysanthemums are at grade 3 and 4. Compared with CK1, the CK2 treatment prolonged the duration of cut chrysanthemums in the above grade 3 and grade 4 by 4 d and 2 d, respectively, with the best ornamental period reached at 8 d for both.
The treatment with different concentrations of MWCNTs combined with CK2 demonstrated a more pronounced effect on shortening the flower development process, maintaining blooming, and delaying senescence in cut chrysanthemums. The cut chrysanthemums treated with T4 quickly proceeded to grade 1 on the second day. Although the effects of T1, T2, and T3 were not as prominent as T4, the duration of grade 2 and 3 in cut chrysanthemums was limited to 2 d, respectively. The four treatments listed above resulted in optimal ornamental periods of 22 d, 24 d, 26 d, and 20 d, respectively, for cut chrysanthemums at grade 3 and grade 4. Compared to CK1 (10 d) and CK2 (16 d), there was a 4–10 d and 10–16 d extension for the optimal ornamental period, respectively. In addition, both treatments delayed the transition to grade 5, sustaining it for 2–8 d. In comparation to CK1 (10 d) and CK2 (8 d), the transition was shortened by 2–8 d and 0–6 d, respectively. Through this comparation, it can be inferred that T3, which include 5 mg L−1 MWCNT, 30 g L−1 sucrose, and 0.2 g L−1 8-hydroxyquinoline, was the most effective among all treatments.
3.2. The Treatment of MWCNTs Combined with Sucrose and 8-Hydroxyquinoline on Inflorescence Morphology of Cut Chrysanthemums
The inflorescence diameters of cut chrysanthemums under different treatments at the same FI are shown in
Figure 3A. It can be discovered that the inflorescence diameter was significantly larger under the combined treatments with the addition of MWCNTs (T1, T2, T3, and T4) compared to distilled water (CK1) and common preservative (CK2) used as the controls. Specifically, at grade 1 and 2, the maximum inflorescence diameter of cut chrysanthemums in T3 treatment solution reached 5.17 cm and 5.85 cm, respectively, which was 2.06 and 2.16 times larger than that of CK1, and 1.70 and 1.62 times more than CK2. At grade 3, 4, and 5, the cut chrysanthemum flowers with T4 treatment exhibited the largest diameter, measuring 6.98, 9.32, and 10.34 cm, respectively. These values were 2.30, 1.84, and 1.53 times greater than those in CK1, as well as being 1.35, 1.10, and 1.20 times those of CK2, respectively. These findings suggested that the addition of MWCNTs into sugar and 8-hydroxyquinoline promoted the increase in the inflorescence diameter of cut chrysanthemums, which, to some extent, facilitated their postharvest flowering process.
The spherical index, indicating the ratio of diameter to longitudinal diameter, reflects the fecundity of cut flowers. The spherical index at this maximum FI (grade 5) was 1.81 and 2.67 when CK1 and CK2 were used as the preservative solution. Conversely, in the treatment with common preservatives supplemented with different concentrations of MWCNTs, this index gradually increased with the rising concentration. Among them, T1, T2, and T3 treatments, respectively, reached 3.17, 3.28, and 3.54, while T4 reached 3.59. The spherical indices of these treatments were 1.75–1.98 and 1.19–1.35 times higher than those of CK1 and CK2 treatments, respectively. This finding further indicates that the incorporation of MWCNTs into the common solution enhances the ornamental value of cut chrysanthemums by causing the inflorescences to appear larger and plumper at the maximum FI (grade 5).
3.3. The Treatment of MWCNTs Combined with Sucrose and 8-Hydroxyquinoline on Physiological Changes of Cut Chrysanthemums
As previously described, the common preservative supplemented with MWCNTs had a significant effect on the promotion of the flowering process compared with CK1 and CK2. This discovery led to further investigation into the underlying physiological causes. Therefore, we compared their physiological changes in water and sugar uptake and some metabolites in the initial stages of cut flower vase plugging.
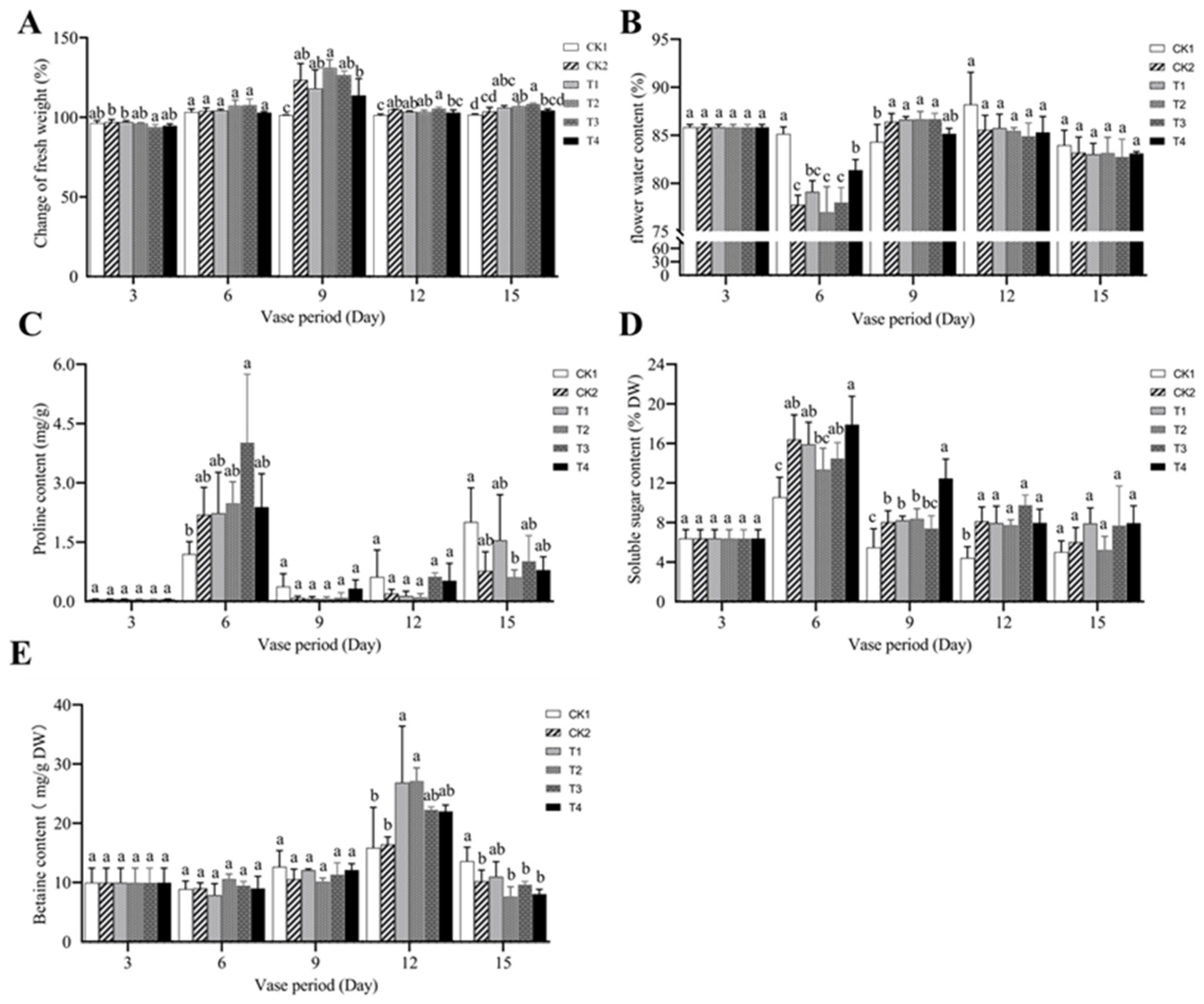
In
Figure 4A, the changes in fresh weight of the entire cut chrysanthemum over 3, 6, 9, 12, and 15 d of the vase period compared with the initial original fresh weight are depicted. The overall trend of each treatment showed an initial increase, reaching a peak at day 9, and then followed by a decrease. On day 6, there was no significant difference in fresh weight between the treatments. However, on day 9, the flower fresh weight of all treatments significantly increased compared with CK1, especially the T2 treatment. On day 12, even though the fresh weight of each treatment decreased compared with day 9, the T3 treatment still displayed a significant increase compared with other treatments. Overall, the fresh weight change of cut chrysanthemums treated with deionized water (CK1) was at the minimum with respect to the common preservative and the common preservative with the addition of MWCNTs.
Figure 4B shows the variation in flower water content of the cut chrysanthemums during the vase period. The flower water content of each treatment exhibited a general trend of decrease on day 6 or day 9, and then increased. On the third day of exposure to the vase solution, the flower water content did not significantly differ among treatments, and by day 6, except for CK1, all treatments reached their lowest values of the flower water content, among which the flower water content of T2 decreased the most. By day 12, CK1 exhibited the highest value for flower water content at 88.21%. However, the overall variation in flower water content in each treatment solution on day 12 and day 15 showed no significant differences.
Figure 4C provides the variation in flower proline content of the cut chrysanthemums during the vase period. It is observed that the flower proline content across all treatments exhibited peak levels on day 6 and day 15. On day 6, the proline content in petals between the CK1 and T3 treatments showed a significant difference, with values of 1.20 mg g
−1 and 4.02 mg g
−1, respectively. On day 15, the proline content between the CK1 and T2 treatments also showed a significant difference. In contrast to the earlier observation, the highest content was found in CK1 with 2.01 mg g
−1 and the lowest in the T2 treatment with 0.62 mg g
−1.
Figure 4D,E illustrate the changes in flower soluble sugar and betaine contents, both of which showed peaks on days 6 and 12, respectively. On day 6, the soluble sugar content in the flowers of the T4 treatment solution was the highest at 17.90%, while the CK1 content was the lowest at 10.6%, making the former 1.83 times higher than the latter. Additionally, on days 9 and 12, the soluble sugar content under the CK1 treatment was significantly lower than that of the other treatments (except the T3 treatment on day 9), at 5.48% and 4.42%, respectively. The flower betaine content was highest under the T1 and T2 treatments on day 12, reaching 27.1 mg g
−1 and 22.3 mg g
−1, respectively, and lowest under CK1 and CK2, at 15.86 mg g
−1 and 16.4 mg g
−1, respectively, with the former being 1.36–1.71 times more abundant than the latter.
3.4. The Treatment of MWCNTs Combined with Sucrose and 8-Hydroxyquinoline on Gene Expression Changes of Cut Chrysanthemums
Aquaporins (AQPs) play an important role in promoting plant water transport and increasing the water permeability of cell membranes [
29]. The sucrose transporter (SUT) also plays an important role in the regulation of sugar accumulation to maintain stable cellular osmotic pressure [
30]. The
BADH gene encodes for betaine aldehyde dehydrogenase, an important enzyme for the biosynthesis of glycine betaine that plays a definite role in stress tolerance through osmoregulation [
31]. Based on the above results of physiological changes in water and sugar uptake and some metabolites in the cut flowers, the expression levels of the relevant genes
CgAQP,
CgBADH, and
CgSUT in the cut chrysanthemums were determined and are presented in
Figure 5. As observed in the figure, the expression of the above three genes rapidly increased during the vase period in both distilled water and common preservatives, while a more pronounced increase was shown in the common preservatives supplemented with MWCNTs. Among them, the most obvious alteration was observed in
CgAQP (
Figure 5A). Under the CK1 and CK2 treatments, the expression level of
CgAQP increased from 1.12 and 1.02 initially to 5.68 and 7.92 at the end, respectively, whereas its expression increased even more exaggeratedly from 0.98–1.13 to 12.06–27.32 in the T1–T4 treatments. The expression levels of
CgBADH in the T3 and T4 treatments were also significantly increased from 0.98–1.04 at the initial stage to 13.08–16.55 in the final stage, respectively (
Figure 5B). In contrast to
CgAQP and
CgBADH, the increase rate of
CgSUT expression under the T1–T4 treatments was not very pronounced, merely increasing from 0.94–1.13 at the initial stage to 3.55–6.31 at the later stage (
Figure 5C). Moreover, it can also be inferred that those three genes are characterized by higher expression levels as the concentration of MWCNTs added in the common preservatives increases.
3.5. Distribution of MWCNTs in the Stem End of Cut Chrysanthemums
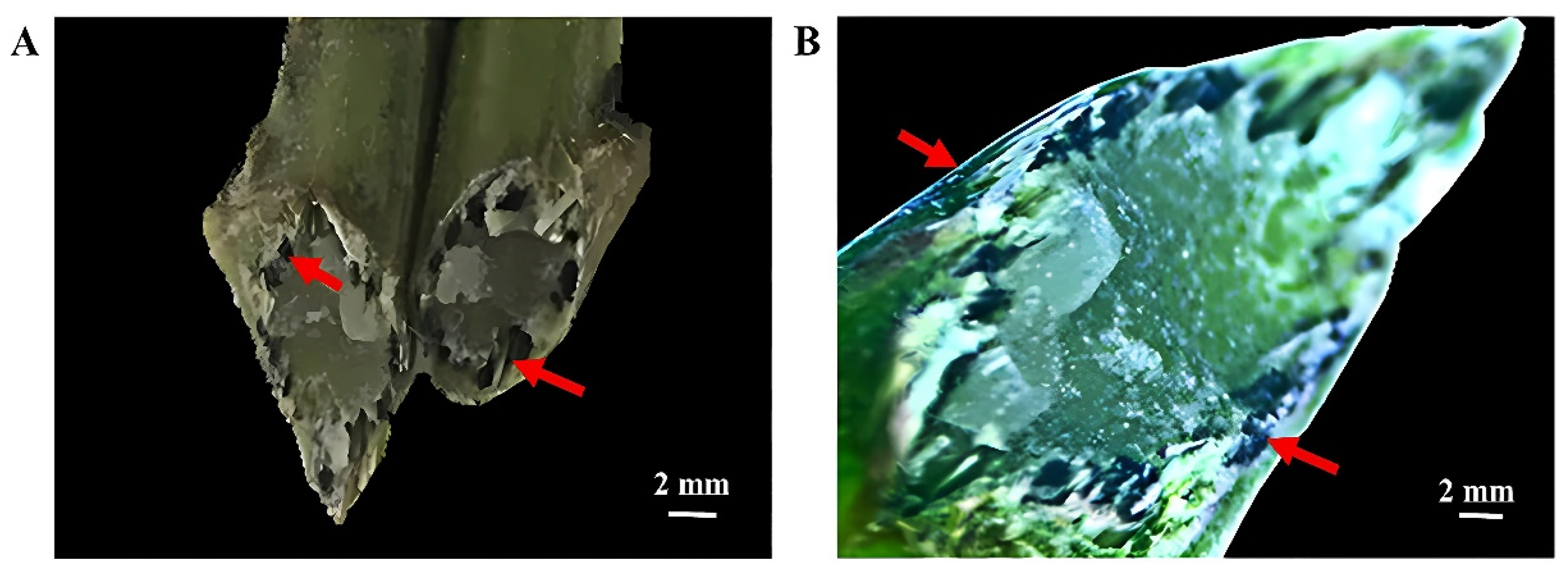
From both perspectives in camera and in stereomicroscope, it is evident that a substantial quantity of MWCNTs aggregated in the microtubular tissues of the roots of the cut chrysanthemums in the vial insertion solution containing a higher concentration of MWCNTs. And the particles of MWCNTs in the pith of the cut surface of the flower stems are clearly visible (
Figure 6).
4. Discussion
The floral index (FI) serves as a visual gauge for assessing floral development and senescence in cut lily and herbaceous peony (
Paeonia). For
Lilium asiaticum ‘Elite’, a six grade FI classification was established, where each grade is characterized as follows: with dense green buds (grade 1); with light orange buds (grade 2); with red but unexpanded flowers (grade 3); with half-opened flowers (grade 4); with fully open flowers (grade 5); and with senescing flowers (grade 6) [
32]. Similarly, for herbaceous peony, seven grades based on the main characteristics observed from the bud stage to petal senescence and withering were defined across 11 cultivars [
33]. The distinctions within each grade were determined by factors such as bud color, sepal adhesion to the bud, petal opening and closing, the curling and shedding of the exterior petals, etc. This study extends the application of the FI to cut chrysanthemums, redefining its six grades with specific morphological characteristics.
After harvesting, fresh-cut flowers are separated from the mother plant, leading to the interruption of water, nutrient, and plant growth regulator (PGR) supply. Metabolic activities such as transpiration and respiration inside the plant result in a series of physiological and biochemical changes, significantly accelerating the aging process [
3]. Among these changes, the imbalance in water metabolism, where the transpiration rate of the flower stems exceeds the absorption rate, is a primary cause of the rapid aging of fresh-cut flowers [
34]. Additionally, postharvest microbial invasion causing blockage in the flower stems’ conducting tissues leads to embolism, reducing water and nutrient absorption and further accelerating the aging of the flowers, severely impacting the ornamental quality of cut chrysanthemums [
7]. Similar to intact plants, cut chrysanthemums must maintain water balance from development to flowering to sustain normal physiological and metabolic activities. In this study, cut chrysanthemums placed in deionized water entered the optimal viewing period (grades 3 and 4) on the 16th day after harvesting, but with smaller inflorescence bud diameters and less full inflorescence shapes. During this period, there were no significant changes in fresh weight and water content. Only after 10 d did the inflorescences gradually age, with loose flower structures and drooping flowers, resulting in a decline in ornamental quality. Physiological and biochemical studies indicate that the transition from flowering to senescence in cut chrysanthemums is closely related to their reduced capacity to absorb water and sugars and a decrease in osmoregulatory substance content.
The composition of the vase solution is closely related to the vase life and ornamental quality of the cut flowers. To address the above issues, the main components of the cut flower preservative solution include water, sugars, fungicides, and PGRs. Among them, sugars and fungicides act as energy sources and inhibitors of bacterial growth, respectively [
35,
36]. In this experiment, a vase solution composed of 30 g L
−1 sucrose and 0.2 g L
−1 β-hydroxyquinoline (CK2) was used. Deionized water and sucrose, as the foundational components of the vase solution, provide ample energy for the cut flowers, promoting their physiological processes. The bactericide, 8-hydroxyquinoline, can reduce the growth of bacteria and microorganisms. When used in conjunction with sucrose, it enhances ROS scavenging capability and alleviates lipid peroxidation, thereby improving the postharvest quality of ethylene-insensitive cut flowers [
37]. The cut chrysanthemums treated with CK2 entered the optimal viewing period 4 d earlier compared to those placed in deionized water, delaying the transition to the late flowering stage by 2 d and exhibiting gradual senescence. The inflorescences were larger in diameter and had fuller shapes (
Figure 3). At the physiological and biochemical levels, the soluble sugar content in the CK2 flowers significantly increased, but excessive water loss occurred on the 9th day, affecting subsequent preservation effects (
Figure 4). It can be observed that the common preservative solution has a certain effect on the postharvest flowering preservation of cut chrysanthemums but requires further improvement.
Some studies suggest that carbon nanomaterials, like common preservatives, can effectively act as fungicides, enhance water balance, and serve as antioxidants to regulate the disrupted metabolism of reactive oxygen species, delaying senescence and ultimately improving the ornamental performance of cut flowers [
13,
14]. In this study, we also found that adding carbon nanomaterials to common preservatives, including carbohydrates and 8-hydroxyquinoline, was more beneficial for enhancing the postharvest flowering quality and preservation performance of cut chrysanthemums. During the postharvest flowering process of cut chrysanthemums, the development of inflorescence buds has essentially been completed, and postharvest flowering is essentially the elongation of flowers. Studies have shown that the elongation growth of chrysanthemum flowers is mainly due to the elongation of cells at the base of the flowers and an increase in the number of cell layers. Transcriptomic sequencing analysis and protein interaction analysis have indicated that genes in the auxin signaling pathway interact with the TCP transcription factor family, jointly regulating the growth of chrysanthemum flowers [
9]. In this study, compared to deionized water and a bottle solution containing only 0.2% 8-hydroxyquinoline + 3% sucrose, ‘Jinba’ exhibited accelerated postharvest flowering in a solution containing MWCNTs. This may be because MWCNTs participate in the auxin signaling pathway, thereby regulating the upregulation of related genes. At the same time, the length and spherical index of the flowers increased, enhancing their ornamental value, mainly due to the intensified elongation growth of the inner layer of the flowers. In contrast to the concentration-dependent improvement in postharvest flowering and vase life of
Dianthus reported [
23], the effect of MWCNTs on ‘Jinba’ in this study showed no significant concentration dependence. Among the four tested solution concentrations, the bottle solution containing 5 mg L
−1 MWCNTs had a better promoting effect on the postharvest inflorescence development of ‘Jinba’ cultivar. Observations under a camera and stereo microscope revealed that MWCNTs were adsorbed on the surface, pith, and pith rays of the inflorescence stem, and also accumulated in the microtubular tissues on the cut surface, without being transferred into the plant tissues. Further research is needed on how MWCNTs participate in the auxin signaling pathway, regulate the elongation growth and increase in cell layers at the base of the petals, and promote flowering and preservation.
Water is a crucial factor in the flowering and preservation of cut flowers, as it is a key substance for maintaining turgor pressure in plant cells. The turgidity of flowers depends on the balance of water absorption and loss to preserve the quality of fresh cut flowers [
2]. In cut roses, the petal aquaporin activity was found to be inhibited by PGRs at the transcriptional level, significantly reducing petal water content, and promoting flower opening [
38]. Sugars have various effects, serving not only as an energy source for metabolism but also playing a crucial regulatory role in the main functions of plant metabolism, growth, and stress response. Additionally, they play an important role in the aging process [
39]. Therefore, the absorption and utilization of exogenous sugars are of great significance for accelerating the flowering process and delaying senescence. In this study, from postharvest to the onset of flowering (grade 1), ‘Jinba’ cultivar inflorescence stems were primarily attributed to the absorption of water and sugars from the vase solution. In comparison to the common preservative solution, the inflorescence stems placed in the MWCNT-containing vase solution exhibited a more significant increase in fresh weight, with soluble sugar content maintained at a higher level. Although MWCNTs could promote the absorption of sugars from the preservative solution during the vase period, their effect was not as pronounced compared to the common preservative solution. The efficient expression of
CgAQP and
CgSUT during the vase period indicates an enhanced functionality of aquaporin and sucrose transporter proteins. This validates that MWCNTs and the common preservative solution complement each other, collectively promoting the absorption of water and sugars from the vase solution by inflorescence stems, laying a material foundation for the acceleration of the flowering process.
The aging pattern of cut chrysanthemums is characterized by an ethylene-insensitive type due to 1-aminocyclopropane-1-carboxylic acid (ACC) deficiency. During the preservation of cut chrysanthemums, maintaining water balance [
40] and membrane system stability, and enhancing the activity of antioxidant protective enzymes can slow down the aging process [
41]. Proline and betaine, as osmotic regulators, play a crucial role in preserving internal water, inhibiting membrane lipid peroxidation to maintain membrane integrity, and delaying aging [
42]. The levels of proline and betaine in the flowers placed in MWCNT-containing vase solutions showed no significant advantage compared to the common preservative solution in the early stage. However, during the full flowering period, they were generally higher, promoting increased water absorption capacity and fresh weight. Although there was a downward trend in the late vase period, it still enhanced the water absorption capacity of the cut chrysanthemums overall, effectively alleviating water loss and maintaining water balance, consistent with the findings of this study on changes in fresh weight and water content. During the vase period, the efficient expression of
CgBADH also indicates the vigorous metabolism of osmotic regulators, positively promoting the flowering and preservation of the cut chrysanthemums. Otherwise, the greater the accumulation of betaine, the stronger its improvement on membrane activity, leading to enhanced stress resistance in cut flowers. During the vase period, the change in betaine induced by MWCNTs followed a unimodal curve, with the peak appearing in the late stage of full flowering (grade 4). This suggests that MWCNTs can promote the release of betaine in the late stage of full flowering, thereby extending the flowering period and delaying senescence. Studies have indicated that imbalances in water during the vase process can significantly increase proline content [
43]. Therefore, further in-depth research analysis is needed to understand the mechanism by which MWCNTs induce a sharp increase in osmotic regulators in the later stages.
In summary, the addition of MWCNTs to the common preservative can enhance the plant’s water absorption capacity throughout the entire bottle insertion period. It also induces the accumulation of osmotic regulators such as betaine and proline in the later stages of bottle insertion. This further accelerates the flowering process, resulting in larger inflorescence diameters and a prolonged flowering period. Therefore, MWCNTs can be considered as a modified component in cut flower preservatives, synergistically acting with sucrose and 8-hydroxyquinoline to improve ornamental performance of cut flowers. Further research is necessary to delve deeper into the physiological mechanisms of promoting flowering and postharvest longevity.