Abstract
Fungal decay is one of the most significant causes of postharvest losses of blueberries, with Botrytis rot caused by Botrytis cinerea and Alternaria rot caused by Alternaria alternata being the two most destructive fungal diseases. Plant essential oil has attracted the extensive attention of scholars due to its natural antifungal and anti-corrosion effects. In this study, the effects of fumigation treatment with plant essential oils on the growth of pathogenic fungi in blueberry fruits in vitro and the activity of defense-related enzymes of fungi-inoculated blueberry were evaluated. The results showed that, of the six natural plant essential oils of cinnamon, oregano, clove, tea tree, pomelo peel, and rosemary, oregano essential oil had the most efficient inhibitory effect on Botrytis cinerea and Alternaria alternata in PDA. After fumigating inoculated blueberry fruits with concentration gradients of 0, 30, 60, and 90 μL/L of oregano essential oil, it was found that the activity of defense-related enzymes such as phenylalanine ammonia lyase (PAL), polyphenol oxidase (PPO), peroxidase (POD), chitinase (CHI), and β-1,3-glucanase (GLU) in the inoculated blueberry fruits was induced and enhanced to varying degrees throughout the entire storage period, effectively enhancing the resistance of blueberry fruits to pathogenic fungi and reducing the postharvest decay caused by Botrytis cinerea and Alternaria alternata. The optimal concentration for the fumigation treatment with oregano essential oil is 60 μL/L. This study provides a theoretical basis for the postharvest application of oregano essential oil in blueberries and other fruits and vegetables.
1. Introduction
Blueberries are of major consumer concern and great market appreciation due to their phenolic compound content and powerful antioxidant properties. However, the postharvest loss rate of fresh blueberries is very high. The main physiological and pathological reasons for this can be attributed to the following main issues: fruit shrinkage, softening, and decay, greatly shortening the storage and shelf life of blueberries. The decay caused by microbial infection is the main factor that limits blueberry storage, transportation, circulation, and marketing [1]. Various pathogens invade the surface of blueberry fruits through naturally open pores, stomata, wounds, and fruit stems, or invade the outer epidermis of the fruit by secreting host cell wall hydrolases and other substances [2]. Numerous studies have documented that the postharvest decay of blueberries was associated mostly with fungal infections, especially of Alternaria alternata (Alternaria rot) and Botrytis cinerea (Botrytis rot), which shortened the blueberries’ shelf life [3]. Botrytis cinerea and Alternaria alternata can continue to grow at very low temperatures, at −2 °C and −3 °C, respectively, which enables them to affect fruit during cold chain transportation and refrigeration [4], typically causing tremendous economic losses of fruit and vegetables. Therefore, microbial infection is considered one of the topics that requires continuous and in-depth research in the blueberry industry.
Botrytis cinerea and Alternaria alternata are the most common fungi that cause Botrytis rot disease and Alternaria rot disease in many fresh fruit and vegetables, respectively, such as strawberries [5], tomatoes [6], and winter jujube [7]. Postharvest fungal infections are mainly controlled through the use of fungicides. However, the widespread use of fungicides can not only lead to them remaining on the surface of fruits and vegetables and thus posing a threat to human health, but also is environmentally unfriendly. In recent decades, natural plant essential oils have received increasing attention as an additive for food preservation due to their non-toxic nature and excellent antimicrobial properties [8,9].
Essential oils, also known as volatile oils or aromatic oils, are naturally derived secondary metabolites in plants. They can be separated from the whole plant or plant flowers, buds, seeds, leaves, tender branches, bark, wood, fruits, and roots through methods such as pressing and distillation, resulting in a volatile oily liquid with an aromatic odor [10,11]. Generally speaking, essential oils are complex mixtures composed of hydrocarbons, often containing dozens to hundreds of organic compounds, which can be roughly divided into terpenes, aromatics, aliphatics, and small amounts of nitrogen-containing and sulfur-containing compounds based on their structures [10,12]. It is precisely because of these organic compounds that essential oils display a broad spectrum of antimicrobial activity. With the help of modern technology, essential oils can be released as plant fumigants onto suitable agricultural products such as fruits and vegetables, gradually replacing the use of chemical fungicides that pose a threat to human health and the environment in the control of postharvest decay and spoilage of fruits and vegetables during storage [13]. Mariadhas et al. found that garlic essential oil has high antifungal activity against Penicillium, Aspergillus flavus, and Aspergillus niger in plum fruits, preventing and controlling the decay of plum fruits [14]. Mahdi et al. found that clove essential oil and thyme essential oil can affect the energy metabolism of pathogen cells by inhibiting glycolysis, disrupt the normal physiological activity of Aspergillus niger in pomegranate fruits, and reduce the growth and germination of Aspergillus niger filaments [15]. Furthermore, Lin et al. found that essential oils such as cinnamon, cloves, star anise, and thyme have strong inhibitory effects on three harmful fungi in blueberries: Aspergillus, Penicillium, and Botrytis [16].
Essential oils contain four of the most active antimicrobial compounds, namely, terpenes (such as paraffins and limonenes), terpenoids (such as thymol and carvacrol), phenylpropene (such as eugenol and vanillin), and other compounds (allicin or isothiocyanates) [9]. These compounds, on the one hand, can directly inhibit the growth of fungi and prevent pathogen infection in fruits; on the other hand, they can also improve the fruit’s disease resistance by inducing the activity of disease-resistant enzymes and the content of disease-resistant substances in the fruit itself, thereby increasing its resistance to pathogenic microorganisms and reducing the fruit’s decay rate. Research by Cindi et al. (2016) has shown that thyme oil and cinnamon oil can increase the activity of defense-related enzymes such as PAL, GLU, and CHI, and effectively inhibit the occurrence of the decay of peaches [17]. Research by Pan et al. (2019) has shown that kiwifruit treated with methyl jasmonate (MeJA) fumigation increases the activity of defense enzymes such as superoxide dismutase (SOD), POD, catalase (CAT), and PPO [18]. Research by the authors Wei et al. (2018) has shown that tea tree oil and hot air treatment has varying degrees of effectiveness on the main pathogens of postharvest strawberry, enhancing the activity of GLU and CHI, which can directly act on the microorganisms themselves, thereby reducing the losses caused by fruit decay and spoilage [19]. However, little information is available regarding the effects of plant essential oil application in the vapor phase on Botrytis cinerea and Alternaria alternata spore germination or on the disease resistance of blueberry fruit.
Accordingly, the objectives of the present study were to investigate (1) the antifungal activities of plant essential oil vapor against Botrytis cinerea and Alternaria alternata in vitro and (2) the effect of plant essential oil treatment on the activities of defense-related enzymes in blueberry fruit. This research will contribute to the practical application of plant essential oils in the vapor phase for preserving postharvest fruit and vegetables.
2. Materials and Methods
2.1. Materials
Blueberries (Vaccinium corymbosum L. “O’Neal”) without mechanical damage on their surfaces were hand-harvested from the blueberry plantation at the Dalian Blueberry Technology Development Co., Ltd. facility in Zhuanghe City (Dalian, China). The fruits with intact white frost were uniform in weight (1.5–2 g) and color (100% blue), and then transported to the laboratory within 3 h of harvest using a refrigerated vehicle (10 °C, S.F. Express, Dalian, Liaoning, China). In the laboratory, the fruits were cooled for 24 h at 0 ± 0.5 °C and 90% RH.
Six plant essential oils (food grade), including cinnamon, oregano, cloves, tea tree, pomelo peel, and rosemary, were purchased from Zhongxiang Natural Plant Co., Ltd. in Ji’an City, China, and Guizhou Miao Medicine Biotechnology Co., Ltd. in Tongren City, China. They were all extracted using hydrodistillation and the purity of each essential oil was 99%.
Botrytis cinerea and Alternaria alternata were obtained from the School of Bioengineering, Dalian University of Technology, China, and were maintained at 4 °C in the dark in Petri dishes containing Potato Dextrose Agar (PDA; Hopebio, Qingdao, China).
2.2. Screening of Plant Essential Oils
The antimicrobial activity of plant essential oils was studied using the filter paper method, and the specific methodology is as follows:
2.2.1. Preparation of Filter Paper Sheets
Qualitative filter paper with uniform texture and strong water absorption were prepared, and circular paper pieces with a diameter of 5 mm were punched out. Then, the circular paper pieces were sealed in a dry and clean culture dish, sterilized under high pressure at 121 °C for 15 min, and then placed on a clean bench for later use.
2.2.2. Preparation of Fungal Suspension
The method is the same as that described by Ji et al. [1]. Specifically, both Botrytis cinerea and Alternaria alternata were cultured on PDA slants at 28 °C for 5 days, and then 5 mL septic NaCl solution (0.9%, v/v) was added to each slant and slowly scraped off the agar surface. The aseptic NaCl solutions containing pathogen spores were each transferred to a sterile triangle bottle and then dispersed with glass beads. After shaking, the spore solutions were filtered using absorbent cotton to remove mycelial fragments and were then washed 2–3 times with aseptic NaCl solution. Finally, 1 × 106 CFU/mL spore suspensions were prepared as needed using a blood cell counting board.
2.2.3. Selection of Essential Oils
Approximately 15–20 mL of melted PDA medium was poured into a culture dish (90 mm × 20 mm), and after cooling, 200 mL of fungal suspension was evenly applied onto the PDA culture substrate. Sterile filter paper with a diameter of 5 mm was placed at the center of each culture dish, 5 μL of cinnamon, oregano, clove, tea tree, pomelo peel, and rosemary essential oil solutions were dropped on the filter paper, respectively, and then these culture dishes were covered quickly. Filter paper without adding essential oil was used as a control. The PDA plates were sealed with cling film and incubated upside down in a constant temperature incubator at 28 °C for 7 days. The diameter of the antimicrobial ring (mm) was measured using the cross-shaped method. This process was repeated three times for each essential oil in the experiment. The criterion for determining the antimicrobial effect of plant essential oils on fungal strains is that if the diameter of the antimicrobial zone (including the diameter of filter paper) is between 7 and 9 mm, this indicates low sensitivity; if it is between 10 and 15 mm, it is moderately sensitive; if the diameter is greater than 15 mm, this indicates high sensitivity; if there is no antimicrobial zone or if the diameter of the antimicrobial zone is less than 5 mm, it is considered insensitive.
2.3. Fungal Inhibition Experiment of Oregano Essential Oil
Based on the results of the antifungal experiment with essential oils and literature review on the application of plant essential oils in fruit and vegetable preservation, 0, 30, 60, and 90 μL/L were selected as the concentration gradient of oregano essential oil for antifungal experiments conducted on PDA medium.
Approximately 15–20 mL of melted PDA medium was poured into a culture dish (90 mm × 20 mm) and a small hole with a diameter of 6 mm was dug in the center of each culture dish, which was then added to 20 μL fungal suspension. An appropriate amount of sterile filter paper of the same size and specification was sequentially pasted on the inner surface of each culture dish cover. Based on the remaining volume of space in the PDA plate, the corresponding volume of oregano essential oil was calculated and dropped onto the filter paper, making the concentration of oregano essential oil in the plate 0 (control), 30, 60, 90 μL/L, respectively. The filter paper without adding essential oil was used as a control. The flat plate was sealed with cling film and incubated upside down in a constant temperature incubator at 28 °C for 7 days. The results were observed and the colony growth diameter was measured using the crossover method.
2.4. Fumigation Treatment of Oregano Essential Oil on Inoculated Blueberries
2.4.1. Blueberry Inoculation
There are 30 blueberry fruits in the same concentration of essential oil treatment group, totaling 120 fruits. Firstly, the blueberries were soaked in a 2% sodium hypochlorite solution for 2 min, rinsed with sterile distilled water for 2 min, and finally, the remaining distilled water on the surface of the blueberries was absorbed with sterile filter paper for later use. A small hole with a diameter of 5 mm and a depth of 3 mm was punctured at the equator of each blueberry, and 20 μL suspension of Botrytis cinerea and Alternaria alternata were inoculated into the small hole, respectively.
2.4.2. Fumigation Treatment
Six uncovered culture dishes containing qualitative filter paper (allowing essential oils to gradually diffuse) were placed at the bottom of photosynthetic controlled atmosphere box (size: 46.3 cm × 27.3 cm × 26.6 cm, Beijing Hengqingyuan Technology Co., Ltd., Beijing, China), and then inoculated blueberry fruits were placed flat on two hollow compartments above the culture dishes. According to the volume of the box, the corresponding volume of oregano essential oil was calculated and dropped onto the filter paper, making the concentration of oregano essential oil in the box 0 (control), 30, 60, 90 μL/L, respectively. Finally, these atmosphere boxes were placed in an environment with a temperature of 0 ± 0.5 °C and a relative humidity of 90% for fumigation treatment for 18 h. Each treatment was repeated three times.
After fumigation, the lids of the controlled atmosphere boxes were opened, and the treated blueberries were ventilated for 2 h. Then, the inoculated blueberries were placed in a commercial PET plastic box (10.5 cm × 10.5 cm × 3 cm) and stored in cold storage at a temperature of 0 ± 0.5 °C and a relative humidity of 90%. On the 0th, 4th, 8th, 12th, and 16th day of storage, random samples were taken from each experimental group, frozen in liquid nitrogen, crushed, and stored in a −80 °C refrigerator for measuring the activity of resistance-related enzymes.
2.5. Determination of Enzyme Activity Related to Disease Resistance
The activity of phenylalanine ammonia lyase (PAL) was determined using the PAL assay kit from Suzhou Keming Biotechnology Co., Ltd., in Suzhou city, China. A total of 0.1 g of inoculated blueberry frozen sample powder was added into a 1.5 mL centrifuge tube and the supernatant was prepared according to the instructions of the reagent kit. Then, the supernatant and various reagents were added into the 1.5 mL centrifuge tube in sequence. After centrifugation, the supernatant was transferred to a UV plate, thoroughly mixed, and allowed to stand for 10 min. The absorbance value at 290 nm was measured, and the result was expressed in U/g (U represents an enzyme activity unit where the absorbance value at 290 nm changes by 0.05 per minute per gram of tissue in each milliliter of reaction system).
Activity determination of polyphenol oxidase (PPO) and peroxidase (POD): a total of 0.1 g of inoculated blueberry frozen sample powder and 1 mL of 0.1 mol/L phosphate-buffered solution (pH = 6.4) were sequentially added into a 1.5 mL centrifuge tube, shaken thoroughly with a vortex instrument, and centrifuged at 4 °C and 12,000× g for 30 min. Then, the supernatant (enzyme extract) was taken for subsequent experiments. For PPO, 100 μL enzyme extract and 600 μL 0.5 mol/L of catechol solution were sequentially added to a 1.5 mL centrifuge tube, and quickly mixed evenly. For POD, 100 μL enzyme extract and 600 μL 25 mmol/L of guaiacol solution were sequentially added to a 1.5 mL centrifuge tube, mixed evenly, and incubated in a 30 °C water bath for 5 min; then, 40 μL of 0.5 mol/L H2O2 solution was added and quickly mixed evenly. After 15 s of the reaction, the absorbance value at a wavelength of 398 nm (PPO) and 470 nm (POD) was recorded, respectively, as the initial value. Then, every 60 s, the absorbance value was measured and recorded continuously. Six data points were taken as a group, and parallel measurements were made three times. The results were expressed in U/g (U represents an enzyme activity unit where the absorbance increases by 1 change per gram of sample per minute).
Activity determination of β-1,3-glucanase (GLU) and chitinase (CHI): The GLU and CHI assay kits produced by Suzhou Keming Biotechnology Co., Ltd., in Suzhou city, China. were used, respectively, for the determination. A total of 0.1 g of inoculated blueberry frozen sample powder and 1 mL of extraction solution were sequentially added to a 1.5 mL centrifuge tube, shaken, and mixed evenly. The mixture was centrifuged to obtain the supernatant for later use. Then, the supernatant and various reagents were added in sequence according to the instructions in the reagent kit, thoroughly mixed, and transferred to a 96-well plate. The absorbance values at 550 nm (GLU) and 585 nm (CHI) were determined, respectively, and the results of both were expressed in mg/h/g.
2.6. Statistical Analysis
All the experiments were performed in triplicates. Data are presented as the mean ± SD (standard deviation), and figures were drawn with Origin 8.0.
3. Results and Discussion
3.1. Antifungal Activities of Six Plant Essential Oils in PDA Cultures
Blueberry fruits are highly vulnerable to multifarious fungi infestation during storage and transportation, and Botrytis rot caused by Botrytis cinerea and Alternaria rot caused by Alternaria alternata are two of the most destructive diseases.
As shown in Figure 1, there was no antifungal zone observed in the control group, while the six selected plant essential oils exhibited varying degrees of antifungal activity against Botrytis cinerea, which is a similar result to that reported in the study of Lin et al. [16]. The diameter of the antifungal circle of the rosemary essential oil was 7 mm, which had a low sensitivity to Botrytis cinerea. The diameter of the antifungal circle of the tea tree essential oil was 10 mm, which had a moderately sensitive antifungal effect on Botrytis cinerea. And the diameters of the antifungal circle of the pomelo peel, cinnamon, clove, and oregano essential oils were 17 mm, 30 mm, 36 mm, and 50 mm, respectively; all were greater than 15 mm, indicating highly sensitive antifungal effects on Botrytis cinerea. It can be seen that the order of the inhibitory effects of the six plant essential oils to Botrytis cinerea is as follows: oregano > clove > cinnamon > pomelo peel > tea tree > rosemary.
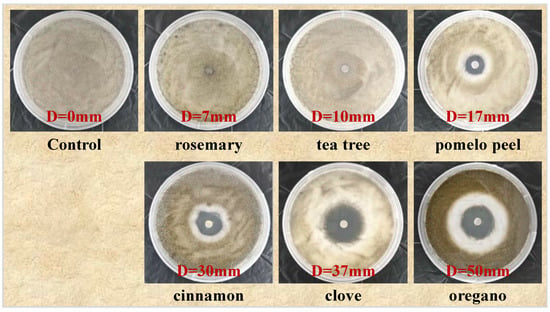
Figure 1.
Inhibitory effects of six essential oils on Botrytis cinerea.
Similarly, as shown in Figure 2, there is no antifungal zone in the control group. The rosemary and tea tree essential oils were used to treat Alternaria alternata, with antifungal ring diameters of 13 and 15 mm, respectively, exhibiting moderate sensitive antibacterial effects; the Alternaria alternata treated with pomelo peel, cinnamon, cloves, and oregano essential oils had antifungal ring diameters of 25, 40, 50, and 60 mm, all higher than 15 mm, and had highly sensitive antifungal effects. Therefore, the inhibitory effect of six plant essential oils to Alternaria alternata is as follows: oregano > clove > cinnamon > pomelo peel > tea tree > rosemary. The above results indicate that among these six plant essential oils, the most significant inhibitory effect on Botrytis cinerea and Alternaria alternata is that found with oregano essential oil.
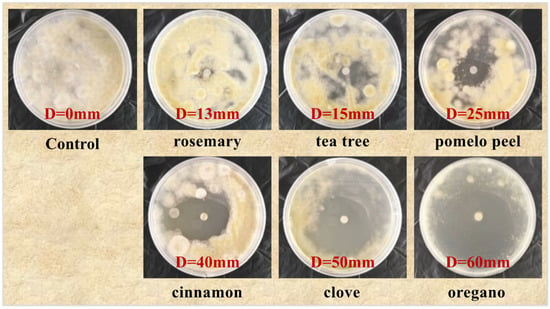
Figure 2.
Inhibitory effects of six essential oils on Alternaria alternata.
3.2. The Antifungal Effect of Oregano Essential Oil
To determine the optimal concentration for processing blueberry fruit with oregano essential oil, antifungal experiments in vitro were conducted using concentration gradients of 0, 30, 60, and 90 μL/L of oregano essential oil. In Table 1 and Figure 3, we can observe that after fumigation with different concentrations of oregano essential oil, the control group showed significant growth of Botrytis cinerea and Alternaria alternata. When the concentration of oregano essential oil was 30 μL/L, there was still a small amount of growth of the two pathogenic fungi, but compared with the control group, 30 μL/L of oregano essential oil had a significant inhibitory effect on both types of fungi. When the oregano essential oil concentration reached 60 μL/L or even higher, both Botrytis cinerea and Alternaria alternata on the PDA plate showed no colony growth, achieving a complete antifungal effect. Based on existing research, the mechanism of action of plant essential oils is related to the degradation of cell walls, damage to cytoplasmic membranes and membrane proteins, leakage of cell contents, cytoplasmic condensation, and depletion of proton dynamics, ultimately leading to cell lysis and death [20,21]. In particular, a specific component of plant essential oils, epoxyethane, interacts with ergosterol, which maintains cell integrity, survival, function, and normal fungal growth, interfering with cell wall synthesis, thereby affecting the morphogenesis and growth of hyphae. In addition, phenolic compounds in plant essential oils interact with proteins in the cytoplasmic membrane, altering the fluidity and permeability of the membrane. Membrane proteins are disrupted, causing the leakage of ions and other cellular contents, ultimately leading to cell collapse [21], achieving the goal of inhibiting fungal growth and reproduction.

Table 1.
Antifungal activity of oregano essential oil with different concentrations against two rot fungi.
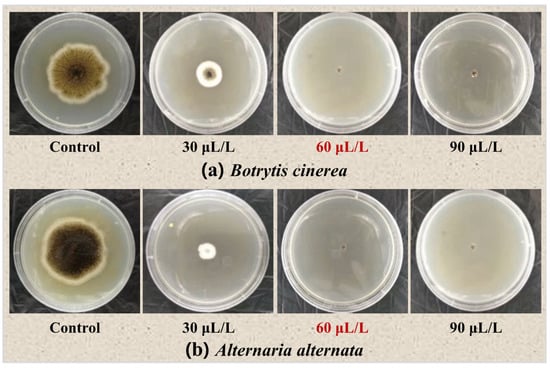
Figure 3.
Inhibitory effect of different concentrations of oregano essential oil on blueberry rot fungi.
3.3. Effect of Oregano Essential Oil on PAL Activity in Blueberry
In addition to directly inhibiting pathogen growth, many studies have also shown that postharvest essential oil treatment can reduce fruit decay by inducing disease resistance [17,19,22].
In the disease resistance response of plants, phenylpropanoid metabolism is an important pathway for producing antimicrobial substances [23], and PAL is the first key enzyme and rate-limiting enzyme in this pathway [24]. PAL is often stimulated by adverse conditions such as cold, injury, and disease and insect invasion, leading to an increase in its activity and it playing a role in disease resistance and defense. As shown in Figure 4, during the storage process of blueberry fruits inoculated with Botrytis cinerea (Figure 4A) and Alternaria alternata (Figure 4B), the PAL activity showed a trend of first increasing and then decreasing. Of the different concentrations of essential oils used, 60 μL/L of oregano essential oil significantly increased the PAL activity in blueberry fruits inoculated with two types of pathogenic fungi, making its activity consistently higher than the control group, and both reached their peak enzyme activity on the eighth day of storage, which was 1.5 times (Botrytis cinerea) and 1.1 times (Alternaria alternata) higher than their respective control groups. Therefore, treatment with 60 μL/L oregano essential oil can effectively induce PAL activity in blueberries and improve their disease resistance.
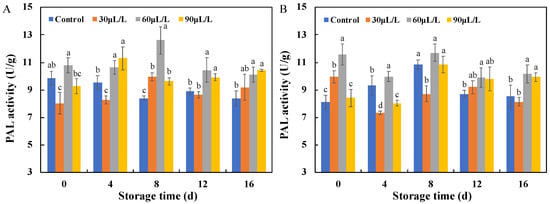
Figure 4.
Effects of different concentrations of oregano essential oil on PAL activities of blueberries inoculated with Botrytis cinerea (A) and with Alternaria alternata (B). Values with different lowercase letter in the same time point are significantly different (p < 0.05).
3.4. Effect of Oregano Essential Oil on PPO Activity in Blueberry
The relationship between polyphenol oxidase (PPO) and disease resistance has been widely studied. In the process of resisting infection by plant pathogenic microorganisms, PPO can form a protective barrier by catalyzing the formation of phenolic oxidation products such as lignin and quinones, protecting cells from pathogen invasion. It can also directly exert antidisease effects by forming quinones [25]. As shown in Figure 5A, the PPO activity of the blueberry fruits inoculated with Botrytis cinerea showed a trend of first increasing and then decreasing during storage. The difference in PPO enzyme activity between each treatment and the control group was not significant during storage for 0–4 days, but after storage for 4 days, the PPO activity in each group continued to increase, reaching its peak at 12 days. Among them, the PPO activity of blueberries treated with 60 μL/L oregano essential oil was significantly higher than that of the control and other treatment groups, and the maximum PPO activity of the fruit can be increased by 1.6 times compared to the control on the eighth day of storage. In Figure 5B, it is shown that during the entire storage period, the PPO activity of blueberry fruits inoculated with Alternaria alternata treated with 30 μL/L and 90 μL/L oregano essential oil was not significantly different from the control group, and even in the later stage of storage, the enzyme activity of the 30 μL/L oregano essential oil-treated group was significantly lower than that of the control group. However, the PPO activity of the 60 μL/L oregano essential oil treatment group remained at a higher level compared to that of the control and other treatment groups. Therefore, fumigation with 60 μL/L oregano essential oil can effectively prevent the serious deterioration of blueberry fruit diseases.
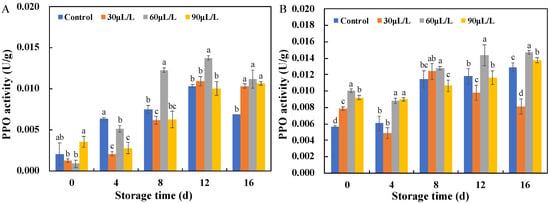
Figure 5.
Effects of different concentrations of oregano essential oil on PPO activities of blueberries inoculated with Botrytis cinerea (A) and with Alternaria alternata (B). Values with different lowercase letter in the same time point are significantly different (p < 0.05).
3.5. Effect of Oregano Essential Oil on POD Activity in Blueberry
POD, like PPO, is considered a crucial enzyme in the process of fruit resistance against pathogen infection [26], and its enhanced activity is one of the markers that induce resistance [2]. As shown in Figure 6, during storage, there was no significant difference in the POD activity between the blueberry fruits inoculated with Botrytis cinerea and Alternaria alternata treated with 30 μL/L and 90 μL/L oregano essential oil and the control group, and even the enzyme activity of the 90 μL/L oregano essential oil treatment group was lower than that of the control group in the early and late stages of storage. However, the blueberry fruits inoculated with Botrytis cinerea and Alternaria alternata showed significantly higher POD activities after being treated with 60 μL/L essential oil compared to the control and other treatment groups, and showed the highest enzyme activity on the 8th (Botrytis cinerea) and 12th (Alternaria alternata) day of storage, 2.7 and 6 times higher than their respective control groups. This indicates that 60 μL/L oregano essential oil induces POD activity in blueberry fruits and can maintain it at a high level, effectively improving the disease resistance of blueberry fruits throughout the storage period.
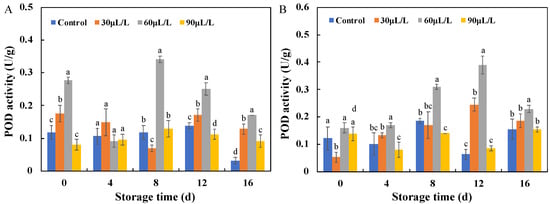
Figure 6.
Effects of different concentrations of oregano essential oil on POD activities of blueberries inoculated with Botrytis cinerea (A) and with Alternaria alternata (B). Values with different lowercase letter in the same time point are significantly different (p < 0.05).
3.6. Effect of Oregano Essential Oil on CHI and GLU Activities in Blueberry
CHI and GLU are two important hydrolytic enzymes in plant disease resistance systems, capable of hydrolyzing the main components of fungal cell walls, chitin and glucan, respectively, to disrupt the cell structure of pathogenic microorganisms and inhibit their growth and reproduction [19,22]. When the two show a synergistic effect, fruit resistance to pathogen infection can be improved by combining the decomposition of the cell wall of the pathogen and the destruction of the structure of the mycelium. Therefore, in the plant disease resistance system, the synthesis speed of the GLU and CHI enzymes has become an important indicator for measuring plant disease resistance [27].
As shown in Figure 7, the CHI activity of the inoculated blueberry fruits showed a fluctuating trend during storage. The CHI activity of the blueberries inoculated with Botrytis cinerea was significantly enhanced by the 60 μL/L oregano essential oil treatment after 8–16 days of storage, and were 3.8 (day 8), 1.4 (day 12), and 1.6 (day 16) times higher than that of the control, respectively. However, 60 μL/L oregano essential oil significantly enhanced the CHI activity of the blueberries inoculated with Alternaria alternata for the entire storage period, reaching its maximum value on the eighth day of storage, which was 3.1 times higher than that of the control group. Like CHI, the GLU activity of the inoculated blueberries in Figure 8 also showed a fluctuating trend during storage, but the 60 μL/L oregano essential oil treatment still performed well in improving the GLU activity and resisting Botrytis cinerea and Alternaria alternata. In this study, oregano essential oil was found to enhance the GLU and CHI activities of the inoculated blueberries, similar to the effects reported for tea tree essential oil, thyme oil, and cinnamon oil described above [17,19].
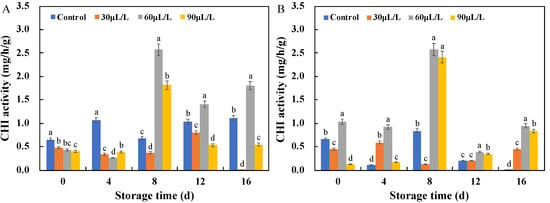
Figure 7.
Effects of oregano essential oil treatment on CHI activities of the blueberry fruit inoculated with Botrytis cinerea (A) and Alternaria alternata (B). Values with different lowercase letter in the same time point are significantly different (p < 0.05).
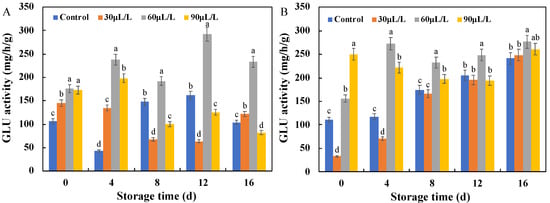
Figure 8.
Effects of oregano essential oil treatment on GLU activities of the blueberry fruit inoculated with Botrytis cinerea (A) and Alternaria alternata (B). Values with different lowercase letter in the same time point are significantly different (p < 0.05).
In this study, it was found that oregano essential oil can enhance the PAL, PPO, POD, GLU, and CHI activities of inoculated blueberries against pathogenic fungi. Similar results have been reported in many studies, such as with thyme oil and cinnamon oil, which significantly reduced the incidence and severity of peach brown rot by increasing the activities of PAL, GLU, and CHI [17]; thyme oil induced the enhancement of CHI and GLU activities to control anthracnose in avocados [28]; and tea tree essential oil enhances the activities of CHI and GLU to control the decay of strawberries [19]. Therefore, we can conclude that oregano essential oil can resist the invasion of Botrytis cinerea and Alternaria alternata by enhancing the activities of disease-resistant enzymes, thereby controlling the occurrence of Botrytis rot and Alternaria rot in postharvest blueberries.
4. Conclusions
In this study, the inhibitory effects of six natural plant essential oils, including cinnamon, oregano, cloves, tea trees, pomelo peel, and rosemary, on the rot-causing fungi Botrytis cinerea and Alternaria alternata in blueberries were explored through in vitro experiments. Among them, the inhibitory effect of oregano essential oil on the two main rot fungi of blueberries was found to be the strongest. Based on the results of the antifungal experiments in vitro and a literature review, the concentration gradients of 0, 30, 60, and 90 μL/L oregano essential oils were selected for the fumigation treatment of fungi-inoculated blueberries.
The activities of defense-related enzymes of fungi-inoculated blueberries were evaluated after fumigation treatment with selected concentration gradients of oregano essential oil. It was found that the treatment with 60 μL/L oregano essential oil could enhance the activities of PAL, PPO, POD, CHI, and GLU in the disease-resistant system of inoculated blueberry fruits to varying degrees throughout the entire storage period, effectively enhancing the disease resistance of inoculated blueberry fruits and reducing postharvest decay caused by Botrytis cinerea and Alternaria alternata.
Author Contributions
Conceptualization, Y.J. and W.H.; data curation, Y.J.; formal analysis, Y.J.; funding acquisition, W.H.; investigation, Y.J.; methodology, Y.J. and W.H.; project administration, W.H.; resources, W.H.; software, Y.J. and Y.G.; supervision, Y.G. and G.S.; validation, Y.J.; visualization, Y.J., Y.G. and G.S.; writing—original draft, Y.J.; writing—review and editing, Y.G. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the ‘Thirteenth Five-Year Plan’ for the National key research and development program (Grant No. 2016YFD0400903) and National Natural Science Foundation (Grant No. 31471923).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author Yuge Guan was employed by the company Zhejiang Hongyuan Agricultural Development Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ji, Y.R.; Hu, W.Z.; Liao, J.; Xiu, Z.L.; Jiang, A.L.; Yang, X.Z.; Guan, Y.G.; Feng, K.; Saren, F. Effect of ethanol vapor treatment on the growth of Alternaria alternata and Botrytis cinerea and defense-related enzymes of fungi-inoculated blueberry during Storage. Front. Microbiol. 2021, 12, 618252. [Google Scholar] [CrossRef]
- Qin, S.W. Isolation, Identification, and Biological Control of Latent Pathogenic Fungi in Blueberry Fruits. Master’s Thesis, Dalian University of Technology, Dalian, China, 2017. [Google Scholar]
- Wang, C.Y.; Chen, C.T.; Yin, J.J. Effect of allyl isothiocyanate on antioxidants and fruit decay of blueberries. Food Chem. 2010, 120, 199–204. [Google Scholar] [CrossRef]
- Sommer, N.F. Role of controlled environments in suppression of postharvest diseases. Can. J. Plant Pathol. 1985, 7, 331–339. [Google Scholar] [CrossRef]
- Alessandra, R.; Camille, E.G.; Joséli, S. Optimization of Bacillus velezensis S26 sporulation for enhanced biocontrol of gray mold and anthracnose in postharvest strawberries. Postharvest Biol. Technol. 2024, 210, 112737. [Google Scholar]
- Wang, P.; Wu, T.; Cheng, Y.; Gao, Y.; Huang, B.; Li, Z. The phytocytokine systemin enhances postharvest tomato fruit resistance to Botrytis cinerea. Postharvest Biol. Technol. 2024, 210, 112774. [Google Scholar] [CrossRef]
- Xu, H.; Li, C.; Wang, M.; Guo, Y.; Zhang, S.; Ge, Y. Enhancement of disease resistance against Alternaria alternata in winter jujube fruit by phenyllactic acid through regulating Ca2+ signaling transduction and mitogen-activated protein kinase cascades. Postharvest Biol. Technol. 2024, 210, 112739. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Moham, M.T.M. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2019, 96, 253–267. [Google Scholar] [CrossRef]
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; Anjos, R.S.D.; Riella, H.G.; Araújo, P.H.H.D.; Oliveira, D.D.; Fiori, M.A. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60. [Google Scholar] [CrossRef]
- Marta, M.; Silvia, B.; Dharini, S. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Gu, N.; Zheng, Y. Research progress on the preservative and fresh-keeping effects of plant essential oils on fruits and vegetables. Food Sci. 2010, 31, 427–430. [Google Scholar]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities—Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Arasu, M.V.; Viayaraghavan, P.; Ilavenil, S.; Al-Dhabi, N.A.; Choi, K.C. Essential oil of four medicinal plants and protective properties in plum fruits against the spoilage bacteria and fungi. Ind. Crops Prod. 2019, 133, 54–62. [Google Scholar] [CrossRef]
- Jahani, M.; Pira, M.; Aminifard, M.H. Antifungal effects of essential oils against Aspergillus niger in vitro and in vivo on pomegranate (Punica granatum) fruits. Sci. Hortic. 2020, 264, 109188. [Google Scholar] [CrossRef]
- Lin, S.; Wang, W.; Zhang, J.; Yang, X. Preliminary screening of in vitro antibacterial activity of 10 plant essential oils against blueberry pathogens. Hunan Agric. Sci. 2018, 2, 73–75. [Google Scholar]
- Cindi, M.D.; Soundy, P.; Romanazzi, G.; Sivakumar, D. Different defense responses and brown rot control in two Prunus persica cultivars to essential oil vapours after storage. Postharvest Biol. Technol. 2016, 119, 9–17. [Google Scholar] [CrossRef]
- Pan, L.Y.; Zhao, X.Y.; Chen, M.; FU, Y.Q.; Xiang, M.L.; Chen, J.Y. Methyl jasmonate regulates defense enzyme activity and induces resistance to postharvest soft rot disease in kiwifruit. Plant Prot. 2019, 45, 75–80. [Google Scholar]
- Wei, Y.; Wei, Y.; Xu, F.; Shao, X. The combined effects of tea tree oil and hot air treatment on the quality and sensory characteristics and decay of strawberry. Postharvest Biol. Technol. 2018, 136, 139–144. [Google Scholar] [CrossRef]
- Gemma, O.; Ma, A.R.G.; Laura, A.G.; Paula, V.; Robert, S.F.; Ma, I.H.H.; Isabel, P.M.; Susana, F. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: A review. Postharvest Biol. Technol. 2010, 57, 139–148. [Google Scholar]
- Carlos, D.G.; Clemencia, C.; Annalisa, S.; Chiara, R.; Antonello, P. Chitosan coatings enriched with essential oils: Effects on fungi involved in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar]
- Shao, X.; Wang, H.; Xu, F.; Cheng, S. Effects and possible mechanisms of tea tree oil vapor treatment on the main disease in postharvest strawberry fruit. Postharvest Biol. Technol. 2013, 77, 94–101. [Google Scholar] [CrossRef]
- Liu, H.X. The Regulatory Effects of 1-MCP, BTH, and PHC on Postharvest Senescence of Peach Fruit (Prunus persica L.) and Their Induced Disease Resistance Mechanisms. Ph.D. Thesis, China Agricultural University, Beijing, China, 2004. [Google Scholar]
- Ozturk, A.; Yildiz, K.; Ozturk, B.; Karakaya, O.; Gun, S.; Uzun, S.; Gundogdu, M. Maintaining postharvest quality of medlar (Mespilus germanica) fruit using modified atmosphere packaging and methyl jasmonate. LWT-Food Sci. Technol. 2019, 111, 117–124. [Google Scholar] [CrossRef]
- Gao, Y.; Kan, C.; Wan, C.; Chen, C.; Chen, M.; Chen, J. Quality and biochemical changes of navel orange fruits during storage as affected by cinnamaldehyde-chitosan coating. Sci. Hortic. 2018, 239, 80–86. [Google Scholar] [CrossRef]
- Xu, H.X. A Study on the Effects and Mechanisms of Different Silicon Source Preparations on the Main Post Harvest Diseases of Grapes. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2004. [Google Scholar]
- He, C.; Zhang, Z.; Li, B.; Xu, Y.; Tian, S. Effect of natamycin on Botrytis cinerea and Penicillium expansum—Postharvest pathogens of grape berries and jujube fruit. Postharvest Biol. Technol. 2019, 151, 134–141. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Beukes, M.; Korsten, L. Expression of pathogenesis-related (PR) genes in avocados fumigated with thyme oil vapours and control of anthracnose. Food Chem. 2016, 194, 938–943. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).