Abstract
As peat (P) demand increases throughout the horticultural industry, alternative fibers must be evaluated. Sugarcane bagasse (B), wood fiber (W), and coconut coir (C) have received interest as domestically available alternatives to P, with demonstrated success in producing greenhouse crops. However, there is limited research comparing these materials to peat. This research evaluated the substrate properties and productivity of Petunia Supertunia Mini Vista ‘Indigo’ in pine bark substrates amended with C, W, B, or P and fertigated weekly at 100, 200, or 300 parts per million (ppm) nitrogen (N) to account for possible N immobilization. The container capacity was lowest and air-filled porosity was highest in W and B substrates. Substrate pH increased in W and B substrates, and C substrates were fertigated at 100 ppm N. Increasing the N rate increased the growth index in all substrates, especially B and W substrates later in the production period. Higher fertilization increased shoot mass, chlorophyll content, and blooms across all substrates, demonstrating that fertilizer supplementation may offset possible N immobilization. While plant growth and quality parameters were greatest in the P blend, increasing N applications produced similar-quality plants using alternative substrates, demonstrating that modifying fertilizer management practices can make alternative fibers a viable horticultural substrate.
1. Introduction
Demand for soilless substrate materials has increased in recent years as competition for resources emerges between expanding, newer horticultural sectors relying on soilless production, such as controlled environment specialty crops and vertical farming, and existing sectors, such as small fruit production and Christmas trees, transition to soilless culture [1,2]. The horticulture industry relies on Sphagnum peat moss as the main constituent of growing substrate as it possesses desirable physical and chemical characteristics for successful plant production and is readily available [3,4]. Much of the peat used in the global horticulture industry is shipped from Canada and several northern European countries [5]. As concerns surrounding the environmental and economic sustainability of peat use mount [6,7,8], U.S. growers are particularly interested in soilless substrate materials that (a) have a wider range of suppliers and (b) have origins closer to their production facilities [1,2]. As substrate demands are anticipated to increase in the coming decades [9], substrate materials with similar characteristics to peat moss, including low density, high water-holding capacity and air-filled porosity, sufficient cation exchange capacity, easily modified pH and nutrient content, reduced pathogen, pest, and weed pressures, and uniformity of material [4,10,11] will need to be explored, evaluated, and made readily available to growers. Several materials such as coconut coir, sugarcane bagasse, and wood fiber are currently being used and/or evaluated for soilless production due to their abundance as byproducts of major agricultural industries.
Coconut coir consists of the husk and pith from the mesocarp of the Cocos nucifera fruit and is generally considered an abundant waste product [12,13]. Coir has been successfully used as a substrate component in plant production since the 1980s [14] and possibly even earlier [15,16]. Coir is a lightweight material with high water-holding capacity and air space, as well as sufficient cation-exchange capacity, although significant variation can exist between sources [17]. In contrast with peat, coir has a slightly higher pH, potentially high salt content, superior wettability, and is generally slower to decompose [13,14]. Coir is one of the more commonly used substrate components in horticulture [4] and has demonstrated successful use in crop production [13,18,19,20].
Wood fiber is an abundant substrate material, which has been gaining momentum in the horticultural industry due to its regional availability, renewability, and low production costs [21,22]. Many forms of wood fiber exist that include byproducts and waste products from the forestry and lumber industries, as well as disc-refined and heat-extruded materials made specifically for soilless substrates. Adding wood fibers to peat-based soilless substrates has shown promise in producing salable crops when incorporated in peat at rates between 10 and 40% by volume [23,24], highlighting opportunities to reduce the pressure on peat supplies without detriment to crops. Properties of wood fiber vary based on how the fiber was produced and the particle size, but the material is generally lightweight, has air-filled porosities around 30%, and container capacities ranging from 45 to 65% by vol. [24,25,26,27].
Sugarcane bagasse (SCB) is a byproduct of the sugar industry and consists of the fibrous remnants of the cane stalk following juice extraction for producing sugar. Despite current use as a fuel to power sugar cane mills and as an unrefined material for the paper industry; significant amounts of bagasse remain [28]. Fortunately, this material is domestically abundant in tropical/subtropical sugarcane-producing regions of the United States (particularly Florida and Louisiana) and does not need to be shipped from overseas [29]. In Louisiana alone, an estimated 350,000 to 700,000 t of SCB remains unused after 80–90% is utilized as fuel for mills [30], and alternative uses are needed to mitigate storage costs. The abundance of SCB and the minimal production and pre-treatment costs of this material provide a valuable opportunity for use as a substrate amendment, particularly in the southeastern United States, where transportation costs can be reduced due to the nearby location of the sugarcane production and refinement industry. Sugarcane bagasse is lightweight, with a lower bulk density, higher air-filled porosity, and similar water-holding capacity as peat [31]. It has a track record of success in improving crop yields in field production [28] as well as in container production [31]. Despite demonstrating promise, rigorous evaluation of the physical/chemical properties of SCB and value for use in soilless plant production systems has been limited.
In contrast with peat moss, which is usually thousands of years into its degradation process by the time it is incorporated into soilless growing substrates, coir, bagasse, and wood fibers are regenerated quickly but have less stable carbon forms and higher carbon/nitrogen (C:N) ratios. These characteristics raise concerns for nitrogen immobilization due to microbially mediated decomposition and subsequent substrate shrinkage, which can reduce available N to the growing plants, hindering finished crop quality or requiring higher fertilizer inputs. For reference, the C:N of peat ranges from 20 to 80 [18,32,33,34]. Coir has a C:N around 75 to 186 [18,35], bagasse ranges from 65 to 213 [36,37,38,39], and wood can be much higher, ranging from 222 to 749 [40] but typically greater than 300 [21]. Previous research [27] has shown increased CO2 efflux rates in wood-based substrates compared to pine bark, indicating enhanced microbial activity and decomposition. However, additional research has also shown that supplementary, higher nitrogen fertilizer rates can alleviate the nitrogen deficiency effects often associated with the decomposition of carbon-rich materials. Jackson et al. [26] determined that increasing N fertigation concentration by 100 parts per million (ppm; mg L−1) in pine tree substrate yielded Euphorbia (Poinsettia) crops with similar growth and bract length as those grown in peat-based mixes. Increases in growth concomitant with increased N application in the pine tree substrate treatment were witnessed up to 300 ppm N. Similar growth improvements with 100 ppm N increases were observed in Chrysanthemum grown in pine tree substrate compared to those grown in peat-based substrates [25] and with increases in controlled release fertilizer with Ilex and Azalea grown in pine tree substrate [27]. Therefore, in evaluating the suitability of alternative substrate components, developing an associated fertility management program can greatly expand the substrate options available to growers. The objective of this study was to evaluate the suitability of coconut coir, sugarcane bagasse, and wood fiber as a substrate component compared to peat in a bark-based, greenhouse floriculture crop substrate. This was accomplished by (1) assessing the impacts of incorporating alternative substrate components on substrate physical and chemical characteristics, (2) evaluating crop quality and growth differences, and (3) determining fertilizer requirements necessary to compensate for the possible increase in nitrogen immobilization compared to peat-based substrates.
2. Materials and Methods
2.1. Substrate Blending and Physical Properties
The experimental substrate blends consisted of a base of 35% aged pine (Pinus taeda) bark (Phillips Bark Processing Co., Brookhaven, MS, USA) amended with 65% by vol. of one of four fibers. The fibers included peat moss ((P); Pro-Moss, Premier Horticulture, Ltd., Quebec, QC, Canada), coconut coir ((C); Fibredust, LLC, Cromwell, CT, USA), aged SCB ((B); stored on site for two years; Louisiana Sugar Cane Cooperative, St. Martin Parish, LA, USA), or wood fiber ((W); EZ-Blend Hydrafiber, Profile Products, Buffalo Grove, IL, USA). After hydrating the fiber materials, clumps were hand-separated prior to blending. Respective substrates were blended for 10 min. utilizing a 0.11 m−3 concrete mixer (Yardmax YM0115, Roselle, IL, USA). Lime (Lime-Rite Pelletized dolomitic lime, Imerys, Roswell, GA, USA) was applied to the C and W substrates at a rate of 2.37 kg·m−3 and the P and B substrates at a rate of 2.97 kg·m−3 to account for the generally higher pH of coir and wood compared to peat and bagasse. Thus, four experimental substrates were utilized in this research, 65:35 peat/bark (P), 65:35 coir/bark (C), 65:35 wood fiber/bark (W), and 65:35 SCB/bark (B), with all ratios being volumetric.
Three replicate samples for each blended substrate were assessed using the North Carolina State University Porometer method, as outlined by Fonteno et al. [41] for bulk density (Db), container capacity (CC), air space (AS), and total porosity (TP). Particle size distribution was conducted on three replicates of each substrate treatment and component by sifting a 100 g oven-dried sample (105 °C for 48 h) through six U.S. standard sieves with apertures of 6.3, 2.0, 0.7, 0.5, 0.3, and 0.1 mm, as well as a bottom pan, by shaking for 5 min. using a sieve shaker operated at 278 oscillations per min. (Ro-Tap RX-29; W.S. Tyler, Mentor, OH, USA). The fractions of substrates within each of the sieves were weighed and represented as a percent of the total sample material.
2.2. Crop Growth and Evaluation
The experimental study design was a multifactorial, randomized block design, where the main effects consisted of substrate and fertigation concentration (100, 200, and 300 ppm N) for a total of 12 treatments. Fifteen 2.5 L plastic containers (C300S, Nursery Supplies, Inc., Kissimmee, FL, USA) were filled per each of the four substrate treatments, with five replications per substrate and fertigation concentration combination and a total of 60 containers. Containers were uniformly filled to the top, followed by dropping from a height of 5 cm in triplicate to facilitate substrate settling, and topped-off to a substrate level just 1 cm below the lip of the container. One asexually propagated plug of Petunia Supertunia® Mini Vista ‘Indigo’ was transplanted to the center of each container on 23 February 2022. Plants were located in an on-site rain-out greenhouse with a plastic sheeting cover at the Louisiana State University AgCenter Hammond Research Station in Hammond, LA, USA. Environmental control was set to maintain temperatures between 15.6 and 32.2 C. Irrigation was supplied via pressure-compensating spray stakes (12.1 L h−1, 160-degree spray pattern; Plum, Netafim Corp., Hatzerim, Israel) installed at the outer edge of each container. Plants were manually irrigated for one week, after which irrigation was supplied in a singular, one-minute cycle of 200 mL per container at 1200 h via spray stakes every three to four days as needed. After four weeks, irrigation was applied every 1–2 days as needed to all plants. Between 4 and 7 weeks, growth differences and wilting were observed, and additional irrigation (+200 mL daily) was applied to plants in the 200 and 300 ppm treatments. At seven weeks after planting, irrigation was increased to 200 mL per container per irrigation event, twice daily, at 0900 h and 1300 h for all plants. Irrigation water samples were assessed on 28 March 2022 with a measured pH of 8.6 and alkalinity of 185.44 ppm CaCO3.
Starting 7 days after planting (DAP), crops were fertigated with 200 mL at one of three fertilizer concentrations every 7 days, replacing the daily 200 mL irrigation event. A liquid stock solution containing water-soluble fertilizer (Peters Professional Peat-Lite Special (20% Total N, 10% P2O5, 20% K2O) Base Formulation, ICL Group Ltd., Tel-Aviv, Israel) was blended at 100, 200, and 300 ppm N and kept in 95 L plastic drums. The irrigation system was engineered to deliver fertigation solution via the spray stakes, supplying 200 mL per fertigation event. As daily irrigation increased to 400 mL per plant, fertigation volume was similarly increased; thus, one month after planting, fertigation volume was increased to 400 mL per plant on all treatments.
At both 26 and 63 DAP, the leachate pH and electrical conductivity (EC) from three randomly selected replicates of each substrate and fertigation treatment were measured following the non-destructive pour-through procedure as described by Wright [42] through the use of a handheld meter (GroLine HI9814, Hanna Instruments, Smithfield, RI, USA). The final data collection event occurred at 63 DAP. The assessed plant growth and quality metrics included growth index [GI; (Widest Width × Perpendicular Width × Height)/3], bloom count (quantity of blooms at least 50% open), and chlorophyll content. The GI and bloom count were measured at 26 and 63 DAP (four and nine weeks, respectively) for each replicate. Foliar chlorophyll content was assessed non-destructively on three randomly selected, fully mature leaves, each on three randomly selected replicates of each treatment type using a soil plant analysis development (SPAD) meter (SPAD 502 Plus, Spectrum Technologies, Aurora, IL, USA). The extent to which substrate shrinkage occurred was assessed as the difference in depth from the top lip of the container to the surface of the substrate at planting and 63 DAP for four replicates to assess comparative degrees of settling and substrate blend decomposition.
Dry plant biomass was assessed for shoots and roots, respectively, via destructive harvest of plant tissue, where shoots were severed from the roots at the substrate surface level from three of the five replicates (n = 3). Shoot and root tissue were separated and dried at 70 °C for 48 h. Before drying the roots, the substrate was separated from the roots manually, followed by washing, drying at 70 °C for 48 h, and weighing.
2.3. Data Analysis
Assessments of analysis of variance (ANOVA) were performed using JMP Pro 17.0.0 (SAS Institute, Cary, NC, USA) to assess the effects on the static physical properties and particle size distribution of the different material blends. A least squares model was employed to evaluate the effects of substrate, fertigation concentration, and the interactive effects between the two. Data collected from the experiment conducted in the greenhouse were evaluated with ANOVA in JMP Pro for substrate and fertigation rate effects on pour-through parameters (pH and EC), blooms, SPAD chlorophyll content, growth index, root dry mass, shoot dry mass, and finally, root/shoot (R:S) ratio. In the event that ANOVA tests were significant, mean separation delineating differences between the substrate and fertigation treatments were assessed utilizing Tukey’s honestly significant difference at α = 0.05.
3. Results and Discussion
3.1. Substrate Properties
Total porosity amongst substrate treatments ranged from 83 to 92% (Table 1). The highest TP was observed in C, followed by W, B, and P. Similarly, C had the highest CC, followed by P, W, and B. The B and W substrates had the highest AS, followed by C, then P. Bulk density was similar among P, B, and W, substrates and lowest in C. Bagasse slightly increased TP compared to peat, improving AS and decreasing CC.

Table 1.
Physical properties of substrate blends utilized to grow Petunia Supertunia Mini Vista ‘Indigo’ crop in this research.
Differences in particle size distribution between substrates were observed (Table 1). Most notably, C had higher proportions of medium and small particles, whereas the other substrates tended to have higher proportions of larger particles. This is likely due to the observation that peat, wood fiber, and bagasse particles tended to clump together, whereas coir particles separated quite easily.
Previous research includes both similarities and differences with these results. Regarding wood fiber, Dickson et al. [24] showed improved TP, AS, and CC on Hydrafiber blended at 60% compared to peat, whereas results herein demonstrate decreases in CC using the same wood fiber at 65%. Regarding coco coir, this research demonstrates coco coir as a lightweight (low Db) substance that improves TP, AS, and CC compared to peat. Dickson et al. [24] showed decreased AS by nearly half compared to peat when evaluating the same on 60% fiber blends. Meerow [13] found little change in AS and CC between bark blends mixed with coir versus sphagnum peat. Fields et al. [43] also found higher TP and AS in coir/bark blends than peat/bark blends at 35% incorporation. This further supports that the physical properties of coir can vary widely depending on the source of the fiber [17].
3.2. Greenhouse Experiment
Between 26 and 63 DAP, substrate pH decreased at all fertilizer concentrations in the P substrate by 0.3 to 0.6 units (Table 2). The other substrates tended to show increases in substrate pH during that timeframe, except for C at 200 ppm and 300 ppm N, which slightly decreased over time. A substrate pH rise was expected during plant production at this research location as a result of the relatively high pH and alkalinity of the irrigation water used and the tendency of petunia species to increase substrate pH over time [44,45]. Dickson et al. [24] also found that pH increased in coir and Hydrafiber blends compared to peat/bark blends, though it was not as high as the results found here. Stamps and Evans [46] found increases over time in 1:1 coir/bark blends that surpassed pH increases in 1:1 peat/bark blends; however, the differences in pH were still less than one unit. In our study, substrate pH levels remained within an acceptable range (5.5 to 6.2 [47]) at all fertigation levels and time points for P and B substrates but remained above 7 for C and W substrates. Similar substrate research [13,20] identified little to no difference in pH in coir substrates over peat. Sugarcane bagasse has an inherently lower pH and has previously shown the capacity to mitigate rising pH in production systems with high pH and alkalinity irrigation water [31]. While the incorporation of SCB at lower percentages (15% and 30%) maintained a pH at or below that of peat in the Thiessen et al. [31] study, the results of this study showed that twice as much bagasse usage maintained a pH at similar or lower levels than peat only up to 30 DAP. Similar increases in pH throughout the production process using Hydrafiber were identified when compared to bagasse when blended with a standard bagged substrate by Thiessen et al. [31]. Iron deficiency is the most common disorder in petunia production and tends to occur at substrate pH levels greater than 6.4 [47]; therefore, although C and W were limed at a lower rate than P and B in this study, even lower rates may be needed to ensure proper substrate pH ranges during production. Pour-through EC values were lower than 1.0 mS/cm for all treatments at all times, below the recommended range [47]. At 26 DAP, changes in EC with fertilizer concentrations were not apparent. At 63 DAP, EC values generally increased with fertilizer concentration, but the effects of fertilizer rates on pour-through EC beyond 200 ppm N were limited in P, W, and C and greater in the B substrate. It is important to remember that changes in EC are affected by differences in plant growth throughout the experiment, which affected plant uptake. Greater increases in pour-through EC with increasing fertilizer indicate less uptake, which was witnessed in all B treatments and in P at 200 ppm N.

Table 2.
Growth index (GI) and pore water fertility assessment over the production cycle of a Petunia Supertunia® Mini Vista ‘Indigo’ crop produced in Trade #1 containers, conducted using the pour-through method.
The growth index improved with the fertigation rate and time in all treatments (Table 2). Growth increases due to the fertilizer effect were most dramatic in B and W substrates at the end of the experiment, suggesting possible N consumption associated with microbial decay of wood and SCB. Petunia growth index was higher at all dates and in all fertilizer treatments in the P substrate than all other blends; however, increased fertilizer concentration (300 ppm N) was associated with a statistically similar final growth index to P in C and W substrates (Table 2), indicating that increased fertilizer can account for losses in fertility due to possible N immobilization. Meerow [13] found no differences in growth index in Pentas grown in coir/bark blends compared to peat but a significant decrease in Ixora. This suggests that sensitivity to the properties of coir varies by species.
Increasing fertilizer concentration was also associated with increased shoot mass, chlorophyll content, and blooms in all substrate treatments by the end of the experiment (Table 3). Plants grown in the P substrate outperformed other substrates in all these measurements at all fertilizer concentrations and were visually superior to all other treatments (Figure 1); however, C shoot weight at 300 ppm N and chlorophyll in all treatments at 300 ppm N were statistically similar to those of the P substrate (Table 3), demonstrating that increased fertilizer application can account for any negative effects associated with N immobilization in these other fibers. Dickson et al. [24] also showed similarly higher values for growth, dry mass, and blooms in peat substrates over coir and wood fiber in petunia. Previous research utilizing large proportions of pine tree substrate [25,26] found that increases in fertilizer of about 100 ppm N were enough to reach growth index and shoot dry weight values similar to plants grown in peat-based mixes. The results herein suggest that increases of at least 200 ppm N for coir and wood fiber materials, and possibly much higher for bagasse, are needed to approach growth and shoot mass values comparable to plants grown in peat substrates. The B substrate had the lowest growth index, shoot mass, and blooms at every fertilizer level. Thiessen et al. [31] found no differences in growth and quality measurements in Osteospermum grown in 15% and 30% bagasse blended with a peat-based mix, suggesting the 65% blend used in this experiment may be too high for adequate plant health or require fertilizer levels that negate potential savings. Despite the decreases in plant growth and blooms, the B substrate had higher chlorophyll content than the C and W substrates at every fertilizer level, possibly due to a slightly higher N and micronutrient availability with lower substrate pH (Table 2). With substrate pH levels around 7, it is quite likely C and W plants were experiencing Fe and Mn deficiencies, which can contribute significantly to leaf chlorosis [47].

Table 3.
Substrate effects on root mass, shoot mass, root/shoot, shrinkage, soil plant analysis development (SPAD) chlorophyll, and bloom count of Petunia Supertunia® Mini Vista ‘Indigo’ grown in different substrate blends at three fertigation levels.
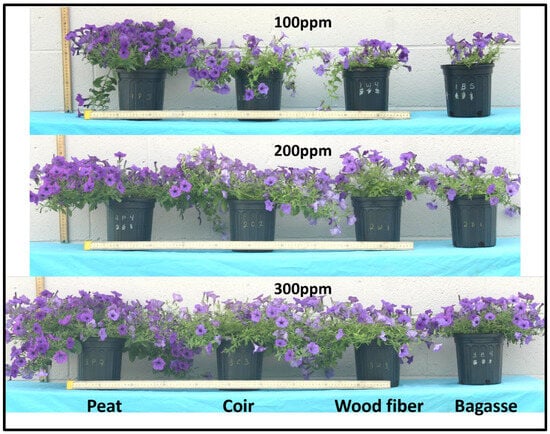
Figure 1.
Crop growth and blooming differences between plants grown in different substrate blends under 100 parts per million (ppm) nitrogen (N; top), 200 ppm N (middle), and 300 ppm N (bottom) fertigation levels. Pictured are the replications most representative of the average growth for each substrate and fertigation combination.
Root mass was generally highest in the C substrates and lowest in B, with the exception of P at 300 ppm (Table 3). Both P and W intermediate at all fertilizer levels with no consistent trend with fertilizer concentration (Table 3). Root quality was visually assessed and ubiquitously considered healthy in all treatments (Figure 2). Meerow [13] also found no difference in pentas root dry mass grown in bark blends amended with coir vs. sphagnum peat. Root/shoot ratios were highest in non-peat substrates at 100 ppm N fertilizer (Table 3), indicating these plants were more stressed than those grown in peat, and putting more resources towards root growth over shoot growth. Root/shoot ratios decreased in non-peat substrates with higher fertilizer concentrations but still generally remained higher than those of peat-grown plants.

Figure 2.
Root growth differences between plants grown in different substrate blends under 100 parts per million (ppm) nitrogen (N; top), 200 ppm N (middle), and 300 ppm N (bottom) fertigation levels. Pictured are the replications most representative of the average growth for each substrate and fertigation combination.
Substrate shrinkage over the two-month growing period ranged from −3.53% to 5.13% (Table 3). Slight substrate expansion occurred in some instances, most notably in C at 300 ppm and W at 200 ppm. No clear trends were witnessed across substrates and fertilizer concentrations. Shrinkage was higher in B substrates but did not significantly change with fertilizer level (p = 0.7498); in fact, fertilizer concentration did not significantly affect shrinkage in any substrate. Shrinkage amounts were about 10× higher in this experiment than those found in 15 and 30% blends of Hydrafiber and SCB blended with a peat-based mix in Osteospermum production [31], illustrating that higher blend percentages of these fibers will lead to greater settling and/or decomposition.
4. Conclusions
Efforts to ensure substrate security may be realized by using alternative materials as an amendment to horticultural substrates. While sphagnum peat moss is the industry standard, several materials of interest displayed promise as sustainably sourced peat alternatives when blended with pine bark. Coco coir, wood fiber, and bagasse all increased total substrate porosity and air space compared to sphagnum peat. Coco coir also slightly improved container capacity while wood fiber and bagasse decreased it, indicating that irrigation practices will likely need further tailoring when switching to these fibers. Substrate pH was maintained within desirable nutrient availability ranges only in the sphagnum peat moss treatment for the entirety of the growing period when blended with pine bark at 65% compared to coir, wood fiber, and bagasse, suggesting further adjustments to liming rates are needed and/or blend percentages need to be adjusted depending on the pH sensitivity of the particular crop species. Bagasse also kept substrate levels within desirable ranges during crop growth; however, the upward trend of pH poses concerns for crops with longer production times. While pH values for wood fiber and bagasse remained higher than that of the other materials investigated, they also remained relatively stable throughout the crop growth period and may likely hold stable, appropriate pH ranges if less lime is incorporated at planting. Supplementing fertilizer improved the growth of petunias grown in coir, wood, and bagasse; at least 200 ppm N is needed for coir and wood fiber and higher amounts for SCB to produce plants with growth and quality parameters that approach those of petunia grown in peat.
Significant variation can occur across types of wood fiber and sources of coco coir and bagasse. As demand for peat continues to rise, the readily available, low-cost, and local nature of both wood fiber and bagasse to U.S. growers substantiates the need to further explore these sources and the pretreatment methods, forms, blend percentages, and production practices that result in adequate plant growth and quality. The blending of the fibers used herein at 65% with bark may adjust the pH too drastically or require additional fertilizer that is economically disadvantageous for sensitive, heavy-feeding crops such as petunia and may be better suited as a replacement for a smaller portion of peat in bark-based mixes or limited to crops with lower nutrient sensitivity and feeding requirements.
Author Contributions
Conceptualization, J.S.F.; methodology, J.S.F. and M.E.T.; formal analysis, M.E.T. and D.E.A.; investigation, M.E.T.; resources, J.S.F.; data curation, M.E.T.; writing—original draft preparation, M.E.T.; writing—review and editing, J.S.F. and D.E.A.; visualization, M.E.T.; supervision, J.S.F.; project administration, J.S.F.; funding acquisition, J.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Ashley Hickman, Research Associate with the Hammond Research Station, for her assistance with project initiation and data collection. We would further like to thank student interns Fidelina Olivia Lemus and Amanda Mizell for their assistance with data collection.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fields, J.; Owen, J.; Lamm, A.; Altland, J.; Jackson, B.; Zheng, Y.; Oki, L.; Fontenot, K.; Samtani, J.; Campbell, B. Soilless substrate science: A North American needs assessment to steer soilless substrate research into the future. In Proceedings of the II International Symposium on Growing Media, Soilless Cultivation, and Compost Utilization in Horticulture, Ghent, Belgium, 22–27 August 2021; Volume 1317, pp. 313–318. [Google Scholar] [CrossRef]
- Jackson, B.E.; Fields, J.; Owen, J.; Altland, J. Soilless Substrate Science for Current and Future Growers. Grower Talks. 2022. Available online: https://www.growertalks.com/Article/?articleid=25658 (accessed on 29 November 2023).
- Maher, M.; Prasad, M.; Raviv, M. Organic Soilless Media Components. In Soilless Culture: Theory and Practice; Raviv, M., Lieth, J.H., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 459–504. [Google Scholar]
- Schmilewski, G. The Role of Peat in Assuring the Quality of Growing Media. Mires Peat 2008, 3, 1–8. [Google Scholar]
- Peat for Horticulture. International Peatland Society. 2019. Available online: https://peatlands.org/peat/peat-for-horticulture/ (accessed on 1 February 2024).
- Alexander, P.D.; Bragg, N.C.; Meade, R.; Padelopoulos, G.; Watts, O. Peat in horticulture and conservation: The UK response to a changing world. Mires Peat 2008, 3, 1–10. [Google Scholar]
- Cleary, J.; Roulet, N.T.; Moore, T.R. Greenhouse Gas Emissions from Canadian Peat Extraction, 1990–2000: A Life-cycle Analysis. AMBIO 2005, 34, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Freeman, C. Peatlands: Our greatest source of carbon credits? Carbon Manag. 2011, 2, 289–301. [Google Scholar] [CrossRef]
- Blok, C.; Eveleens, B.; van Winkel, A. Growing media for food and quality of life in the period 2020–2050. Acta Hortic. 2021, 1305, 341–356. [Google Scholar] [CrossRef]
- Krucker, M.; Hummel, R.L.; Cogger, C. Chrysanthemum Production in Composted and Noncomposted Organic Waste Substrates Fertilized with Nitrogen at Two Rates Using Surface and Subirrigation. HortScience 2010, 45, 1695–1701. [Google Scholar] [CrossRef]
- Yu, P.; Li, Q.; Huang, L.; Niu, G.; Gu, M. Mixed Hardwood and Sugarcane Bagasse Biochar as Potting Mix Components for Container Tomato and Basil Seedling Production. Appl. Sci. 2019, 9, 4713. [Google Scholar] [CrossRef]
- Mariotti, B.; Martini, S.; Raddi, S.; Tani, A.; Jacobs, D.F.; Oliet, J.A.; Maltoni, A. Coconut Coir as a Sustainable Nursery Growing Media for Seedling Production of the Ecologically Diverse Quercus Species. Forests 2020, 11, 522. [Google Scholar] [CrossRef]
- Meerow, A.W. Growth of Two Subtropical Ornamentals Using Coir (Coconut Mesocarp Pith) as a Peat Substitute. HortScience 1994, 29, 1484–1486. [Google Scholar] [CrossRef]
- Newman, J. Core Facts about Coir. Nursery Management. 2007. Available online: https://www.nurserymag.com/news/core-facts-about-coir/ (accessed on 29 November 2023).
- Pryce, S. Alternatives to peat. Prof. Hort. 1991, 5, 101–106. [Google Scholar]
- Reynolds, S. Preliminary Studies in Western Samoa Using Various Parts of the Coconut Palm (Cocos Nucifera L.) as Growing Media. Acta Hortic. 1973, 37, 1983–1991. [Google Scholar] [CrossRef]
- Abad, M.; Fornes, F.; Carrión, C.; Noguera, V.; Noguera, P.; Maquieira, A.; Puchades, R. Physical Properties of Various Coconut Coir Dusts Compared to Peat. HortScience 2005, 40, 2138–2144. [Google Scholar] [CrossRef]
- Arenas, M.; Vavrina, C.; Cornell, J.; Hanlon, E.; Hochmuth, G. Coir as an Alternative to Peat in Media for Tomato Transplant Production. HortScience 2002, 37, 309–312. [Google Scholar] [CrossRef]
- Evans, M.R.; Stamps, R. 486 PB 128 Growth of Annual Species in Coconut Coir Substrates. HortScience 1994, 29, 501a–501. [Google Scholar] [CrossRef]
- Scagel, C.F. Growth and Nutrient Use of Ericaceous Plants Grown in Media Amended with Sphagnum Moss Peat or Coir Dust. HortScience 2003, 38, 46–54. [Google Scholar] [CrossRef]
- Harris, C.N.; Dickson, R.W.; Fisher, P.R.; Jackson, B.E.; Poleatewich, A.M. Evaluating Peat Substrates Amended with Pine Wood Fiber for Nitrogen Immobilization and Effects on Plant Performance with Container-grown Petunia. HortTechnology 2020, 30, 107–116. [Google Scholar] [CrossRef]
- Durand, S.; Jackson, B.E.; Fonteno, W.C.; Michel, J.-C. The Use of Wood Fiber for Reducing Risks of Hydrophobicity in Peat-Based Substrates. Agronomy 2021, 11, 907. [Google Scholar] [CrossRef]
- Jackson, B.E. Substrates on Trial: Wood Fiber in the Spotlight. 2018. Available online: https://www.greenhousemag.com/article/substrates-on-trial-wood-fiber-in-the-spotlight/ (accessed on 13 November 2023).
- Dickson, R.W.; Helms, K.M.; Jackson, B.E.; Machesney, L.M.; Lee, J.A. Evaluation of Peat Blended with Pine Wood Components for Effects on Substrate Physical Properties, Nitrogen Immobilization, and Growth of Petunia (Petunia × hybrida Vilm.-Andr.). HortScience 2022, 57, 304–311. [Google Scholar] [CrossRef]
- Wright, R.D.; Jackson, B.E.; Browder, J.F.; Latimer, J.G. Growth of Chrysanthemum in a Pine Tree Substrate Requires Additional Fertilizer. HortTechnology 2008, 18, 111–115. [Google Scholar] [CrossRef]
- Jackson, B.E.; Wright, R.D.; Barnes, M.C. Pine Tree Substrate, Nitrogen Rate, Particle Size, and Peat Amendment Affect Poinsettia Growth and Substrate Physical Properties. HortScience 2008, 43, 2155–2161. [Google Scholar] [CrossRef]
- Jackson, B.E.; Wright, R.D.; Browder, J.F.; Harris, J.R.; Niemiera, A.X. Effect of Fertilizer Rate on Growth of Azalea and Holly in Pine Bark and Pine Tree Substrates. HortScience 2008, 43, 1561–1568. [Google Scholar] [CrossRef]
- Bhadha, J.H.; Xu, N.; Khatiwada, R.; Swanson, S.; LaBorde, C. Bagasse: A Potential Organic Soil Amendment Used in Sugarcane Production. 2020. Available online: https://edis.ifas.ufl.edu/pdf/SS/SS69000.pdf (accessed on 10 March 2024).
- Background. USDA ERS—Background. (2021, October 19). Available online: https://www.ers.usda.gov/topics/crops/sugar-and-sweeteners/background/#production (accessed on 10 March 2024).
- Webber, C.L., III; White, P.M., Jr.; Spaunhorst, D.J.; Lima, I.M.; Petrie, E.C. Sugarcane Biochar as an Amendment for Greenhouse Growing Media for the Production of Cucurbit Seedlings. J. Agric. Sci. 2018, 10, 104–115. [Google Scholar] [CrossRef]
- Thiessen, M.; Fields, J.S.; Abdi, D.; Beasley, J. Sugarcane Bagasse Is an Effective Soilless Substrate Amendment in Quick-turn Osteospermum Production. HortScience 2023, 58, 1170–1177. [Google Scholar] [CrossRef]
- Loisel, J.; Yu, Z.; Beilman, D.W.; Camill, P.; Alm, J.; Amesbury, M.J.; Anderson, D.; Andersson, S.; Bochicchio, C.; Barber, K.; et al. A database and synthesis of northern peatland soil properties and Holocene carbon and nitrogen accumulation. Holocene 2014, 24, 1028–1042. [Google Scholar] [CrossRef]
- Peat, Inc. Analysis Summary. 2022. Available online: https://www.peatinc.com/about/ (accessed on 29 November 2023).
- Watmough, S.; Gilbert-Parkes, S.; Basiliko, N.; Lamit, L.J.; Lilleskov, E.A.; Andersen, R.; del Aguila-Pasquel, J.; Artz, R.E.; Benscoter, B.W.; Borken, W.; et al. Variation in carbon and nitrogen concentrations among peatland categories at the global scale. PLoS ONE 2022, 17, e0275149. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Puchades, R.; Maquieira, A.; Noguera, V. Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants. Bioresour. Technol. 2002, 82, 241–245. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, J.; Vig, A.P. Potential utilization of bagasse as feed material for earthworm Eisenia fetida and production of vermicompost. SpringerPlus 2015, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Nisaren, B.N.; Wogi, L.; Tamiru, S. Effect of filter cake and bagasse on selected physicochemical properties of calcareous sodic soils at Amibara, Ethiopia. Int. J. Agron. Agric. Res. 2019, 14, 20–28. [Google Scholar]
- Uchimiya, M.; Hay, A.G.; LeBlanc, J. Chemical and microbial characterization of sugarcane mill mud for soil applications. PLoS ONE 2022, 17, e0272013. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Bhadha, J.H.; Rabbany, A.; Swanson, S.; McCray, J.M.; Li, Y.C.; Strauss, S.L.; Mylavarapu, R. Crop Nutrition and Yield Response of Bagasse Application on Sugarcane Grown on a Mineral Soil. Agronomy 2021, 11, 1526. [Google Scholar] [CrossRef]
- Domeño, I.; Irigoyen, I.; Muro, J. New wood fibre substrates characterization and evaluation in hydroponic tomato culture. Eur. J. Hortic. Sci. 2010, 75, 89–94. [Google Scholar]
- Fonteno, W.C.; Hardin, C.T.; Brewster, J.P. Procedures for Determining Physical Properties of Horticultural Substrates Using the NCSU Porometer; Horticultural Substrates Laboratory, North Carolina State University: Raleigh, NC, USA, 1995. [Google Scholar]
- Wright, R.D. The Pour-through Nutrient Extraction Procedure. HortScience 1986, 21, 227–229. [Google Scholar] [CrossRef]
- Fields, J.S.; Owen, J.S., Jr.; Scoggins, H.L. The Influence of Substrate Hydraulic Conductivity on Plant Water Status of an Ornamental Container Crop Grown in Suboptimal Substrate Water Potentials. HortScience 2017, 52, 1419–1428. [Google Scholar] [CrossRef]
- Argo, B.; Fisher, P. Understanding Plant Nutrition: Irrigation Water Alkalinity and pH. 2008. Available online: https://www.greenhousegrower.com/production/fertilization/understanding-plant-nutrition-irrigation-water-alkalinity-ph/ (accessed on 13 January 2023).
- Dickson, R.W.; Fisher, P.R.; Argo, W.R. Quantifying the Acidic and Basic Effects of Fifteen Floriculture Species Grown in Peat-based Substrate. HortScience 2017, 52, 1065–1072. [Google Scholar] [CrossRef]
- Stamps, R.H.; Evans, M.R. Growth of Dieffenbachia maculata ‘Camille’ in Growing Media Containing Sphagnum Peat or Coconut Coir Dust. HortScience 1997, 32, 844–847. [Google Scholar] [CrossRef]
- Owen, W.G.; Henry, J.; Whipker, B. Fert, Dirt, and Squirt: Monitoring pH and EC of Greenhouse Crops. E-Gro Diagnostic Series, 12. 2018. Available online: http://www.fertdirtandsquirt.com/books.php (accessed on 19 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).