A Review on Flower Bulb Micropropagation: Challenges and Opportunities
Abstract
1. Ornamental Geophyte History
2. World Ornamental Plants Sector: Situation of Flower Bulbs in the Sector
3. Methodology
4. Flower Bulbs: Traditional Propagation and Challenges
5. Micropropagation
5.1. In Vitro Regeneration Pathways
5.2. Micropropagation of Flower Bulbs
5.3. The Key Factors Affecting Micropropagation of Flower Bulbs
5.3.1. Explant Choice
5.3.2. Culture Medium
5.3.3. Environmental Conditions
5.4. Stages of the Micropropagation of the Flower Bulbs
5.4.1. Stage 0: Preparation of Mother Stock Plant Material
5.4.2. Stage 1: Establishment of Aseptic Culture
5.4.3. Stage 2: Multiplication
5.4.4. Stage 3: Bulb Growth
5.4.5. Stage 4: Dormancy Breaking
5.4.6. Stage 5: Ex Vitro Acclimatization and Growth
6. Somaclonal Variation
7. New Approaches and Future Perspectives for Flower Bulb Micropropagation
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benschop, M.; Kamenetsky, R.; Le Nard, M.; Okubo, H.; De Hertogh, A. 1 The global flower bulb industry: Production, utilization, research. Hortic. Rev. 2010, 36, 1–115. [Google Scholar]
- Correvon, H.; Massé, H. Les Iris Dans les Jardins; Aux Jardins Correvon: Geneva, Switzerland, 1907; p. 214. [Google Scholar]
- Reynolds, M.; Meachem, W. The Garden Bulbs of Spring; Funk & Wagnalls: Madison, WI, USA, 1967; p. 237. [Google Scholar]
- Doerflinger, F. The Bulb Book; David & Charles: Newton Abbot, UK, 1973; p. 309. [Google Scholar]
- Margaris, N. Flowers in Greek mythology. In Proceedings of the IV International Symposium on New Floricultural Crops 541, Crete, Greece, 22–27 May 1999; pp. 23–29. [Google Scholar]
- Warren, P. The fresco of the garlands from Knossos. Bull. Corresp. Hellénique 1985, 11, 187–208. [Google Scholar] [CrossRef]
- Day, J. Crocuses in context: A diachronic survey of the crocus motif in the Aegean Bronze Age. Hesperia J. Am. Sch. Class. Stud. Athens 2011, 80, 337–379. [Google Scholar]
- Janick, J.; Kamenetsky, R.; Puttaswamy, S.H. Horticulture of the Taj Mahal: Gardens of the imagination. Chron. Hortic. 2010, 50, 30–33. [Google Scholar]
- Bryan, J.E. Bulbs; Timber Press, Inc.: Portland, OR, USA, 1989; p. 451. [Google Scholar]
- De Hertogh, A.; Schepeen, J.; Kamenetsky, R.; Le Nard, M.; Okubo, H. The Globalization of Flower Bulb Industry. In Ornamental Geophytes: From Basic Science to Sustainable Production; Kamenetsky, R., Hiroshi, O., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–16. [Google Scholar]
- Pavord, A. The Tulip: The Story of a Flower That Has Made Men Mad; Bloomsbury Publishing: London, UK, 1999; p. 480. [Google Scholar]
- Marasek-Ciolakowska, A.; Sochacki, D.; Marciniak, P. Breeding Aspects of Selected Ornamental Bulbous Crops. Agronomy 2021, 11, 1709. [Google Scholar] [CrossRef]
- Orlikowska, T.; Podwyszyńska, M.; Marasek-Ciołakowska, A.; Sochacki, D.; Szymański, R. Tulip. In Ornamental Crops; Springer: Cham, Switzerland, 2018; pp. 769–802. [Google Scholar] [CrossRef]
- Saraç, Y.; Balkaya, A.; Deligöz, İ. Lale Islahı. In Süs Bitkileri Islahı (Türler); Kazaz, S., Mendi, Y.Y., Eds.; Gece Publishing: Ankara, Turkey, 2021; pp. 375–414. [Google Scholar]
- Rees, A. The physiology of ornamental bulbous plants. Bot. Rev. 1966, 32, 1–23. [Google Scholar] [CrossRef]
- Bailey, L.H. Manual of Cultivated Plants—Most Commonly Grown in the Continental United States and Canada, 2nd ed.; Macmillan Company: New York, NY, USA, 1949; ISBN 0025055208. [Google Scholar]
- Gabellini, S.; Scaramuzzi, S. Evolving consumption trends, marketing strategies, and governance settings in ornamental horticulture: A grey literature review. Horticulturae 2022, 8, 234. [Google Scholar] [CrossRef]
- Kim, K.-W.; De Hertogh, A. Tissue culture of ornamental flowering bulbs (geophytes). Hortic. Rev. 1997, 18, 87–169. [Google Scholar]
- Pizano, M. Innovation and sustainability in South American floriculture. In Proceedings of the XXXI International Horticultural Congress (IHC2022): International Symposium on Innovations in Ornamentals: From Breeding to Market, Angers, France, 14–20 August 2022; pp. 77–84. [Google Scholar] [CrossRef]
- Sener, B.; Sevim, D. Discovery of bioactive drug candidates from some Turkish medicinal plants-neuroprotective potential of Iris pseudacorus L. Pure Appl. Chem. 2020, 92, 1175–1179. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R. Plant growth regulators for the cultivation and vase life of geophyte flowers and leaves. Agriculture 2023, 13, 855. [Google Scholar] [CrossRef]
- Kocak, M.; Sevindik, B.; Izgu, T.; Tutuncu, M.; Mendi, Y.Y. Synthetic seed production of flower bulbs. In Synthetic Seeds: Germplasm Regeneration, Preservation and Prospects; Springer: Cham, Switzerland, 2019; pp. 283–299. [Google Scholar] [CrossRef]
- Seyidoglu, N.; Zencirkiran, M.; Ayasligil, Y. Position and application areas of geophytes within landscape design. Afr. J. Agric. Res. 2009, 4, 1351–1357. [Google Scholar]
- Ziv, M.; Lilien-Kipnis, H. Bud regeneration from inflorescence explants for rapid propagation of geophytes in vitro. Plant Cell Reports 2000, 19, 845–850. [Google Scholar] [CrossRef]
- WHO. Monographs on Selected Medicinal Plants Volume 1; World Health Organization: Geneva, Switzerland, 1999; 297p, Available online: https://www.who.int/publications/i/item/9241545178 (accessed on 18 February 2024).
- WHO. Monographs on Selected Medicinal Plants Volume 2; World Health Organization: Geneva, Switzerland, 2002; 375p, Available online: https://iris.who.int/bitstream/handle/10665/42052/9241545372.pdf?sequence=2&isAllowed=y (accessed on 18 February 2024).
- WHO. Monographs on Selected Medicinal Plants Volume 3; World Health Organization: Geneva, Switzerland, 2007; 390p, Available online: https://iris.who.int/bitstream/handle/10665/42052/9789241547024_eng.pdf?sequence=3&isAllowed=y (accessed on 18 February 2024).
- WHO. Monographs on Selected Medicinal Plants Volume 4; World Health Organization: Geneva, Switzerland, 2009; 456p, Available online: https://iris.who.int/bitstream/handle/10665/42052/9789241547055_eng.pdf?sequence=4&isAllowed=y (accessed on 18 February 2024).
- WHO. Monographs on Medicinal Plants Commonly Used in the Newly Independent States (NIS); World Health Organization: Geneva, Switzerland, 2010; 441p, Available online: https://www.e-lactancia.org/media/papers/0-Fitoterapia-Plantas-WHO-O5NIS_2010.pdf (accessed on 18 February 2024).
- Das, R.; Barman, A.; Ray, S. Traditional knowledge, phytochemistry, and pharmacological properties of the African genus Scadoxus (Amaryllidaceae). South Afr. J. Bot. 2022, 151, 565–577. [Google Scholar] [CrossRef]
- Pautasso, M. Ten simple rules for writing a literature review. PLoS Comput. Biol. 2013, 9, e1003149. [Google Scholar] [CrossRef]
- Rees, A.R. Ornamental Bulbs, Corms and Tubers; CABI International: Wallingford, UK, 1992; Volume 1, p. 220. [Google Scholar]
- Kamenetsky, R. Biodiversity of geophytes, phytogeography, morphology and survival strategies. In Ornamental Geophytes: From Basic Science to Sustainable Production; Kamenetsky, R., Okubo, H., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 57–76. [Google Scholar]
- Dafni, A.; Cohen, D.; Noy-Mier, I. Life-cycle variation in geophytes. Ann. Mo. Bot. Gard. 1981, 68, 652–660. [Google Scholar] [CrossRef]
- Woodhead, T.W. Ecology of woodland plants in the neighbourhood of Huddersfield. Bot. J. Linn. Soc. 1906, 37, 333–406. [Google Scholar] [CrossRef][Green Version]
- De Klerk, G. Micropropagation of bulbous crops: Technology and present state. Floric. Ornam. Biotechnol. 2012, 6, 1–8. [Google Scholar]
- Leeggangers, H.C.F.; Moreno-Pachon, N.; Gude, H.; Immink, R.H. Transfer of knowledge about flowering and vegetative propagation from model species to bulbous plants. Int. J. Dev. Biol. 2013, 57, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Sochacki, D. Propagation of Ornamental Geophytes:Physiology and Management Systems. In Ornamental Geophytes from Basic Science to Sustainable Production; Kamenetsky, R., Okubo, H., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 261–286. [Google Scholar]
- Ulus, A.; Seyidoğlu, N. Bazi Doğal Geofitlerin Doku Kültürü İle Üretimi. J. Fac. For. Istanb. Univ. 2006, 56, 71–80. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination; Academic Press: London, UK, 1998; p. 666. [Google Scholar]
- Anderson, N. Selection tools for reducing generation time of geophytic herbaceous perennials. In Proceedings of the XIII International Symposium on Flower Bulbs and Herbaceous Perennials 1237, Seoul, Republic of Korea, 1–3 May 2019; pp. 53–66. [Google Scholar] [CrossRef]
- Langens-Gerrits, M.M.; De Klerk, G.-J.M. Micropropagation of flower bulbs: Lily and Narcissus. In Plant Cell Culture Protocols; Humana Press: Totowa, NJ, USA, 1999; pp. 141–147. [Google Scholar] [CrossRef]
- Luna, T. Vegetative propagation. In Nursery Manual for Native Plants: A Guide for Tribal Nurseries; Dumroese, R.K., Luna, T., Landis, T.D., Eds.; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 2009; Volume 1, pp. 153–175. [Google Scholar]
- Podwyszyńska, M.; Orlikowska, T.; Trojak-Goluch, A.; Wojtania, A. Application and Improvement of In Vitro Culture Systems for Commercial Production of Ornamental, Fruit, and Industrial Plants in Poland. Acta Soc. Bot. Pol. 2022, 91, 914. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E. Plant Propagation: Principles and Practice; Soil Science: New York, NY, USA, 1959; p. 662. [Google Scholar]
- Debergh, P.; Maene, L. A scheme for commercial propagation of ornamental plants by tissue culture. Sci. Hortic. 1981, 14, 335–345. [Google Scholar] [CrossRef]
- Debergh, P.C.; Read, P. Micropropagation. In Micropropagation: Technology and Application; Debergh, P.C., Zimmerman, R.H., Eds.; Springer: Cham, Switzerland, 1991; pp. 1–13. [Google Scholar] [CrossRef]
- Ozzambak, M.E.; Senel, U.; Gulden, H.; Zeybekoglu, E.; Baser, S. Mikroçoğaltım teknikleri. In Süs Bitkileri Islahı (Klasik ve Biyoteknolojik Yöntemler); Yalcin Mendi, Y.K.S., Ed.; Gece Publishing: Ankara, Turkey, 2021; Volume 341–360. [Google Scholar]
- Bidabadi, S.S.; Jain, S.M. Cellular, molecular, and physiological aspects of in vitro plant regeneration. Plants 2020, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Yang, Y.; Pan, G.; Shen, Y. New insights into tissue culture plant-regeneration mechanisms. Front. Plant Sci. 2022, 13, 926752. [Google Scholar] [CrossRef]
- Von Arnold, S. Somatic embryogenesis. In Plant Propagation by Tissue Culture, 3rd ed.; George, E.F., Hall, M.A., de Klerk, G.J., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 1, pp. 335–354. [Google Scholar]
- Williams, E.; Maheswaran, G. Somatic embryogenesis: Factors influencing coordinated behaviour of cells as an embryogenic group. Ann. Bot. 1986, 57, 443–462. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X. Regulation of Somatic Embryogenesis in Higher Plants. Crit. Rev. Plant Sci. 2010, 29, 36–57. [Google Scholar] [CrossRef]
- Zimmerman, J.L. Somatic embryogenesis: A model for early development in higher plants. Plant Cell 1993, 5, 1411. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.; Sondahl, M.; Caldas, L.; Maraffa, S. The physiology of in vitro asexual embryogenesis. Hortic. Rev. 1980, 2, 268–310. [Google Scholar] [CrossRef]
- Green, P.B. Organogenesis-a biophysical view. Annu. Rev. Plant Physiol. 1980, 31, 51–82. [Google Scholar] [CrossRef]
- Hicks, G.S. Patterns of organ development in plant tissue culture and the problem of organ determination. Bot. Rev. 1980, 46, 1–23. [Google Scholar] [CrossRef]
- Irish, V.F. The Arabidopsis petal: A model for plant organogenesis. Trends Plant Sci. 2008, 13, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M. Organogenesis in vitro. Curr. Opin. Plant Biol. 1999, 2, 61–64. [Google Scholar] [CrossRef]
- Tutuncu, M.; Basar, S.; Yalcin Mendi, N.Y. Organogenesis. In Süs Bitkileri Islahı (Klasik ve Biyoteknolojik Yöntemler); Yalcin Mendi, Y.K.S., Ed.; Gece Publishing: Ankara, Turkey, 2021; pp. 361–392. [Google Scholar]
- Hnatuszko-Konka, K.; Gerszberg, A.; Weremczuk-Jeżyna, I.; Grzegorczyk-Karolak, I. Cytokinin signaling and de novo shoot organogenesis. Genes 2021, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, O.J.; Beaty, R.M. Organogenesis. In Plant Tissue Culture Concepts and Laboratory Exercises; Routledge: Abingdon, UK, 2018; pp. 125–138. [Google Scholar] [CrossRef]
- Sevindik, B.; Tutuncu, M.; Evci Çürük, P.; Mendi, Y.Y. Somatik embryogenesis. In Süs Bitkileri Islahı (Klasik ve Biyoteknolojik Yöntemler); Yalcin Mendi, Y.K.S., Ed.; Gece Publishing: Ankara, Turkey, 2021; pp. 413–440. [Google Scholar]
- Von Arnold, S.; Sabala, I.; Bozhkov, P.; Dyachok, J.; Filonova, L. Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ. Cult. 2002, 69, 233–249. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Vemmos, S.N. Season and explant origin affect phenolic content, browning of explants, and micropropagation of × Malosorbus florentina (Zucc.) Browicz. HortScience 2013, 48, 102–107. [Google Scholar] [CrossRef]
- Ascough, G.D.; van Staden, J.; Erwin, J.E. In Vitro Storage Organ Formation of Ornamental Geophytes. Hortic. Rev. 2008, 34, 417–445. [Google Scholar]
- Robb, S.M. The Culture of Excised Tissue 3 Lilium speciosum Thun. J. Exp. Bot. 1957, 8, 348–352. [Google Scholar] [CrossRef]
- Ziv, M. The Contribution Of Biotechnology To Breeding, Propagation And Disease Resistance In Geophytes. In Proceedings of the VII International Symposium on Flowerbulbs, Herzliya, Israel, 1 December 1997. [Google Scholar] [CrossRef]
- Hussey, G. The application of Tissue Culture to the Vegetative Propagation of Plants; Science Progress: Norwich, UK, 1978; pp. 185–208. [Google Scholar]
- Mehbub, H.; Akter, A.; Akter, M.A.; Mandal, M.S.H.; Hoque, M.A.; Tuleja, M.; Mehraj, H. Tissue Culture in Ornamentals: Cultivation Factors, Propagation Techniques, and Its Application. Plants 2022, 11, 3208. [Google Scholar] [CrossRef]
- Dhooghe, E.; Reheul, D.; Van Labeke, M.C. Overcoming Pre-Fertilization Barriers in Intertribal Crosses between Anemone coronaria L. and Ranunculus asiaticus L. Horticulturae 2021, 7, 529. [Google Scholar] [CrossRef]
- Sevindik, B.; İzgü, T.; Tütüncü, M.; Çürük, P.; Sarı, N.; Mendi, Y.Y. Double-haploid plant production through anther and ovule culture of wild Cyclamen persicum Mill. and Melody F1 cyclamen cultivar. Vitr. Cell. Dev. Biol. Plant 2023, 59, 711–723. [Google Scholar] [CrossRef]
- Sevindik, B.; İzgü, T.; Tütüncü, M.; Çürük, P.; Söğüt, Z.; Mendİ, Y.Y. Effects of different plant growth regulators on ovule culture of Turkish Cyclamen persicum Mill. and commercial variety "Melody F1". Alatarım 2017, 16, 1–9. [Google Scholar]
- Tütüncü, M.; Mendi, Y.Y. Effect of pollination with gamma irradiated pollen on in vitro regeneration of ovule culture in Cyclamen. Turk. J. Agric. Food Sci. Technol. 2022, 10, 2415–2420. [Google Scholar] [CrossRef]
- Van Creij, M.; Kerckhoffs, D.; De Bruijn, S.; Vreugdenhil, D.; Van Tuyl, J. The effect of medium composition on ovary-slice culture and ovule culture in intraspecific Tulipa gesneriana crosses. Plant Cell Tissue Organ. Cult. 2000, 60, 61–67. [Google Scholar] [CrossRef]
- Van Tuyl, J.; Van Creij, M.; Van Dien, M. In vitro pollination and ovary culture as a breeding tool in wide hybridization of Lilium and Nerine. In Proceedings of the VI International Symposium on Flower Bulbs 325, Skiernieweice, Poland, 12 May 1992; pp. 461–466. [Google Scholar] [CrossRef]
- Van Tuyl, J.M.; Van Diën, M.P.; Van Creij, M.G.M.; Van Kleinwee, T.C.M.; Franken, J.; Bino, R.J. Application of in vitro pollination, ovary culture, ovule culture and embryo rescue for overcoming incongruity barriers in interspecific Lilium crosses. Plant Sci. 1991, 74, 115–126. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Bao, M.-z.; Liu, G.-f. Production of interspecific hybrids between Lilium longiflorum and L. lophophorum var. linearifolium via ovule culture at early stage. Euphytica 2009, 167, 45–55. [Google Scholar] [CrossRef]
- Tütüncü, M.; Mendi, Y. Evaluation of pollen tube growth and fertilization via histological analysis in Cyclamen persicum. In Proceedings of the XXVI International Eucarpia Symposium Section Ornamentals: Editing Novelty 1283, Erfurt, Germany, 1–4 September 2019; pp. 21–26. [Google Scholar] [CrossRef]
- Karamian, R.; Ebrahimzadeh, H. Plantlet regeneration from protoplast-derived embryogenic calli of Crocus cancellatus. Plant Cell Tissue Organ. Cult. 2001, 65, 115–121. [Google Scholar] [CrossRef]
- Koetle, M.; Finnie, J.; Balázs, E.; Van Staden, J. A review on factors affecting the Agrobacterium-mediated genetic transformation in ornamental monocotyledonous geophytes. South Afr. J. Bot. 2015, 98, 37–44. [Google Scholar] [CrossRef]
- Babu, P.; Chawla, H. In vitro regeneration and Agrobacterium mediated transformation in gladiolus. J. Hortic. Sci. Biotechnol. 2000, 75, 400–404. [Google Scholar] [CrossRef]
- Chib, S.; Thangaraj, A.; Kaul, S.; Dhar, M.K.; Kaul, T. Development of a system for efficient callus production, somatic embryogenesis and gene editing using CRISPR/Cas9 in Saffron (Crocus sativus L.). Plant Methods 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Cohen, A.; Meredith, C.P. Agrobacterium-mediated transformation of Lilium. In Proceedings of the VI International Symposium on Flower Bulbs 325, Skierniewice, Poland, 12 May 1992; pp. 611–618. [Google Scholar] [CrossRef]
- Hoshi, Y.; Kondo, M.; Mori, S.; Adachi, Y.; Nakano, M.; Kobayashi, H. Production of transgenic lily plants by Agrobacterium-mediated transformation. Plant Cell Rep. 2004, 22, 359–364. [Google Scholar] [CrossRef]
- Kamo, K.; Jordan, R.; Guaragna, M.A.; Hsu, H.-t.; Ueng, P. Resistance to Cucumber mosaic virus in Gladiolus plants transformed with either a defective replicase or coat protein subgroup II gene from Cucumber mosaic virus. Plant Cell Rep. 2010, 29, 695–704. [Google Scholar] [CrossRef]
- Kondo, T.; Hasegawa, H.; Suzuki, M. Transformation and regeneration of garlic (Allium sativum L.) by Agrobacterium-mediated gene transfer. Plant Cell Rep. 2000, 19, 989–993. [Google Scholar] [CrossRef]
- Lu, G.; Zou, Q.; Guo, D.; Zhuang, X.; Yu, X.; Xiang, X.; Cao, J. Agrobacterium tumefaciens-mediated transformation of Narcissus tazzeta var. chinensis. Plant Cell Rep. 2007, 26, 1585–1593. [Google Scholar] [CrossRef]
- Popowich, E.; Firsov, A.; Mitiouchkina, T.; Filipenya, V.; Dolgov, S.; Reshetnikov, V. Agrobacterium-mediated transformation of Hyacinthus orientalis with thaumatin II gene to control fungal diseases. Plant Cell Tissue Organ. Cult. 2007, 90, 237–244. [Google Scholar] [CrossRef]
- Suzuki, S.; Supaibulwatana, K.; Mii, M.; Nakano, M. Production of transgenic plants of the Liliaceous ornamental plant Agapanthus praecox ssp. orientalis (Leighton) Leighton via Agrobacterium-mediated transformation of embryogenic calli. Plant Sci. 2001, 161, 89–97. [Google Scholar] [CrossRef]
- Wilmink, A.; Van de Ven, B.; Dons, J. Expression of the GUS-gene in the monocot tulip after introduction by particle bombardment and Agrobacterium. Plant Cell Rep. 1992, 11, 76–80. [Google Scholar] [CrossRef]
- Van Aartrijk, J.; Van der Linde, P. In vitro propagation of flower-bulb crops. In Proceedings of the Tissue culture as a plant production system for horticultural crops: Conference on Tissue Culture as a Plant Production System for Horticultural Crops, Beltsville, MD, USA, 20–23 October 1985; pp. 317–331. [Google Scholar] [CrossRef]
- Preece, J. Stock plant physiological factors affecting growth and morphogenesis. In Plant Propagation by Tissue Culture. Volume 1: The Background; Springer: Dordrecht, The Netherlands, 2008; pp. 403–422. [Google Scholar] [CrossRef]
- Yasemin, S.; Koksal, N.; Buyukalaca, S. Indirect organogenesis and in vitro bulb formation of Pancratium maritimum. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 154, 713–727. [Google Scholar] [CrossRef]
- Murashige, T. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Chu, C. Establishment of an efficient medium for another culture of rice through comparative experiments on the nitrogen sources. Sci. Sin. 1975, 18, 223–231. [Google Scholar]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Propagators Soc. 1980, 30, 421–427. [Google Scholar]
- Gamborg, O.L.c.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- George, E.; de Klerk, G. The components of plant tissue culture media I: Macro-and micro-nutrients. In Plant Propagation by Tissue Culture. Volume 1: The Background; Springer: Dordrecht, The Netherlands, 2008; pp. 65–113. [Google Scholar] [CrossRef]
- Thorpe, T.; Stasolla, C.; Yeung, E.; de Klerk, G.; Roberts, A.; George, E. The components of plant tissue culture media II: Organic additions, osmotic and pH effects, and support systems. In Plant Propagation by Tissue Culture. Volume 1: The Background; Springer: Dordrecht, The Netherlands, 2008; pp. 115–173. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Kosson, R.; Treder, J. Polyamines and methyl jasmonate in bulb formation of in vitro propagated tulips. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 123, 591–605. [Google Scholar] [CrossRef]
- Debergh, P.C. Effects of agar brand and concentration on the tissue culture medium. Physiol. Plant. 1983, 59, 270–276. [Google Scholar] [CrossRef]
- Beruto, M.; Curir, P.; Debergh, P. Influence of agar on in vitro cultures: II. Biological performance of Ranunculus on media solidified with three different agar brands. Vitr. Cell. Dev. Biol. Plant 1999, 35, 94–101. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. Plant Propagation by Tissue Culture: The Background, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Bornwaßer, T.; Tantau, H.-J. Evaluation of LED lighting systems in in vitro cultures. In Proceedings of the VII International Symposium on Light in Horticultural Systems 956, Wageningen, The Netherlands, 15–18 October 2012; pp. 555–562. [Google Scholar] [CrossRef]
- Pundir, R.K.; Pathak, A.; Upadhyaya, D.C.; Muthusamy, A.; Upadhyaya, C.P. Red and Blue Light-Emitting Diodes Significantly Improve Tuberization of Potato (L.). J. Hortic. Res. 2021, 29, 95–108. [Google Scholar] [CrossRef]
- Bach, A.; Świderski, A. The effect of light quality on organogenesis of Hyacinthus orientalis L. in vitro. Acta Biol. Cracoviensia Ser. Bot. 2000, 42, 115–120. [Google Scholar]
- Bakhshaie, M.; Khosravi, S.; Azadi, P.; Bagheri, H.; van Tuyl, J.M. Biotechnological advances in Lilium. Plant Cell Rep. 2016, 35, 1799–1826. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Chakrabarty, D.; Paek, K. Growth and uptake of sucrose and mineral ions by bulblets of Lilium oriental hybrid ‘Casablanca’during bioreactor culture. J. Hortic. Sci. Biotechnol. 2002, 77, 253–257. [Google Scholar] [CrossRef]
- Pałka, P.; Cioć, M.; Hura, K.; Szewczyk-Taranek, B.; Pawłowska, B. Adventitious organogenesis and phytochemical composition of Madonna lily (Lilium candidum L.) in vitro modeled by different light quality. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 152, 99–114. [Google Scholar] [CrossRef]
- Van Aartrijk, J.; Blom-Barnhoorn, G.J. Adventitious Bud Formation from Bulb-scale Explants of Lilium speciosum Thunb. in vitro Interacting effects of NAA, TIBA, wounding, and temperature. J. Plant Physiol. 1984, 116, 409–416. [Google Scholar] [CrossRef]
- Amaki, W.; Shinohara, Y.; Hayata, Y.; Sano, H.; Suzuki, Y. Effects of bulb desiccation and storage on the in vitro propagation of hyacinth. Sci. Hortic. 1984, 23, 353–360. [Google Scholar] [CrossRef]
- Gavinlertvatana, P.; Read, P.E.; Wilkins, H.; Heins, R. Influence of Photoperiod and Daminozide Stock Plant Pretreatments on Ethylene and CO2 Levels and Callus Formation from Dahlia Leaf Segment Cultures1. J. Am. Soc. Hortic. Sci. 1979, 104, 849–852. [Google Scholar] [CrossRef]
- Hosoki, T.; Sagawa, Y. Clonal Propagation of Ginger (Zingiber officinale Roscoe) through Tissue Culture1. HortScience 1977, 12, 451–452. [Google Scholar] [CrossRef]
- Stimart, D.P.; Ascher, P.D. Developmental Responses of Lilium longiflorum Bulblets to Constant or Alternating Temperatures in Vitro1. J. Am. Soc. Hortic. Sci. 1981, 106, 450–454. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Sochacki, D. Micropropagation of tulip: Production of virus-free stock plants. In Protocols for In Vitro Propagation of Ornamental Plants; Humana Press: Totowa, NJ, USA, 2010; pp. 243–256. [Google Scholar] [CrossRef]
- Muraseva, D.S.; Novikova, T.I. Efficient protocol for in vitro propagation from bulb scale explants of Fritillaria ruthenica Wikstr. (Liliaceae), a rare ornamental species. Rendiconti Lincei. Sci. Fis. Nat. 2018, 29, 491–497. [Google Scholar] [CrossRef]
- Kumar, V.; Moyo, M.; Van Staden, J. Enhancing plant regeneration of Lachenalia viridiflora, a critically endangered ornamental geophyte with high floricultural potential. Sci. Hortic. 2016, 211, 263–268. [Google Scholar] [CrossRef]
- Mirici, S.; Parmaksız, İ.; Özcan, S.; Sancak, C.; Uranbey, S.; Sarıhan, E.O.; Gümüşcü, A.; Gürbüz, B.; Arslan, N. Efficient in vitro bulblet regeneration from immature embryos of endangered Sternbergia fischeriana. Plant Cell Tissue Organ. Cult. 2005, 80, 239–246. [Google Scholar] [CrossRef]
- Buckley, P.M.; Reed, B.M. 045 Antibiotic susceptibility of plant-associated bacteria. HortScience 1994, 29, 434. [Google Scholar] [CrossRef]
- George, E.F. Plant Propagation by Tissue Culture Part 1. The Technology; CABI: Wallingford, UK, 1993. [Google Scholar]
- Silva, J.A.T.d.; Winarto, B.; Dobránszki, J.; Zeng, S. Disinfection procedures for propagation of. Folia Hortic. 2015, 27, 3–14. [Google Scholar] [CrossRef]
- Yasemin, S.; Köksal, N.; Büyükalaca, S. Effects of disinfection conditions and culture media on in vitro germination of sea daffodil (Pancratium maritimum). J. Biol. Environ. Sci. 2018, 34, 13–22. [Google Scholar]
- Barrett, C.; Cassells, A.C. An evaluation of antibiotics for the elimination of Xanthomonas campestris pv. pelargonii (Brown) from Pelargonium x domesticum cv.‘Grand Slam’explants in vitro. Plant Cell Tissue Organ. Cult. 1994, 36, 169–175. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Mavrommati, E.; Kartsonas, E.; Petropoulos, S.A. Effect of Temperature and Sucrose on In Vitro Seed Germination and Bulblet Production of Pancratium maritimum L. Agronomy 2022, 12, 2786. [Google Scholar] [CrossRef]
- Nikopoulos, D.; Alexopoulos, A.A. In vitro propagation of an endangered medicinal plant: Pancratium maritimum L. J. Food Agric. Environ. 2008, 6, 393–398. [Google Scholar]
- Lagram, K.; El Merzougui, S.; Boudadi, I.; Ben El Caid, M.; El Boullani, R.; El Mousadik, A.; Serghini, M.A. In vitro shoot formation and enrooted mini-corm production by direct organogenesis in saffron (Crocus sativus L.). Vegetos 2023, 1–6. [Google Scholar] [CrossRef]
- Sochacki, D.; Orlikowska, T. The obtaining of narcissus plants free from potyviruses via adventitious shoot regeneration in vitro from infected bulbs. Sci. Hortic. 2005, 103, 219–225. [Google Scholar] [CrossRef]
- Redhwan, A.; Acemi, A.; Özen, F. Effects of plant growth regulators on in vitro seed germination, organ development and callogenesis in Pancratium maritimum L. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 154, 97–110. [Google Scholar] [CrossRef]
- Rafiq, S.; Rather, Z.; Bhat, R.A.; Nazki, I.; Al-Harbi, M.S.; Banday, N.; Farooq, I.; Samra, B.N.; Khan, M.; Ahmed, A.F. Standardization of in vitro micropropagation procedure of Oriental Lilium Hybrid Cv.‘Ravenna’. Saudi J. Biol. Sci. 2021, 28, 7581–7587. [Google Scholar] [CrossRef]
- Rather, Z.; Nazki, I.; Qadri, Z.; Mir, M.; Bhat, K.; Hussain, G. In vitro propagation of herbaceous peony (Paeonia lactiflora Pall.) cv. Sara Bernhardt using shoot tips. Indian J. Hortic. 2014, 71, 385–389. [Google Scholar]
- Farooq, I.; Banday, N.; Nazki, I.; Malik, A.A.; Khan, F.; Mir, M.; Yaseen, T. In vitro sterilization of Lilium LA Hybrids “Indian Summerset” and “Nashville” as influenced by different sterilant combinations. Pharma Innov. J. 2022, 11, 1795–1798. [Google Scholar]
- Appleton, M.; Ascough, G.; Van Staden, J. In vitro regeneration of Hypoxis colchicifolia plantlets. South Afr. J. Bot. 2012, 80, 25–35. [Google Scholar] [CrossRef][Green Version]
- Devi, K.; Sharma, M.; Ahuja, P. Direct somatic embryogenesis with high frequency plantlet regeneration and successive cormlet production in saffron (Crocus sativus L.). South Afr. J. Bot. 2014, 93, 207–216. [Google Scholar] [CrossRef]
- Lapiz-Culqui, Y.K.; Meléndez-Mori, J.B.; Mállap-Detquizán, G.; Tejada-Alvarado, J.J.; Vilca-Valqui, N.C.; Huaman-Human, E.; Oliva, M.; Goñas, M. In Vitro Bulbification of Five Lily Varieties: An Effective Method to Produce Quality Seeds and Flowers. Int. J. Agron. 2022, 2022, 8775989. [Google Scholar] [CrossRef]
- Patil, A.M.; Gunjal, P.P.; Das, S. In vitro micropropagation of Lilium candidum bulb by application of multiple hormone concentrations using plant tissue culture technique. Int. J. Res. Appl. Sci. Biotechnol. 2021, 8, 244–253. [Google Scholar] [CrossRef]
- Youssef, N.M.; Shaaban, S.A.; Ghareeb, Z.F.; Taha, L.S. In vitro bulb formation of direct and indirect regeneration of Lilium orientalis cv. “Starfighter” plants. Bull. Natl. Res. Cent. 2019, 43, 211. [Google Scholar] [CrossRef]
- Ozel, C.A.; Khawar, K.M.; Unal, F. Factors affecting efficient in vitro micropropagation of Muscari muscarimi Medikus using twin bulb scale. Saudi J. Biol. Sci. 2015, 22, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Fidalgo, F.; Santos, I.; Salema, R. In vitro bulb formation of Narcissus asturiensis, a threatened species of the Amaryllidaceae. J. Hortic. Sci. Biotechnol. 2002, 77, 149–152. [Google Scholar] [CrossRef]
- Kukulczanka, K.; Kromer, K.; Czastka, B. Propagation of Fritillaria meleagris L. through tissue culture. In Proceedings of the III International Symposium on Growth Regulators in Ornamental Horticulture 251, Skierniewice, Poland, 5–10 September 1988; pp. 147–154. [Google Scholar] [CrossRef]
- Kumar, P.; Partap, M.; Ashrita; Rana, D.; Kumar, P.; Warghat, A.R. Metabolite and expression profiling of steroidal alkaloids in wild tissues compared to bulb derived in vitro cultures of Fritillaria roylei—High value critically endangered Himalayan medicinal herb. Ind. Crops Prod. 2020, 145, 111945. [Google Scholar] [CrossRef]
- Sevindik, B.; Mendi, Y.Y. Somatic embryogenesis in Crocus sativus L. In In Vitro Embryogenesis in Higher Plants; Humana Press: New York, NY, USA, 2016; pp. 351–357. [Google Scholar] [CrossRef]
- Taheri-Dehkordi, A.; Naderi, R.; Martinelli, F.; Salami, S.A. A robust workflow for indirect somatic embryogenesis and cormlet production in saffron (Crocus sativus L.) and its wild allies; C. caspius and C. speciosus. Heliyon 2020, 6, e05841. [Google Scholar] [CrossRef] [PubMed]
- Slimani, C.; El Goumi, Y.; Chaimae, R.; El Ghadraoui, L.; Benjelloun, M.; Lazraq, A. Micropropagation and potential of bioactive compounds of saffron (Crocus sativus L.) for nutrition and health. Not. Sci. Biol. 2022, 14, 11278. [Google Scholar] [CrossRef]
- Kritskaya, T.; Kashin, A.; Kasatkin, M.Y. Micropropagation and Somaclonal Variation of Tulipa suaveolens (Liliaceae) in vitro. Russ. J. Dev. Biol. 2019, 50, 209–215. [Google Scholar] [CrossRef]
- Pierik, R.; Sprenkels, P.; Van Der Harst, B.; Van Der Meys, Q. Seed germination and further development of plantlets of Paphiopedilum ciliolare Pfitz. in vitro. Sci. Hortic. 1988, 34, 139–153. [Google Scholar] [CrossRef]
- Tubić, L.; Savić, J.; Mitić, N.; Milojević, J.; Janošević, D.; Budimir, S.; Zdravković-Korać, S. Cytokinins differentially affect regeneration, plant growth and antioxidative enzymes activity in chive (Allium schoenoprasum L.). Plant Cell Tissue Organ Cult. (PCTOC) 2015, 124, 1–14. [Google Scholar] [CrossRef]
- Ziv, M. Morphogenesis of gladiolus buds in bioreactors—Implication for Scaled-up Propagation of Geophytes. In Progress in Plant Cellular and Molecular Biology. Current Plant Science and Biotechnology in Agriculture; Nijkamp, H.J.J., Van Der Plas, L.H.W., Van Aartrijk, J., Eds.; Springer: Dordrecht, The Netherlands, 1990; Volume 9. [Google Scholar] [CrossRef]
- Pence, V.C. Evaluating costs for the in vitro propagation and preservation of endangered plants. Vitr. Cell. Dev. Biol. Plant 2011, 47, 176–187. [Google Scholar] [CrossRef]
- Backs-Hüsemann, D.; Reinert, J. Embryobildung durch isolierte Einzelzellen aus Gewebekulturen von Daucus carota. Protoplasma 1970, 70, 49–60. [Google Scholar] [CrossRef]
- Sochacki, D.; Marciniak, P.; Ciesielska, M.; Zaród, J.; Sutrisno. The Influence of Selected Plant Growth Regulators and Carbohydrates on In Vitro Shoot Multiplication and Bulbing of the Tulip (Tulipa L.). Plants 2023, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Plessner, O.; Ziv, M.; Negbi, M. In vitro corm production in the saffron crocus (Crocus sativus L.). Plant Cell Tissue Organ. Cult. 1990, 20, 89–94. [Google Scholar] [CrossRef]
- Santos, J.; Santos, I.; Salema, R. In vitro production of bulbs of Narcissus bulbocodium flowering in the first season of growth. Sci. Hortic. 1998, 76, 205–217. [Google Scholar] [CrossRef]
- Wu, X.Q.; Liao, Z.P.; Luo, P.; Luan, A.Y.; Zeng, L.H. In vitro induction and regeneration of callus from three inflorescence organs of Chinese narcissus (Narcissus tazetta L. var. chinensis Roem). J. Hortic. Sci. Biotechnol. 2014, 89, 619–624. [Google Scholar] [CrossRef]
- Bahr, L.R.; Compton, M.E. Competence for in vitro bulblet regeneration among eight Lilium genotypes. HortScience 2004, 39, 127–129. [Google Scholar] [CrossRef]
- Sultana, J.; Sutlana, N.; Siddique, M.; Islam, A.; Hossain, M.; Hossain, T. In vitro bulb production in Hippeastrum (Hippeastrum hybridum). J. Cent. Eur. Agric. 2010, 11, 469–474. [Google Scholar]
- Kizil, S.; Khawar, K.M. The effects of plant growth regulators and incubation temperatures on germination and bulb formation of Fritillaria persica L. Propag. Ornam. Plants 2014, 14, 133–138. [Google Scholar]
- Stanišić, M.; Raspor, M.; Ninković, S.; Milošević, S.; Ćalić, D.; Bohanec, B.; Trifunović, M.; Petrić, M.; Subotić, A.; Jevremović, S. Clonal fidelity of Iris sibirica plants regenerated by somatic embryogenesis and organogenesis in leaf-base culture—RAPD and flow cytometer analyses. South Afr. J. Bot. 2015, 96, 42–52. [Google Scholar] [CrossRef]
- Maślanka, M.; Mazur, J.; Kapczyńska, A. In Vitro Organogenesis of Critically Endangered Lachenalia viridiflora. Agronomy 2022, 12, 475. [Google Scholar] [CrossRef]
- Doğan, S.; Çağlar, G.; Palaz, E.B. The effect of different applications on in vitro bulb development of an endemic hyacinth plant (Hyacinthus orientalis L. subsp. chionophyllus Wendelbo) grown in Turkey. Turk. J. Agric. Food Sci. Technol. 2020, 8, 1713–1719. [Google Scholar] [CrossRef]
- Çakmak, D.; Karaoglu, C.; Aasim, M.; Sancak, C.; Özcan, S. Advancement in protocol for in vitro seed germination, regeneration, bulblet maturation, and acclimatization of Fritillaria persica. Turk. J. Biol. 2016, 40, 878–888. [Google Scholar] [CrossRef]
- Ebrahimzadeh, H.; Karamian, R.; Nouri, D.M. Somatic Embryogenesis and Regeneration of Plantlet in Saffron, Crocus sativus L. 2000. Available online: https://www.sid.ir/paper/536838/en (accessed on 3 January 2024).
- Sheibani, M.; Nemati, S.H.; Davarinejad, G.H.; Azghandi, A.V.; Habashi, A.A. Induction Of Somatic Embryogenesis In Saffron Using Thidiazuron (TDZ). In Proceedings of the ISHS Acta Horticulturae 739: II International Symposium on Saffron Biology and Technology, Masshad, Iran, 28 October 2006; pp. 259–267. [Google Scholar] [CrossRef]
- Marković, M.; Trifunović-Momčilov, M.; Radulović, O.; Paunović, D.M.; Antonić Reljin, D.D.; Uzelac, B.; Subotić, A. The Effects of Different Auxin–Cytokinin Combinations on Morphogenesis of Fritillaria meleagris Using Bulb Scale Sections In Vitro. Horticulturae 2023, 9, 910. [Google Scholar] [CrossRef]
- Kocak, M.; Izgu, T.; Sevindik, B.; Tutuncu, M.; Curuk, P.; Simsek, O.; Aka Kacar, Y.; Teixeira da Silva, J.A.; Yalcin Mendi, Y. Somatic embryogenesis of Turkish Cyclamen persicum Mill. Scientia Horticulturae 2014, 172, 26–33. [Google Scholar] [CrossRef]
- Bach, A.; Ptak, A. Somatic embryogenesis and plant regeneration from ovaries of Tulipa gesneriana L. in in vitro cultures. Acta Hortic. 2001, 391–394. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Marasek-Ciolakowska, A. Micropropagation of tulip via somatic embryogenesis. Agronomy 2020, 10, 1857. [Google Scholar] [CrossRef]
- İzgü, T.; Sevindik, B.; Çürük, P.; Şimşek, Ö.; Aka Kaçar, Y.; Teixeira da Silva, J.A.; Yalçın Mendi, Y. Development of an efficient regeneration protocol for four Cyclamen species endemic to Turkey. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 127, 95–113. [Google Scholar] [CrossRef]
- Kiviharju, E.; Tuominen, U.; Törmälä, T. The effect of explant material on somatic embryogenesis of Cyclamen persicum Mill. Plant Cell Tissue Organ. Cult. 1992, 28, 187–194. [Google Scholar] [CrossRef]
- Otani, M.; Shimada, T. Somatic embryogenesis and plant regeneration from Cyclamen persicum Mill. leaf cultures. Plant Tissue Cult. Lett. 1991, 8, 121–123. [Google Scholar] [CrossRef]
- Takamura, T.; Miyajima, I.; Matsuo, E. Somatic embryogenesis of Cyclamen persicum Mill. ‘Anneke’from aseptic seedlings. Plant Cell Rep. 1995, 15, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Wicart, G.; Mouras, A.; Lutz, A. Histological study of organogenesis and embryogenesis in Cyclamen persicum Mill. tissue cultures: Evidence for a single organogenetic pattern. Protoplasma 1984, 119, 159–167. [Google Scholar] [CrossRef]
- Fersing, J.; Mouras, A.; Lutz, A. First stage in the industrial vegetative propagation of Cyclamen by in vitro culture. Pepinier. Hortic. Maraichers 1982, 224, 27–30. [Google Scholar]
- Kumari, A.; Baskaran, P.; Van Staden, J. In vitro propagation via organogenesis and embryogenesis of Cyrtanthus mackenii: A valuable threatened medicinal plant. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 131, 407–415. [Google Scholar] [CrossRef]
- Nautiyal, A.; Ramlal, A.; Agnihotri, A.; Rashid, A. Stress-induced somatic embryogenesis on seedlings of Azadirachta indica A. Juss. by thidiazuron and its inhibition by ethylene modulators. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 153, 357–366. [Google Scholar] [CrossRef]
- Yu, Y.; Qin, W.; Li, Y.; Zhang, C.; Wang, Y.; Yang, Z.; Ge, X.; Li, F. Red light promotes cotton embryogenic callus formation by influencing endogenous hormones, polyamines and antioxidative enzyme activities. Plant Growth Regul. 2019, 87, 187–199. [Google Scholar] [CrossRef]
- Mendi, N.Y.Y.; Özdemir, Ş.; Ugur, S.; Sevindik, B.; İzgu, T.; Kaya, E.; Tutuncu, M.; Çuruk, P.; Şimsek, Ö.; Koyuncu, O. In vitro Regeneration and Synthetic Seed Production of Colchicum cilicicum Grown Naturally in Turkey. Alatarım 2022, 21, 18–26. [Google Scholar]
- Beruto, M.; Curir, P.; Debergh, P. Callus growth and somatic embryogenesis in thalamus tissue of Ranunculus asiaticus L. cultivated in vitro: Cytokinin effect and phenol metabolism. Vitr. Cell. Dev. Biol. Plant 1996, 32, 154–160. [Google Scholar] [CrossRef]
- Azeri, F.N.; Öztürk, G. Microbulb and plantlet formation of a native bulbous flower, Lilium monodelphum M. Bieb, var. Armenum, through tissue culture propagation. Biotechnol. Rep. 2021, 32, e00665. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Bioreactor systems for micropropagation of plants: Present scenario and future prospects. Front. Plant Sci. 2023, 14, 1159588. [Google Scholar] [CrossRef]
- Akita, M.; Takayama, S. Induction and development of potato tubers in a jar fermentor. Plant Cell Tissue Organ. Cult. 1994, 36, 177–182. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Alsadon, A.; Al-Aizari, A.A.; Al-Mohidib, M. In vitro floral emergence and improved formation of saffron daughter corms. Horticulturae 2022, 8, 973. [Google Scholar] [CrossRef]
- Gao, R.; Wu, S.-Q.; Piao, X.-C.; Park, S.-Y.; Lian, M.-L. Micropropagation of Cymbidium sinense using continuous and temporary airlift bioreactor systems. Acta Physiol. Plant. 2014, 36, 117–124. [Google Scholar] [CrossRef]
- Hao, Z.; Ouyang, F.; Geng, Y.; Deng, X.; Hu, Z.; Chen, Z. Propagation of potato tubers in a nutrient mist bioreactor. Biotechnol. Tech. 1998, 12, 641–644. [Google Scholar] [CrossRef]
- Jiménez, E.; Pérez, N.; de Feria, M.; Barbón, R.; Capote, A.; Chávez, M.; Quiala, E.; Pérez, J.C. Improved production of potato microtubers using a temporary immersion system. Plant Cell Tissue Organ. Cult. 1999, 59, 19–23. [Google Scholar] [CrossRef]
- Jo, U.; Murthy, H.; Hahn, E.; Paek, K. Micropropagation of Alocasia amazonica using semisolid and liquid cultures. Vitr. Cell. Dev. Biol. Plant 2008, 44, 26–32. [Google Scholar] [CrossRef]
- Kim, E.; Hahn, E.; Murthy, H.; Paek, K. Enhanced shoot and bulblet proliferation of garlic (Allium sativum L.) in bioreactor systems. J. Hortic. Sci. Biotechnol. 2004, 79, 818–822. [Google Scholar] [CrossRef]
- Lian, M.L.; Piao, X.C.; Park, S.Y. Mass Production of Lilium Bulblets in Bioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.Y., Murthy, H., Zhong, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 389–415. [Google Scholar] [CrossRef]
- Lian, M.; Chakrabarty, D.; Paek, K. Bulblet formation from bulbscale segments of Lilium using bioreactor system. Biol. Plant. 2003, 46, 199–203. [Google Scholar] [CrossRef]
- Lim, S.; Seon, J.; Paek, K.; Han, B.; Son, S. Development of pilot scale process for mass production of Lilium bulblets in vitro. In Proceedings of the International Symposium on Biotechnology of Tropical and Subtropical Species Part 2 461, Brisbane, QLD, Australia, 29 September–3 October 1997; pp. 237–242. [Google Scholar] [CrossRef]
- Piao, X.C.; Chakrabarty, D.; Hahn, E.J.; Paek, K.Y. A simple method for mass production of potato microtubers using a bioreactor system. Curr. Sci. 2003, 84, 1129–1132. [Google Scholar]
- Teisson, C.; Alvard, D. In vitro production of potato microtubers in liquid medium using temporary immersion. Potato Res. 1999, 42, 499–504. [Google Scholar] [CrossRef]
- Yu, W.-C.; Joyce, P.; Cameron, D.; McCown, B. Sucrose utilization during potato microtuber growth in bioreactors. Plant Cell Rep. 2000, 19, 407–413. [Google Scholar] [CrossRef]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Being the Collected Papers of C. Raunkiaer; CABI: Wallingford, UK, 1934. [Google Scholar]
- Sheikh, F.R.; Jose-Santhi, J.; Kalia, D.; Singh, K.; Singh, R.K. Sugars as the regulators of dormancy and sprouting in geophytes. Ind. Crops Prod. 2022, 189, 115817. [Google Scholar] [CrossRef]
- Borochov, A.; Spiegelstein, H.; Weiss, D. Dormancy and storage of geophytes. In Proceedings of the VII International Symposium on Flowerbulbs 430, Herzliya, Israel, 10 March 1996; pp. 405–410. [Google Scholar] [CrossRef]
- Marković, M.; Trifunović Momčilov, M.; Uzelac, B.; Jevremović, S.; Subotić, A. Bulb Dormancy In Vitro—Fritillaria meleagris: Initiation, Release and Physiological Parameters. Plants 2021, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, C.; Sui, J.; Liang, J.; Ge, J.; Li, J.; Pan, W.; Yi, M.; Du, Y.; Wu, J. A wake-up call: Signaling in regulating ornamental geophytes dormancy. Ornam. Plant Res. 2022, 2, 8. [Google Scholar] [CrossRef]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, para-, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar] [CrossRef]
- Takayama, S.; Misawa, M. Differentiation in Lilium bulbscales grown in vitro. Effects of activated charcoal, physiological age of bulbs and sucrose concentration on differentiation and scale leaf formation in vitro. Physiol. Plant. 1980, 48, 121–125. [Google Scholar] [CrossRef]
- Langens-Gerrits, M.M.; Miller, W.B.; Croes, A.F.; De Klerk, G.-J. Effect of low temperature on dormancy breaking and growth after planting in lily bulblets regenerated in vitro. Plant Growth Regul. 2003, 40, 267–275. [Google Scholar] [CrossRef]
- Carasso, V.; Mucciarelli, M. In vitro bulblet production and plant regeneration from immature embryos of Fritillaria tubiformis Gren. & Godr. Propag. Ornam. Plants 2014, 14, 101–111. [Google Scholar]
- Santos, A.; Fidalgo, F.; Santos, I. In vitro propagation of Hyacinthus orientalis cv. Jan Boss from bulb twin-scale explants. Floric. Ornam. Plant Biotechnol. 2006, 2, 561–563. [Google Scholar]
- Nower, A. Micropropagation and Bulblet Formation In vitro of Dutch Iris cv. Blue Magic. J. Product. Dev. 2007, 12, 329–340. [Google Scholar] [CrossRef]
- Staikidou, I.; Watson, S.; Harvey, B.M.; Selby, C. Narcissus bulblet formation in vitro: Effects of carbohydrate type and osmolarity of the culture medium. Plant Cell Tissue Organ. Cult. 2005, 80, 313–320. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Ross, H. Formation of tulip bulbs in vitro. Acta Hortic. 2003, 616, 413–419. [Google Scholar] [CrossRef]
- Islam, M.S.; Roni, M.Z.K.; Shimasaki, K. Factors affecting bulblet growth of ‘Lilium sp. in vitro’ and ‘in vivo’. Plant Omics 2017, 10, 263–268. [Google Scholar] [CrossRef]
- Langens-Gerrits, M.; De Klerk, G.J.; Croes, A. Phase change in lily bulblets regenerated in vitro. Physiol. Plant. 2003, 119, 590–597. [Google Scholar] [CrossRef]
- Kamo, K.; Roh, M.; Blowers, A.; Smith, E.; Van Eck, J. Transgenic Gladiolus. In Transgenic Crops III. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 48. [Google Scholar] [CrossRef]
- Van Tuyl, J.M.; Arens, P.; Marasek-Ciolakowska, A. Breeding and genetics of ornamental geophytes. In Ornamental Geophytes: From Basic Science to Sustainable Horticultural Production, 1st ed.; Kamenetsky, R., Okubo, H., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 131–158. [Google Scholar]
- Van Harmelen, M.; Löffler, H.; Van Tuyl, J. Somaclonal variation in lily after in vitro cultivation. In Proceedings of the VII International Symposium on Flowerbulbs 430, Herzliya, Israel, 10 March 1996; pp. 347–350. [Google Scholar] [CrossRef]
- Abdolinejad, R.; Shekafandeh, A.; Jowkar, A.; Gharaghani, A.; Alemzadeh, A. Indirect regeneration of Ficus carica by the TCL technique and genetic fidelity evaluation of the regenerated plants using flow cytometry and ISSR. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 143, 131–144. [Google Scholar] [CrossRef]
- Konar, S.; Adhikari, S.; Karmakar, J.; Ray, A.; Bandyopadhyay, T.K. Evaluation of subculture ages on organogenic response from root callus and SPAR based genetic fidelity assessment in the regenerants of Hibiscus sabdariffa L. Ind. Crops Prod. 2019, 135, 321–329. [Google Scholar] [CrossRef]
- Vitamvas, J.; Viehmannova, I.; Cepkova, P.H.; Mrhalova, H.; Eliasova, K. Assessment of somaclonal variation in indirect morphogenesis-derived plants of Arracacia xanthorrhiza. Pesqui. Agropecuária Bras. 2019, 54, e00301. [Google Scholar] [CrossRef]
- Memon, N.; Yasmin, A.; Pahoja, V.; Hussain, Z.; Ahmad, I. In vitro regeneration of gladiolus propagules. J. Agr. Technol. 2012, 8, 2331–2351. [Google Scholar]
- Asadi, N.; Zarei, H.; Hashemi-Petroudi, S.H.; Mousavizadeh, S.J. Micropropagation and assessment of somaclonal variation in Galanthus transcaucasicus in vitro plantlets. Ornam. Hortic. 2021, 27, 505–515. [Google Scholar] [CrossRef]
- Hesami, M.; Jones, A.M.P. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl. Microbiol. Biotechnol. 2020, 104, 9449–9485. [Google Scholar] [CrossRef] [PubMed]
- Kucukrecep, A.; Tekdal, D. Machine Learning Applications for Plant Biotechnology: Modeling of The Plant Tissue Culture Procedures with Artificial Neural Networks. Kadirli Uygulamalı Bilim. Fakültesi Derg. 2022, 2, 306–315. [Google Scholar]
- Prasad, V.; Gupta, S.D. Photometric clustering of regenerated plants of gladiolus by neural networks and its biological validation. Comput. Electron. Agric. 2008, 60, 8–17. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Avilez-Montalvo, R.N. Plant Tissue Culture: A Battle Horse in the Genome Editing Using CRISPR/Cas9. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: New York, NY, USA, 2018; pp. 131–148. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Jin, G.; Han, D.; Xue, J.; Du, Y.; Chen, X.; Yang, F.; Zhao, C.; Zhang, X. A Modified Method for Transient Transformation via Pollen Magnetofection in Lilium Germplasm. Int. J. Mol. Sci. 2023, 24, 15304. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Naderi, R.; Kafi, M.; Azadi, P.; Shakh-Asadi, M.; Okazaki, K. Effect of ‘Chloroxynil’ on Agrobacterium-mediated transformation efficiency of Lilium cv ‘Manissa’. Sci. Hortic. 2020, 271, 109404. [Google Scholar] [CrossRef]


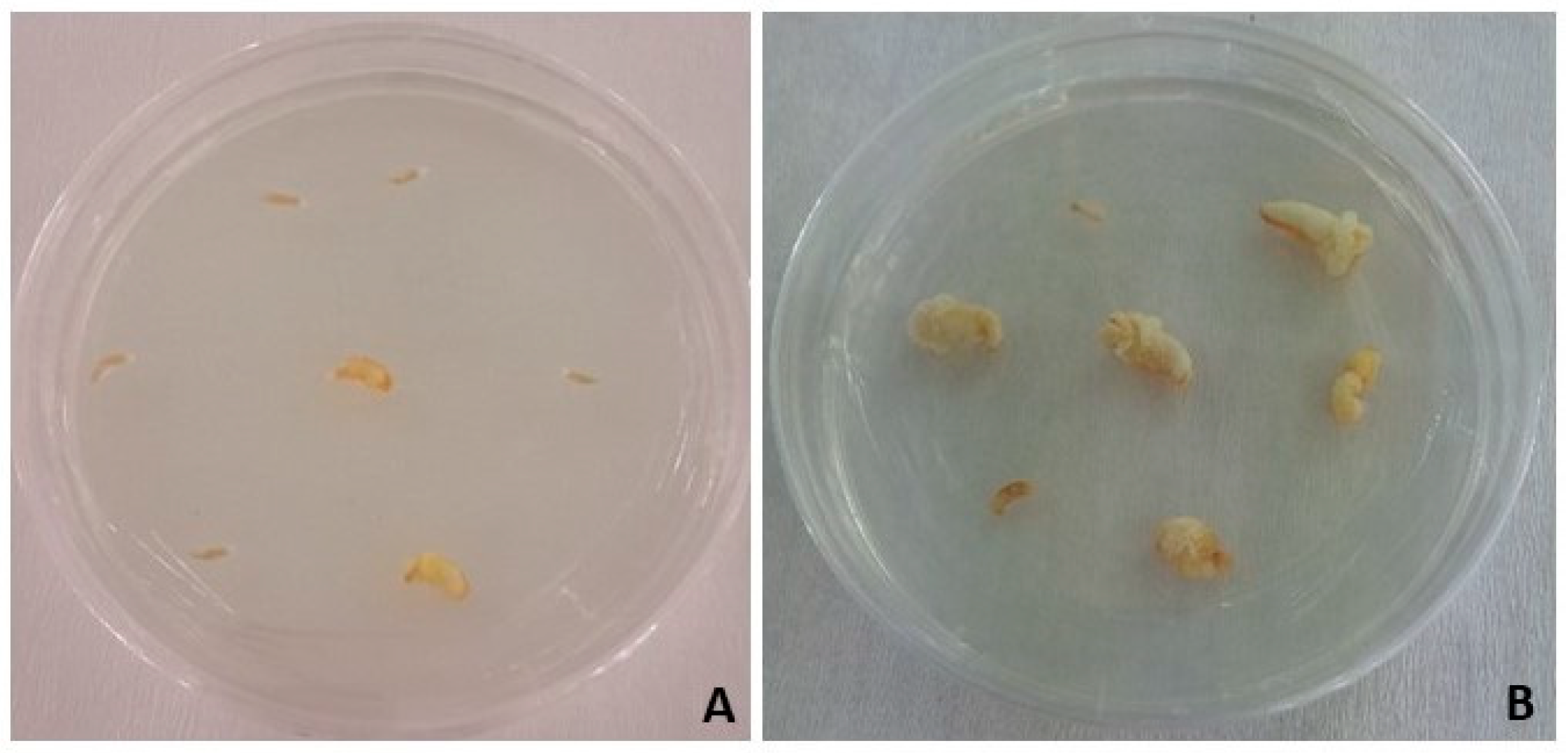
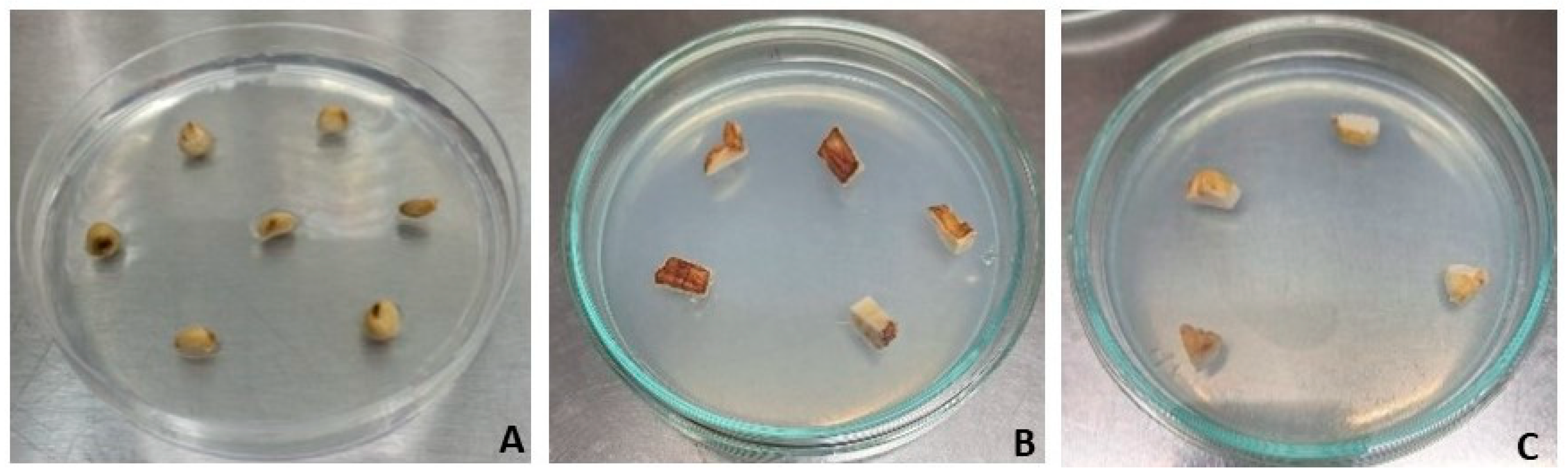

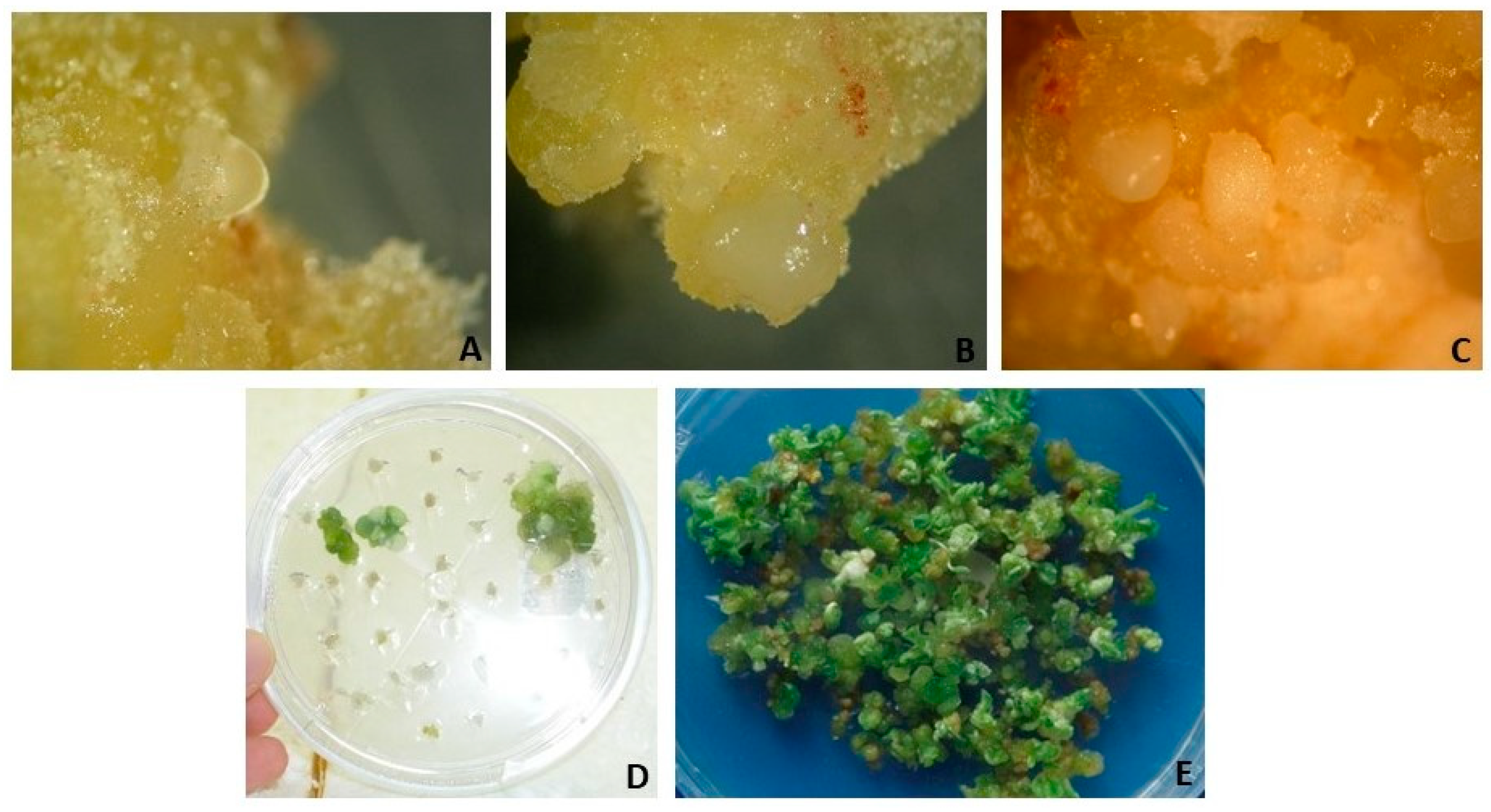

| Inputs | Outputs |
|---|---|
| Research Question | Micropropagation methodologies, challenges, and opportunities in geophytes, flower bulbs |
| Databases | Web of Science, Google Scholar, PubMed, ScienceDirect, Scopus |
| Sectioning | The basic flow (history, economy, flower bulb propagation and challenges, micropropagation, flower bulb micropropagation, stages, challenges, opportunities, conclusion) was determined for the literature review, and titles were added |
| Searching | For each section, keywords were determined |
| Screening literature | First, titles and abstracts; selecting the quality and relevance Second, full text assessment |
| Paper Management | Endnote |
| Sections | Keywords |
|---|---|
| History | Geophytes history Flower bulbs history |
| World Ornamental Plants Sector–Flower Bulbs situation in the sector | Flower bulbs production rate Flower bulbs marketing Flower bulbs sector Ornamental plants sector Ornamental industry Flower market Ornamental plants sector reports |
| Flower bulb propagation and challenges | Geophytes propagation Flower bulbs propagation Flower bulbs traditional propagation Storage organs Flower bulbs seed propagation Flower bulbs vegetative propagation Flower bulbs micropropagation |
| Micropropagation | Micropropagation Somatic embryogenesis Organogenesis Plant regeneration Factors affecting plant regeneration Explant choice for plant regeneration Culture medium for plant regeneration Environmental conditions for plant regeneration |
| Micropropagation of Flower Bulbs | Geophytes micropropagation Flower bulbs micropropagation Flower bulbs tissue culture Flower bulbs in vitro culture Flower bulbs micropropagation stages Flower bulbs plant material preparation Flower bulbs mother stock material Flower bulbs surface sterilization Flower bulbs disinfection Flower bulbs multiplication Flower bulbs dormancy breaking Flower bulbs acclimatization Flower bulbs planting Tissue culture and artificial intelligence |
| Somaclonal variation | Flower bulbs somaclonal variation Geophytes somaclonal variation |
| Conclusion | Flower bulbs genome editing Flower bulbs CRISPR Flower bulbs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasemin, S.; Beruto, M. A Review on Flower Bulb Micropropagation: Challenges and Opportunities. Horticulturae 2024, 10, 284. https://doi.org/10.3390/horticulturae10030284
Yasemin S, Beruto M. A Review on Flower Bulb Micropropagation: Challenges and Opportunities. Horticulturae. 2024; 10(3):284. https://doi.org/10.3390/horticulturae10030284
Chicago/Turabian StyleYasemin, Sara, and Margherita Beruto. 2024. "A Review on Flower Bulb Micropropagation: Challenges and Opportunities" Horticulturae 10, no. 3: 284. https://doi.org/10.3390/horticulturae10030284
APA StyleYasemin, S., & Beruto, M. (2024). A Review on Flower Bulb Micropropagation: Challenges and Opportunities. Horticulturae, 10(3), 284. https://doi.org/10.3390/horticulturae10030284






