Abstract
The purple-leaf phenotype in pak choi is due to the accumulation of anthocyanin. The main regulated genes are unclear. The gene controlling the purple-leaf phenotype was mapped on A03 using BSA-seq, but the candidate interval was not further narrowed with InDel markers. Based on our previous study, we hypothesized that the candidate gene that regulates purple leaves in pak choi may also be the Dark_Pur gene from B. juncea. Using the Dark_Pur-1 marker to identify P1, P2, F1, and F2, it was confirmed that the purple trait in purple-leaf pak choi was controlled by the Dark_Pur gene from B. juncea through distant hybridization. A DNA segment of approximately 514 Kb containing the Dark_Pur gene was reintroduced into pak choi from B. juncea. Meanwhile, a new purple pak choi germplasm line was created with green pak choi × purple B. juncea via distant hybridization, which proved that distant hybridization is an effective method for creating new germplasms. Furthermore, the purple-leaf phenotypes of 20 pak choi varieties were identified, and the purple-leaf traits of all lines were derived from B. juncea via distant hybridization. At present, few studies have focused on the background of the purple trait in pak choi; however, in this study, our results suggest that there is a high probability that the purple trait in pak choi may be completely derived from purple B. juncea via distant hybridization. This study also lays a good foundation for research on the creation of new germplasms through distant hybridization among the Brassica species.
1. Introduction
Brassica rapa contains many types of vegetables that play an important role in balancing the supply of vegetables in the market, including different subspecies, such as Chinese cabbage, zicaitai, pak choi, and turnip. Pak choi (Brassica campestris L. ssp. chinensis) is a subspecies of B. rapa, and its main edible parts are its young leaves. Because of its short growing period and strong adaptability, it is planted across the country, especially in the Yangtze River basin, and it can be planted and supplied annually, which has important economic value. In recent years, the consumption of pak choi has increased in Southeast Asian and European countries because of its delicious taste and nutritiousness [1].
Anthocyanins accumulate in plant tissues and can make plants appear purple, red, blue, and other colors [2,3]. Flavonoid biosynthesis is an important pathway leading to anthocyanin accumulation and has been extensively studied in many horticultural crops [4,5,6]. At present, the related research on anthocyanin function is relatively comprehensive. In plants, anthocyanins can protect plants from UV damage and cold stress by absorbing light [7]. As one of the most important secondary metabolites of plants, anthocyanins not only have physiological functions in plants but are also beneficial to human health. Anthocyanins can treat and prevent diabetes [8] and are the most effective free radical scavengers, protecting the human body from damage by harmful free radical substances and helping the human body prevent various diseases related to free radicals [9]. Purple vegetables are rich in anthocyanins and have high economic value; thus, breeding various types of purple vegetables will become a primary goal for breeders in the future. Purple resources are scarce in Brassica crops, and some cultivated species include purple cauliflower (the CC genome) [10] and purple-leaf B. juncea (the AABB genome) [11]. The epidermis of the stems or roots in some types of vegetables in B. rapa (the AA genome), such as zicaitai [12] and purple turnip [13], appear to be purple due to anthocyanin accumulation. More than 600 kinds of anthocyanins have been identified in nature [14]. The six most common anthocyanins are cyanidin, pelargonidin, peonidin, malvidin, petunia, and delphinidin [15]. In past reports using high-performance liquid chromatography (HPLC), the anthocyanin species of pak choi have been identified mainly as cornflower derivatives [16,17].
Based on related studies, genes related to anthocyanin accumulation are mainly classified into three categories, including genes involved in transcriptional regulation in the nucleus (the MYB-bHLH-WD40 complex), structural genes related to the biosynthetic pathway (CHS, FLS, DFR, and ANS), and transporter genes that transfer anthocyanins from the cytoplasm to the vacuole [18,19,20]. Among them, MYB transcription factors (TFs) dominate the regulatory network of anthocyanin biosynthesis. The role of MYB transcription factors in the regulatory network of anthocyanin metabolism has been reported in several horticultural crops. In studies related to anthocyanin synthesis in eggplant, when SmTT8 was co-expressed with SmCBFs and SmMYB113 in tobacco, the anthocyanin content was significantly increased compared with SmCBFs and SmMYB113, suggesting that SmMYB113 significantly increased the expression level of anthocyanin structural genes [21]. In another study, MYB75 (PAPI), MYB90 (PAP2), MYB113, and MYB114 were silenced in Arabidopsis, and anthocyanin synthesis was blocked, suggesting that these four genes play an important role in anthocyanin synthesis [22]. In a study on cabbage and cauliflower, the function of BoMYB2 in regulating anthocyanin synthesis was validated. The transcriptional expression of structural genes in purple cabbage was positively correlated with the expression of BoMYB2, indicating that BoMYB2 had a positive regulatory effect on the expression of structural genes in the anthocyanin synthetic pathway [23]. BrMYBL2.1 was thought to regulate anthocyanin synthesis in the stem epidermis of zicaitai [24]. The R2R3-MYB transcript from B. juncea regulates anthocyanin synthesis in purple Chinese cabbage [25]. These studies show that MYB transcription factors are closely related to anthocyanin synthesis in Brassica crops.
There are many reports on the gene locus of anthocyanins in B. rapa, and it is mainly localized in A02, A03, A07, and A09. The anthocyanin-synthesis-related gene anl was mapped onto A09 in Chinese cabbage using the F2 population [26]. Zhang et al. [27] used the hybridization of Chinese cabbage and zicaitai to construct an F2 population and located the main gene controlling the purple trait on A09 in B. rapa. Yu et al. [28] used BSA-seq to obtain three QTLs related to purple traits from the backcross population of a cross between purple pak choi and green Chinese cabbage. Hayashi et al. [29] hybridized purple turnip with Chinese cabbage to construct an F2 population and positioned the locus Anp that controlled anthocyanin accumulation on chromosome A07. Guo et al. [30] hybridized zicaitai with green cabbage to construct an F2 population and found that BrEGL3.1 and BrEGL3.2, the major genes controlling anthocyanin accumulation, were located on chromosome A09. Zhang et al. [31] used BSA-seq to locate the purple gene Anm in Chinese cabbage on chromosome A02. Liu et al. [32] located BrPur, the gene that controls purple pak choi, at the end of chromosome A03. Wang et al. [33] located the purple gene BrPur in the region of 54.87 kb at the end of the A03 linkage group. These results indicate that the inheritance of the purple trait in Brassica is relatively complex. Although the purple trait genes were mainly located in chromosomes A07 and A03 in the study on purple localization in pak choi, there are few background studies on the purple locus. Through localization and further study, it has been speculated that the background of purple pak choi is a distant cross material.
Currently, the distant hybridization of plants is an effective technique to create and develop excellent germplasm resources. This technique has been applied to improve plant resistance, stress resistance, and plant quality. The Brassica crop species provides an excellent example of the targeted use of interspecific hybridization for crop improvement [34]. This is particularly evident in the Brassica “Triangle of U” species, of which B. rapa, B. nigra, and B. oleracea (which are diploids with the A, B, and C genomes, respectively) were found to be the extant progenitors of the allotetraploid species B. juncea (A and B genomes), B. napus (A and C genomes), and B. carinata (B and C genomes) [35]. A hexaploid hybrid between B. juncea and B. oleracea was obtained via an embryo culture [36]. A new germplasm resource resistant to cystitis nematodes was created by crossing radish (RR) with B. napus (AACC) [37].
In this paper, the pak choi DH line with purple leaves was used, and the leaf color regulation gene, which was an MYB transcript derived from B. juncea via distant hybridization, was located. We described the development of a new germplasm that expands the genetic variation of purple pak choi by infiltrating the B. juncea gene into pak choi. We also demonstrated pak choi introgression lines carrying more B. juncea chromosome fragments using newly developed markers. This study may help infer the interactions between the A genome and B genome in Brassica crops, which would provide evidence for introgression breeding in Brassica species.
2. Materials and Methods
2.1. Plant Materials
The parent ‘2150912’ is a pak choi of the DH line with deep purple leaves (Figure 1), and the parent ‘2150913’ is a pak choi highly inbred line with green leaves (Figure 1). F1 was obtained from P1 × P2 (Figure 1). F2 individuals were obtained from the F1 line self-fertilization. The F1 was back-crossed with P2 to produce the BC1 populations (F1 × P2). All these materials were cultivated at the Shunyi farm of the Institute of Vegetables and Flowers, the Chinese Academy of Agricultural Sciences.
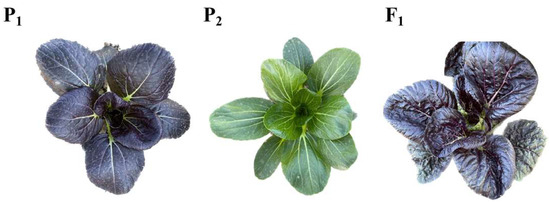
Figure 1.
The phenotypic investigation of the P1, P2, and F1 lines. P1: male parent with purple leaves. P2: female parent with green leaves. F1: F1 (P1 × P2) with purple leaves.
2.2. Identification and Genetic Analysis of the Purple-Leaf Phenotype
The experimental materials were planted in an experimental field at the Vegetable and Flower Research Institute, and the leaf colors of all the P1, P2, F1, F2, and BC1 populations in the adult plant stage were observed and counted. The statistical results were used for the genetic analysis.
2.3. Bulked Segregant Analysis Sequence (BSA-Seq) for Primary Mapping
Combined with the phenotypic results of the leaf color trait survey, the F2 population was used to construct the extreme pools of purple leaf color and green leaf color. Forty single plants exhibiting purple leaves were selected from the F2 population, and their DNA was extracted and mixed in equal amounts to construct an extreme purple pool (P-Mix). Forty single plants exhibiting green leaves were extracted and mixed in equal amounts to construct an extreme green pool (G-Mix). Two mixed pools and two parent pools were used for the association analyses. Approximately 40× genome sequences for each pool were generated using an Illumina HiSeq 2500 platform (Illumina, San Diego, CA, USA). The total plant genomic DNA was extracted from the leaves using the TPS method at a total DNA sample concentration of 100 ng/μL. DNA samples from the extreme purple pool, the extreme green pool, and the parents were sent to Wuhan Benagen Technology Co., Ltd., Wuhan, China for resequencing.
Qubit 2.0 was used to construct the extreme pools for the initial quantification and dilution of the libraries. Insert fragments of the libraries were detected using Agilent 2100 (Agilent 2100, Agilent Technologies Inc., Santa Clara, CA, USA). Sequencing was performed using an Illumina NovaSeq (Illumina, CA, USA), an Illumina high-throughput sequencing platform. Variant detection was performed using B. rapa version V3.0 (http://39.100.233.196/#/, accessed on 4 January 2024) as the reference genome based on the number of extreme mixing pool samples and sequencing depths. The SNP/InDel loci in the purple mixed pools and green mixed pools were compared with P2 as the reference parent, respectively. The mean value of the ΔSNP index was calculated and combined with the upper and lower boundary thresholds of the 95% and 99% confidence intervals obtained from the simulated hypothesis testing to screen the candidate regions associated with the trait and plot the SNP/InDel index map.
2.4. Molecular Marker Development
Insertion/deletion (InDel) markers were used to identify the molecular markers associated with the purple locus. All gene sequences were obtained from the B. rapa database, and markers were designed using Primer3 Plus (https://www.primer3plus.com/index.html, accessed on 4 January 2024). PCR primers were synthesized by Shanghai Shenggong Bioengineering Technology Service Co., Ltd., Shanghai, China. The primer sequences of the molecular markers developed in this study are shown in Table S1.
The screening and identification of the markers associated with the purple loci were carried out as follows: InDel markers of 80–150 bp were developed based on the results of the BSA-seq, separated via 1.5% agarose gel electrophoresis, and visualized using UV light. The detection of heterologous B. juncea gene infiltration fragment markers was performed as follows: markers were designed with specific primers using the B. juncea genome v2.0 sequence as the reference genome. PCR amplicons were subjected to 150 V via 8% polyacrylamide gel electrophoresis for 80 min, followed by silver staining. The PCR amplification conditions and protocol were as follows: 95 °C for 3 min; 30–35 cycles of 95 °C for 30 s, 55–60 °C for 15 s, and 72 °C for 40 s; and a final extension step of 72 °C for 5 min.
2.5. Embryo Rescue from Distant Hybridization
The pak choi and B. juncea parent materials were grown in greenhouses and pollinated during flowering, with green pak choi as the maternal parent and purple B. juncea as the paternal parent. Ten to twelve days after pollination, several ovaries of each F1 plant were first treated with 75% anhydrous ethanol for 1 min, then with 8% NaClO for 15 min, and finally, rinsed with sterile water three times. The ovules in the ovaries were removed with tweezers and then inoculated into a B5 medium. The inoculated ovules were cultured at approximately 25 °C for 30–50 days with a light intensity of 2000 lx for 10 h per day.
3. Results
3.1. Inheritance Pattern Analysis of the Purple-Leaf Phenotype in Pak Choi
In this study, P1 was a DH line, ‘2150912’, with purple leaves, and P2 was a highly inbred line, ‘2150913’, with green leaves. The F1 line was obtained from P1 × P2 and had purple leaves (Figure 1). A total of 502 F2 individuals were obtained from the self-fertilization of the F1 line, and 378 of these plants were purple and 124 were green. The separation ratio of leaf color in the F2 populations was 3:1 (χ2 = 0.024 < χ20.05,1 = 3.841). Meanwhile, 209 BC1 individuals were obtained from F1 × P2, and the separation ratio of the purple (107 plants) and green (102 plants) leaves was 1:1 (χ2 = 0.02 < χ20.05,1 = 3.841) (Table 1). From the phenotypic investigation of the F2 and BC1 populations, it was inferred that the purple-leaf trait was controlled by one dominant gene pair in pak choi.

Table 1.
Inheritance pattern analyses of the purple trait in pak choi.
3.2. Primary Mapping of the Purple-Leaf Phenotype via BSA-Seq
The P1, P2, and 40 F2 purple plants pool (P-Mix) and the 40 F2 green plants pool (G-Mix) were re-sequenced for 40× to excavate the genome variations resulting in the purple phenotype, and the BSA-seq technique was used to map the regulating purple-leaf gene. P2 was used as the reference parent, and the SNP/InDel loci between the P-Mix and G-Mix pools were aligned, respectively. The P-Mix pool contained 21.1 Gb of data (92.797% coverage), and the G-Mix obtained 19.6 Gb of data (92.244% coverage). The sequencing quality Q20 was greater than 97% and the Q30 was greater than 92%, which indicated that the data quality control met the requirements for further analysis. The InDel index values between the P-Mix and G-Mix pools were calculated, and the candidate interval of the regulating purple-leaf genes was located in the 18 Mb region from 20,200,001 bp to 38,152,257 bp on A03 (Figure 2).
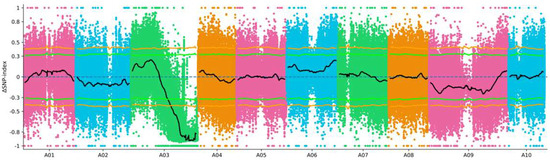
Figure 2.
Primary mapping of the purple-leaf phenotype via BSA-seq, and the candidate interval of regulating purple-leaf genes was located by calculating the ∆InDel index in pak choi. The line indicates that 1M was taken as the window, each slide was 100 kb, and the average ΔSNP index of the SNP site in the window was counted.
3.3. Fine Mapping and Candidate Gene Excavation for the Purple-Leaf Phenotype in Pak Choi
Ten InDel primers were designed in the initial mapping region between the P-Mix and G-Mix pools, which identified P1 and P2, to map the candidate genes for the purple-leaf phenotype. The 10 InDel markers were also used to distinguish the 502 F2 individuals, and 10 recombinant individuals were identified. According to their phenotypes and genotypes, the target genes were initially located in the 30,067,746–31,915,855 bp region on chromosome A03 (Figure 3).
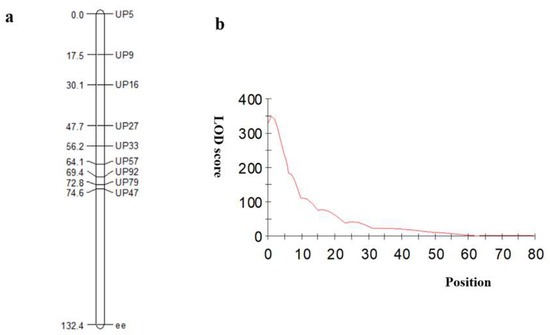
Figure 3.
The genetic map was constructed according to the combination phenotype and InDel markers. In (a), Joinmap 4.0 was used for the linkage analysis of the genotype results, and an integrated calculation of the genetic distance and the F2 population phenotypic information of the genetic map was performed. In (b), Ici Mapping 4.1 was used to map the location interval to build the genetic map and location.
In our previous study, a new purple B. rapa variety was obtained via distant hybridization from green B. rapa × purple B. juncea, and the purple-leaf-regulating gene Dark_Pur was mapped, which encoded the transcription factor R2R3-MYB from B05 in B. juncea [31,38]. The BjPur gene in B. juncea was also mapped, and it encoded a new R2R3-MYB transcription factor [11], while the Dark_Pur gene and the BjPur gene were the same. The transformed purple Chinese cabbage plant was obtained via the particle bombardment of isolated microspores from Chinese cabbage to transform the Dark_Pur gene, thus verifying the function of the Dark_Pur gene [38]. In this study, the candidate interval was not further narrowed with more markers, and we hypothesized that the candidate gene regulating purple in pak choi may be the Dark_Pur gene from B. juncea. The molecular marker Dark_Pur-1 (Table S1) was designed based on the Dark_Pur gene to prove our hypothesis. The Dark_Pur-1 marker identified the P1, P2, F1, and 502 F2 individuals, as well as 209 BC1 individuals (Figure 4). The results from the Dark_Pur-1 (Table S1) marker identification were consistent with the phenotype, from which we inferred that the candidate gene for the purple-leaf phenotype in pak choi was from the Dark_Pur gene on B05 in B. juncea.
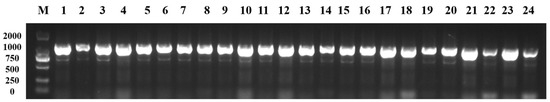
Figure 4.
The molecular marker of the Dark_Pur gene identified P1, F1, F2, and BC1 individuals (partial data shown). 1, P1 individual; 2, F1 individual; 3–5, BC1 individuals; and 6–24, F2 individuals.
3.4. The Purple-Leaf Gene in Pak Choi Was Obtained from B. juncea through Distant Hybridization
How did the purple-leaf gene in B. juncea enter pak choi? We hypothesized that the Dark_Pur gene may have been introduced into pak choi via distant hybridization. How were long genome segments infiltrated into pak choi from B. juncea through distant hybridization? Via blasting and alignment in the Brassica database (http://39.100.233.196/#/, accessed on 4 January 2024), the Dark_Pur gene was found to be highly homologous with BraA02g017040.3C and BraA03g040840.3C from B. rapa, which were homologous with the Arabidopsis thaliana gene ATMYB75, which was a key TF in activating anthocyanin biosynthetic genes [39]. The BraA03g040840.3C gene was located in the candidate interval, which may be exchanged with the Dark_Pur gene. The BraA03g040840.3C gene was not swapped as much as expected, and it could be detected in P1 and P2.
The Dark_Pur gene was used as the origin to explore how the long fragments from B. juncea were introduced into pak choi via distant hybridization. Primers were designed with a step size of 0.5 Mb based on the B. juncea genome (V2.0) upstream and downstream of the Dark_Pur gene (Figure 5), which amplified similar PCR productions in purple B. juncea. In the downstream of the Dark_Pur gene, the marker at 0.5 Mb detected the same PCR production in P1 as that detected in purple B. juncea, but the marker at 1.0 Mb detected a different PCR production from that detected in purple B. juncea. Therefore, we designed 11 pairs of primers between the 0.5 Mb and 1.0 Mb intervals (Table S1). However, the markers could not detect the same PCR production in P1 as that in purple B. juncea, and so 0.505 Mb in purple pak choi downstream of Dark_Pur was identified in purple B. juncea. The markers at −0.5/−1.0/−1.5 Mb upstream of the Dark_Pur gene could not detect the same PCR production in P1 as that in purple B. juncea, and so we also designed nine pairs of primers (Table S1) between 0 and -0.5 Mb. The marker at -7.8 kb detected the same PCR production in P1 as that detected in purple B. juncea. Therefore, 7.8 kb upstream of Dark_Pur was identified in purple B. juncea. In conclusion, the length of the fragment containing the Dark_Pur gene in B. juncea was determined to be approximately 514 kb (7.8 kb + 1 kb + 0.505 Mb) via distant hybridization (Figure 5).
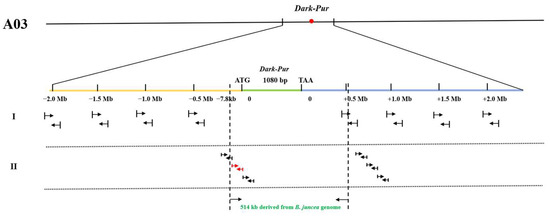
Figure 5.
Model illustrating the introgression of an exogenous fragment from B. juncea into a pak choi chromosome. Molecular markers were used to identify the lengths of the genome fragments from B. juncea entering pak choi through distant hybridization. The arrows represent the physical locations of the designed marker.
3.5. A New Pak Choi Germplasm Line Was Created with Green Pak Choi × Purple B. juncea through Distant Hybridization
To further verify that the purple-leaf phenotype of B. juncea could be infiltrated into green pak choi through distant hybridization, P2, a highly inbred pak choi line (the AA genome) with green leaves, was hybridized with P3, a highly inbred B. juncea line (the AABB genome) with purple leaves. Via hand-pollination and embryo rescue, F1 (the AAB genome) was obtained, and they were dwarf plants with relatively low fertility. The purple-leaf F1 plants were selected to self-fertilize, and offspring were obtained via embryo rescue. After three generations of phenotypic screening, marker screening, self-cross, and embryo rescue, the stable-inheritance plants with purple leaves were obtained (Figure 6). The B genome of F1 (the AAB genome) was lost in the self-fertilization process, which verified that the purple-leaf phenotype of pak choi was derived from B. juncea via distant hybridization.
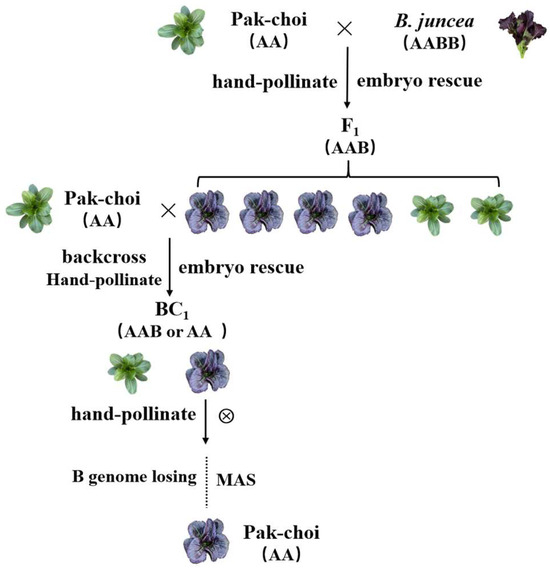
Figure 6.
A new pak choi germplasm line was created with green pak choi × purple B. juncea through distant hybridization, and the purple-leaf phenotype was stably inherited in pak choi.
3.6. The Purple-Leaf Phenotype of 20 Pak Choi Varieties Were All Derived from B. juncea through Distant Hybridization
The purple-leaf phenotype of pak choi was dominant, and the homozygous and heterozygous genotypes both showed purple, which would be necessary to develop markers to distinguish it for breeding. Based on the B. juncea reference genome (V2.0), a series of InDel primers were designed rounding the corner of the Dark_Pur gene, and the DN1 marker (DN1-F: TTCTTGTGCCGAGCATGGAT; DN1-R: GTGTGTTTTCAGGACGGTGC) accurately identified P1 (1063 bp), P2 (250 bp), and F1 (1063 bp and 250 bp) (Figure 7a). Meanwhile, the DN1 marker was utilized to identify the homozygous and heterozygous genotypes in 48 F2 plants (Figure 7b,c), and the homozygous purple individuals were effectively screened. Increasing numbers of purple pak choi varieties are being favored in the market; however, where the purple-leaf phenotype of these purple pak choi varieties comes from is unclear. Using the DN1 marker to identify 20 pak choi varieties (zibao, zixiu, etc.), the PCR amplification product included the 1063 bp fragment in all varieties (Figure 8), which indicated that all 20 purple pak choi varieties obtained the purple-leaf phenotype from purple B. juncea via distant hybridization.
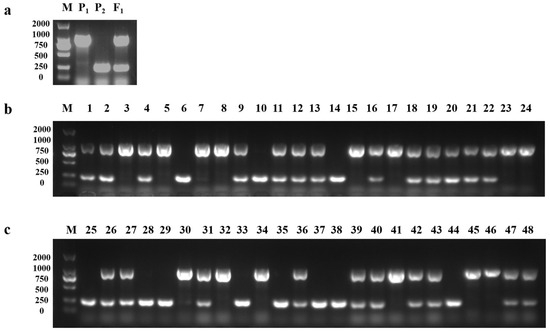
Figure 7.
An InDel marker was developed to identify the P1, P2, F1, and F2 individuals. (a) The DN1 marker identified P1 (1063 bp), P2 (250 bp), and F1 (1063 bp and 250 bp). (b,c) The homozygous and heterozygous genotypes of 48 purple F2 plants were identified with DN1 markers. Numbers 3, 5, 7, 8, 15, 17, 23, 24, 30, 32, 34, 42, 45, and 46 are homozygous purple plants; numbers 1, 2, 4, 9, 11–13, 16, 18–22, 26, 27, 31, 36, 39, 40, 42, 43, 47, and 48 are hybrid purple plants; and numbers 6, 10, 14, 25, 28, 29, 33, 35, 37, 38, and 44 are green plants.
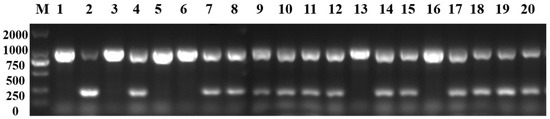
Figure 8.
The purple-leaf phenotype of the pak choi varieties is derived from purple B. juncea through distant hybridization, as identified by the DN1 markers. Numbers 1, 3, 5, 6, 13, and 16 are homozygous purple plants and numbers 2, 4, 7–12, 14, 15, and 17–20 are hybrid purple plants.
4. Discussion
4.1. MYB Transcription Factor Regulated the Purple Phenotype
Purple varieties are excellent for human health because they are rich in anthocyanins. In Arabidopsis, the spatial and temporal expression of structural genes involved in anthocyanin biosynthesis was determined by individual R2R3-MYB transcription factors (MYB11, MYB12, and MYB111) or a protein complex comprising the basic helix–loop–helix (bHLH), R2R3-MYB, and WD40-type transcription factors or the interaction complex [40,41]. MYB, bHLH, and WD40 form one ternary complex (MBW) for regulating the expression of anthocyanin biosynthesis genes in which MYB plays the main role [42]. Two MYB types, R2R3- and R3-MYB, which increase or inhibit anthocyanin biosynthesis, respectively, compete to generate the MBW complex [43,44]. The activation of positive R2R3-MYB factors results in anthocyanin accumulation, and their functional loss leads to color loss in plants [45]. For example, a loss of MYB promoter function resulted from a retrotransposon insert in grapes [46] and via methylation in pears [47]. Meanwhile, purple-leaf cabbage was obtained via either the substitution of the promoter or deletion of BoMYBL2-1 [48]. In this study, it was found that the accumulation of anthocyanins was caused by the exogenous R2R3-MYB transcription factor (the Dark_Pur gene).
4.2. Distant Hybridization Is a Very Effective Method for Creating New Germplasms
Distant hybridization plays an important role in improving crops and breeding new varieties and has been widely used to transfer target traits into new germplasms. The CR genes were transferred from a B. rapa AA genome to a B. oleracea CC genome through distant hybridization, and a 3.42 Mb fragment containing the CRa gene from the B. rapa AA genome to the B. oleracea CC genome was identified [49]. In addition, the B. rapa AA genome was infiltrated into the B. juncea AA genome (AABB), and 59.2% of the B. juncea AA genome in the new B. juncea introgression lines was covered by the B. rapa AA genome [50]. In our previous study, a new purple B. rapa variety was obtained through distant hybridization (green Chinese cabbage × purple B. juncea) [31,38], and new allopolyploids (AACC) were obtained with Cai-xin × Chinese kale [51]. Radish and kohlrabi were subjected to intergeneric distant hybridization using tissue culture and chromosome-doubling technology, and five hybrid individuals were obtained via a combination of artificial pollination and embryo rescue, three of which were identified as hybrids with a hybrid frequency of 0.85% [52]. The parent line GRG2462, with a high thousand-seed weight and low seed-oil content, was also derived from B. napus and S. alba by distant hybridization [53]. In this study, a new pak choi germplasm line was created with green pak choi × purple B. juncea via distant hybridization. The length of the fragment containing the Dark_Pur gene from B. juncea was approximately 514 kb in the B. rapa AA genome, which proved the distant hybridization event. A relatively large fragment—not a single gene—was transferred into the genome.
4.3. The Genomic Similarity of Brassica Crops Is the Basis of Distant Hybridization
Based on the U’s triangle [35], three diploids (B. rapa, the AA genome; B. nigra, the BB genome; and B. oleracea, the CC genome) and three allotetraploids (B. napus, the AACC genome; B. juncea, the AABB genome; and B. carinata, the BBCC genome) were obtained from the three-diploid pairwise cross. The genomic information of Brassica species such as B. rapa [54], B. oleracea [55,56], and B. napus [57] has been released. There is a high degree of covariance between the B. rapa A genome and the B. nigra B genome on chromosomes A04 to B04, A05 to B05, and A06 to B06 [58].
It has been proven that there is a high degree of similarity between the A genomes of B. rapa and B. juncea (>98%), and the similarity between the B. rapa A genome and the B. juncea B genome was in the range of 90% to 95% [25]. The high homology of the A, B, and C genomes between Brassica species provides the possibility of distant hybridization for crop improvement and creating new germplasm accessions. In our previous study, a new purple B. rapa variety was obtained using distant hybridization from green Chinese cabbage × purple B. juncea, and the purple-leaf-regulating gene Dark_Pur was mapped on A02, which encoded the transcription factor R2R3-MYB from B05 in B. juncea [31,38]. In this study, the purple-leaf phenotype in pak choi was also controlled by the Dark_Pur gene, and the Dark_Pur gene was mapped on A03 through a mapping analysis. Meanwhile, a new pak choi germplasm line was created with green pak choi × purple B. juncea via distant hybridization, and the Dark_Pur gene was also identified in the A03 chromosome of the new purple pak choi line. Chinese cabbage and pak choi are two subspecies belonging to B. rapa (the AA genome), and the genome recombination was favorable in the distant hybridization with B. juncea (the AABB genome), which indicates that the chromosome exchange in the distant hybridization was more likely to have occurred in the high-similarity places, and the chromosome exchange was not fixed in one event.
4.4. The Purple-Leaf Phenotype of the Pak Choi Varieties Is Derived from Purple B. juncea through Distant Hybridization
Distant hybridization can be classified into intergeneric hybridization and interspecific hybridization according to the genetic relationship between the parents. By exploring beyond the boundaries of species and expanding genetic variation, researchers can combine the elite agronomical characteristics of different species and create new species or new crop types in the Brassica genus. The purple-leaf phenotypes of Chinese cabbage and pak choi are gaining increasing attention in the market. However, the purple Chinese cabbage and pak choi varieties mostly come from foreign companies, and the backgrounds of the purple-leaf phenotypes are not clear. We collected the seeds of 20 purple-leaf pak choi varieties from seed markets, and the DN1 marker was used to identify whether they contained the gene controlling the purple-leaf phenotype in purple B. juncea. All the collected purple pak choi varieties contained the Dark_Pur gene from B. juncea. There may have been a lack of wild purple pak choi germplasm resources. The purple phenotype of the purple pak choi derives from B. juncea via distant hybridization. In addition, distant hybridization was proven to be an efficient method for innovating germplasms.
5. Conclusions
This study showed that MYB transcription factors from Brassica juncea regulate purple leaves in pak choi. The gene controlling the phenotype of purple leaves was localized in the A03 1.8 Mb region using BSA-seq combining phenotyping and genotyping. The B. juncea genome infiltrated 514 Kb of DNA containing the Dark_Pur gene into pak choi through distant hybridization. Based on this result, we developed molecular markers to identify the purple pak choi commercial species in the market derived from Brassica juncea. In addition, we created purple pak choi containing Dark_Pur through distant hybridization and embryo rescue techniques. The Dark_Pur gene can be stably infiltrated into different Brassica species through distant hybridization and maintain its function, which will make purple-trait breeding more efficient. This will be used to produce pak choi containing high anthocyanin levels that can be used for functional food and production purposes. How this gene plays a stable role in regulating anthocyanin synthesis in plant tissues requires further study. The results of this study provide a reference for identifying the introgression breeding of Brassica, reveal the recombination of genes in different genomes in Brassica crops, and clarify the Brassica juncea genomic events to improve the leaf color of pak choi.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10030276/s1, Table S1: Details of the primers used in the article.
Author Contributions
X.W. and Y.Z. drafted the manuscript; X.W., Y.Z., B.S., S.Z. (Shujiang Zhang), S.Z. (Shifan Zhang), H.Z., R.S., J.Z., Z.L., G.L. and F.L. performed the experiments; and G.L. and F.L. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China (grant number 2023YFD1200101), the China Agriculture Research System (CARS-23-A-14), the National Natural Science Foundation of China (32102373 and 32172562), and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-IVFCAAS). This work was performed at the State Key Laboratory of the Vegetable Biobreeding, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing 100081, China, and the Key Laboratory of Biology and Genetic Improvement of Horticultural Crops, Ministry of Agriculture, Beijing, China.
Data Availability Statement
The data are contained within the article and in the Supplementary Materials.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Tan, C.; Chen, H.D.; Dai, G.Q.; Liu, Y.; Shen, W.J.; Wang, C.C.; Liu, D.; Liu, S.J.; Xu, S.Q.; Zhu, B.; et al. Identification and characterization of the gene BraANS.A03 associated with purple leaf color in pak choi (Brassica rapa L. ssp. chinensis). Planta 2023, 258, 14. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Wu, Y.; Zheng, L.; Zhang, G. Regulatory Mechanisms of Anthocyanin Biosynthesis in Apple and Pear. Int. J. Mol. Sci. 2021, 22, 8441. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.C.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications123. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Pang, Q.; Chen, X.; Li, T.; Fang, J.; LIN, S.; Jia, H. Transcriptome analysis of strawberry fruit in response to exogenous arginine. Planta 2020, 252, 82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, M.D.; Wen, C.X.; Xie, X.L.; Tian, W.; Wen, S.Q.; Lu, R.K.; Liu, L.D. Integrated metabolomic and transcriptomic analysis of the anthocyanin regulatory networks in Salvia miltiorrhiza Bge. flowers. BMC Plant Biol. 2020, 20, 349. [Google Scholar] [CrossRef]
- Li, W.F.; Mao, J.; Yang, S.J.; Guo, Z.G.; Ma, Z.H.; Dawuda, M.M.; Zuo, C.W.; Chu, M.Y.; Chen, B.H. Anthocyanin accumulation correlates with hormones in the fruit skin of ‘Red Delicious’ and its four generation bud sport mutants. BMC Plant Biol. 2018, 18, 363. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, X.; Gao, J.; Guo, Y.; Huang, Z.; Du, Y. The tomato hoffman’s anthocyaninless gene encodes a bHLH transcription factor involved in anthocyanin biosynthesis that is developmentally regulated and induced by low temperatures. PLoS ONE 2016, 11, e0151067. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Fernandes, A.F.; Brás, N.; Mateus, N.; de Freitas, V.; Fernandes, I. Anthocyanins as Antidiabetic Agents—In Vitro and In Silico Approaches of Preventive and Therapeutic Effects. Molecules 2020, 25, 3813. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Hu, N.; Ding, C.; Zhang, Q.; Li, W.; Suo, Y.; Wang, H.; Bai, B.; Ding, C. In vitro and in vivo biological activities of anthocyanins from Nitraria tangutorun Bobr. fruits. Food Chem. 2016, 194, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Deng, Z.Y.; Zhu, H.H.; Hu, C.L.; Liu, R.H.; Young, J.C.; Tsao, R. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res. Int. 2012, 46, 250–259. [Google Scholar] [CrossRef]
- Heng, S.P.; Cheng, Q.Q.; Zhang, T.; Liu, X.J.; Huang, H.; Yao, P.J.; Liu, Z.X.; Wan, Z.J.; Fu, T.D. Fine-mapping of the BjPur gene for purple leaf color in Brassica juncea. Theor. Appl. Genet. 2020, 133, 2989–3000. [Google Scholar] [CrossRef]
- Li, G.H.; Chen, H.C.; Liu, J.L.; Luo, W.L.; Xie, D.S.; Luo, S.B.; Wu, T.Q.; Akram, W.; Zhong, Y. A high-density genetic map developed by specific-locus amplified fragment (SLAF) sequencing and identification of a locus controlling anthocyanin pigmentation in stalk of Zicaitai (Brassica rapa L. ssp. chinensis var. purpurea). BMC Genom. 2019, 20, 343. [Google Scholar] [CrossRef]
- Zhuang, H.; Lou, Q.; Liu, H.; Han, H.; Wang, Q.; Tang, Z.; Ma, Y.; Wang, H. Differential Regulation of Anthocyanins in Green and Purple Turnips Revealed by Combined De Novo Transcriptome and Metabolome Analysis. Int. J. Mol. Sci. 2019, 20, 4387. [Google Scholar] [CrossRef]
- He, J.A.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Jeon, J.; Lim, C.J.; Kim, J.K.; Park, S.U. Comparative Metabolic Profiling of Green and Purple Pakchoi (Brassica rapa Subsp. Chinensis). Molecules 2018, 23, 1613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Chen, G.P.; Dong, T.T.; Pan, Y.; Zhao, Z.P.; Tian, S.B.; Hu, Z.L. Anthocyanin Accumulation and Transcriptional Regulation of Anthocyanin Biosynthesis in Purple Bok-Choy (Brassica rapa var. chinensis). J. Agric. Food Chem. 2014, 62, 12366–12376. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cui, Y.; Yao, Y.; An, L.; Bai, Y.; Li, X.; Yao, X.; Wu, K. Genome-wide identification of WD40 transcription factors and their regulation of the MYB-bHLH-WD40 (MBW) complex related to anthocyanin synthesis in Qingke (Hordeum vulgare L. var. nudum Hook. f.). BMC Genom. 2023, 24, 166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, H.; Huang, J.-R. Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts. Mol. Plant 2012, 5, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; He, Y.J.; Li, J.; Liu, Y.; Chen, H.Y. CBFs Function in Anthocyanin Biosynthesis by Interacting with MYB113 in Eggplant (Solanum melongena L.). Plant Cell Physiol. 2020, 61, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Mendenhall, J.; Huo, Y.; Lloyd, A. TTG1 complex MYBs, MYB5 and TT2, control outer seed coat differentiation. Dev. Biol. 2009, 325, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.X.; Chiu, L.W.; Li, L. Transcriptional regulation of anthocyanin biosynthesis in red cabbage. Planta 2009, 230, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, K.; Wu, J.; Guo, N.; Liang, J.L.; Wang, X.W.; Cheng, F. QTL-Seq and Sequence Assembly Rapidly Mapped the Gene BrMYBL2.1 for the Purple Trait in Brassica rapa. Sci. Rep. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.L.; Li, F.; Zhang, S.F.; Zhang, H.; Qian, W.; Li, P.R.; Zhang, S.J.; Sun, R.F. Mining for Candidate Genes in an Introgression Line by Using RNA Sequencing: The Anthocyanin Overaccumulation Phenotype in Brassica. Front. Plant Sci. 2016, 7, 1245. [Google Scholar] [CrossRef]
- Burdzinski, C.; Wendell, D.L. Mapping the Anthocyaninless (anl) Locus in Rapid-Cycling Brassica rapa (RBr) to Linkage Group R9. BMC Genet. 2007, 8, 64. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Gong, Z.; Hui, M. Screening RAPD markers linked to purple trait of Chinese cabbage and its chromosome location. Acta Bot. Boreali-Occident. Sin. 2008, 28, 901–906. [Google Scholar]
- Yu, Y.; Zhang, Y.; Zhang, D. SRAP Markers Linked to Purple Trait in Chinese Cabbage. Mol. Plant Breed. 2009, 7, 573–578. [Google Scholar] [CrossRef]
- Hayashi, K.; Matsumoto, S.; Tsukazaki, H.; Kondo, T.; Kubo, N.; Hirai, M. Mapping of a novel locus regulating anthocyanin pigmentation in Brassica rapa. Breed Sci. 2010, 60, 76–80. [Google Scholar] [CrossRef]
- Guo, N.; Wu, J.; Zheng, S.N.; Cheng, F.; Liu, B.; Liang, J.L.; Cui, Y.; Wang, X.W. Anthocyanin profile characterization and quantitative trait locus mapping in zicaitai (Brassica rapa L. ssp chinensis var. purpurea). Mol. Breed. 2015, 35, 11. [Google Scholar] [CrossRef]
- Zhang, S.J.; Li, P.R.; Qian, W.; Zhang, S.F.; Li, F.; Zhang, H.; Wang, X.W.; Sun, R.F. Mapping and expression profiling reveal an inserted fragment from purple mustard involved anthocyanin accumulation in Chinese cabbage. Euphytica 2016, 212, 83–95. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Zhang, D.; Yu, S.; Zhang, F.; Zhao, X.; Yu, Y.; Xu, J.; Lu, G. Primary Mapping of pur, a Gene Controlling Purple Leaf Color in Brassica rapa. Acta Agric. Boreali-Sin. 2013, 28, 49–53. [Google Scholar]
- Wang, W.H.; Zhang, D.S.; Yu, S.C.; Liu, J.; Wang, D.; Zhang, F.L.; Yu, Y.J.; Zhao, X.Y.; Lu, G.X.; Su, T.B. Mapping the BrPur gene for purple leaf color on linkage group A03 of Brassica rapa. Euphytica 2014, 199, 293–302. [Google Scholar] [CrossRef]
- Katche, E.; Quezada-Martinez, D.; Katche, E.I.; Vasquez-Teuber, P.; Mason, A.S. Interspecific Hybridization for Brassica Crop Improvement. Crop Breed. Genet. Genom. 2019, 1, e190007. [Google Scholar]
- Nagaharu, U. Genome-analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn. J. Bot. 1935, 7, 389–452. [Google Scholar]
- Li, J.X.; Rao, L.L.; Chen, L.P. Production and characterization of interspecific hybrids between tuber mustard (Brassica juncea) and red cabbage (Brassica oleracea) through embryo culture. Acta Hortic. 2016, 1127, 431–436. [Google Scholar] [CrossRef]
- Peterka, H.; Budahn, H.; Schrader, O.; Ahne, R.; Schütze, W. Transfer of resistance against the beet cyst nematode from radish (Raphanus sativus) to rape (Brassica napus) by monosomic chromosome addition. Theor. Appl. Genet. 2004, 109, 30–41. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, G.L.; Zhang, S.J.; Zhang, S.F.; Zhang, H.; Sun, R.F.; Li, F. Comprehensive transcriptome-metabolome analysis and evaluation of the Dark_Pur gene from Brassica juncea that controls the differential regulation of anthocyanins in Brassica rapa. Genes 2022, 13, 283. [Google Scholar] [CrossRef]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005, 139, 1840–1852. [Google Scholar] [CrossRef]
- Ramsay, N.A.; Glover, B.J. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005, 10, 63–70. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, T.; Lyu, L.; Li, W.; Wu, W. Research progress in understanding the biosynthesis and regulation of plant anthocyanins. Sci. Hortic. 2023, 321, 112374. [Google Scholar] [CrossRef]
- Wang, L.J.; Lu, W.X.; Ran, L.Y.; Dou, L.W.; Yao, S.; Hu, J.; Fan, D.; Li, C.F.; Luo, K.M. R2R3-MYB transcription factor MYB6 promotes anthocyanin and proanthocyanidin biosynthesis but inhibits secondary cell wall formation in Populus tomentosa. Plant J. 2019, 99, 733–751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, J.X.; Chen, K.L.; Yao, W.K.; Zhang, B.X.; Wang, J.Y.; Tian, S.T.; Liu, H.L.; Wang, Y.Q.; Liu, Y.L.; et al. A novel R3 MYB transcriptional repressor, MaMYBx, finely regulates anthocyanin biosynthesis in grape hyacinth. Plant Sci. 2020, 298, 12. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.H.; Kim, C.K. Roles of R2R3-MYB transcription factors in transcriptional regulation of anthocyanin biosynthesis in horticultural plants. Plant Mol. Biol. 2018, 98, 1–18. [Google Scholar] [CrossRef]
- Walker, A.R.; Lee, E.; Bogs, J.; McDavid, D.A.J.; Thomas, M.R.; Robinson, S.P. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. 2007, 49, 772–785. [Google Scholar] [CrossRef]
- Wang, Z.G.; Meng, D.; Wang, A.D.; Li, T.L.; Jiang, S.L.; Cong, P.H.; Li, T.Z. The Methylation of the PcMYB10 Promoter Is Associated with Green-Skinned Sport in Max Red Bartlett Pear. Plant Physiol. 2013, 162, 885–896. [Google Scholar] [CrossRef]
- Song, H.; Yi, H.; Lee, M.; Han, C.T.; Lee, J.; Kim, H.; Park, J.I.; Nou, I.S.; Kim, S.J.; Hur, Y. Purple Brassica oleracea var. capitata F-rubra is due to the loss of BoMYBL2-1 expression. BMC Plant Biol. 2018, 18, 16. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Yang, L.M.; Zhang, Y.Y.; Zhuang, M.; Ji, J.L.; Hou, X.L.; Li, Z.S.; Han, F.Q.; Fang, Z.Y.; Lv, H.H.; et al. Introgression of clubroot resistant gene into Brassica oleracea L. from Brassica rapa based on homoeologous exchange. Hortic. Res. 2022, 9, 16. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Chang, L.; Wang, T.; Liang, J.; Lin, R.; Wu, J.; Wang, X. Expanding the genetic variation of Brassica juncea by introgression of the Brassica rapa Genome. Hortic. Res. 2022, 9, uhab054. [Google Scholar] [CrossRef]
- Wei, Y.X.; Zhu, M.Z.; Qiao, H.Y.; Li, F.; Zhang, S.J.; Zhang, S.F.; Zhang, H.; Sun, R.F. Characterization of interspecific hybrids between flowering Chinese cabbage and broccoli. Sci. Hortic. 2018, 240, 552–557. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Z.Y.; Chen, F.F.; Zhu, Y.Q.; Guo, X.C.; Fu, M.J.; Chen, J.H.; Wu, J.G.; Zhu, Z.J. Production and identification of x Brassicoraphanus distant hybrids between radish (Raphanus sativus L.) and kohlrabi (Brassica oleracea L. var. Caulorapa DC.). N. Z. J. Crop Hortic. Sci. 2023, 51, 341–354. [Google Scholar] [CrossRef]
- Zhao, C.J.; Xie, M.L.; Liang, L.B.; Yang, L.; Han, H.S.; Qin, X.R.; Zhao, J.X.; Hou, Y.; Dai, W.D.; Du, C.F.; et al. Genome-Wide Association Analysis Combined with Quantitative Trait Loci Mapping and Dynamic Transcriptome Unveil the Genetic Control of Seed Oil Content in Brassica napus L. Front. Plant Sci. 2022, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Wang, H.Z.; Wang, J.; Sun, R.F.; Wu, J.; Liu, S.Y.; Bai, Y.Q.; Mun, J.H.; Bancroft, I.; Cheng, F.; et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Liu, Y.M.; Yang, X.H.; Tong, C.B.; Edwards, D.; Parkin, I.A.P.; Zhao, M.X.; Ma, J.X.; Yu, J.Y.; Huang, S.M.; et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 11. [Google Scholar] [CrossRef]
- Parkin, I.A.P.; Koh, C.; Tang, H.B.; Robinson, S.J.; Kagale, S.; Clarke, W.E.; Town, C.D.; Nixon, J.; Krishnakumar, V.; Bidwell, S.L.; et al. Transcriptome and methylome profiling reveals relics of genome dominance in the mesopolyploid Brassica oleracea. Genome Biol. 2014, 15, 18. [Google Scholar] [CrossRef]
- Chalhoub, B. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef]
- Priya, P.; Arun, J.; Bisht, N.C.; Padmaja, K.L.; Sarita, S.; Vibha, G.; Pradhan, A.K.; Deepak, P. Comparative mapping of Brassica juncea and Arabidopsis thaliana using Intron Polymorphism (IP) markers: Homoeologous relationships, diversification and evolution of the A, B and C Brassica genomes. BMC Genom. 2008, 9, 113. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).