An Evaluation of Insecticidal Trunk Injections for the Control of the European Cherry Fruit Fly Rhagoletis cerasi L. (Diptera: Tephritidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites
2.2. Trunk Injection Method
2.3. Sampling and Evaluation of Insecticidal Effects
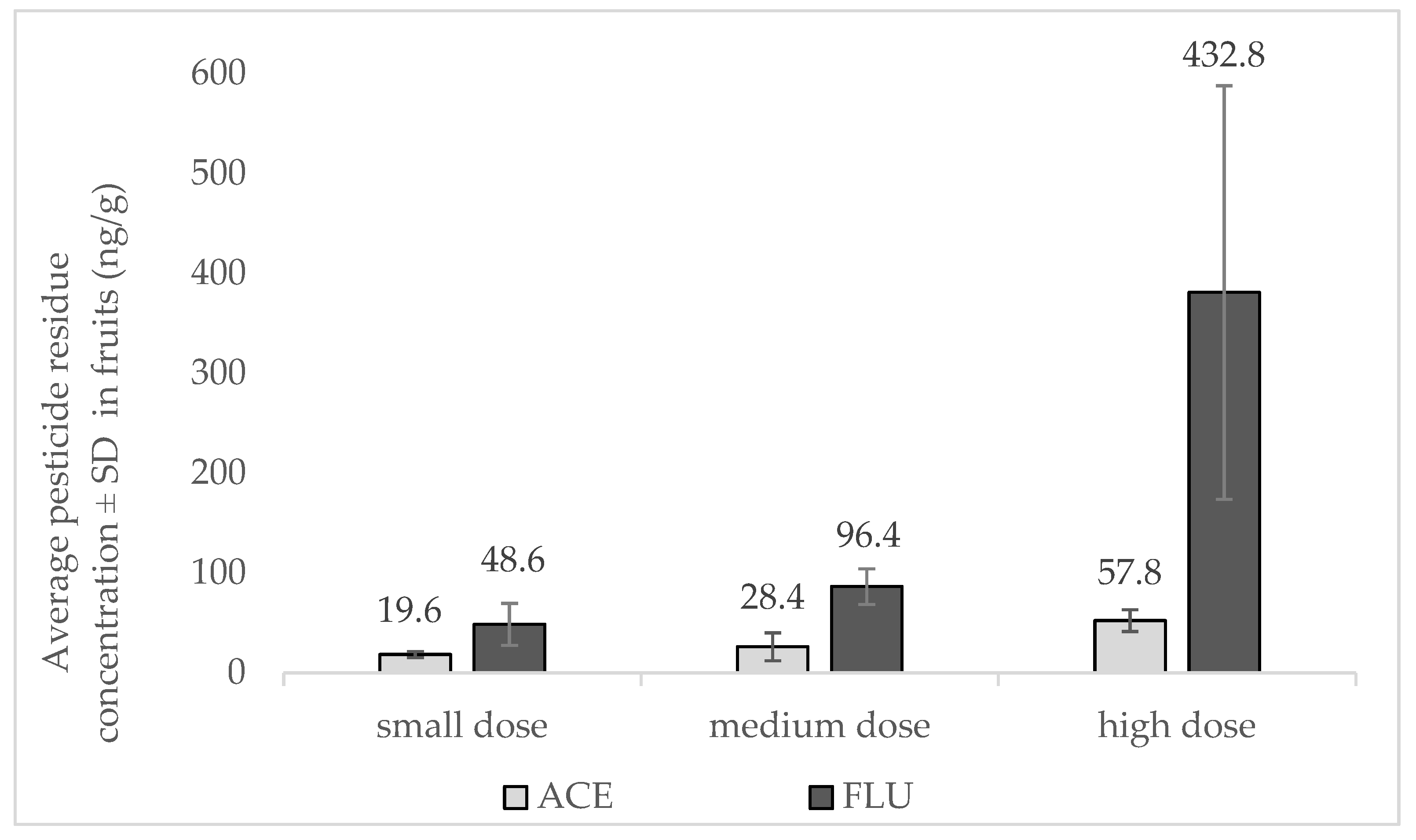
2.4. Analyses of Pesticide Residue
2.5. Statistical Analyses
3. Results
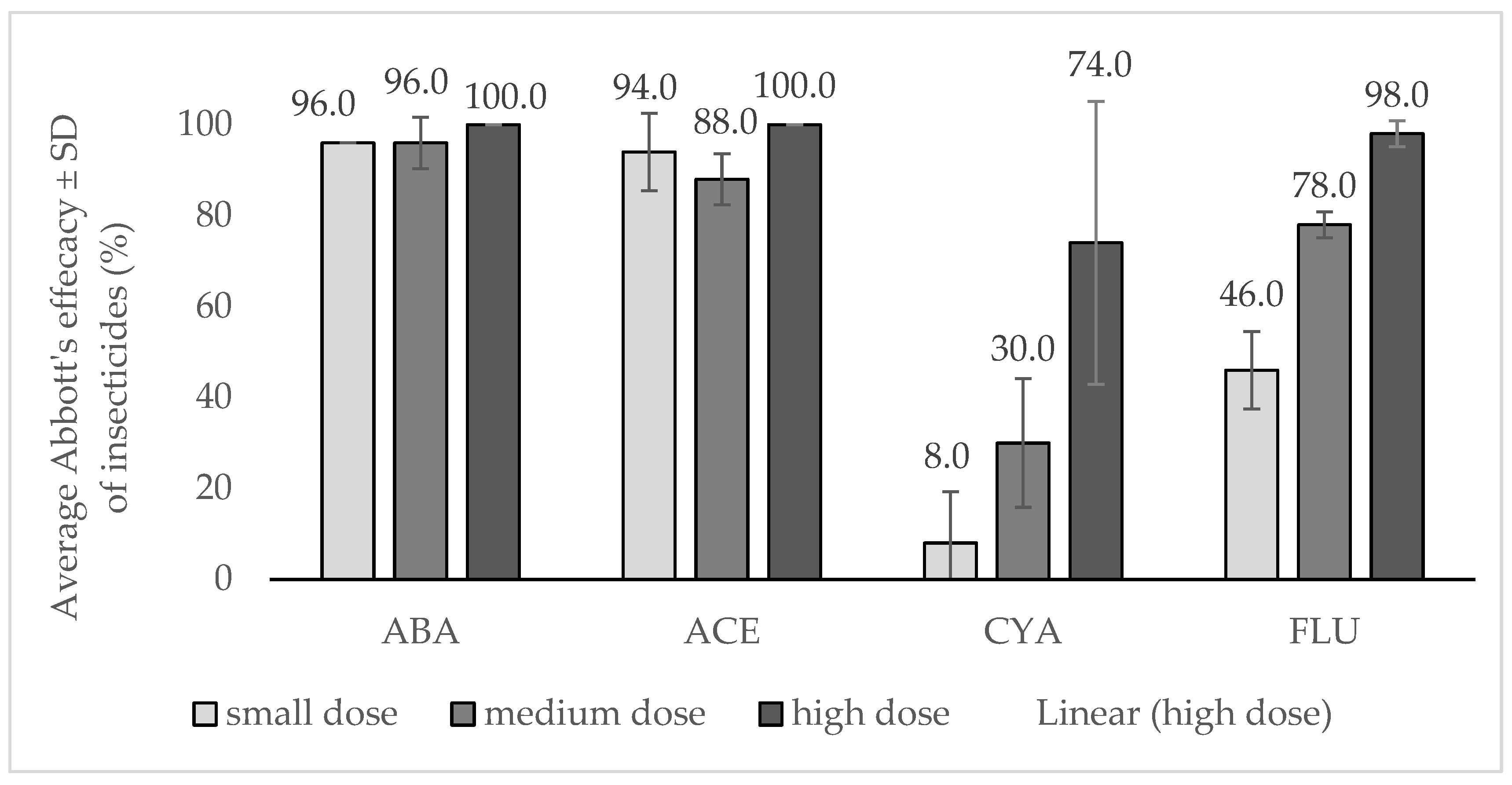
3.1. Efficacy Test in 2021
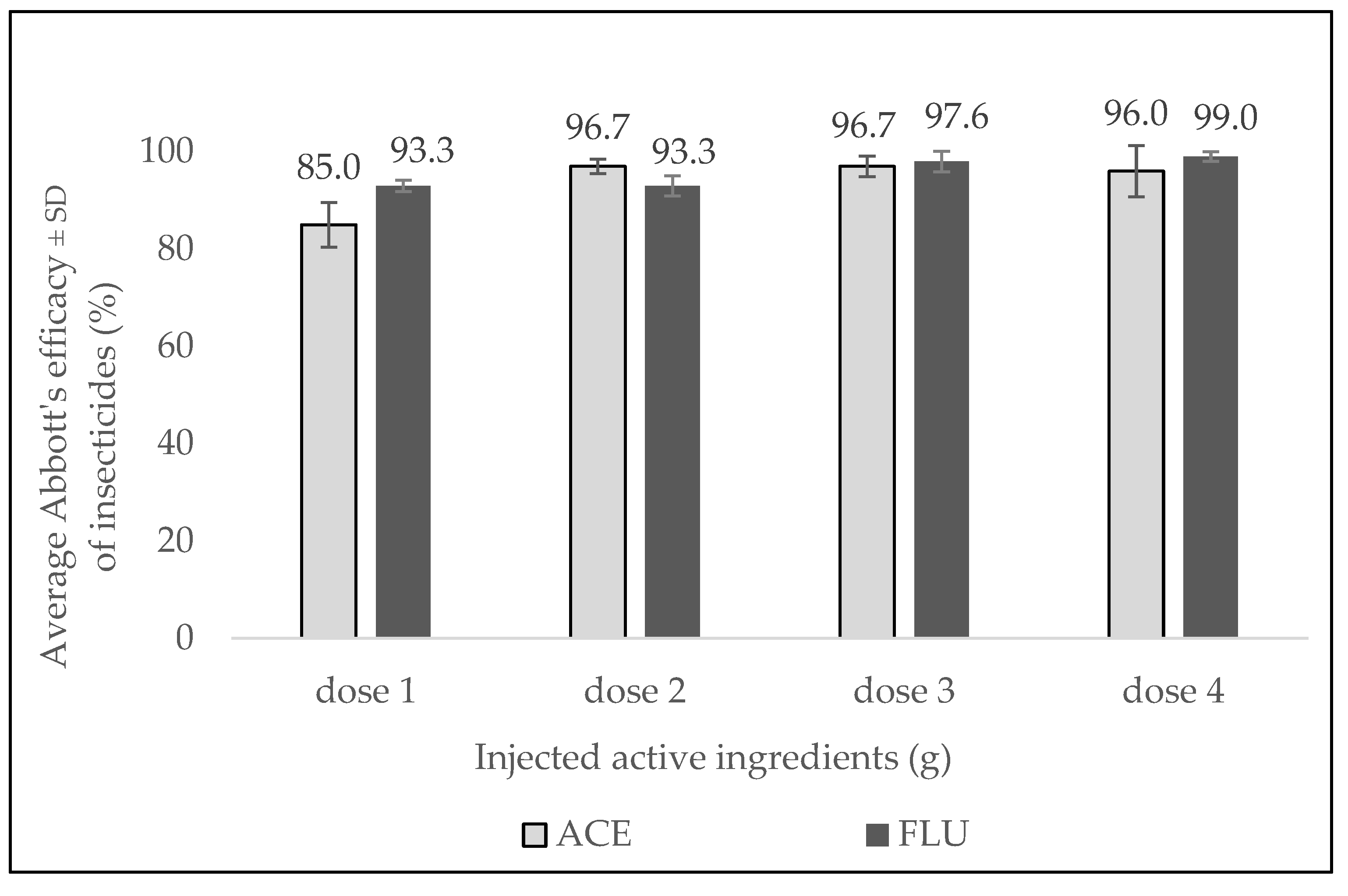
3.2. Dosage Evaluation 2022
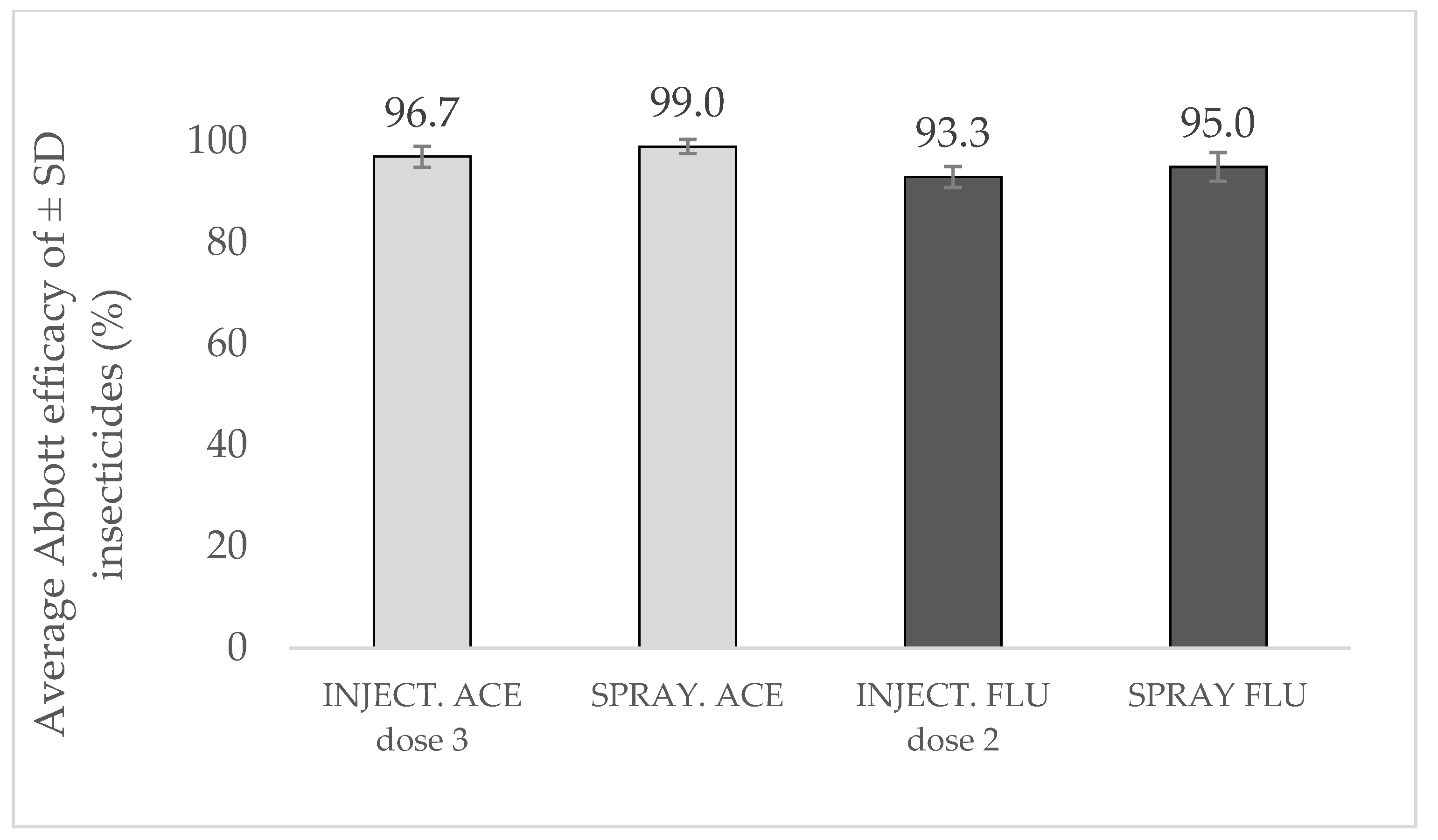
3.3. Spray Control Comparison
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hungarian Central Statistical Office. Agriculture. Available online: https://www.ksh.hu/stadat_eng (accessed on 11 May 2022).
- Food and Agriculture Organization of the United Nations. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en (accessed on 20 July 2023).
- Stamenković, S.; Perić, P.; Milošević, D. Rhagoletis cerasi Loew (Diptera: Tephritidae)—Biological Characteristics, Harmfulness and Control. Pestic. Phytomed. 2012, 27, 269–281. [Google Scholar] [CrossRef]
- Fimiani, P. Multilarval infestations by Rhagoletis cerasi L. (Diptera: Trypetidae) in cherry fruits. In Fruit Flies of Economic Importance; Cavalloro, R., Ed.; Balkema: Rotterdam, The Netherlands, 1983; pp. 52–59. [Google Scholar]
- Daniel, C.; Wyss, E. Susceptibility of different life stages of the European cherry fruit fly, Rhagoletis cerasi, to entomopathogenic fungi. J. Appl. Entomol. 2009, 133, 473–483. [Google Scholar] [CrossRef]
- Alaserhat, İ. Investigation of Distribution, Population Development, Flight Period, Infestation and Damage Rate of Cherry Fruit Fly, Rhagoletis cerasi L. (Diptera: Tephritidae), Associated with Cherry and Sour Cherry Orchards. Erwerbs-Obstbau 2022, 64, 155–164. [Google Scholar] [CrossRef]
- Büda, V.; Radžiutė, S.; Apšegaitė, V.; Blažytė-Čereškienė, L.; Čepulytė, R.; Bumbulytė, G.; Mozūraitis, R. Electroantennographic and Behavioural Responses of European Cherry Fruit Fly, Rhagoletis cerasi, to the Volatile Organic Compounds from Sour Cherry, Prunus cerasus, Fruit. Insects 2022, 13, 114. [Google Scholar] [CrossRef]
- AliNiazee, M.T.; Long, L.E. Biology and Control of the Cherry Fruit Flies: A Worldwide Perspective, Agricultural Experiment Station, Oregon State University, Special Report 971. In Proceedings of the International Cherry Fruit Fly Symposiumon, The Dalles, OR, USA, 3 March 1995. [Google Scholar]
- Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics 1995, 8, 17–29. [Google Scholar] [CrossRef]
- Zhu, H.; Derksen, R.C.; Guler, H.; Krause, C.R.; Ozkan, H.E. Foliar deposition and off-target loss with different spray techniques in nursery applications. Trans. ASABE 2006, 49, 325–334. [Google Scholar] [CrossRef]
- Wise, J.C.; VanWoerkom, A.H.; Aćimović, S.G.; Sundin, G.W.; Cregg, B.M. Trunk Injection: A Discriminating Delivering System for Horticulture Crop IPM. Entomol. Ornithol. Herpetol. 2014, 3, 126. [Google Scholar]
- Aćimović, S.G.; Cregg, B.M.; Sundin, G.W.; Wise, J.C. Comparison of drill- and needle-based tree injection technologies in healing of trunk injection ports on apple trees. Urban For. Urban Green. 2016, 19, 151–157, ISSN 1618–8667. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Cregg, B.M.; McCullough, D.G.; Poland, T.M.; Hol-lingworth, R.M. Distribution of trunk-injected 14C-imidacloprid in ash trees and effects on emerald ash borer (Coleoptera: Buprestidae) adults. Crop Prot. 2009, 28, 655–661. [Google Scholar] [CrossRef]
- VanWoerkom, A.H.; VanWoerkom, S.G.; Aćimović, G.W.; Sundin, B.M.; Cregg, D.; Mota-Sanchez, C.; Vandervoort, J.C.; Wise, J.C. Trunk injection: An alternative technique for pesticide delivery in apples. Crop Prot. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Acimovic, S.G. Disease Management in Apples Using Trunk Injection Delivery of Plant Protective Compounds. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2014. [Google Scholar] [CrossRef]
- Archer, L.; Crane, J.H.; Albrecht, U. Trunk injection as a tool to deliver plant protection materials—An overview of basic principles and practical considerations. Horticulturae 2022, 8, 552. [Google Scholar] [CrossRef]
- Berger, C.; Laurent, F. Trunk injection of plant protection products to protect trees from pests and diseases. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Doccola, J.J.; Wild, P.M. Tree Injection as an Alternative Method of Insecticide Application. In Insecticides—Basic and Other Applications; Soloneski, S., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 61–78. [Google Scholar]
- Burkhard, R.; Binz, H.; Roux, C.A.; Brunner, M.; Ruesch, O.; Wyss, P. Environmental fate of emamectin benzoate after tree micro injection of horse chestnut trees. Environ. Toxicol. Chem. 2015, 34, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Kiss, M.; Hachoumi, I.; Ladányi, M.; Nagy, V. Preliminary results about the efficacy of abamectin trunk injection against the walnut husk fly (Rhagoletis completa). J. Plant Dis. Prot. 2020, 128, 333–338. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Skourti, A.; Nika, E.P.; Papadoulis, G.T. Trunk Injection with Insecticides Manages Xylotrechus chinensis (Chevrolat) (Coleoptera: Cerambycidae). Insects 2022, 13, 1106. [Google Scholar] [CrossRef]
- Khosroshahi, M.Z.; Sareh, N.; Abolmohsen, H. Rapid correction of iron deficiency in peach trees by direct injection of ferrous sulfate into the trunk. Arch. Agron. Soil Sci. 2022, 69, 1405–1418. [Google Scholar] [CrossRef]
- Archer, L.; Kunwar, S.; Alferez, F.; Batuman, O.; Albrecht, U. Trunk Injection of Oxytetracycline for Huanglongbing Management in Mature Grapefruit and Sweet Orange Trees. Phytopathology 2023, 113, 1010–1021. [Google Scholar] [CrossRef]
- Kiss, M.; Sörös, C.; Gutermuth, Á.; Ittzés, A.; Szabó, Á. Avermectin Trunk Injections: A Promising Approach for Managing the Walnut Husk Fly (Rhagoletis completa). Horticulturae 2023, 9, 655. [Google Scholar] [CrossRef]
- Denoirjean, T.; Belhassen, D.; Doury, G.; Ameline, A.; Werrie, P.Y.; Fauconnier, M.L.; Hance, T.; Le Goff, G.J. Essential Oil Trunk Injection Into Orchard Trees: Consequences on the Performance and Preference of Hemipteran Pests. J. Econ. Entomol. 2023, 116, 389–398. [Google Scholar] [CrossRef]
- Wheeler, C.E.; Vandervoort, C.; Wise, C.J. Organic Control of Pear Psylla in Pear with Trunk Injection. Insects 2020, 11, 650. [Google Scholar] [CrossRef]
- Skalský, M.; Niedobová, J.; Popelka, J. The efficacy of European fruit Lecanium, Parthenolecanium corni (Bouché, 1844) control using natural products. Hort. Sci. 2019, 46, 195–200. [Google Scholar] [CrossRef]
- EN 15662:2018; European Standard—Foods of Plant Origin—Multimethod for the Determination of Pesticide Residues Using GC- and LC-Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE—Modular QuEChERS-Method. European Commission: Brussels, Belgium, 2018. Available online: https://standards.cen.eu/ (accessed on 22 August 2022).
- Végh, R.; Sörös, C.; Majercsik, N.; Sipos, L. Determination of Pesticides in Bee Pollen: Validation of a Multiresidue High-Performance Liquid Chromatography-Mass Spectrometry/Mass Spectrometry Method and Testing Pollen Samples of Selected Botanical Origin. J. Agric. Food Chem. 2022, 70, 1507–1515. [Google Scholar] [CrossRef]
- Guidance SANTE 11312/2021—Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. Available online: https://www.accredia.it/en/documento/guidance-sante-11312-2021-analytical-quality-control-and-method-validation-procedures-for-pesticide-residues-analysis-in-food-and-feed/ (accessed on 11 October 2023).
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The aligned rank transform for nonparametric factorial analyses using only ANOVA procedures. In Proceedings of the ACM Conference on Human Factors in Computing Systems (CHI ’11), Vancouver, BC, Canada, 7–12 May 2011; Honorable Mention Paper. ACM Press: New York, NY, USA, 2011; pp. 143–146. [Google Scholar]
- Durner, E. Effective Analysis of Interactive Effects with Non-Normal Data Using the Aligned Rank Transform, ARTool and SAS University Edition. Horticulturae 2019, 5, 57. [Google Scholar] [CrossRef]
- Dobson, A.J. An Introduction to Generalized Linear Models, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2002. [Google Scholar]
- Cody, R. Biostatistics by Example Using SAS Studio; SAS Institute: Tokyo, Japan, 2016. [Google Scholar]
- Osborne, J. Improving your data transformations: Applying the Box-Cox transformation. Pract. Assess. Res. Eval. 2010, 15, 12. [Google Scholar]
- Öztuna, D.; Elhan, A.H.; Tüccar, E. Investigation of Four Different Normality Tests in Terms of Type 1 Error Rate and Power under Different Distributions. Turk. J. Med. Sci. 2006, 36, 7. [Google Scholar]
- Elkin, L.A.; Kay, M.; Higgins, J.J.; Wobbrock, J.O. An aligned rank transform procedure for multifactor contrast tests. In Proceedings of the ACM Symposium on User Interface Software and Technology (UIST ’21), Virtual Event, 10–14 October 2021; ACM Press: New York, NY, USA, 2021; pp. 754–768. [Google Scholar]
- Regulation (EC) No 2019/88 of the European Parliament and of the Council as Regards Maximum Residue Levels for Acetamiprid in Certain Products. (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0088&from=EN (accessed on 2 January 2024).
- EFSA (European Food Safety Authority); Bellisai, G.; Bernasconi, G.; Carrasco Cabrera, L.; Castellan, I.; del Aguila, M.; Ferreira, L.; Santonja, G.G.; Greco, L.; Jarrah, S.; et al. Modification of the existing maximum residue levels and setting import tolerances for flupyradyfurone and difluoroacetic acid (DFA) in various crops. EFSA J. 2023, 21, e8423. [Google Scholar] [PubMed]
- Lasota, J.A.; Dybas, R.A. Abamectin as a pesticide for agricultural use. Acta Leiden. 1990, 59, 217–225. [Google Scholar] [PubMed]
- Dai, Y.J.; Ji, W.W.; Chen, T.; Zhang, W.J.; Liu, Z.H.; Ge, F.; Yuan, S. Metabolism of the Neonicotinoid insecticides acetamiprid and thiacloprid by the yeast Rhodotorula mucilaginosa strain IM-2. J. Agric. Food Chem. 2010, 58, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Jeschke, P.; Velten, R.; Beck, M.E.; Ebbinghaus-Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Flupyradifurone: A brief profile of a new butenolide insecticide. Pest Manag. Sci. 2015, 71, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.D.; Portillo, H.E.; Annan, I.B.; Cameron, R.A.; Clagg, D.G.; Dietrich, R.F.; Watson, L.J.; Leighty, R.M.; Ryan, D.L.; McMillan, J.A.; et al. Movement of cyantraniliprole in plants after foliar applications and its impact on the control of sucking and chewing insects. Pest Manag. Sci. 2015, 71, 395–403. [Google Scholar] [CrossRef]
- Daniel, C.; Grunder, J. Integrated Management of European Cherry Fruit Fly Rhagoletis cerasi (L.): Situation in Switzerland and Europe. Insects 2012, 3, 956–988. [Google Scholar] [CrossRef] [PubMed]
- Kolyaee, R.; Kamali, H. Control Management of Cherry Fruit Fly (Rhagoletis cerasi L.) by Bait Spray Method. Pestic. Plan Prot. Sci. 2014, 1, 40–49. [Google Scholar]
- Jelani, A.R. Development and field evaluation of a tractor mounted oil palm trunk injector. J. Oil Palm Res. 2018, 30, 101–110. [Google Scholar] [CrossRef][Green Version]


| Location and Date of Injection | Active Ingredient | Sampling Date | ||||
|---|---|---|---|---|---|---|
| (DAT: Days after Treatment) | ||||||
| Name | Dose | g/Tree | mL/Tree | Fruit Samples | Leave Samples | |
| Budapest, | Abamectin | small | 0.18 | 10 | 23 June 2021 (DAT 42) | - |
| 12 May 2021 | (ABA) | medium | 0.36 | 20 | - | |
| (efficacy test) | high | 0.72 | 40 | - | ||
| Acetamiprid | small | 0.2 | 10 | 23 June 2021 (DAT 42) | - | |
| (ACE) | medium | 0.4 | 20 | - | ||
| high | 0.8 | 40 | 23 June 2021 (DAT 42) | |||
| Cyantraniliprole | small | 0.2 | 10 | 23 June 2021 (DAT 42) | - | |
| (CYA) | medium | 0.4 | 20 | - | ||
| high | 0.8 | 40 | - | |||
| Flupyradifurone | small | 0.2 | 10 | 23 June 2021 (DAT 42) | - | |
| (FLU) | medium | 0.4 | 20 | - | ||
| high | 0.8 | 40 | 23 June 2021 (DAT 42) | |||
| Injection control | - | Water | 40 | 23 June 2021 (DAT 42) | - | |
| Budapest | Acetamiprid | dose 1 | 0.056 | 40 | 15 June 2022 (DAT 34) | - |
| 12 May 2022 | (ACE) | dose 2 | 0.56 | 40 | - | |
| (dosage | dose 3 | 1.12 | 40 | - | ||
| evaluation) | dose 4 | 2.25 | 40 | 15 June 2022 (DAT 34) | ||
| Flupyradifurone | dose 1 | 0.33 | 40 | 15 June 2022 (DAT 34) | - | |
| (FLU) | dose 2 | 0.66 | 40 | - | ||
| dose 3 | 1.21 | 40 | - | |||
| dose 4 | 3.96 | 40 | 15 June 2022 (DAT 34) | |||
| Injection control | Water | 40 | 15 June 2022 (DAT 34) | - | ||
| Budapest 12 May 2022 | Spray Control | A. I. (g/tree) | Fruit Sampling | Leaves Sampling | ||
| (spray control) | Acetamiprid (ACE) | spraying | 1 | - | 29 June 2022 (DAT 17) | 29 June 2022 (DAT 17) |
| Flupyradifurone (FLU) | spraying | 0.6 | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyuris, R.; Szabó, Á.; László, A.M.; Gutermuth, Á.; Sörös, C. An Evaluation of Insecticidal Trunk Injections for the Control of the European Cherry Fruit Fly Rhagoletis cerasi L. (Diptera: Tephritidae). Horticulturae 2024, 10, 278. https://doi.org/10.3390/horticulturae10030278
Gyuris R, Szabó Á, László AM, Gutermuth Á, Sörös C. An Evaluation of Insecticidal Trunk Injections for the Control of the European Cherry Fruit Fly Rhagoletis cerasi L. (Diptera: Tephritidae). Horticulturae. 2024; 10(3):278. https://doi.org/10.3390/horticulturae10030278
Chicago/Turabian StyleGyuris, Rita, Árpád Szabó, Anna M. László, Ádám Gutermuth, and Csilla Sörös. 2024. "An Evaluation of Insecticidal Trunk Injections for the Control of the European Cherry Fruit Fly Rhagoletis cerasi L. (Diptera: Tephritidae)" Horticulturae 10, no. 3: 278. https://doi.org/10.3390/horticulturae10030278
APA StyleGyuris, R., Szabó, Á., László, A. M., Gutermuth, Á., & Sörös, C. (2024). An Evaluation of Insecticidal Trunk Injections for the Control of the European Cherry Fruit Fly Rhagoletis cerasi L. (Diptera: Tephritidae). Horticulturae, 10(3), 278. https://doi.org/10.3390/horticulturae10030278






