Abstract
The total land used for land-based food farms is less than 1% in Singapore. As a result, more than 90% of Singapore’s food needs are imported. To strengthen food security, Singapore has set a target to develop the capability and capacity of the agri-food industry to locally produce 30% of its nutritional needs by 2030. To achieve this goal, technology is the key to helping farms to “grow more with less”. This review first discusses how aeroponic systems have been adapted for growing all kinds of leafy vegetables in the tropics through the manipulation of root-zone temperature and heat priming to save power energy. Growing vegetable crops indoors and in greenhouses not only allows the growers to achieve high productivity but also enables them to enhance nutritional values. The second part of this paper emphasizes how to achieve substantial yield through deficit irrigation with higher nutritional quality in a cost-effective manner. Growing crops vertically has become increasingly popular, as it increases land use. We establish a commercially viable LED-integrated aeroponic system to grow vegetables vertically. The last part of the paper discusses the impacts of LED spectral quality, quantity, and duration on vegetable production.
1. Introduction
In Singapore, a city-state characterized by its high urban population, less than 1% of its land is allocated to land-based food farms [1]. This scarcity of land for agricultural purposes is compounded by Singapore’s hot and humid climate, which poses significant challenges for vegetable cultivation, as plants are sensitive to temperature fluctuations [2]. Consequently, over 90% of Singapore’s demand for subtropical and temperate vegetable crops, such as Chinese broccoli and lettuce, is met through imports from other countries [3,4]. Climate change, moreover, exacerbates these challenges, altering traditional growing conditions and placing strain on existing agricultural practices [5,6]. Coupled with population growth and the stockpiling of food reserves by countries with large populations, Singapore’s food security has been under pressure for decades [1,7,8]. The COVID-19 pandemic further underscored vulnerabilities in food supply chains, particularly in a highly urbanized context like Singapore [9,10]. To mitigate these risks and ensure a steady food supply, Singapore has pursued diversification strategies, reducing reliance on a single food source [11]. The Singapore Food Agency has set an ambitious target titled “30 by 30”, aiming to locally produce 30% of the nation’s nutritional needs by 2030 [12]. Given Singapore’s limited land availability and water scarcity, technology plays a pivotal role in achieving this goal, enabling farms to maximize productivity while minimizing resource usage by “growing more with less” [12].
High temperatures under tropical climates inhibit the growth of temperate and subtropical crops and decrease their production via a number of physiological mechanisms, especially photosynthesis [13,14,15]. Aeroponic farming systems have been adapted by our research team to grow subtropical and temperate leafy vegetables in a tropical greenhouse through the manipulation of root-zone temperature (RZT) [3,16,17,18] in order to save power energy. For a tropical greenhouse, even when cooling only the RZ of the vegetable crops, the power energy requirement is still a major production cost. Our recent studies show that RZ heat priming could serve as a promising strategy to reduce the energy costs of growing temperate crops in a tropical greenhouse [19,20]. Thus, the first part of this review discusses the mechanisms responsible for the manipulation of RZT on the productivity of aeroponically grown leafy vegetables in the tropical greenhouse.
Growing vegetable crops indoors and in greenhouses allows growers to achieve not only high productivity but also high accumulations of nutritional compounds through manipulating growth conditions [21,22,23,24,25]. Plants are able to detect and react to alterations in their immediate surroundings, termed microclimate changes. Apart from changing the light environments [21,22,23], the availability of water not only influences plants’ growth and development but also impacts their nutritional composition [24,25]. The second part of this paper focuses on strategies for attaining significant crop yields while enhancing nutritional value in a cost-efficient manner through deficit irrigation using aeroponic farming systems both indoors and in a greenhouse. Our studies have shown that the nutritional quality of leafy vegetables could be improved under mild water deficit through deficit irrigation [24,25]. Achieving substantial reductions in energy and water consumption in urban agriculture and doing so without sacrificing yield while improving nutritional quality could greatly benefit society through resource conservation.
To increase areas of cultivation, growing leafy vegetable crops vertically indoors under controlled environments and in greenhouses has become increasingly popular in Singapore [1,8]. Light plays a crucial role in the cultivation of vegetables [26,27,28]. My team has developed a commercially viable vertical aeroponic farming system integrated with LED technology to cultivate leafy greens both indoors and in greenhouses. Our findings indicate that for the indoor cultivation of leafy vegetables, a combination of red (R)- and blue (B)-LEDs proves more effective in enhancing photosynthesis, thereby increasing productivity compared to using either R- or B-LEDs alone [29,30]. More and more studies have highlighted the importance of LED spectral quality on plants grown indoors [21,22,23,31,32,33,34]. However, the optimal LED spectral combinations vary depending on the plant species [26,30,35,36]. The success of indoor vertical farming illuminated by LEDs also hinges on local energy costs and environmental factors such as temperature, humidity, nutrient availability, and CO2 levels [35]. Relying solely on energy-intensive indoor vertical farming, which necessitates controlled lighting and environmental conditions, is not sustainable for achieving the “30 by 30” goal, which emphasizes achieving more with fewer resources [35]. Therefore, the final part of this review examines the influence of LED spectral quality, intensity, and duration on various leafy vegetables grown aeroponically in tropical greenhouse environments, aiming to reduce cooling costs. Additionally, this review paper also discusses the supplementation of natural sunlight with LED lighting to enhance vegetable production [37,38] and improve nutritional quality in tropical greenhouse environments.
2. Enhancing Productivity of Aeroponically Grown Leafy Vegetables in a Tropical Greenhouse through Manipulation of Root-Zone Temperature (RZT)
Vegetables provide essential nutrients for human nutrition and are a fundamental constituent of the daily diet [39,40,41]. In Singapore, local produce usually costs much more than imports. In Singapore’s “30 by 30” strategy, vegetables are one of the most important food items to focus on. This is because vegetables are easily perishable and have a limited shelf life after harvest. Singapore has a tropical climate with relatively high and uniform day and night temperatures and high humidity throughout the year. In the tropics, the average daily temperature fluctuates between 25 and 34 °C, though it can occasionally soar up to 40 °C in greenhouse environments [17]. It is well known that air temperature is a main factor affecting vegetable growth and yield. The optimum growth temperature for vegetable crops is species-dependent [41]. According to Wien [42], the temperature optimums for hot, warm, and cold season vegetables are, respectively, 25–27 °C, 20–25 °C, and 18–25 °C. High temperatures inhibit the growth and development of subtropical and temperate vegetable crops under tropical conditions. Thus, Singapore has to import about 90% of its needs in subtropical crops, for example, Chinese broccoli, capsicum, and Chinese cabbage, and all its needs in temperate crops such as lettuce [3,4]. Climate changes further affect our ability to farm. Among the types of climate changes, increases in temperature have the most negative impact on crops, including vegetable crops [43,44,45]. In their recent publication, Dumitru et al. [43] conducted a review utilizing the Web of Science and Scopus databases, examining 219 scientific articles out of a total of 107,799 concerning “climate change impacts” from 1990 to 2023. Their analysis revealed that only 53 out of the 219 reviewed publications specifically addressed the impacts of climate change on vegetable crops. Thus, there is a need for more specialized research on the impacts of climate change on vegetable crops. While crop breeders will have to provide the farmers with heat-resistant cultivars, it is also necessary for crop growers to adopt better farming practices to combat climate change [46]. Controlled environment agriculture (CEA), including either simple greenhouses or indoor farming, is a powerful way to produce fresh food that reduces the impacts of climate changes on agriculture [47,48].
Both air temperature and root zone temperature (RZT) play crucial roles as environmental factors influencing plant growth and eventual production yield. Shoots can moderate their temperature through transpiration, while roots are unable to do so [49]. Compared to shoot responses, root responses to high temperatures are understudied. Nonetheless, roots are pivotal for plant growth and development, as they supply the necessary water and essential minerals required for survival. Efficient nutrient uptake and water absorption by roots lead to enhanced growth and improved yield [50,51]. Not only ambient temperature but also RZT can strongly affect plant growth and development. Roots can adjust their morphological, biochemical, and physiological traits in response to RZT [52,53,54,55]. It was reported that the response of plant growth rates to high air temperatures was influenced by RZT [56]. Since the late 1970s, a few studies have even demonstrated that RZT is more critical than air temperature in enhancing plant growth [57,58,59,60,61]. Using aeroponic systems, our research team has successfully grown subtropical and temperate vegetable crops in the tropical greenhouse by cooling the RZ independently of hot ambient temperatures [53]. In aeroponic systems, plants with their roots are suspended in the air, where a nutrient solution is sprayed on the roots’ surface [53]. Our results show that RZ cooling provides great benefits to the root growth and development of subtropical vegetables such as Chinese broccoli (Brassica alboglabra L.) [62] and temperate lettuce (Lactuca sativa L.) in tropical greenhouses [63]. Subtropical and temperate vegetable crops cultivated in cool RZTs exhibited longer total root lengths, higher numbers of root tips, expanded root surface areas, and smaller root diameters in contrast to those grown in hot ambient RZTs [62,63,64]. Abscisic acid (ABA) and ethylene synthesized in the roots play major roles in regulating root growth and development [65,66,67,68,69]. Cool RZT decreased ABA concentration and alleviated ABA-induced stomatal closure [65,66,69]. Our studies also revealed that cooling the RZT promoted root elongation, increased number of root tips, and decreased root diameter, which is associated with lower RZ ethylene accumulation and corresponds to higher stomatal conductance and shoot growth [67,68]. The effects of RZT on root morphology may have modified root hydraulic conductivity and affected shoot water potential [69]. Grown in tropical greenhouses under cool RZTs, cool-season vegetable crops with well-developed root systems could enhance water and mineral uptake, thus, preventing water deficit and mineral nutrient deficiency [53,63]. The effects of RZT on water and mineral nutrient uptake have also been reported by other teams in strawberry [70], tomato, muskmelon, and honey locust crops [71].
Under cool-RZT, the photosynthetic efficiency and final production yield of subtropical and temperate vegetable crops were enhanced remarkably compared to the whole plants grown under hot ambient temperatures [16,17,18]. Our studies showed that cool-RZT alternates the partitioning of mineral nutrients uptake by the roots [63]. In response to the changes in RZT, the partitioning of photoassimilates produced in the leaves was also altered to meet changes in shoot and root nutrient demands [72]. Cool-RZT also alleviates the negative effects of hot-ambient-RZT on NO3− assimilation and the coordination of carbon and nitrogen metabolism [53]. Studies conducted in the tropical greenhouse revealed that cooling the RZT of subtropical and temperate vegetable crops alleviated both stomatal and non-stomatal constraints on photosynthesis, including reductions in chlorophyll content, photosynthetic light use efficiency, Rubisco protein content, and maximum Rubisco activity, irrespective of hot ambient temperatures [17,18]. Studies conducted by other researchers have also shown that RZT affects photosynthesis and assimilate partitioning [61,73]. Through the mitigation of high-temperature stress on root and shoot growth and development and various physiological processes (Figure 1), subtropical and temperate vegetable crops can now be grown all year round through cooling only the RZ in a tropical greenhouse.
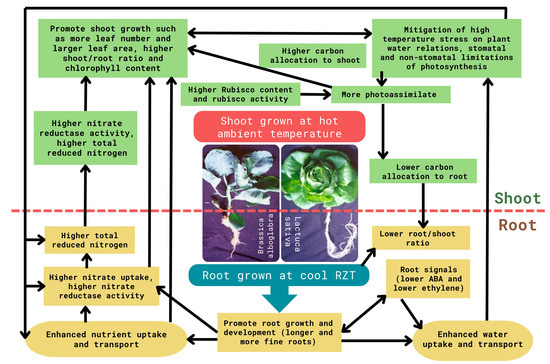
Figure 1.
Changes of various physiological parameters of subtropical (Brassica alboglabra L.) and temperate vegetable crops (Lactuca sativa L.) grown with aeroponic systems by exposing their roots to cooling temperatures (15–25 °C) while their aerial parts were maintained at fluctuating hot ambient temperatures (25–40 °C) in the tropical greenhouse. The changes of different physiological parameters are in comparison to those whole plants grown under hot fluctuating ambient temperatures.
3. Root-Zone (RZ) Heat Priming Effects on Growth, Productivity, Physiology, and Nutritional Quality
Recently, there has been an increasing number of reports on the phenomenon of plant stress memory responses to abiotic stress including heat stress, which has provided new strategies for improving crop heat resilience [74,75,76]. According to the literature, plants have mitotic stress memory (somatic memory, in the same generation) and meiotic stress memory (in the next generations) [76]. Many plants can remember past stress events, leading to increased tolerance for future stressors, which is referred to stress priming [76,77,78]. Plants naturally cope with heat stress, which is defined as basal heat tolerance. Most plants have the capacity to enhance their resistance to higher temperatures following a short period of exposure to heat stress, a phenomenon known as acquired heat tolerance or heat priming, also known as thermopriming [79,80,81]. Species with low heat tolerance are predicted to be most vulnerable to global warming [76]. It was reported that for each degree rise in average temperature, there is an estimated decrease in yield by 3.1%, 3.2%, and 6.0% respectively for soybeans, maize, and rice [82]. Extensive literature has detailed the diverse mechanisms through which various crop species have evolved to perceive ambient temperature signals to facilitate heat priming as an adaptive response to hotter environments [76]. Heat priming protects plants from the damage of different physiological responses, especially photosynthesis upon subsequent more frequent and severe heat stress, by increasing the accumulation of protective chemicals, including heat shock proteins, antioxidants, and secondary metabolites [77,83,84]. For instance, in the study with Origanum vulgare, it was reported that heat stress induced stomatal and non-stomatal limitation of photosynthesis, but heat priming enhanced photosynthetic heat tolerance [84].
Heat-priming treatment on aerial parts of the plants and their responses has been well studied [76,83]. Reports have discussed seed and whole plant priming [76,85]. However, there is scant information regarding the effects of RZ heat priming on photosynthetic performance, productivity, and root morphology. Although aeroponic systems have been adapted for growing all kinds of vegetables in the tropics through the manipulation of RZT to save power energy, high energy costs were still incurred, even when cooling only the RZ of the vegetable crops. Increases in both the average temperatures and frequency of heat waves are likely to happen severely in the tropics [84]. Thus, RZ heat priming could serve as one of the promising means to further reduce high energy consumption in cultivating vegetable crops in tropical greenhouses [19,20]. In our previous study, two different leafy greens, Eruca sativa (cv. arugula) and Lactuca sativa (cv. canasta), were first grown under 25 °C in a tropical greenhouse for 10 days. They were then exposed to sublethal RZT of 38 °C for RZ heat priming for 10 days. Our results showed that RZ heat priming did not have any negative impact on root morphology [19]. RZ heat-primed canasta plants had better photosynthetic performance and higher productivity after subjecting to subsequent RZT of 42 °C for 10 days compared to non-primed canasta plants. However, RZ-heat-priming-enhanced thermotolerance was not observed in arugula during the same periods of experiments [19]. This result suggests that the effect of RZ heat priming is largely species-dependent.
In another recent study, we carried out the same RZ heat priming for 10 days for the same species in the same greenhouse, but plants were exposed to a subsequent RZT of 42 °C for 16 days. It was found that RZ heat priming at RZT of 38 °C resulted in the slower leaf expansion of canasta after six days of RZ heat priming compared to those grown under RZT of 25 °C. However, RZ heat priming did not affect the leaf expansion of arugula compared to 25 °C-RZT plants [20]. During RZ heat priming, dynamic photoinhibition measured by lowered midday Fv/Fm ratios in canasta leaves [20] could lead to a decline in overall photosynthetic performance [17,86], which could be partially responsible for the slower leaf growth. Improvements in heat resistance to prevent damage to the photosynthetic apparatus may be coupled with reduced photosynthetic performance during heat priming. This could be due to the development and maintenance of the primed effect [84]. Despite its significantly lower midday Fv/Fm ratio, RZ heat priming did not affect the leaf expansion of arugula [20]. Both plant species had predawn Fv/Fm ratios greater than 0.8, indicating that no chronic photoinhibition occurred in any plants during RZ heat priming [20]. After being exposed to a subsequent 42 °C-RZT for 16 days following RZ heat priming, arugula had similar higher fresh shoot weights (Figure 2A) and larger root systems with a higher total root length (Figure 2B), larger total root surface area, and a similar total number of root tips [20] as those of 25 °C-RZT plants compared to those of non-primed arugula. However, RZ heat priming did not enhance the shoot productivity of canasta (Figure 2A) after exposure to 42 °C-RZT for 16 days compared to those non-primed plants. Arugula seems to retain and sustain stressful memories for a longer period, allowing for longer responses to subsequent stressful situation compared to canasta. At harvest, arugula had higher accumulations of dietary minerals such as Ca (Figure 2C) and Mg (Figure 2D) in RZ-heat-primed plants than in 25 °C-RZT plants, which could be due its larger root system [3]. However, RZ heat priming did not promote the root development of canasta (Figure 2B). RZ-heat-primed and non-primed canasta plants had higher concentrations of Ca and Mg (Figure 2C,D) compared to those grown at 25 °C-RZT, suggesting that the minerals were concentrated within small plants.
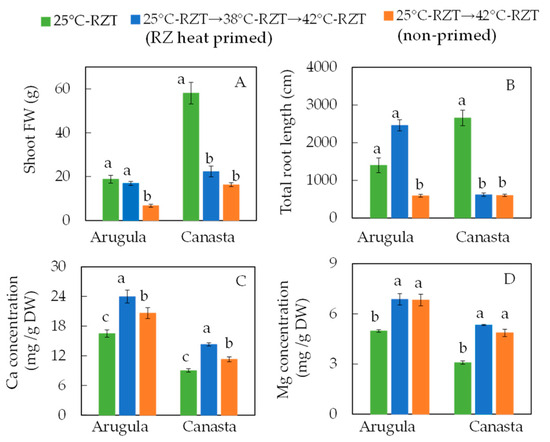
Figure 2.
Shoot FW (A), total root length (B), concentrations of Ca (C) and Mg (D) of arugula and canasta at harvest (36 days after transplanting). The vertical lines through the means represent standard errors (n = 4). Different lowercase letters are statistically different (p < 0.05, Tukey’s multiple comparison test). Each species was analyzed separately. 25 °C-RZT: plants were kept at a constant 25 °C-RZT for 24 h daily for 36 DAT (days after transplanting); 25 °C-RZT→38 °C-RZT→42 °C-RZT (RZ heat priming): plants were grown at a constant 25 °C-RZT, 24 h daily for the first 10 days, then subjected to fluctuating hot A-RZT with a constant 38 °C-RZT maintained from 1100 to 1700 h for 6 h daily from 11 to 20 DAT and then exposed to fluctuating hot A-RZT with a constant 42 °C-RZT maintained for 16 days from 1100 to 1700 h for 6 h daily; 25 °C-RZT→42 °C-RZT (non-priming): plants were grown at a constant 25 °C-RZT 24 h daily for 20 days and for the last 16 days were transferred to fluctuating hot A-RZT with a constant 42 °C-RZT maintained from 1100 to 1700 h for 6 h daily (Modified from He et al. [20].
During heat stress, the chloroplast is the major site that produces reactive oxygen species (ROS) such as H2O2, which may induce oxidative stress [87]. Heat priming protected plants from heat damage to the photosynthetic apparatus and enhanced their photosynthetic capacity compared to non-primed plants via increased antioxidant activity [78,84,88]. Ascorbic acid, an antioxidant, confers resistance to heat stress to lower the H2O2 concentration [88,89]. Phenolic compounds are also natural antioxidants that protect plants from photodamage. It was reported that coriander (Coriandrum sativum L.) accumulated higher levels of total phenolic compounds under a combination of PPFD of 300 μmol m−2 s−1 and 30 °C-RZT than under lower PPFD and lower RZT [89]. In our study, RZ-heat primed arugula and canasta plants had higher concentrations of total ascorbic acids (Figure 3A) and total phenolic compounds (Figure 3B) compared to non-primed plants after being subjected to a subsequent 42 °C-RZT for 16 days. Although RZ heat priming also enhanced its nutritional quality, the productivity of canasta was compromised (Figure 3). The results of our second study [20] imply that RZ heat priming effects depend on not only species but also the duration of subsequent high RZT. Our results also suggest that it is feasible to enhance the productivity and nutritional quality of temperate leafy green at lower production costs in a tropical greenhouse through RZ heat priming strategy.
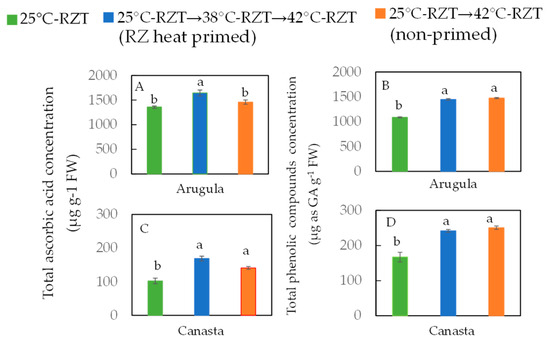
Figure 3.
Total ascorbic acid (A,C) and total phenolic compounds (C,D) concentration of arugula and canasta at harvest (36 days after transplanting). The vertical lines through the means represent standard errors (n = 4). Different lowercase letters are statistically different (p < 0.05, Tukey’s multiple comparison test). Each species was analyzed separately. Refer to Figure 2 for the details of different RZT treatments (Modified from He et al. [20]).
4. Deficit Irrigation Effects on Productivity and Nutritional Quality of Aeroponically Grown Leafy Vegetables Indoors and in a Tropical Greenhouse
Due to its land scarcity, Singapore lacks natural freshwater resources. Although Singapore has four “national taps” (local catchment area, imported water, NEWater, and desalinated water) that have helped mitigate water scarcity, Singapore remains vulnerable. Agricultural activities account for about 70% of freshwater withdrawals. Furthermore, global warming will result in severe water shortages [90]. An integrated approach, therefore, is imperative for increasing food security. For instance, using aeroponic farming systems for vegetable production could be the most promising water-efficient approach to urban agriculture [26,91]. The adoption of innovative technologies has the potential to decrease water consumption while simultaneously preserving or even enhancing crop yields [92]. Grown with the aeroponic farming systems, plant roots are sprayed with a fine mist of nutrient solution at different spraying intervals depending on plant species. Aeroponic farming systems allow for the reuse of water and circulate nutrients back to the nutrient tank. Both water and nutrients, which have not been absorbed by plants, will be recycled after adjusting the EC and pH to the optimal levels. Thus, water use efficiency could be maximized [53,91].
Deficit irrigation (DI), defined as the application of water below the evapotranspiration requirements, is a practical irrigation strategy to enhance water productivity. Using DI strategy, the crop is exposed to a mild drought stress level either during the whole growing period or during a particular growth stage [93]. Studies have reported that DI strategies present significant opportunities for conserving water without sacrificing crop yield, or with only a minor reduction in yield, while also improving the quality of the produce [94,95,96,97]. Using aeroponic farming systems, irrigation durations and frequencies could be manipulated to simulate DI [24,25]. In our previous study on common ice plants (M. crystallinum) grown aeroponically indoors under LED lighting, different degrees of drought stress were induced with different nutrient spraying intervals of 5, 30, 60, and 240 min. The long spraying interval of 240 min resulted in a reduction in shoot and root biomass accumulation. However, drought stress induced by longer nutrient spraying intervals (simulating DI) enhanced the nutritional quality, leading to the higher accumulation of total ascorbic acid, total phenolic compounds, total soluble sugar, and proline of common ice plants [24]. In a study with hot peppers, (Capsicum annuum cv. Battle) under different DI treatments, including 85%, 70%, and 55% of water-holding capacity, it was found that 85% of water-holding capacity resulted in the high accumulation of vitamin C production and saved a large amount of water compared 100% of water-holding capacity (control) [98]. Increased total phenolic compounds and proline of Achillea species were reported after the moderate drought stress treatment (50% field capacity) [99]. In a study with two green leafy lettuce cultivars (Lollo Bionda and Vera), it was found that cultivar Vera could produce desirable fresh mass with improved nutritional quality, increasing dietary phytochemicals, ascorbic acid, antioxidant activity, glucose, fructose, and reducing browning under higher DI levels [100]. In our recent study with aeroponically grown Tuscan kale (B. oleracea L.) in a tropical greenhouse, DI was induced through prolonging nutrient spraying interval (NSI) one week prior to harvest after growing under 5 minNSI for four weeks [25]. There was no significant decrease in shoot FW in plants transferred from 5 min to 60 minNSI (5 minNSI→60 minNSI), while the shoot FW of plants transferred from 5 min to 90 minNSI (5 minNSI→90 minNSI), was significantly lower compared to those grown under 5 minNSI (Figure 4B). These results were supported by the size of Tuscan kale plants shown in Figure 4A. Can nutritional quality be improved in aeroponically grown Tuscan kale under DI prior to harvesting without a substantial yield penal? The results of 5 minNSI → 60 minNSI plants were promising, as the concentrations of their proline and total soluble sugars increased (Figure 4C,D) after NSI transfer for one week without a substantial yield penalty (Figure 4B). However, no increase in total ascorbic acid was observed from the same plants (Figure 4E), which could be due to a reduction in photosynthetic capacity under DI. Plants under severe DI treatment such as 5 minNSI → 90 minNSI had a much lower total ascorbic acid concentration than that of 5 minNSI plants, resulting from their much lower photosynthetic capacity [25]. This is because the synthesis of ascorbic acid is highly dependent on the newly fixed carbon in the leaves [101]. Induced DI prior to harvest did not increase the total phenolic compounds (Figure 4F), which may also be due to the shortage of newly fixed carbon [102]. However, it was reported that pre-harvest DI enhanced ascorbic acid and sugars in sugar apples prior to harvest at harvest and during storage in sugar apples [103]. The optimization of deficit irrigation to increase water production with enhanced nutritional values of leafy vegetables at pre-harvest can yield significant advantages for both consumers and growers.
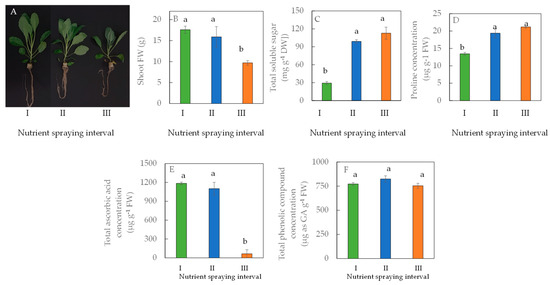
Figure 4.
Tuscan kale grown under different NSI at harvest (A). Proline (B), total soluble sugar (C), total ascorbic acid (D) and total phenolic compounds (E) concentrations of Tuscan kale after transferring the four-week-old 5 minNSI plants to different NSIs (F) concentrations of Tuscan kale grown at different NSIs. I, 5 minNSI, II, 5 minNSI, four weeks→60 minNSI, 1 Week; III, 5 minNSI, 4 weeks→90 minNSI, 1 week. For (B–F), values are means ± standard errors. Means sharing the same letters are not significantly different from each other (p> 0.05) as determined by Tukey’s multiple comparison test (Modified from He et al. [25]).
5. Impacts of LED Spectral Quality, Intensity, and Photoperiod on Productivity and Nutritional Quality of Leafy Vegetables Grown Indoors and in the Tropical Greenhouse
To increase Singapore’s food security and boost the efficient use of farmland, Singapore’s government tendered out some new plots of farmland on 20-year leases from 2017 to 2019. Priority was given to the high-tech farmers. Vertical farming enables year-round production in Singapore’s high-tech farms while using less land to produce more in CEA [104]. Light is a key factor for the success of vertical vegetable cultivation. To my best knowledge, we are the first team to elucidate the effect of LED lighting on vegetable crops in Singapore. Providing sufficient and effective lighting to plants not only allows fast growth but also minimizes energy utilization [105,106,107]. A commercially viable LED integrated farming systems has been established by my team to grow leafy vegetables both indoors [29,30,108,109,110,111] and in a tropical greenhouse [112,113,114].
In the indoor cultivation of leafy vegetables, the ideal combination of red (R)- and blue (B)-LEDs, or red (R)- and green (G)-LEDs proves more effective in enhancing productivity compared to using red (R)-LEDs or blue (B)-LEDs alone. This was supported by our studies on Chinese broccoli (B. alboglabra Bailey) [108], common ice plants (Mesembryanthemum crystallinum) [29], and lettuce (L. sativa L. cv. canasta) [30] grown indoors under different combinations of LED spectral qualities. The optimal combination of RB-LED promoting the growth of vegetable crops has also been reported by other researchers in lettuce, [105,115], radish and spinach [105], cucumber [106,116], rapeseed rosette leaves [117], and sweet basil [118]. For certain crops such as Chinese broccoli [108], cucumber [106,116], sweet basil [118], and ice plants [29], the effective LED spectral quality increasing vegetable production is closely linked to the enhanced photosynthetic performance on a leaf area basis. Under optimal combinations of LED lighting, higher photosynthetic performance was achieved by a higher light-saturated photosynthetic CO2 assimilation rate and stomatal conductance and greater concentrations of photosynthetic pigments and soluble and Rubisco proteins compared to plants grown under R-LED or B-LED alone or other ineffective combinations of R- and B-LED [30]. However, for lettuce (L. sativa L. cv. canasta), the effect of LED spectral quality on productivity is not associated with photosynthetic performance on a leaf area basis but is closely linked to leaf traits [30]. For example, when grown under 100% R-LED or 20% green (G)-LED and 80% R-LED, lettuce plants had the lowest maximal photosynthetic rate on a leaf area basis due to the inhibitory effects on maximum quantum efficiency of photosystem II (PS II) with lower photosynthetic pigments, total soluble protein, and total Rubisco protein. However, lettuce plants grown under 100% R-LED or 20% G-LED and 80% B-LED had the largest total leaf area and light interception area but the highest shoot biomass compared to other plants [30]. To sum up the above, shoot productivity is closely related to photosynthetic performance on a leaf area basis when plants have the maximum light interception per unit leaf area. This is because light interception by plant canopy is a main factor determining plant biomass production [119]. On the other hand, light absorption and light interception area play important roles in whole plant photosynthetic capability, which is associated with shoot productivity [30,120,121]. However, the optimal combinations of LED spectral quality on crop production are species-dependent [122]. According to the mega-analysis from 350 articles using ISI Web of Science, Ma et al. [122] found that the impact of LED lighting on plant growth and quality characteristics varied depending on the plant species and cultivation conditions. They noted that existing research on LED lights lacks comprehensive information for horticultural production. They then recommended that future studies incorporate secondary and respiratory metabolism, which are closely linked to crop quality, to provide more valuable insights.
Indoor farming requires a temperature- and light-controlled environment. To achieve the “30 by 30” goal in Singapore using a “grow more with less” strategy, it is not possible to totally rely on high energy-cost indoor vertical farming systems. Thus, LED-integrated vertical aeroponic farming systems [112,114] and supplemental LED lighting in addition to natural sunlight for greenhouse vegetable production [37,38,113] are alternative approaches to reduce energy consumption. Our study showed that both LED spectral quality and light intensity are important for enhancing the shoot productivity of three subtropical vegetables grown with a vertical aeroponic farming system in a tropical greenhouse, namely Kai Lan (B. alboglabra), Nai Bai (B. chinensis L.), and mizuna (B. juncea var. japonica) [114]. In a study of six different vegetable crops, Nájera and Urrestarazu also found that both LED spectral quality and intensity influenced productivity [123]. In another study on heat-resistant (HR) and heat-sensitive (HS) recombinant inbred lines (RILs) of lettuce (L. sativa) grown with vertical aeroponic farming systems, we found that both lettuce RILs had higher shoot and root productivity when they were grown under longer photoperiods [112]. However, higher shoot and root productions were not associated with higher photosynthetic CO2 assimilation rate on a leaf area basis. Instead, both lettuce RILs exposed to longer photoperiods promoted the development of additional and larger sinks supported by increased leaf numbers and total leaf area [112]. The whole-plant carbon gain depends on the total photosynthetic area. An increase in the total leaf area enhanced the carbon gain at the whole-plant level resulting from high light interception and absorption [30,122,124]. For example, the total CO2 assimilation of both lettuce RILs per plant under 18 h was more than twofold compared to those grown under 12 h, regardless of their similar photosynthetic rates on a leaf basis [112]. Furthermore, the intercepted light energy must be transduced into the biomass area [124]. Due to the increased leaf number and total leaf area, there were decreases in the soluble and insoluble sugar concentrations in lettuce leaves exposed to longer photoperiods, implying no feedback inhibition of photoassimilates [112]. When photoassimilates could be effectively transported out from the source leaves during the photoperiod, most of the new fixed carbon could be traduced into biomass [125]. These findings indicate that extending the photoperiod at a consistent moderate level of PPFD, such as 300 µmol photon m−2 s−1, has the potential to enhance the production of lettuce plants [112].
Light, temperature, water, and nutrients are the main factors that determine crop productivity [126]. In greenhouses, it is not difficult to control the temperature, water, and nutrients. As mentioned earlier, cooling the RZ allows the production of subtropical and temperate vegetable crops in Singapore all year round [3,16,17,18]. However, greenhouse crops have experienced frequent cloudy or rainy days in Singapore. The intensity of natural sunlight (SL) is low in greenhouses, which affects plant growth and productivity, even for a horizontal single-layer growing system. It is not easy to control the growth irradiation [126,127]. There are numbers of papers that demonstrated that supplemental LED to natural SL could efficiently compensate for a shortage of light for photosynthesis and plant growth [37,38,113,128,129]. In a study on HR and HS lettuce RILs grown under natural SL supplemented with different combinations of red (R)- and blue B-LED ratios: 100R:0B (0B), 92R:8B (8B), 84R:16B (16B), and 76R:24B (24B) with an equal PPFD of 100 µmol m−2 s−1, it was found that 16B supplementary light treatment is optimal for HR-RIL, while HS-RIL had higher shoot FW under the 8B condition. The different responses of HR and HS lettuce RIL could be due to their different susceptibilities to heat stress in a tropical greenhouse [113]. Apart from light spectral quality, light intensity highly influences plant physiological processes, plant growth, and plant development [129]. In a tropical greenhouse, we studied the effects of two different intensities of supplementary LED (PPFDs of 150 and 300 µmol m−2 s−1) and natural SL on sweet potato (Ipomoea batatas L.) leaves, which are consumed as fresh tropical vegetables [38], as well as a temperate Cos lettuce (L. sativa L. cv. CL—2741) [37]. Sweet potato leaves grown under natural SL with supplemental LED lighting had higher leaf FW and higher photosynthetic pigment concentrations compared to those grown under natural SL. The enhanced leaf biomass was due to the increased thickness of leaves, which improved their photosynthetic capacity under their growth temperature and irradiances. However, there were no significant differences in these parameters between leaves exposed to natural-SL-supplemented LED lighting under 150 and 300 µmol m−2 s−1) [38]. With Cos lettuce grown in the tropical greenhouse under natural sunlight, it was found that total leaf area, shoot FW, photosynthetic rate, and the concentrations of photosynthetic pigments, cytochrome bf, leaf total soluble protein, and Rubisco protein were higher in plants supplemented with LED lighting of 300 µmol m−2 s−1 than with LED lighting of 150 µmol m−2 s−1 [37]. The different responses of sweet potato leaves and temperate Cos lettuce to supplementary LED intensity are mainly due to different levels of sunlight during different months of the year. In the study on sweet potato leaves [38], the natural SL inside the greenhouse was much higher (average maximum PPFD ~ 800 μmol m−2 s−1) than in the study with Cos lettuce study (average PPFD ~500 μmol m−2 s−1). The daily light integral (DLI, mol m−2 day−1), which describes the number of photosynthetically active photons that are delivered to a specific area over a 24-h period, has been shown to better relate many plant growth traits and physical performances than instantaneous PPFD levels at any given time [130]. To save energy, the optimization of supplemental LED lighting intensity must take the DLI inside the greenhouse into consideration.
Under LED lighting, adjustments can be made to control both light intensity and photoperiod in order to optimize DLI, thereby enhancing both crop productivity and nutritional quality. In the meta-analyses, Poorter et al. [130] reported that 70 traits, including morphology, anatomy, physiology, and growth, are better related to DLI than to instantaneous PPFD levels at any given time. In a study with sweet basil (Ocimum basilicum), it was reported that shoot FW, photosynthesis, soluble sugar, and phenolic contents were higher in plants grown under higher DLIs of 12.9, 16.5, or 17.8 mol m−2 day−1 than under lower DLI of 9.3 mol m−2 day−1 [131]. Similar results of biomass accumulation with increasing DLI were also reported in lettuce (L. sativa L. cv. Ziwei) [132], “Red Russian” kale (B. napus pabularia), “All Star” lettuce mix (L. sativa) and spinach (Spinacia oleracea) [133], and cucumber (Cucumis sativus L.) [134]. There are two different ways to achieve the same DLI: a low PPFD with a long photoperiod or a short photoperiod with a high PPFD. Recently, our team studied the effects of light intensity and duration, including 24 h of continuous lighting (CL) and different DLIs on C4 halophyte purslane (Portulaca oleracea L.) grown indoors. Compared to purslane plants grown under lower PPFD, shorter photoperiods, and thus lower DLI (240 μmol photon m−2 s−1, 12 h, DLI = 10.368 mol m−2 day−1), higher DLIs (20.736 mol m−2 day−1) via different combinations of PPFD and photoperiods promoted root and shoot growth and thus increased shoot productivity. Higher DLIs also enhanced nutritional quality, resulting in higher accumulations of proline and total phenolic compounds as well as dietary minerals such as K, Ca, Mg, and Fe [135]. However, under the same higher DLI (20.736 mol m−2 day−1), different combinations of specific PPFD and photoperiod could have different effects on plant growth and the nutritional quality [136]. Overall, growing purslane under a PPFD of 320 µmol m−2 s−1 with 18 h photoperiod (DLI = 20.736 mol m−2 day−1) is the most suitable strategy for enhancing the productivity and nutritional quality of purslane [135]. However, purslane grown under the CL condition with the same DLI (240 μmol photon m−2 s−1, 24 h photoperiod) had lower shoot and root biomass, which resulted from the lower maximal quantum efficiency of PS II (Fv/Fm ratios < 0.8) and lower electron transport rate, indicating photoinhibition and low light use efficiency [137]. Both the photoperiod and light intensity supply to plants are directly related to the electricity cost. Hence, more research needs to be carried out on DLI strategies via different combinations of PPFD and photoperiod by studying their impacts on growth, yield, and nutritional quality [138].
To sum up the work done by our team over the past 10 years, Table 1 summarises the impacts of LED spectral quality, intensity and photoperiod on productivity, physiology and nutritional quality of different vegetable crops grown in doors and in the tropical greenhouse.

Table 1.
Impacts of LED spectral quality, intensity and photoperiod on leafy vegetables grown indoors and in the tropical greenhouse in Singapore.
6. Conclusions
The article presented a review on how to cultivate all kinds of leaf vegetable crops all year round using innovative indoor and greenhouse growing systems in a land-limited and a water-scarce country such as Singapore. First, through root-zone cooling while the aerial parts were maintained at fluctuating hot ambient temperatures in a tropical greenhouse, subtropical and temperate vegetable crops were successfully grown aeroponically in Singapore. Cooling only the RZ promotes root growth and adjusts their morphology. With well-developed root systems, vegetable crops enhance water and mineral uptake, thus preventing water deficit and mineral nutrient deficiency. Subtropical and temperate vegetable crops grown under cool-RZT also enhanced photosynthetic efficiency, partitioned newly fixed carbon to the additional sinks, and thus increased final yield compared to the whole plants grown under hot ambient temperature. Our studies also indicate that RZ heat priming is a viable approach to augment both the productivity and nutritional value of temperate leafy greens, all while reducing production costs in the tropical greenhouse. However, the positive effects of RZ heat priming depend on species and the duration of subsequent high RZT. Apart from a lack of land, Singapore is also one of the most water-stressed countries in the world. Deficit irrigation could be easily simulated using aeroponic farming systems as irrigation durations, and frequencies could be regulated. Deficit irrigation, which was induced by prolonging the nutrient spraying intervals at pre-harvest, enhanced the nutritional values of Tuscan kale without a yield penalty. Our findings suggest that the optimization of deficit irrigation to enhance nutritional values without a substantial yield penalty of leafy vegetables can bring benefits to both consumers and growers. To increase land productivity, LED integrated vertical farming systems have been established to grow different kinds of leafy vegetables indoors or in the greenhouse. However, the responses of leafy vegetables to LED spectral quality, intensity, duration, and DIL depend on species and other environmental factors. To reduce energy costs, supplementing natural sunlight with LED lighting for greenhouse vegetable production is an alternative strategy. However, the prevailing DLI inside the greenhouse should be considered in order to optimize the intensity of supplemental LED lighting and thus save power energy.
Funding
This is a review article. The data presented are re-organized from the author’s own published works. The projects were funded by the teaching materials’ vote of National Institute of Education, Nanyang Technological University, Singapore; Meod Pte. Ltd., (MEOD 2/20 HJ), Singapore; the Ministry of Education, Singapore, under its Academic Research Fund Tier 1 (2018-T1-001-008) and the Singapore Millennium Foundation (SMF-Farming System), Singapore.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Diehl, J.A.; Sweeney, E.; Wong, E.; Sia, C.S.; Yao, H.; Prabhudesai, M. Feeding cities: Singapore’s approach to land use planning for urban agriculture. Glob. Food Secur. 2020, 26, 100377. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- He, J. Impact of root-zone temperature on photosynthetic efficiency of aeroponically grown temperate and subtropical vegetable crops in the tropics. In Theory and Applications in Energy, Biotechnology and Nanotechnology; Buchner, T.B., Ewingen, N.H., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2009; pp. 111–144. [Google Scholar]
- Ludher, E.K. Singapore’s smart governance of food. In The Governance of City Food Systems: Case Studies from Around the World; Deakin, M.M., Davide, D., Nunzia, B., Eds.; Fondazione Giangiacomo Feltrinelli: Milan, Italy, 2016; pp. 131–154. [Google Scholar]
- Carotti, L.; Graamans, L.; Puksic, F.; Butturini, M.; Meinen, E.; Heuvelink, E.; Stanghellini, C. Plant factories are heating up: Hunting for the best combination of light intensity, air temperature and root-zone temperature in lettuce production. Front. Plant Sci. 2021, 11, 592171. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Klerkx, L.; Rose, D. Dealing with the game-changing technologies of Agriculture 4.0: How do we manage diversity and responsibility in food system transition pathways? Glob. Food Secur. 2020, 24, 100347. [Google Scholar] [CrossRef]
- Ritzel, C.; Ammann, J.; Mack, G.; El Benni, N. Determinants of the decision to build up excessive food stocks in the COVID-19 crisis. Appetite 2022, 176, 106089. [Google Scholar] [CrossRef]
- Tortajada, C.; Lim, N.S.W. Food Security and COVID-19: Impacts and resilience in Singapore. Front. Sustain. Food Syst. 2021, 5, 740780. [Google Scholar] [CrossRef]
- Singapore Parliament. Impact of COVID-19 Restrictions on Singapore’s Economy and Robustness of National Stockpile of Essential Items. 2020. Available online: https://sprs.parl.gov.sg/search/sprs3topic?reportid=oral-answer-2191 (accessed on 23 October 2023).
- Teng, P. Assuring food security in Singapore, a small island state facing COVID-19. Food Sec. 2020, 12, 801–804. [Google Scholar] [CrossRef]
- Singapore Food Agency. 30 by 30—Our Food Future. 2019. Available online: https://www.ourfoodfuture.gov.sg/30by30/ (accessed on 23 October 2023).
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant responses to heat stress: Physiology, transcription, noncoding RNAs, and epigenetics. Int. J. Mol. Sci. 2020, 22, 117. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.T. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef]
- He, J.; Lee, S.K. Growth and photosynthetic characteristics of lettuce (Lactuca sativa L.) grown under fluctuating hot ambient temperatures with the manipulation of cool rootzone temperature. J. Plant Physiol. 1998, 152, 387–391. [Google Scholar]
- He, J.; Lee, S.K.; Dodd, I.C. Limitations to photosynthesis of lettuce grown under tropical conditions: Alleviation by root-zone cooling. J. Exp. Bot. 2001, 52, 1323–1330. [Google Scholar] [CrossRef]
- He, J.; Lee, S.K. Relationship among photosynthesis, ribulose-1,5-bisphosphate carboxylase (Rubisco) and water relations of subtropical vegetable Chinese broccoli grown in the tropics by manipulation of root-zone temperature. Environ. Exp. Bot. 2001, 46, 119–128. [Google Scholar] [CrossRef]
- He, J.; Lai, C.-H.; Lim, Y.J.; Qin, L. Heat priming impacts on root morphology, productivity and photosynthesis of temperate vegetable crops grown in the tropics. J. Adv. Agric. Technol. (JOAAT) 2019, 6, 14–19. [Google Scholar] [CrossRef]
- He, J.; Tan, C.; Qin, L. Root-zone heat priming effects on maximum quantum efficiency of PSII, productivity, root Morphology and nutritional quality of two aeroponically grown leafy greens in a tropical greenhouse. Plants 2022, 11, 1684. [Google Scholar] [CrossRef]
- Alrajhi, A.A.; Alsahli, A.S.; Alhelal, I.M.; Rihan, H.Z.; Fuller, M.P.; Alsadon, A.A.; Ibrahim, A.A. The effect of LED light spectra on the growth, yield and nutritional value of red and green lettuce (Lactuca sativa). Plants 2023, 12, 463. [Google Scholar] [CrossRef]
- Tang, Y.; Mao, R.; Guo, S. Effects of LED spectra on growth, gas exchange, antioxidant activity and nutritional quality of vegetable species. Life Sci. Space Res. 2020, 26, 77–84. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X.; Li, J.; Zhang, Y.; Yang, Y.; Zheng, W.; Xue, X. Effects of light-emitting diode spectral combinations on growth and quality of pea sprouts under long photoperiod. Front. Plant Sci. 2022, 13, 978462. [Google Scholar] [CrossRef]
- He, J.; Chua, E.L.; Qin, L. Drought does not induce crassulacean acid metabolism (CAM) but regulates photosynthesis and enhances nutritional quality of Mesembryanthemum crystallinum. PLoS ONE 2020, 15, e0229897. [Google Scholar] [CrossRef]
- He, J.; Chang, C.; Qin, L.; Lai, C.H. Impacts of deficit irrigation on photosynthetic performance, productivity and nutritional quality of aeroponically grown Tuscan Kale (Brassica oleracea L.) in a tropical greenhouse. Int. J. Mol. Sci. 2023, 24, 2014. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, H.; Mozafari, V.; Roosta, H.R. Optimizing growth conditions in vertical farming: Enhancing lettuce and basil cultivation through the application of the Taguchi method. Sci. Rep. 2023, 13, 6717. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.G.; Mickens, M.A.; Aronne, G.; Gómez, C. Spectral effects of blue and red light on growth, anatomy, and physiology of lettuce. Physiol. Plant. 2021, 172, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the lights for leafy greens in indoor vertical farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Chong, E.L.C.; Choong, T.W.; Lee, S.K. Plant growth and photosynthetic characteristics of Mesembryanthemum crystallinum grown aeroponically under different blue- and red-LEDs. Front. Plant Sci. 2017, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qin, L.; Chow, W.S. Impacts of LED spectral quality on leafy vegetables: Productivity closely linked to photosynthetic performance or associated with leaf traits? Int. J. Agric. Biol. Eng. 2019, 12, 16–25. [Google Scholar] [CrossRef]
- Niu, Y.; Lyu, H.; Zhang, L.M.; Li, H. Photosynthesis prediction and light spectra optimization of greenhouse tomato based on response of red–blue ratio. Sci. Hortic. 2023, 318, 112065. [Google Scholar] [CrossRef]
- Rabara, R.C.; Behrman, G.; Timbol, T.; Rushton, P.J. Effect of spectral quality of monochromatic LED lights on the growth of artichoke seedlings. Front. Plant Sci. 2017, 8, 190. [Google Scholar] [CrossRef]
- Cavallaro, V.; Muleo, R. The effects of LED light spectra and intensities on plant growth. Plants 2022, 11, 1911. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Aljafer, N.; Jbara, M.; McCallum, L.; Lengger, S.; Fuller, M.P. The Impact of LED lighting spectra in a plant factory on the growth, physiological traits and essential oil content of lemon balm (Melissa officinalis). Plants 2022, 11, 342. [Google Scholar] [CrossRef]
- He, J.; Qin, L. Microclimate control to increase productivity and nutritional quality of leafy vegetables in a cost-effective manner. Int. J. Agric. Biol. Eng. 2022, 15, 55–61. [Google Scholar] [CrossRef]
- Van Gerrewey, T.; Boon, N.; Geelen, D. Vertical Farming: The only way is up? Agronomy 2022, 12, 2. [Google Scholar] [CrossRef]
- He, J.; Bte Jawahir, N.K.; Qin, L. Quantity of supplementary LED lightings regulates photosynthetic apparatus, improves photosynthetic capacity and enhances productivity of Cos lettuce grown in a tropical greenhouse. Photosyn. Res. 2021, 149, 187–199. [Google Scholar] [CrossRef]
- He, J.; Qin, L. Growth and photosynthetic characteristics of sweet potato (Ipomoea batatas) leaves grown under natural sunlight with supplemental LED lighting in a tropical greenhouse. J. Plant Physiol. 2020, 252, 153–239. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Chaudhry, U.K.; Raza, A.; Charagh, S.; Bakhsh, A.; Bohra, A.; Ali, S.; Chitikineni, A.; Saeed, Y.; Visser, R.G.F.; et al. Developing future heat-resilient vegetable crops. Funct. Integr. Genom. 2023, 23, 47. [Google Scholar] [CrossRef]
- Dumitru, E.A.; Sterie, C.M.; Rodino, S.; Butu, M. Consumer preferences in the purchase of agri-food products: Implications for the development of family farms. Agriculture 2023, 13, 1478. [Google Scholar] [CrossRef]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality—A review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Wien, H.C. The Physiology of Vegetable Crops; Cab International: Oxon, NY, USA, 1997. [Google Scholar]
- Dumitru, E.A.; Berevoianu, R.L.; Tudor, V.C.; Teodorescu, F.-R.; Stoica, D.; Giucă, A.; Ilie, D.; Sterie, C.M. Climate change impacts on vegetable crops: A systematic review. Agriculture 2023, 13, 1891. [Google Scholar] [CrossRef]
- Porter, J.R.; Challinor, A.J.; Henriksen, C.B.; Howden, S.M.; Martre, P.; Smith, P. Invited review: Intergovernmental panel on climate change, agriculture, and food—A case of shifting cultivation and history. Global Chang. Biol. 2019, 25, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Scheelbeek, P.F.D.; Birda, F.A.; Tuomisto, H.L.; Green, R.; Harris, F.B.; Joy, E.J.M.; Chalabi, Z.; Allen, E.; Haines, A.; Dangour, A.D. Effect of environmental changes on vegetable and legume yields and nutritional quality. Proc. Nat. Acad. Sci. USA 2018, 115, 6804–6809. [Google Scholar] [CrossRef]
- Mylonas, I.; Stavrakoudis, D.; Katsantonis, D.; Korpetis, E. Chapter 1—Better farming practices to combat climate change. In Climate Change and Food Security with Emphasis on Wheat; Ozturk, M., Gul, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–29. [Google Scholar]
- Kozai, T. Plant factory in Japan—Current situation and perspectives. Chron. Hortic. 2013, 53, 8–11. [Google Scholar]
- Wang, R.; Isozaki, M.; Iwasaki, Y.; Muramatsu, Y. Root-zone temperature effects on spinach biomass production using a nutrient film technique system. HortSci. 2022, 57, 532–540. [Google Scholar] [CrossRef]
- Yamori, N.; Levine, C.P.; Mattson, N.S.; Yamori, W. Optimum root zone temperature of photosynthesis and plant growth depends on air temperature in lettuce plants. Plant Mol. Biol. 2022, 110, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, B.; McCormack, M.L.; Ma, Z.; Guo, D. Diverse belowground resource strategies underlie plant species coexistence and spatial distribution in three grasslands along a precipitation gradient. New Phytol. 2017, 216, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tian, Q.; Michelsen, A.; Lu, M.; Huang, L.; Zhao, R. The effect of experimental warming on fine root functional traits of woody plants: Data synthesis. Sci. Total Environ. 2023, 894, 165003. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.C.; Tachibana, S. Effect of supraoptimal root temperature on the growth, root respiration and sugar content of cucumber plants. Sci. Hortic. 1994, 58, 289–301. [Google Scholar] [CrossRef]
- He, J. Mineral nutrition of aeroponically grown subtropical and temperate crops in the tropics with manipulation of root-zone temperature at different growth irradiances. Plant Stress 2010, 4, 14–30. [Google Scholar]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Stokes, A. Root traits as drivers of plant and ecosystem functioning: Current understanding, pifalls and future research needs. New Phytol. 2021, 232, 1123–1158. [Google Scholar] [CrossRef]
- Luo, H.; Xu, H.; Chu, C.; He, F.; Fang, S. High temperature can change root system architecture and intensify root interactions of plant seedlings. Front. Plant Sci. 2020, 11, 160. [Google Scholar]
- Monje, O.; Anderson, S.; Stutte, G.W. The effects of elevated root zone temperature on the development and carbon partitioning of spring wheat. J. Amer. Soc. Hort. Sci. 2007, 132, 178–184. [Google Scholar] [CrossRef]
- Aldous, D.E.; Kaufmann, J.E. Role of root temperature on shoot growth of two Kentucky bluegrass cultivars. Agron. J. 1979, 71, 545–547. [Google Scholar] [CrossRef]
- Kuroyanagi, T.; Paulsen, G.M. Mediation of high-temperature injury by roots and shoots during reproductive growth of wheat. Plant Cell Environ. 1998, 11, 517–523. [Google Scholar] [CrossRef]
- Paulsen, G.M. High temperature responses of crop plants. In Physiology and Determination of Crop Yield; Boote, K.J., Bennett, J.M., Sinclair, T.R., Paulsen, G.M., Eds.; American Society of Agronomy, Crop Science Society of America and Soil Science Society of America: Madison, WI, USA, 1994; pp. 365–389. [Google Scholar]
- Sakamoto, M.; Suzuki, T. Effect of rootzone temperature on growth and quality of hydroponically grown red leaf lettuce (Lactuca sativa L. cv. Red Wave). Am. J. Plant Sci. 2015, 6, 2350–2360. [Google Scholar] [CrossRef]
- Levine, C.P.; Hayashi, S.; Ohmori, Y.; Kusano, M.; Kobayashi, M.; Nishizawa, T.; Kurimoto, I.; Kawabata, S.; Yamori, W. Controlling root zone temperature improves plant growth and pigments in hydroponic lettuce. Ann. Bot. 2023, 132, 455–469. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chua, N.Y.A.; Qin, L. Interaction between iron stress and root-zone temperature on physiological aspects of aeroponically grown Chinese broccoli (Brassica alboglabra). J. Plant Nutri. 2008, 31, 1–20. [Google Scholar]
- Tan, L.P.; He, J.; Lee, S.K. Effects of root-zone temperature on the root development and nutrient uptake of Lactuca sativa L. cv ‘Panama’ grown in an aeroponic system in the tropics. J. Plant Nutr. 2002, 25, 297–314. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Li, S.; Zhang, W.; Yin, C.; Lin, Y. Regulation of phytohormones on the growth and development of plant root hair. Front. Plant Sci. 2022, 13, 865302. [Google Scholar] [CrossRef] [PubMed]
- Nada, K.; He, L.; Tachibana, S. Impaired photosynthesis in cucumber (Cucumis sativus L.) by high root-zone temperature involves ABA-induced stomatal closure and reduction in ribulose-1,5-biphosphate carboxylase/oxygenase activity. J. Jpn. Soc. Hortic. Sci. 2003, 72, 504–510. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Qiao, Y.X.; Zhang, Y.L.; Zhou, Y.H.; Yu, J.Q. Effects of root temperature on leaf gas exchange and xylem sap abscisic acid concentrations in six Cucurbitaceae species. Photosynthetica 2008, 46, 356–362. [Google Scholar] [CrossRef]
- Qin, L.; He, J.; Lee, K.; Dodd, I.C. An assessment of ethylene mediation of lettuce (Lactuca sativa) root growth at high temperatures. J. Exp. Bot. 2007, 58, 3017–3024. [Google Scholar] [CrossRef]
- Choong, T.W.; He, J.; Lee, S.K.; Dodd, I.C. Growing different Lactuca genotypes aeroponically within a tropical greenhouse—Cool rootzone temperatures decreased rootzone ethylene concentrations and increased shoot growth. Front. Physiol. 2016, 7, 405. [Google Scholar] [CrossRef]
- Dodd, I.C.; He, J.; Turnbull, C.G.N.; Lee, S.K.; Critchley, C. Influence of supra-optimal root temperatures on growth and stomatal conductance in Capsicum annuum L. J. Exp. Bot. 2000, 51, 239–248. [Google Scholar] [CrossRef]
- Udagawa, Y.; Ito, T.; Gomi, K. Effects of root temperature on the absorption of water and mineral nutrients by strawberry plants ‘Reiko’ grown hydroponically. J. Jpn. Soc. Hortic. Sci. 1991, 59, 711–717. [Google Scholar] [CrossRef]
- Klock, K.A.; Graves, W.R.; Taber, H.G. Growth and phosphorus, zinc, and manganese content of tomato, muskmelon, and honey locust at high root-zone temperatures. J. Plant Nutr. 1996, 19, 795–806. [Google Scholar] [CrossRef]
- He, J.; Tan, L.P.; Lee, S.K. Root-zone temperature effects on photosynthesis, 14C-photoassimilate partitioning and growth of temperate lettuce (Lactuca sativa cv. Panama) grown in the tropics. Photosynthetica 2009, 47, 95–103. [Google Scholar] [CrossRef]
- Ruter, J.M.; Ingram, D.L. High root-zone temperatures influence rubisco activity and pigment accumulation in leaves of ‘Rotundifolia’ holly. J. Am. Soc. Hortic. Sci. 1992, 117, 154–157. [Google Scholar] [CrossRef]
- Lämke, J.; Brzezinka, K.; Altmann, S.; Bäurle, I. A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J. 2016, 35, 162–175. [Google Scholar] [CrossRef]
- Andriy, B.; Ilnytskyy, Y.; Wóycicki, R.; Kepeshchuk, N.; Fogen, D.; Kovalchuk, I. The elucidation of stress memory inheritance in Brassica rapa plants. Front. Plant Sci. 2015, 6, 5. [Google Scholar]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Schmülling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kumar, P.; Verma, V.; Sharma, R.; Bhargava, B.; Irfan, M. Understanding plant stress memory response for abiotic stress resilience: Molecular insights and prospects. Plant Physiol. Biochem. 2022, 179, 10–24. [Google Scholar] [CrossRef]
- Yeh, C.H.; Kaplinsky, N.J.; Hu, C.; Charng, Y.Y. Some like it hot, some like it warm: Phenotyping to explore thermotolerance diversity. Plant Sci. Int. J. Exp. Plant Biol. 2012, 195, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, L.; Rusalepp, L.; Kaurilind, E.; Sulaiman, H.Y.; Püssa, T.; Niinemets, Ü. Heat priming improved heat tolerance of photosynthesis, enhanced terpenoid and benzenoid emission and phenolics accumulation in Achillea millefolium. Plant Cell Environ. 2021, 44, 2365–2385. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; McLoughlin, F.; Eman Basha, E.; Vierling, E. Assessing plant tolerance to acute heat stress. Bio-Protocol 2017, 7, e2405. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, M.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. Camb. Phil. Soc. 2016, 91, 1118–1133. [Google Scholar] [CrossRef]
- Sulaiman, H.Y.; Liu, B.; Abiola, Y.O.; Kaurilind, E.; Niinemets, Ü. Impact of heat priming on heat shock responses in Origanum vulgare: Enhanced foliage photosynthetic tolerance and biphasic emissions of volatiles. Plant Physiol. Biochem. 2023, 196, 567–579. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sita, K.; Sehgal, A.; Bhandari, K.; Kumar, S.; Prasad, P.V.V.; Jha, U.; Kumar, J.; Siddique, K.H.M.; Nayyar, H. Heat priming of lentil (Lens culinaris Medik.) seeds and foliar treatment with γ-aminobutyric acid (GABA), confers protection to reproductive function and yield traits under high-temperature stress environments. Int. J. Mol. Sci. 2021, 22, 5825. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lee, S.K. Photosynthetic utilization of radiant energy by temperate lettuce grown under natural tropical condition with manipulation of root-zone temperature. Photosynthetica 2004, 42, 457–463. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of reactive oxygen species and hormones in plant responses to temperature changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Nguyen, D.T.P.; Lu, N.; Kagawa, N.; Takagaki, M. Optimization of photosynthetic photon flux density and root-zone temperature for enhancing secondary metabolite accumulation and production of coriander in plant factory. Agronomy 2019, 9, 224. [Google Scholar] [CrossRef]
- Agovino, M.; Casaccia, M.; Ciommi, M.; Ferrara, M.; Marchesano, K. Agriculture, climate change and sustainability: The case of EU-28. Ecol. Indic. 2019, 105, 525–543. [Google Scholar] [CrossRef]
- Carotti, L.; Pistillo, A.; Zauli, I.; Meneghello, D.; Martin, M.; Pennisi, G.; Gianquinto, G.; Orsini, F. Improving water use efficiency in vertical farming: Effects of growing systems, far-red radiation and planting density on lettuce cultivation. Agric. Water Manag. 2023, 285, 108365. [Google Scholar] [CrossRef]
- Incrocci, L.; Thompson, R.B.; Fernandez-Fernandez, M.D.; De Pascale, S.; Pardossi, A.; Stanghellini, C.; Rouphael, Y.Y.; Gallardo, M. Irrigation management of European greenhouse vegetable crops. Agric. Water Manag. 2020, 242, 106393. [Google Scholar] [CrossRef]
- Mahmoud, M.M.A.; Fayad, A.M. The effect of deficit irrigation, partial root drying and mulching on tomato yield, and water and energy saving. Irrig. Drain. 2022, 71, 295–309. [Google Scholar] [CrossRef]
- Asres, L.A. Alternative techniques of irrigation water management for improving crop water productivity. Rev. Agric. Sci. 2023, 11, 36–53. [Google Scholar] [CrossRef]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2007, 58, 147–159. [Google Scholar] [CrossRef]
- Yazgan, S.; Ayas, S.; Demirtas, C.; Büyükcangaz, H.; Candogan, B.N. Deficit irrigation effects on lettuce (Lactuca sativa var. Olenka) yield in unheated greenhouse condition. J. Food Agric. Environ. 2008, 6, 357–361. [Google Scholar]
- Geerts, S.; Raes, D. Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Yu, H.; Yang, X.; Jiang, W. Deficit irrigation affects growth, yield, vitamin C content, and irrigation water use efficiency of hot pepper grown in soilless culture. HortScience 2014, 49, 722–728. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.; Saeidi, G.; Goli, S.A. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Malejane, D.N.; Tinyani, P.; Soundy, P.; Sultanbawa, Y.; Sivakumar, D. Deficit irrigation improves phenolic content and antioxidant activity in leafy lettuce varieties. Food Sci. Nutr. 2018, 6, 334–341. [Google Scholar] [CrossRef]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; González, E.M.; Larrainzar, E. Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front. Plant Sci. 2017, 8, 1042. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech. f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Kowitcharoen, L.; Wongs-Aree, C.; Setha, S.; Komkhuntod, R.; Kondo, S.; Srilaong, V. Pre-harvest drought stress treatment improves antioxidant activity and sugar accumulation of sugar apple at harvest and during storage. Agric. Nat. Resour. 2018, 52, 146–154. [Google Scholar] [CrossRef]
- Tatum, M. Inside Singapore’s Huge Bet on Vertical Farming. 2020. Available online: https://www.technologyreview.com/2020/10/13/1009497/singapore-vertical-farming-food-security (accessed on 18 December 2023).
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Weaver, G.; van Iersel, M.W. Photochemical characterization of greenhouse-grown lettuce (Lactuca sativa L. ‘Green Towers’) with applications for supplemental lighting control. HortScience 2019, 54, 317–322. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Liu, Y.; Choong, T.W. Photosynthetic capacities and productivity of indoor hydroponically grown Brassica alboglabra Bailey under different light sources. Am. J. Plant Sci. 2015, 6, 554–563. [Google Scholar] [CrossRef]
- He, J.; Qin, L. Productivity and photosynthetic characteristics of the facultative halophyte Mesembryanthemum crystallinum grown indoors with LED lighting under different salinities. Acta Hortic. 2020, 1296, 219–226. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Koh, J.Q.D. LED spectral quality and NaCl salinity interact to affect growth, photosynthesis and phytochemical production of Mesembryanthemum crystallinum. Funct. Plant Biol. 2022, 49, 483–495. [Google Scholar] [CrossRef]
- He, J.; Leng, S.Y.; Qin, L. Growth, physiology and nutritional quality of C4 halophyte Portulaca oleracea L. grown aeroponically in different percentages of artificial seawater under different light-emitting diode spectral qualities. Plants 2023, 12, 3214. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Kong, S.M.; Choong, T.W.; Qin, L. Productivity and photosynthetic characteristics of heat-resistant and heat-sensitive recombinant inbred lines (RILs) of Lactuca sativa in response to different durations of LED lighting. Acta Hortic. 2016, 1134, 187–194. [Google Scholar] [CrossRef]
- Choong, T.W.; He, J.; Qin, L.; Lee, S.K. Quality of supplementary LED lighting effects on growth and photosynthesis of two different Lactuca recombinant inbred lines (RILs) grown in a tropical greenhouse. Photosynthetica 2018, 54, 1278–1286. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Alahakoon, P.K.D.T.; Chua, B.L.J.; Choong, T.W.; Lee, S.K. LED-integrated vertical aeroponic farming system for vegetable production in Singapore. Acta Hortic. 2018, 1227, 599–606. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, X.; Yang, S.; Ding, X.; Zou, J. Effect of red and blue light on cucumber seedlings grown in a plant factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Shengxin, C.; Chunxia, L.; Xuyang, Y.; Song, C.; Xuelei, J.; Xiaoying, L.; Zhigang, X.; Rongzhan, G. Morphological, photosynthetic, and physiological responses of rapeseed leaf to different combinations of red and blue lights at the rosette stage. Front. Plant Sci. 2016, 7, 1144. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red:blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Bai, Z.; Mao, S.; Han, Y.; Feng, L.; Wang, G.; Yang, B.; Zhi, X.; Fan, Z.; Lei, Y.; Du, W. Study on light interception and biomass production of different cotton cultivars. PLoS ONE 2016, 11, e0156335. [Google Scholar] [CrossRef]
- Koester, R.P.; Skoneczka, J.A.; Cary, T.R.; Diers, B.W.; Ainsworth, E.A. Historical gains in soybean (Glycine max Merr.) seed yield are driven by linear increases in light interception, energy conversion, and partitioning efficiencies. J. Exp. Bot. 2014, 65, 3311–3321. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, A.; Cheng, Z.-M. (Max). Effects of light emitting diode lights on plant growth, development and traits a meta-analysis. Hortic. Plant J. 2021, 7, 552–564. [Google Scholar] [CrossRef]
- Nájera, C.; Urrestarazu, M. Effect of the intensity and spectral quality of LED light on yield and nitrate accumulation in vegetables. HortScience 2019, 54, 1745–1750. [Google Scholar] [CrossRef]
- Keller, B.; Zimmermann, L.; Rascher, U.; Matsubara, S.; Steier, A.; Muller, O. Toward predicting photosynthetic efficiency and biomass gain in crop genotypes over a field season. Plant Physiol. 2022, 188, 301–317. [Google Scholar] [CrossRef]
- Aluko, O.O.; Li, C.; Wang, Q.; Liu, H. Sucrose utilization for improved crop yields: A review article. Int. J. Mol. Sci. 2021, 22, 4704. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Hanan, J.J. Greenhouses: Advanced Technology for Protected Horticulture; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Tewolde, F.T.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Supplemental LED inter-lighting compensates for a shortage of light for plant growth and yield under the lack of sunshine. PLoS ONE 2018, 13, e0206592. [Google Scholar] [CrossRef]
- Brito, C.; Ferreira, H.; Dinis, L.-T.; Trindade, H.; Marques, D.; Correia, C.M.; Moutinho-Pereira, J. Different LED light intensity and quality change perennial ryegrass (Lolium perenne L.) physiological and growth responses and water and energy consumption. Front. Plant Sci. 2023, 14, 1160100. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T.L. A meta-analysisof plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Baumbauer, D.A.; Schmidt, C.B.; Burgess, M.H. Leaf lettuce yield is more sensitive to low daily light integral than kale and spinach. HortScience 2019, 54, 2159–2162. [Google Scholar] [CrossRef]
- Cui, J.; Song, S.; Yu, J.; Liu, H. Effect of daily light integral on cucumber plug seedlings in artificial light plant factory. Horticulturae 2021, 7, 139. [Google Scholar] [CrossRef]
- He, J.; Gan, J.H.S.; Qin, L. Productivity, photosynthetic light-use efficiency, nitrogen metabolism and nutritional quality of C4 halophyte Portulaca oleracea L. grown indoors under different light intensities and durations. Front. Plant Sci. 2023, 14, 106394. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of lettuce growth under an increasing daily light integral depends on the combination of the photosynthetic photon flux density and photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Rosenqvist, E.; van Kooten, O. Chlorophyll fluorescence: A general description and nomenclature. In Practical Applications of Chlorophyll Fluorescence in Plant Biology; Springer: Boston, MA, USA, 2003; pp. 31–77. [Google Scholar]
- Gavhane, K.P.; Hasan, M.; Singh, D.K. Determination of optimal daily light integral (DLI) for indoor cultivation of iceberg lettuce in an indigenous vertical hydroponic system. Sci. Rep. 2023, 13, 10923. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).