Abstract
Echeveria, classified in the Crassulaceae family, possesses unique adaptive strategies with xeromorphic features to withstand semi-arid environments. The diversity and ecological adaptation of succulent plants offer valuable insights into addressing climate change challenges. In particular, the epidermis, hypodermis, vascular bundles arrangement, and stomata characteristics are commonly used to investigate light, humidity, temperature, and water availability adaptations. While leaf anatomical analysis is a common approach, limited studies have been conducted on Echeveria, especially among cultivars. To understand how succulents cope with environmental stress, leaf morpho-anatomical features were analyzed using the free-hand sectioning method with methanol fixation of fifteen Echeveria cultivars. The finding revealed a robust correlation between epidermis and hypodermis size (r = 0.362–0.729), and a positive association between leaf thickness and the epidermis (r = 0.362–0.536), suggesting implications for water storage. Most cultivars displayed a 3D vascular arrangement, with minor vascular bundles surrounding the main vascular bundle at the center, along with small stomata size, and low stomata frequency in the adaxial surface. Moreover, these cultivars grown under controlled conditions maintain their xeromorphic characteristics with the presence of epicuticular wax and thick and fully expanded small leaves. Likewise, the features of cultivars ultimately suggest that these succulents are tolerant to high temperatures and limited water supply. This study provides a fundamental understanding of Echeveria plants’ leaf anatomy and the correlation of their leaf structures toward environmental stress. Likewise, the methods and results of this study will serve as a benchmark for other research in related species.
1. Introduction
Succulent plants are primarily found in semi-arid regions, exposing them to hot, dry air and limited water supply, which reinforces the development of xeromorphic features with thick cuticles and low stomata density to allows them to adapt to their stressful environment [1]. Plants are considered succulents when specialized enlarged tissues (leaves, stems, and/or roots) are utilized to store water and maintain their activity temporarily when precipitation is scarce [2]. Regarding these xeromorphic characteristics, succulents are suitable to grow in urban areas where rapid civilization and industrialization cause limited green spaces, not only due to their aesthetic and visual appeal but also because of variation purposes [3,4,5,6]. However, these heat-resistant plants strongly display succulence at the cellular level rather than exhibiting their morphology [7,8].
Plants modify their leaf morphology and anatomical structure in response to temperature [9], light intensity [10], and water stress [11]. Modified changes in their leaf anatomy, including epidermis, stomata, and mesophyll structures, have been reported [12,13,14,15]. This is especially true for succulent plants with a special mechanistic relationship where they evolve their anatomical structures in two types: (a) all-cell succulence, wherein water is stored in undifferentiated parenchyma cells, and (b) storage succulence, which utilizes water in specialized achlorophyllous hydrenchyma cells [16]. Nobel [17] reported that succulence is primarily comprised of a thin parenchymal cell wall, enabling the cells to enlarge their vacuoles, occupying up to >95% of the cell volume for water storage [1], which makes their water-use efficiency up to eight times higher in succulents compared with C3 and C4 plants [18,19]. Indeed, many succulents still exhibit thick cuticles and low stomata density regardless of occurring in a mesic or hydric environment [1]. Thus, succulence is considered to employ binary traits rather than a complex syndrome in terms of water-storage capacity that enables succulents to tolerate arid environment [20]. However, in some cases, the same species growing under different environments may evolve varying structural characteristics and thus, plants may look morphologically alike, but they may have variations based on environmental stresses encountered or cultivation conditions [8].
To visualize these leaf-morpho-anatomical characteristics, a light microscope (LM) is widely employed in different aspects due to its easy handling [21,22,23], but the samples must be cut uniformly and thin enough [24]. Normally, a microtome is used to produce a quality section. It can cut samples up to 500 nm without damage to the inner structure [25], but it also requires a multistep for preparation, involving the use of toxic reagents [26]. Furthermore, microtome may not be accessible to all laboratories, while the use of a razor blade, known as free-hand sectioning, is easy to obtain, requiring only skill and patience [27]. Regarding staining techniques, toluidine blue O (TBO), a metachromatic dye capable of staining a variety of colors based on cell wall compounds, is applicable for animal and plant material [28,29]. Its versatility makes it suitable for both research and teaching purposes [30,31]. However, the studies of succulent morpho-anatomical leaves are historically challenged due to their highly unspecialized water-storage parenchyma and necessitating the selection of an appropriate method to approach such water-rich tissues [32].
Up to 13,000 species representing more than 80 families are recognized as succulent plants [33]. Among them, the Echeveria genus, belonging to the Crassulaceae family, is primarily distributed in vulnerable regions with geological changes, with about 85% of their endemic in Mexico, which forces rapid evolutionary changes for environmental adaptation [34]. The plants of this genus are favored for cultivation with various applications due to their attractive geometrical features and ability to thrive over the long term with minimal care, even in stressful conditions [35,36,37,38]. Echeveria is widely hybridized to meet the demands of floricultural markets and has numerous cultivars as they are easy to cross with related or non-related species [34], which results in homoplastic morphology [39]. Although these plants have similar morphology, they may cope differently within varying physiological conditions, and those with different morphology may often have comparable water-use efficiency [8,36]. Succulents in this genus are often recognized as monolithic in terms of water-use strategies. However, their coping strategies may differ between species despite being cultivated in environmental factors [40], which triggers an interest in investigating and evaluating their leaf morpho-anatomy.
Leaf morphologies are reported to reflect environmental variables [41,42]. Likewise, the relationship between leaf morphology and anatomy with environmental variables varies between species and their exposure to different conditions [43]. The leaf anatomy analysis of this genus is still challenging because the leaf blades are thick, the presence of mucilage, and additional chlorophyll content in mesophyll cells, limit anatomical studies and herbarium species preservation [16,32,37,44]. Despite these difficulties, morpho-anatomical attributes of active tissues (i.e., epidermis thickness, stomata density, and vascularization arrangements) are used to understand the adaptive mechanisms in specific environments for Echeveria cultivars. This study uses free-hand sectioning and methanol fixative to characterize their morpho-anatomy and detect possible correlations between leaf features in response to physiological conditions of ornamental Echeveria plants, which are essential beneficial data for both taxonomy and cultivation management.
2. Materials and Methods
2.1. Plant Materials
Fifteen (15) cultivars of Echeveria species were used in this study, namely E. ‘Benbadis’, E. ‘Brave’, E. colorata E. Walther [45], E. ‘Cubic Frost’, E. ‘Dark Ice’, E. ‘Doterang’, E. ‘Glam Pink’, E. ‘Loy’, E. ‘Milk Rose’, E. ‘Fire Pillar’, E. ‘Peerless’, E. ‘Silhouette’, E. ‘Snow Bunny’, E. ‘Tippy’, and E. ‘Viyant’ (Figure 1). Succulent plants were obtained from a succulent nursery in Goyang City, Gyeonggi-do, Republic of Korea. The plants used were of the same age with fully expanded leaves.

Figure 1.
Fifteen (15) Echeveria cultivars subjected to leaf anatomical evaluation: (a) E. ‘Benbadis’; (b) E. ‘Brave’; (c) E. colorata E. Walther; (d) E. ‘Cubic Frost’; (e) E. ‘Dark Ice’; (f) E. ‘Doterang’; (g) E. ‘Fire Pillar’; (h) E. ‘Glam Pink’; (i) E. ‘Loy’; (j) E. ‘Milk Rose’; (k) E. ‘Peerless’; (l) E. ‘Silhouette’; (m) E. ‘Snow Bunny’; (n) E. ‘Tippy’; and (o) E. ‘Viyant’.
2.2. Morphological Evaluation
Quantitative and qualitative features of the cultivars were evaluated. Leaf growth parameters (i.e., leaf length, leaf thickness, basal leaf width, and the number of leaves) were measured, and morphological characteristics (i.e., leaf shape, apex, margin, edge pattern, and presence of trichomes and epicuticular wax) were examined according to the description by Ash et al. [46].
2.3. Free-Hand Sectioning and Fixation Method
Pure methanol was used as a fixative, according to the methods of Neinhuis and Edelmann [47], with some modifications to preserve the cell morphologies. The whole leaf tissues were immersed in methanol overnight, and on the following day, the leaves were sectioned and then subjected to an ethanol series for 30 min in each concentration (50%, 70%, and 100%) before scanning.
After the fixation, leaves were sectioned using a double-edged razor blade. Most succulent leaves are sufficiently rigid; thus, there is no need for additional support like polystyrene layers [25]. Due to succulent plants’ abundant tannin and mucilage, blades were changed after two to three sections.
Afterward, the fixed sample sections were washed with distilled water and were stained by 0.5% the toluidine blue (TBO) pH 4.0 and washed about 3 times for 5 min each in distilled water and then samples were mounted onto slides before observation under a light microscope using cellSens imaging software platform (Model BX53F, Olympus, Japan). The transverse section of each examined plant was observed under a stereomicroscope (SZ 51, Olympus, Tokyo, Japan). The width and the length of the epidermis and hypodermis cells were measured using Image J (v 1.52a, [48]).
2.4. Leaf-Morpho-Anatomical Observation
The upper epidermis was peeled off from the leaf blades with tweezers. Then, the epidermis was spread flat in the water and pressed slightly in slides with the assistance from coverslips, and then it was observed under a light microscope (Model BX53F, Olympus, Japan). Stomatal data (i.e., number and size) were taken from both the adaxial and abaxial areas of the leaves and measured using Image J (v 1.52a, [48]). The stomatal density and index were calculated as described by Salisbury [49].
2.5. Moisture Content Measurement
Plants were thoroughly watered to ensure a comparable water status among plants before the experiment. Moisture content calculations were carried out according to the method used by Lee and Nam [50]. Plants were washed clean to remove soil and air-dried for 24 h at room temperature (RT) and weighed to find their fresh weight. Plants were dried in a dry oven for 24 h at 85 °C and weighed again to find their dry weight.
2.6. Statistical and Cluster Analysis
Numerical data obtained from the morpho-anatomical evaluation were subjected to analysis of variance (ANOVA). The significant differences between means were analyzed using Duncan’s Multiple Range Test (DMRT) at a 5% significance level. Spearman’s correlation of anatomical features, plant morphological data, and moisture content was calculated using SPSS (SPSS version 22, IBM Statistics, Japan).
3. Results
3.1. Leaf Morphology Evaluation
Fifteen Echeveria cultivars possess visual variations that allow us to differentiate one from another (Figure 1 and Table 1). Regarding leaf morphological characteristics, they all have an entire type of leaf margin, and most of them have leaf edge color patterns, except for E. ‘Loy’. Most cultivars are obovate-shaped, except for E. ‘Brave’, E. colorata E. Walther, E. ‘Dark Ice’, E. ‘Peerless’, E. ‘Tippy’, and E. ‘Viyant’ which are cuneate-shaped leaves. Four types of leaf apexes were found among the examined cultivars namely acuminate (E. ‘Benbadis’, E. colorata E. Walther, E. ‘Dark Ice’, E. ‘Doterang’, E. ‘Fire Pillar’, E. ‘Loy’, and E. ‘Tippy’), acute (E. ‘Brave’, E. ‘Cubic Frost’, E. ‘Peerless’, E. ‘Silhouette’, and E. ‘Snow Bunny’), apiculate (E. ‘Viyant), and obtuse with prominent tips (E. ‘Glam Pink’ and E. ‘Milk Rose’). Cultivars did not possess trichomes or exhibit branching, but they varied in the amount of epicuticular wax on leaf surfaces, which ranged from average to abundant.

Table 1.
Morphological characteristics of Echeveria cultivars.
As seen in Table 2, Echeveria plant growth parameters show evident differences in their plant height, diameter, and number of leaves. It is worth noting that all cultivars have swollen leaves, as seen by their leaf thickness, which was about 4.85–7.34 mm. This indicated that leaves are their primary storage organ. Hence, anatomical evaluation using the leaf tissues is essential in understanding their physiological characteristics and adaptive features.

Table 2.
Plant growth parameters of Echeveria cultivars.
3.2. Leaf Morpho-Anatomical Evaluation and Its Environmental Implication
3.2.1. Epidermis and Hypodermis
Epidermis and hypodermis parameters significantly varied between species and cultivars, as shown in Table 3. The largest epidermal width was found in E. colorata E. Walther, E. ‘Doterang’, E. ‘Glam Pink’, and E. ‘Snow Bunny’, ranging from 38.44 to 40.18 μm. The cultivars with small-sized epidermal widths were those of E. ‘Benbadis’, E. ‘Viyant’, and E. ‘Fire Pillar’, ranging from 16.65 to 19.82 μm.

Table 3.
Epidermis and hypodermis parameters of Echeveria cultivars (μm).
The hypodermis, which forms a row of slightly rounded and smaller cells located underneath the epidermal layers, was found in most cultivars. The width of the hypodermis ranged from 44.85 to 133.20 μm, in which the most significant value was found in E. ‘Brave’, while the smallest value was found in E. ‘Tippy’. Likewise, cultivars had no visible trend in their epi- and hypodermis size. For instance, E. ‘Viyant’ had the smallest epidermal width (16.65 μm), but its hypodermal width increased about four times (68.54 μm). On the other hand, E. ‘Snow Bunny’, which had the largest epidermal width (40.18 μm), had a hypodermal width that increased by 1.8 times (74.57 μm).
Transverse sections taken from the middle region of the leaves had a relatively uniform monolayer (uniseriate epidermis) comprising of tightly adhering and rectangular-shaped cells (Figure 2). Hypodermis cells exhibited enlarged cells, having a spherical or ellipsoidal shape. The chlorenchyma tissues were found to be undifferentiated into palisade and spongy layers.
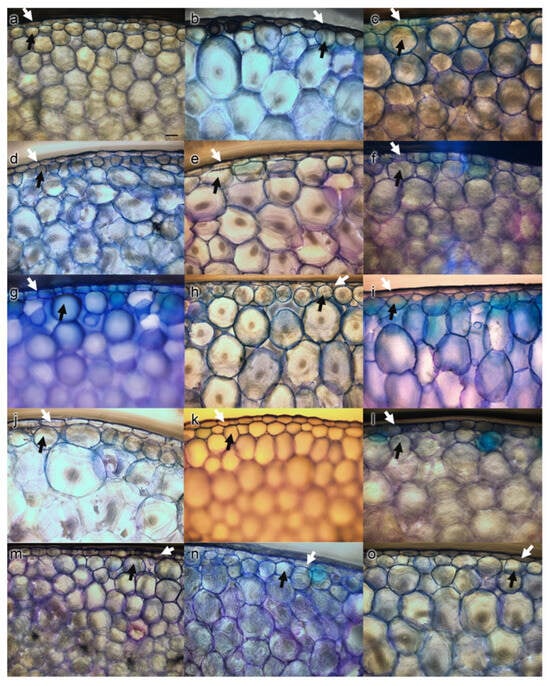
Figure 2.
Leaf anatomical cross-sections of the epidermis (white arrow) and hypodermis layer (dark arrow) of Echeveria cultivars fixed in methanol observed under light microscope: (a) E. ‘Benbadis’; (b) E. ‘Brave’; (c) E. colorata E. Walther; (d) E. ‘Cubic Frost’; (e) E. ‘Dark Ice’; (f) E. ‘Doterang’; (g) E. ‘Fire Pillar’; (h) E. ‘Glam Pink’; (i) E. ‘Loy’; (j) E. ‘Milk Rose’; (k) E. ‘Peerless’; (l) E. ‘Silhouette’; (m) E. ‘Snow Bunny’; (n) E. ‘Tippy’; and (o) E. ‘Viyant’. (100× magnification, scale bar = 50 µm).
Epidermal cells observed in LM showed the boundaries of epidermis cells with clear outlines in most cultivar images (Figure 2). The epidermal cell shapes were observed as polygonal or irregular with different levels of thickness. Periclinal walls exhibited non-reticulate (Figure 3a–c,f,h–m,o) to reticulate (Figure 3d,e,g,n) while anticlinal walls were nearly straight (Figure 3d), straight (Figure 3c,f,h,o) and sinuous (Figure 3a,b,e,g,i–n) (Table 4).
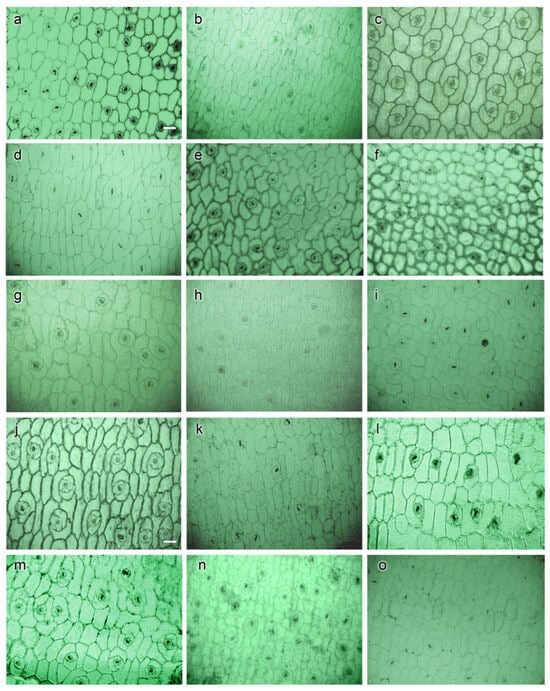
Figure 3.
Observation periclinal and anticlinal walls of epidermis cells of Echeveria cultivars were observed using a light microscope (LM): (a) E. ‘Benbadis’; (b) E. ‘Brave’; (c) E. colorata E. Walther; (d) E. ‘Cubic Frost’; (e) E. ‘Dark Ice’; (f) E. ‘Doterang’; (g) E. ‘Fire Pillar’; (h) E. ‘Glam Pink’; (i) E. ‘Loy’ (j) E. ‘Milk Rose’; (k) E. ‘Peerless’; (l) E. ‘Silhouette’; (m) E. ‘Snow Bunny’; (n). E. ‘Tippy’; and (o) E. ‘Viyant’ (100× magnification, scale bar = 50 µm).

Table 4.
Leaf micro-morphology observed under LM of Echeveria cultivars.
3.2.2. Stomata Characteristics
Fifteen Echeveria cultivars exhibited amphistomatous leaves, which indicate stomata on both adaxial and abaxial surfaces. Individual stomata displayed an anisocytic type comprising three subsidiary cells, one considerably smaller than the others (Figure 4). Stomatal data for Echeveria succulents are presented in Table 5. Most cultivars have bigger stomatal sizes on the adaxial (upper) surface than those on the abaxial (lower) surface, except for those of E. ‘Brave’ (17.06 µm and 18.64 µm) and E. ‘Peerless’ (17.37 and 18.53 µm). The size of stomata ranges from 16.93 ± 0.07 µm (E. ‘Snow Bunny’) to 26.56 ± 0.20 µm (E. ‘Tippy’). Additionally, some have no differences in stomatal size on both leaf surfaces (E. colorata E. Walther, E. ‘Dark Ice’, E. ‘Glam Pink’, E. ‘Loy’, E. ‘Silhouette’, E. ‘Snow Bunny’, and E. ‘Viyant’).
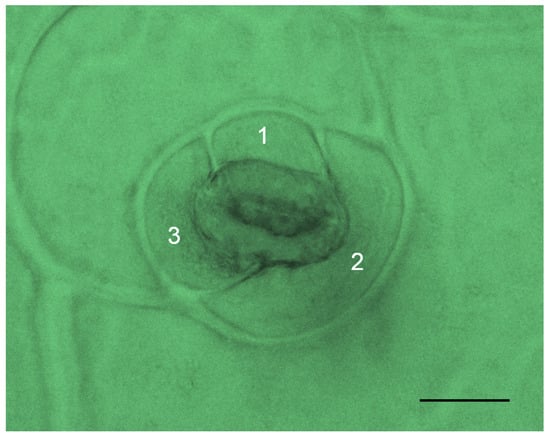
Figure 4.
Stomatal evaluation of E. ‘Brave’ shows an anisocytic stomatal type comprising three subsidiary cells (800× magnification, scale bar = 10 µm).

Table 5.
Stomata size, density, and index of Echeveria cultivars.
Based on the stomatal density, more stomates were found on the adaxial than the abaxial leaf surface. The highest frequency of stomatal distribution was found in E. ‘Doterang’ (33.67 mm−2), and the lowest density was found in E. ‘Fire Pillar’ (11.67 mm−2). The stomata index was also higher in the adaxial surface, especially those in E. ‘Peerless’ (24.25%), E. colorata E. Walther (22.96%), and E. ‘Doterang’ (20.87%), while the lowest stomata index was found in E. ‘Viyant’ (10.00%), E. ‘Cubic Frost’ (10.39%), E.’Glam Pink’ (11.11%), and E. ‘Benbadis’ (11.66%).
3.2.3. Vascular Bundles
Through transverse sectioning (Figure 5), two types of vascularization patterns were observed among cultivars: A. two-dimensional pattern (2D), which indicates a central bundle, and small lateral bundles in a single horizontal plane which was found in E. colorata E. Walther, E. ‘Milk Rose’, E. ‘Fire Pillar’, E. ‘Glam Pink’ and E. ‘Viyant’; and B. three-dimensional pattern (3D) which exhibits a central bundle with smaller peripheral vascular bundles surrounding the central bundle, was found among remaining cultivars. The diagram in Figure 6 presents these anatomical vascularization patterns more vividly.
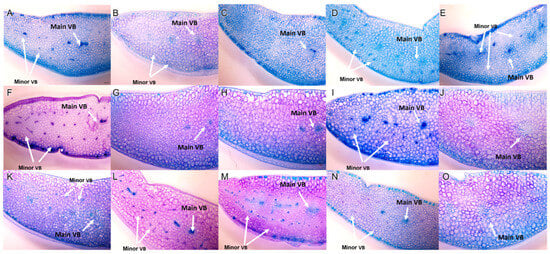
Figure 5.
Transverse section of Echeveria cultivars observed under stereomicroscope showing a 2D vascular bundle with main vascular (Main VB) in the center, and a 3D vascular bundle arrangement with main vascular (Main VB) in the middle of the leaves and minor vascular (Minor VB) surrounding (A) E. ‘Benbadis’; (B) E. ‘Brave’; (C) E. colorata E. Walther; (D) E. ‘Cubic Frost’; (E) E. ‘Dark Ice’; (F) E. ‘Doterang’; (G) E. ‘Fire Pillar’; (H) E. ‘Glam Pink’; (I) E. ‘Loy’; (J) E. ‘Milk Rose’; (K) E. ‘Peerless’; (L) E. ‘Silhouette’; (M) E. ‘Snow Bunny’; (N) E. ‘Tippy’; and (O) E. ‘Viyant’ (0.67× magnification, scale bar = 100 µm).
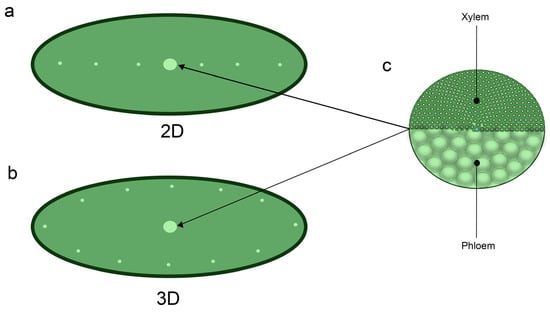
Figure 6.
Diagram of the types of vascularization patterns: (a) 2D type, showing the linear formation of minor vascular bundles (small circles) and the main vascular bundle (big circle) in the center; and the (b) 3D type, exhibiting minor vascular bundles surrounding the edges and the main vascular bundle at the center, while the (c) main vascular bundle is determined to be a collateral type indicating that both the xylem and phloem are adjacent with each other.
Despite having a different vascular arrangement, all plants showed the presence of the primary vesicle at the center of the leaf section and exhibited a collateral type, indicating enclosed formation with xylem cells oriented toward the upper side while phloem cells are arranged on opposite sides. Parenchyma cells were observed with the cellular type of vascular bundle sheaths being well-defined and fairly presented along with the companion cells showing intense metachromasy, which displayed a purple color, while lignified cells (i.e., fibers and vessel elements) presented in a slight purple or greyish-bluish color (Figure 7).
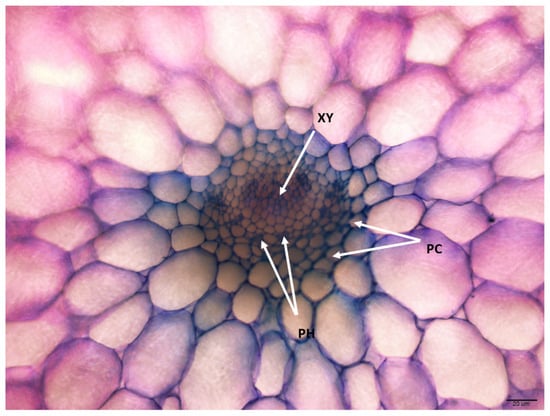
Figure 7.
The transverse section from E. ‘Loy’ shows the collateral vascular bundle as the xylem (XY) is surrounded by the phloem (PH) and parenchyma cells (PC) (200× magnification, 20 μm).
3.3. Moisture Content
Table 6 shows each cultivar’s fresh and dry weight and moisture content analysis. The highest fresh weight was taken from E. ‘Milk Rose’ with 115.13 g, while the lowest was from E. ‘Snow Bunny’ with 14.40 g. However, the trends in the fresh weight were not evident in the dry weight, wherein the values ranged from 0.75 g to 4.55 g, taken from E. ‘Snow Bunny’ and E. ‘Loy,’, respectively. The moisture content ranged from about 92.59% to 97.11%, wherein the highest moisture content was found in E. ‘Milk Rose,’ which also had the thickest leaves (8.21 mm), while the lowest moisture content was found in E. ‘Cubic Frost’ with an average leaf thickness (5.50 mm). It was noted that E. ‘Benbadis’ (4.65 mm), which had the most negligible leaf thickness, had a moisture content of 96.59%.

Table 6.
Moisture content analysis of Echeveria cultivars.
3.4. Correlation Analysis
Spearman’s correlation analysis was performed with the morphological, anatomical, and pertinent data collected to investigate their relationship, which may provide further implications on their environment (Table 7).

Table 7.
Spearman’s correlation of leaf-morphology, leaf characteristics and moisture content of Echeveria cultivars.
Several anatomical characteristics showed significant correlations, mainly those related to leaf thickness, epidermis, hypodermis, epicuticular wax with FW, and DW, which are relevant to water storage. There was a significant correlation between leaf thickness and epidermal length (r = 0.536), epidermal width (r = 0.306), and hypodermal width (r = 0.322). Likewise, the epidermal width and length had a relatively strong association with hypodermis width and length (r = 0.362–0.729). Among anatomical data, the hypodermis width was positively correlated with FW (r = 0.386) and DW (r = 0.348).
In terms of the presence of wax, the abundance of EW was negatively correlated with stomatal density on the abaxial surface (r = −0.294), epidermis length (r = −0.383), and hypodermis width (r = −0.407) and length (r = −0.437).
When considering the stomatal density at the abaxial surface with the epidermal cells (r = 0.328 and 0.376), epidermis (r = 0.502) and hypodermis length (r = 0.402), FW and DW (r = 0.363 and 0.299), there was a positively strong correlation. This correlation was similar to those observed from the adaxial surface as well.
4. Discussion
Succulent plants grow in semi-arid climates where they are exposed to intense sun exposure and harsh conditions which impose evolutionary adaptations to avoid water loss, which is mainly evident with the changes in their morphology (i.e., presence of trichomes, papilla, and epicuticular wax), tissue reinforcements (such as thickening cell walls), and anatomical structure modifications (such as reduction of leaf airspace and stomata density) [2,16].
Most of the investigated Echeveria cultivars have relatively small leaves that are either cuneate or obtuse in shape and are reportedly related to their original habitats in arid regions in Mexico. As these are exposed to high irradiance, these cultivars’ expanded apex and tapered base can help optimize light capture and increase water-use efficiency [51]. In addition, these cultivars possessed the presence of epicuticular wax (EW) in different abundances on leaf surfaces. These powdery EWs are the plant’s outermost barrier and protective component from high sunlight exposure [52]. The abundance of EW is considered one of the most distinctive characteristics of species belonging to the Crassulaceae family [53,54]. Echeveria leaves have naturally thick and smooth cuticles, indicated by their entire leaf margin, which helps release water more quickly than undulating surfaces [55,56].
The adaptive strategies that co-occur in succulent plants thriving in limited water environments encompass various aspects of function and morphology. They include (a) thickening succulent leaves to reduce transpiration (morphology), (b) independently utilizing water by temporarily storing water in one or several organs (function), and (c) ensuring survival during periods of water scarcity (ecology) [2]. Water ability is the most crucial environmental factor influencing morpho-anatomical traits [56] and water-use efficiency. They may either display desiccation (poikilohydric) or non-desiccation tolerance (homoiohydric) [57]. Poikilohydric plants, like lichens or some mosses, equilibrate the humidity of the air during drought periods, while homoiohydric plants can store water in their tissues independently [58,59,60].
In this study, the latter water strategy is exhibited by Echeveria cultivars, shown in determining the epidermal characteristics. The increase in epidermal layers has been reported to enhance cell expansion and water-use efficiency. This is because enlarged epidermal cells facilitate their evapotranspiration, which enables a reduction of the temperature on the leaf surfaces, subsequently lowering internal leaf temperatures [57]. These expanded cells reflect the succulence or the thickness of their leaves, which are prominent in all cultivars. Likewise, these large epidermal cell sizes were observed in E. aff. gigantea accessions, and when grown under a high annual temperature and low annual rainfall, they exhibited a different anatomical feature compared to other accessions, and these adaptive changes of the accession were found to resemble another taxonomic group [42]. Dickison [61] suggested that a subdermal hypodermis with either single or multiple layers of cells is a distinct structural feature and a distinguishing factor in some taxa. Echeveria cultivars have been found to have one single layer of hypodermis in their leaves. A hypodermis has been significantly associated with the rate reduction of outward water movements, and preventing water loss [58,61]. The correlation analysis revealed a positive relationship between epidermis and hypodermis cells, as both cell layers are involved in water storage efficiency [58,59].
The leaf micro-morphology of the epidermal layer is also used as a diagnostic characteristic, being more useful for taxonomical analysis than external morphology alone [62,63]. The consistent polygonal shape is an adaptive mechanism. However, E. ‘Dark Ice’ and E. ‘Tippy,’ which have an irregular shape, are exceptions. This epidermal cell shape ensures efficient packing, allowing cells to fit tightly and minimize gaps, ultimately lessening water loss and allowing optimal organized cell division and growth [64,65]. Likewise, the variation of the epidermal periclinal walls has been used to define clades in Cinnamomum [66], Orobanche [67], and Simira [68] species.
Most of the ornamental succulent cultivars in this study were identified to have non-reticulate periclinal walls. This suggests that parallel-oriented surface tissues do not have a net-like or reticulated pattern signifying structural integrity, enhanced water-impermeable barrier, and specific cell functions more conducive to adaptation or an ecological niche [69]. On the other hand, the pattern of the anticlinal wall (ACW) was suggested to be closely related to their habitat environment, which explains why plants in dry habitats exhibit straight-to-curved ACW, while species that inhabit more humid and wet environments exhibit undulous to sinuous ACW [70]. In some rare cases, like Houttuynia cordata and Saurururs chinensis, straight-to-curved ACW may occur in plants that grow in humid and wet habitats [71].
Ornamental succulent cultivars had amphistomic leaves, indicating stomata presence in both adaxial and abaxial surfaces mainly due to ecotype variations among different individuals or adaptations to differential light stress [72]. The distribution of stomata on leaves surfaces indicates an adaptive advantage under certain environmental conditions [73]. There may appear to be a contradiction when Willmer and Fricker [74] stated that hypostomatic leaves, which have stomata restricted to the lower leaves surface, demonstrate better ability to associate with dry conditions than amphistomatous ones. Many studies indicated that amphistomary leaves are dominant in coping with water-deficit environments, and plants with increased stomata density tend to become more amphistomatic [75]. In this study, nine cultivars namely E. ‘Brave,’ E. Colorata E. Walther, E. ‘Fire Pillar,’ E. ‘Glam Pink,’ E. ‘Loy,’, E. ‘Milk Rose,’ E. ‘Peerless,’ E. ‘Snow Bunny,’ and E. ‘Viyant’, exhibited a range of leaf thickness from 6.01 to 8.21 mm, and Parkhurst [76] found that amphistomaty positively related to the thickness of leaves. In addition, the stomates were identified to have an anisocytic type where the stomata are encircled by three cells of varying sizes [77]. True anisocytic stomata are those made by Crassulaceae species [78], which exhibit a mesogenous origin and have evident size differences, wherein these Echeveria species belong. This type of stomatal arrangement has been attributed to helping reduce water loss and enhance photosynthetic efficiency, which creates a favorable microenvironment and adaptation to diverse environments, especially in drought conditions, that help regulate temperature [77,78,79,80].
Despite being grown in controlled environments, Echeveria cultivars maintain anisocytic features but have varied stomatal distribution. It was noted that Echeveria cultivars had a generally low distribution of stomata (<50 stomata/mm2) compared to xerophytes or mesophytes, wherein the frequency of stomata is 2–10 times higher [81]. The prevailing trend in most plant species indicates that stomatal densities are typically higher on the lower (abaxial) leaf surface compared to the upper (adaxial) surface [72,82]. However, the findings of this study revealed a contrasting observation concerning evaluated ornamental succulents and significant differences, except for those of E. ‘Silhouette.’ This difference can give advice to plant collectors and growers to avoid direct lighting exposure to E. ‘Silhouette’.
The study of Wall et al. [82] reported that higher stomatal density in the adaxial surface had higher photosynthetic capacity, facilitating greater carbon assimilation coupled with higher stomatal conductance and supporting optimal leaf temperatures, essential contributors to overall gas exchange. This mainly explains how succulents can tolerate drought environments. In this study, a higher stomata density is observed in plants with thicker leaves among those examined, such as E. colorata E. Walther (20.33 ± 4.06), E. ‘Loy’ (24.33 ± 1.67), E. ‘Peerless’ (29.67 ± 1.20), and E. ‘Tippy’ (30.67 ± 2.33), showing a positive correlation (r = 0.366). Additionally, the increase in stomatal density was positively associated with the decrease in water availability [83]. However, this trend is not consistent across all studied plants, for example, E. ‘Milk Rose’ and E. ‘Glam Pink’ do not follow this pattern due to differences in stomata distribution and leaf thickness.
On the contrary, occlusive cells of the adaxial epidermis are longer than those of the abaxial epidermis concerning stomata size. This study observed that E. ‘Brave’ and E. ‘Peerless’ had shorter occlusive cells in the adaxial surface. Plants with shorter occlusive stomata on the abaxial surface than on the adaxial surface are often found in rocky regions with deficient annual precipitation and high annual temperatures [39]. Likewise, plants with small stomata size and low stomata frequency distribution indicate they can prevent water loss in semi-arid areas [74]. This finding can offer guidance to both plant collectors and consumers on how to care for their ornamental plants. For instance, E. ‘Milk Rose’, and E. ‘Glam Pink’ prefer to grow in shaded environments; and E. ‘Peerless’ can tolerate more exposure to extreme light conditions compared to E. ‘Brave’.
Regarding vascular bundles, two types of arrangements were found among Echeveria cultivars, namely 2D and 3D. The majority of succulents in this study were found to possess 3D patterns wherein the main bundle is found in the central area, and several smaller bundles are scattered. According to Ogburn and Edwards [84], succulent plants are commonly found to form a 3D arrangement. Furthermore, Sandoval-Zapotitla et al. [39] suggest that this type of broadly branched vascular system allows for faster and more efficient transmission of water to all mesophyll cells, allowing for an adaptive advantage for various habitats, whether under low rainfall conditions or high temperature [85]. In addition, most of the cultivars had a collateral type of vascularization commonly observed in succulent plants [86]. This type of arrangement shows overall poor plant development, similarly, found in other reports for Kalanchoe and Sedum species [39,53,87]. In addition, an amphicribal-type arrangement, characterized by phloem surrounding the xylem, was also discovered in two accessions of E. aff. gigantea, which is considered an uncommon vascular formation in angiosperm leaves [87]. However, this dimorphic feature is also prevalent in species such as Aeonium canariense, A. lindleyi, and A. haworthii, belonging to the Crassulaceae family, and have been reported to exhibit collateral vascular bundles in the middle of the leaf and amphicribal vascular bundles in the basal region [85].
Fifteen Echeveria cultivars were found with expanded chlorenchyma cells, forming ‘all-cell succulence.’ This term, coined by Ihlenfeldt [88], indicates that all cells perform photosynthetic and water-storing capabilities, as reported in Crassulaceae [86,88]. This explains how well they adapt to their natural habitat where they naturally occupy and grow in so-called ‘live fast, die young’ conditions, cliff or rocky regions, with low respiration and low nutrient concentrations, because these environments are characterized to be slow growing with higher stress tolerance and more conservative compared to plants with storage succulence [1,86]. Succulent species, either all-cell succulence or storage succulence, contain gelatinous mucilage, which increases the water-retentive capacity but often leads to difficulties in cross-sectioning studies [37,44].
The succulence of Echeveria cultivars is evident in their moisture content, which was more than 90%. Despite the simplicity, the water content of plant tissues with their dry biomass has notably functional, physiological, and ecological significance [89]. With the help of anatomical and biochemical adaptations, this succulence of Echeveria allows them to maintain high water potential during drought conditions in semi-arid conditions [6]. Consistent with our results, the study of Bousselot et al. [90] concluded that succulent species retain more moisture, not just in their leaves, but also in their substrate for a longer duration than other herbaceous species, including having high dieback and revival rates that allow them to have better chances of survival in environments where water is intermittently available. From a holistic perspective, the moisture content of the succulent cultivars reaffirms the correlation between its anatomy, from the stomata, epidermal and hypodermal cells to their outward characteristics.
5. Conclusions
Fifteen Echeveria cultivars shared xeromorphic features in morphology and similarity in leaf anatomical structures with hypodermis and epidermis. In addition, the small stomatal size, low stomatal density, 3D vascular arrangement, and mesophyll structures explain these cultivars’ water storage capabilities and reflect how they adapt to harsh environments. This study provides knowledge about Echeveria cultivars living in controlled conditions but maintaining xeromorphic features in their wild habitats. Breeders and growers can use this information to optimize greenhouse conditions of ornamental succulents and provide key identification features for cultivars in these taxa.
Author Contributions
Conceptualization, M.K.T.H.T. and Y.-J.H.; methodology, M.K.T.H.T. and R.A.M.C.-B.; formal analysis, M.K.T.H.T. and R.A.M.C.-B.; writing—original draft preparation, M.K.T.H.T. and R.A.M.C.-B.; review and editing, M.K.T.H.T., R.A.M.C.-B. and Y.-J.H.; supervision, Y.-J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2020R1F1A1075112).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- von Willert, D.J.; Eller, B.M.; Werger, M.J.A.; Brinckmann, E.; Ihlenfeldt, H.D. Life strategies of succulents in deserts. Vegetation 1990, 90, 131–143. [Google Scholar] [CrossRef]
- Eggli, U.; Nyffeler, R. Living under temporarily arid conditions succulence as an adaptive strategy. Bradleya 2009, 27, 13–36. [Google Scholar] [CrossRef]
- Van Woert, N.D.; Rowe, D.B.; Andresen, J.A.; Rugh, C.L.; Xiao, L. Watering regime and green roof substrate design affect Sedum plant growth. HortScience 2005, 40, 659–664. [Google Scholar] [CrossRef]
- Velasco, E.; Roth, M.; Norford, L.K.; Molina, L.T. Does urban vegetation enhance carbon sequestration? Landsc. Urban Plan. 2016, 148, 99–107. [Google Scholar] [CrossRef]
- Kim, I.H.; Huh, K.Y.; Huh, M.R. Cold tolerance assessment of Sedum species for shallow extensive green roof system. Korean J. Hortic. Sci. Technol. 2010, 28, 22–30. [Google Scholar]
- Francini, A.; Romano, D.; Toscano, S.; Ferrante, A. The contribution of ornamental plants to urban ecosystem services. Earth 2022, 3, 1258–1274. [Google Scholar] [CrossRef]
- Kluge, M.; Lange, O.L.; Eichmann, M.V.; Schmid, R. CAM in Tillandsia usneoides: Studies on the pathway of carbon and the dependency of CO2–exchange on light intensity, temperature and water content of the plant. Planta 1973, 112, 357–372. [Google Scholar] [CrossRef]
- Ogburn, R.M.; Edwards, E.J. The ecological water-use strategies of succulent plants. Adv. Bot. Res. 2010, 55, 179–225. [Google Scholar]
- Li, F.L.; Bao, W.K. Elevational trends in leaf size of Campylotropis polyantha in the arid Minjiang River Valley, SW China. J. Arid Environ. 2014, 108, 1–9. [Google Scholar] [CrossRef]
- Larbi, A.; Vázquez, S.; El-Jendoubi, H.; Msallem, M.; Abadia, J.; Abaia, A.; Morales, F. Canopy light heterogeneity drives leaf anatomical, ecophysiological, and photosynthetic changes in olive trees grown in a high-density plantation. Photosynth. Res. 2014, 123, 141–155. [Google Scholar] [CrossRef]
- Jones, L.A. Anatomical adaptations of four Crassula species to water availability. Biosci. Horiz. 2011, 4, 13–22. [Google Scholar] [CrossRef]
- Royer, D.L.; McElwain, J.C.; Adams, J.M.; Wilf, P. Sensitivity of leaf size and shape to climate within Acer rubrum and Quercus kelloggii. New Phytol. 2008, 179, 808–817. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, L.; Gao, C.; Liao, D.S.; Long, L.; Jie, Q.; Wei, H.L.; Deng, Q.N.; Zhou, Y.C. A comparative study on the leaf anatomical structure of Camellia oleifera in a low-hot valley area in Guizhou Province, China. PLoS ONE 2021, 17, e0262509. [Google Scholar] [CrossRef]
- Wyka, T.P.; Bagniewska-Zadworna, A.; Kuczyńska, A.; Mikołajczak, K.; Ogrodowicz, P.; Żytkowiak, M.; Surma, M.; Adamski, T. Drought-induced anatomical modifications of barley (Hordeum vulgare L.) leaves: An allometric perspective. Environ. Exp. Bot. 2019, 166, 103798. [Google Scholar] [CrossRef]
- Taratima, W.; Ritmaha, T.; Jongrungklang, N.; Maneerattanarungroj, P.; Kunpratum, N. Effect of stress on the leaf anatomy of sugarcane cultivars with different drought tolerance (Saccharum officinarum, Poaceae). Rev. Biol. Trop. 2020, 68, 1159–1170. [Google Scholar] [CrossRef]
- Griffiths, H.; Males, J. Succulent plants. Curr. Biol. 2017, 27, 890–896. [Google Scholar] [CrossRef]
- Nobel, P.S. Environmental Biology of Agaves and Cacti; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Kluge, M.; Ting, I.P. Crassulacean Acid Metabolism: Analysis of an Ecological Adaptation; Springer: Berlin, Germany, 1978; pp. 5–44. [Google Scholar]
- Nobel, P.S. Environmental influences on CO2 uptake by agaves, CAM plants with high productivities. Econ. Bot. 1990, 44, 488–502. [Google Scholar] [CrossRef]
- Grace, O.M. Succulent plant diversity as natural capital. Plant People Planet 2019, 1, 336–345. [Google Scholar] [CrossRef]
- Manokari, M.; Cokulraj, M.; Badhepuri, M.K.; Dey, A.; Faisal, M.; Alatar, A.A.; Singh, R.K.; Shekhawat, M.S. Microstructural and histochemical modifications in leaves at successive stages of in vitro development of the terrestrial orchid Spathoglottis plicata Blume. Hortic. Environ. Biotechnol. 2023, 64, 497–510. [Google Scholar] [CrossRef]
- Cabahug-Braza, R.A.; Tran, M.K.T.H.; Lim, K.B.; Hwang, Y.J. Phenotypic evaluation and nuclear DNA content analysis of oryzalin-induced Echeveria mutant cultivars. Hortic. Sci. Technol. 2023, 41, 315–328. [Google Scholar] [CrossRef]
- Khan, M.N.E.A.; Hassan, J.; Biswas, M.S.; Khan, H.I.; Sultana, H.; Suborna, M.N.; Rajib, M.M.R.; Akter, J.; Gomasta, J.; Anik, A.A.M. Morphological and anatomical characterization of colchicine-induced polyploids in watermelon. Hortic. Environ. Biotechnol. 2023, 64, 461–474. [Google Scholar] [CrossRef]
- Yeung, E. A beginner’s guide to the study of plant structure. In Tested Studies for Laboratory Teaching; Karche, S.J., Ed.; Purdue University: West Lafayette, IN, USA, 1998; pp. 125–141. [Google Scholar]
- Mohammed, F.; Arishiya, T.F.; Mohamed, S. Microtomes and microtome knives—A review and proposed classification. Ann. Dent. 2012, 19, 43–50. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company Inc.: London, UK, 1940; p. 530. [Google Scholar]
- Ribeiro, V.C.; Leitão, C.A.E. Utilisation of toluidine blue O pH 4.0 and histochemical inferences in plant sections obtained by free-hand. Protoplasma 2020, 257, 993–1008. [Google Scholar] [CrossRef]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Thierry, R.; Marie-Christine, R.; Marie’Le, M.; Richard, B.; Anca, M. Modified technique of Toluidine blue staining in rapid on-site evaluation. Diagn. Cytopathol. 2011, 40, 847–848. [Google Scholar] [CrossRef]
- Parker, A.J.; Haskins, E.F.; Deyrup-Olsen, I. Toluidine blue: A simple, effective stain for plant tissues. Am. Biol. Teach. 1982, 44, 487–489. [Google Scholar] [CrossRef]
- Koller, A.L.; Rost, T.L. Structural analysis of water-storage tissue in leaves of Sansevieria (Agavaceae). Bot. Gaz. 1988, 149, 260–274. [Google Scholar] [CrossRef]
- Fradera-Soler, M.; Rudall, P.J.; Prychid, C.J.; Grace, O.M. Evolutionary success in arid habitats: Morpho-anatomy of succulent leaves of Crassula species from southern Africa. J. Arid Environ. 2021, 185, 104319. [Google Scholar] [CrossRef]
- Nyffeler, R.; Eggli, U. An up-to-date familial and suprafamilial classification of succulent plants. Bradleya 2010, 28, 125–144. [Google Scholar] [CrossRef]
- Uhl, C.H. Polyploidy, dysploidy, and chromosome pairing in Echeveria (Crassulaceae) and its hybrid. Am. J. Bot. 1992, 79, 556–566. [Google Scholar] [CrossRef]
- Cabahug, R.A.M.; Nam, S.Y.; Lim, K.B.; Jeon, J.K.; Hwang, Y.J. Propagation techniques for ornamental succulents. Flower Res. J. 2018, 26, 90–101. [Google Scholar] [CrossRef]
- Borys, M.W.; Leszczyńska-Borys, H. The genus Echeveria as a potential new floral crop. Acta Hortic. 2013, 1000, 91–96. [Google Scholar] [CrossRef]
- Males, J. Secrets of succulence. J. Exp. Bot. 2017, 68, 2121–2134. [Google Scholar] [CrossRef]
- Razzaghmanesh, M.; Beechman, S.; Kazemi, F. The growth and survival of plants in urban green roofs in a dry climate. Sci. Total Environ. 2014, 476, 288–297. [Google Scholar] [CrossRef]
- Sandoval-Zapotitla, E.; Martínez-Quezada, D.M.; Reyes-Santiago, J.; Islas-Luna, M.; Rosas, U. Leaf morpho-anatomical diversity in Echeveria aff. gigantea (Crassulaceae). Bot. Sci. 2019, 97, 218–235. [Google Scholar] [CrossRef]
- Matimati, I.; Musil, C.F.; Raitt, L.; February, E. Non-rainfall moisture interception by dwarf succulents and their relative abundance in an inland arid South African ecosystem. Ecohydrology 2013, 6, 818–825. [Google Scholar] [CrossRef]
- Teskey, R.O. Canopy Processes. In Tree Physiology (Encyclopedia of Forest Sciences); Elsevier Ltd.: Amsterdam, The Netherlands, 2004; pp. 1622–1628. [Google Scholar]
- Francis, B.; Gilman, R.T. Light intensity affects leaf morphology in a wild population of Adenostyles alliariae (Asteraceae). Ital. Bot. 2019, 8, 35–45. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef]
- Rost, T.L. Vascular pattern and hydathodes in leaves of Crassula argentea (Crassulaceae). Bot. Gaz. 1969, 130, 267–270. [Google Scholar] [CrossRef]
- Pilbeam, J. The Genus Echeveria; The British Cactus & Succulent Society: Great Malvern, UK, 2008. [Google Scholar]
- Ash, A.; Ellis, B.; Hickey, L.J.; Johnson, K.; Wilf, P.; Wing, S. Manual of Leaf Architecture; Smithsonian Institution: Washington, DC, USA, 1999; pp. 12–67. [Google Scholar]
- Neinhuis, C.; Edelmann, H.G. Methanol as a rapid fixative for the investigation of plant surfaces by SEM. J. Microsc. 1996, 184, 14–16. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, E.J. On the causes and ecological significance of stomata frequency with special reference to the woodland flora. Philos. Trans. R. Soc. Lond. 1927, 216, 1–65. [Google Scholar]
- Lee, J.H.; Nam, S.Y. Analysis of growth and leaf color changes of Sedum album cv. Athoum according to the spectral power distribution of several white LEDs. Flower Res. J. 2022, 30, 184–193. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Long, L.M.; Patel, H.P.; Cory, W.C.; Stapleton, A.E. The maize epicuticular wax layer provides UV protection. Funct. Plant Biol. 2003, 30, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chernetskyy, M.; Weryszko-Chmielewska, E. Structure of Kalanchoë pumila Bak. leaves (Crassulaceae DC). Acta Agrobot. 2008, 61, 11–24. [Google Scholar] [CrossRef]
- Denton, M.F. SEM Analysis of leaf epicuticular waxes of Sedum Section Gormania (Crassulaceae). Brittonia 1994, 46, 296–308. [Google Scholar] [CrossRef]
- Miroslavov, E.A. Structure and Function of Leaf Epidermis of Angiosperms Plants; Nauka: St. Petersburg, Russia, 1974; pp. 12–54. [Google Scholar]
- Niklas, K.J. Evolution of plant shape: Design constraints. Trends Ecol. Evol. 1986, 1, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Schulze, E.D.; Beck, E.; Muller-Hohenstein, K. Plant Ecology; Springer: Berlin, Germany, 2005; pp. 61–79. [Google Scholar]
- Tomlinson, P.B. Commelinales-Zingiberales. In Anatomy of the Monocotyledons; Metcalfe, C.R., Ed.; Oxford Press: Sydney, Australia, 1969; pp. 81–92. [Google Scholar]
- Kaul, R.B. The role of the multiple epidermis in foliar succulence in Peperomia (Piperaceae). Bot. Gaz. 1977, 138, 213–218. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G. Morpho–anatomical traits for plant adaptation to drought. In Plant Responses to Drought Stress; Springer: Berlin, Germany, 2012; pp. 37–61. [Google Scholar]
- Dickison, W. Integrative Plant Anatomy; Academic Press: San Diego, CA, USA, 2000; p. 533. [Google Scholar]
- Metcalfe, C.R.; Chalk, L. Anatomy of the Dicotyledones, 2nd ed.; Clarendon Press: Oxford, UK, 1979; pp. 456–473. [Google Scholar]
- Stace, C.A. The taxonomic importance of the leaf surface. In Current Concepts in Plant Taxonomy; Heywood, V.H., Moore, D.M., Eds.; Academic Press: London, UK, 1984; pp. 67–93. [Google Scholar]
- Sapala, A.; Runions, A.; Routier-Kierzkowska, A.-L.; Gupta, M.D.; Hong, L.; Hofhuis, H.; Verger, S.; Mosca, G.; Li, C.-B.; Hay, A.; et al. Why plants make puzzle cells, and how their shape emerges. eLife 2018, 7, e32794. [Google Scholar] [CrossRef]
- Sapala, A.; Runions, A.; Smith, R.S. Mechanics, geometry and genetics of epidermal cell shape regulation: Different pieces of the same puzzle. Curr. Opin. Plant Biol. 2019, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gang, Z.; Liu, B.; Rohwer, J.G.; Ferguson, D.K.; Yang, Y. Leaf epidermal micromorphology defining the clades in Cinnamomum (Lauraceae). PhytoKeys 2021, 4, 125–148. [Google Scholar] [CrossRef]
- Plaza, L.; Fernandez, I.; Juan, R.; Pastor, J.; Pujadas, A. Micromorphological studies on seeds of Orobanche species from the Iberian Peninsula and the Balearic Islands, and their systematic significance. Ann. Bot. 2004, 94, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Moraes, T.M.; Barros, C.F.; Silva Neto, S.J.; Gomes, V.M.; Da Cunha, M. Leaf blade anatomy and ultrastructure of six Simira species (Rubiaceae) from the Atlantic rain forest, Brazil. Biocell 2009, 33, 155–165. [Google Scholar] [CrossRef]
- Riederer, M.; Muller, C. Biology of the Plant Cuticle; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 12–56. [Google Scholar]
- Stace, C.A. Cuticular studies as an aid to plant taxonomy. Bull. Br. Mus. Bot. 1965, 4, 3–78. [Google Scholar]
- Song, J.H.; Yang, S.G.; Choi, G.Y. Taxonomic implications of leaf micromorphology using microscopic analysis: A tool for identification and authentication of Korean Puperales. Plants 2020, 9, 566. [Google Scholar] [CrossRef]
- Mott, K.A.; Michaelson, O. Amphistomy as an adaptation to high light intensity in Ambrosia cordifolia (Compositae). Am. J. Bot. 1991, 78, 76–79. [Google Scholar] [CrossRef]
- Jordan, G.J.; Carpenter, R.J.; Brodribb, T.J. Using fossil leaves as evidence for open vegetation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 395, 168–175. [Google Scholar] [CrossRef]
- Willmer, C.; Fricker, M. Stomata; Springer: Dordrecht, The Netherlands, 1996; pp. 73–76. [Google Scholar]
- Pérez, V.; Arévalo, A.; Villanueva-Almanza, L.; Ezcurra, E. Variation in leaf xeromorphism in the desert palm genus Washingtonia (Arecaceae). J. Arid Environ. 2021, 186, 104412. [Google Scholar] [CrossRef]
- Parkhurst, D.F. The adaptive significance of stomatal occurence on one or both surfaces of leaves. J. Ecol. 1978, 66, 367–383. [Google Scholar] [CrossRef]
- Prabhakar, M. Structure, delimitation, nomenclature and classification of stomata. Acta Bot. Sin. 2004, 46, 242–252. [Google Scholar]
- Nunes, T.D.G.; Zhang, D.; Raissig, M.T. Form, development and function of grass stomata. Plant J. 2020, 101, 780–799. [Google Scholar] [CrossRef]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Mori, I.C.; Munemasa, S. Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 2015, 66, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Sunberg, W.; Marshall, D. A comparison of stomatal distribution and length in succulent and non-succulent desert plants. Phytomorphology 1986, 36, 53–66. [Google Scholar]
- Wall, S.; Vialet-Chabrand, S.; Davey, P.; Van Rie, J.; Galle, A.; Cockram, J.; Lawson, T. Stomata on the abaxial and adaxial leaf surfaces contribute differently to leaf gas exchange and photosynthesis in wheat. New Phytol. 2022, 235, 1743–1756. [Google Scholar] [CrossRef]
- Buttery, B.R.; Tan, C.S.; Buzzell, R.I.; Gaynor, J.D.; MacTavish, D.C. Stomatal numbers of soybean and response to water stress. Plant Soil 1993, 149, 283–288. [Google Scholar] [CrossRef]
- Ogburn, R.M.; Edwards, E.J. Repeated origin of three-dimensional leaf venation releases constraints on the evolution of succulence in plants. Curr. Biol. 2013, 23, 722–726. [Google Scholar] [CrossRef]
- Caballero-Ruano, A.; Jiménez-Parrondo, M.S. A contribution to the leaf anatomy studies of Canarian Crasuláceas. Vieraea 1978, 7, 115–132. [Google Scholar]
- Melo-de-Pinna, G.F.A.; Hernades-Lopes, J.; Ogura, A.S.; Santos, L.K.; Silva, D.C.; Haevermans, T. Growth patterns and different arrangements of vascular tissues in succulent leaves. Int. J. Plant Sci. 2016, 177, 643–660. [Google Scholar] [CrossRef]
- Ardelean, M.; Stanescu, I.; Cachita-Cosma, D. Sedum telephium spp. Maximum (L.) Krock. histo-anatomical aspects on the vegetative organs. J. Plant Dev. 2009, 55, 75–80. [Google Scholar]
- Ihlenfeldt, H.-D. Life forms and survival strategies in succulents. Rep. Ger. Bot. Soc. 1985, 98, 409–423. [Google Scholar]
- Ievinsh, G. Water content of plant tissues: So simple that almost forgotten? Plants 2023, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Bousselot, J.M.; Klett, J.E.; Koski, R.D. Moisture content of extensive green roof substrate and growth response of 15 temperate plant species during dry down. HortScience 2011, 46, 518–522. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).