Abstract
Microgreens represent a valuable agrifoods niche. Their cropping cycle is shorter than that of baby leaf greens, but the sowing density is typically much higher, and this has important cost implications for the grower. The current research demonstrates that the choice of sowing density strongly influences yield, as well as developmental stage and other quality parameters. Results also depended on the choice of the species and landrace. Considering the cost of seed, the option of accessing locally available landraces becomes particularly intriguing, again with relevant implications in choosing seed density. Rapini (landraces Cima grande and Fasanese), kale (landraces Barese and Altamura), and commercial cress were grown in an indoor environment. The effects of the three sowing densities (from 3 to 5 seeds∙cm−2) and the growing cycle (earlier harvest, 11 days from sowing, or later harvest, 14 days from sowing) on the microgreen yield and quality were studied. Sowing density affected yield (+19% at highest vs. lowest density), dry matter (but only with a longer cycle, and variable by landrace, with Fasanese rapini landrace 7% more than the Cima grande landrace), developmental stage, and soil coverage. The effects of sowing density can be modulated by cycle duration. Crop heights were 25% and 44% greater for the longer cycle of the Cima grande and Fasanese rapini landraces, respectively. In conclusion, the choices of the species/landrace and seed density must be carefully evaluated given costs and outcomes, with potential for the production of different final products (e.g., microgreens at earlier or later stages, other characteristics) and also for control over costs.
1. Introduction
Although it was in the late 1980s that chefs operating in the San Francisco area first began experimenting with microgreens [1], it is only recently that producers have given them more attention, along with nutritionists and consumers, for their characteristics of colours and flavours, tenderness and crunchiness, as well as high concentrations of vitamins, minerals, antioxidants and possibly other nutraceutical compounds [2,3].
Microgreens can be defined as young and tender edible seedlings [4], harvested for consumption at 10–20 days of emergence [5], and usually cultivated with soilless systems with or without fertilisers and agrochemicals [6,7]. Differently from the “baby leaf” greens [8], “microgreens”, is only a marketing term that refers to the commercial category of product for the consumers without a current legal definition [1].
Crop types and production are diversified through different cultivation techniques and environments, e.g., polytunnel, greenhouse, growing media, artificial and/or natural light [7,9,10,11]. Significant attention must be paid to the specifics of cultivation techniques, among which the most important include selection of species [12], substrates [13], light regimes [11,14,15], irrigation and fertilisation [16,17], as well as sowing density [18,19].
The importance of sowing density lies in the fact that the rates may be greater than 20,000 seeds∙m−2 [20], leading to this factor emerging as a potential economic or technical constraint. Before the experimental phase of the current research, we therefore queried the Scopus database on articles concerning seed density for microgreens, running “(TITLE-ABS-KEY (seed AND density) OR TITLE-ABS-KEY (sowing AND density) AND TITLE-ABS-KEY (microgreen))”. This query yielded few articles: for example, only eight documents were returned by performing research on 4 March 2024 in Scopus for “Microgreens” “density” and “seed(s)”. This indicates the lack of information regarding such a topic. This is surprising considering that microgreen production lends itself to harnessing a huge pool of biodiversity contained in landraces and otherwise underutilised or completely neglected vegetable species [21,22].
Furthermore, microgreens are typically characterised by high contents of minerals, polyphenols and other bioactive compounds and, therefore, may serve as functional foods [23,24]. Expanding cultivation into previously neglected or wild species could thus expand [25,26] the offer in such functional roles. Once again, the question of sowing density for the boost of the industry becomes important. Various Italian regions maintain great traditions in the use of wild species and ones that, in other global contexts, are little used or completely neglected. Such species, many of which were never scientifically investigated, can thus be viewed as a reservoir for potential variation and expansion in microgreen production [27,28].
On these bases, the key agronomic parameters of sowing density and cycle duration were investigated to evaluate the impact on the production of microgreens. In addition, the adaptation of local vegetable landraces of Brassicaceae species to microgreens was investigated.
2. Materials and Methods
Three experiments were carried out in the Department of Soil, Plants and Food Sciences facilities of the University of Bari Aldo Moro in February and March 2023. Prior to the experiments, the germination capacity of seeds of the different species and landraces was preliminarily assessed according to the “International Rules for Seed Testing” of the International Seed Testing Association guidelines [29]. The first experiment explored three brassicaceous crops (kale, cress and rapini) and three sowing densities (3.5, 4.0, and 4.5 seeds∙cm−2). The second and third experiments were focused on two landraces of rapini and two landraces of kale, which were the most promising species selected after the first experiment. For them, larger intervals of sowing densities (3.0, 4.0, and 5.0 seeds∙cm−2) and the harvest time for rapini were tested.
2.1. Common Elements of the Three Experiments
The substrate used for microgreens cultivation was peat “Brill 3 special” (Brill Substrate GmbH & Co. KG, Georgsdorf, Germany). The seeds were sown in plastic perforated-base square trays (17.5 × 17.5 cm, height 3 cm) placed in turn inside larger plastic trays (60 × 40 cm, height 6 cm). Within the growth chamber, the trays were sprayed with water and covered with mulch cloth for germination. Soon after germination, the mulch cloth was removed, and the seedlings were fertigated via subirrigation. Within the larger trays, the perforated ones were given a daily application of two minutes duration, using a half-strength Hoagland nutrient solution [30] with concentrations (mg∙L−1): N 105, P 16, K 117, Ca 100, and Mg 12, resulting in a pH of 6.5 and an electric conductivity of 1.65 dS∙m−1. Subsequently, again by application to the small trays within larger ones, irrigation continued for two minutes per day, using the nutrient solution.
At the harvest times, the parameters hereafter described were measured to study the plant growth (microgreens stage for commercial purposes), yield and the appearance of the microgreens canopy for its commercial and aesthetic value. These included a developmental stage in terms of an integer number, 1—cotyledonary, 2—first true leaf length less than 5 mm, 3—first true leaf length greater than 5 mm (measured by digital calliper); number and length of the true leaves (measured by digital calliper); the average height of microgreens, measured with respect to the substrate surface, at two sides and the centre of each tray (using a ruler; Figure 1); crop coverage, 1—poor (incomplete coverage), 2—good (complete coverage), 3—excessive overlapping (Figure 2); uniformity of canopy, 1—irregular favouring canopy centre, 2—uniform, 3—irregular favouring outwards (Figure 3); fresh and dry weight (obtained through ventilated oven at 65 °C until constant weight was reached). An aliquot of fresh material was also freeze-dried (Scan Vac CoolSafe Freeze Dryers, LaboGene, Denmark) for subsequent chemical analysis.

Figure 1.
Microgreen height above the substrate calculated as an average of three measurements at the sides and centre of the canopy (in this image, an example of cress).

Figure 2.
Crop distribution and coverage, showing examples of cress. Top row—sowing at different densities; central row—resulting crop coverage; bottom row—schematisation of crop coverage: 1—poor (incomplete coverage); 2—good (complete coverage); 3—excessive overlapping.
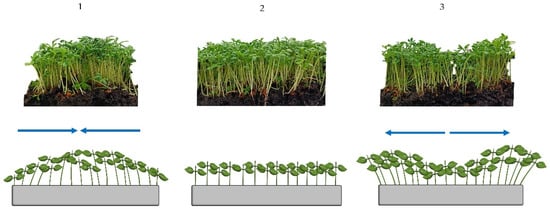
Figure 3.
Uniformity of the microgreen canopy, showing examples of cress. Top row—canopy at harvesting; bottom row—schematisation: 1—irregular favouring centre; 2—uniform; 3—irregular favouring outwards. The arrows indicate the type of irregular distribution of plants.
The experimental design was a randomised block with three replications. The data were analysed using SAS software’s general linear model procedure (SAS Version 9.1, SAS Institute, Cary, NC, USA). All means were compared using the Student–Newman–Keuls (SNK) test at p = 0.05.
2.2. First Experiment: Species and Sowing Densities
Three species of the Brassicaceae family were considered: Brassica rapa L. subsp. Sylvestris L. Janch. var. esculenta Hort., which is commonly known in Italy as “cima di rapa” (literally meaning turnip tops) or rapini; Brassica oleracea var. acephala, a kale known in Italy as “cavolo riccio”; and Lepidium sativum L., cress. The seeds provided by a local nursery were sown at the three sowing densities of 3.5, 4.0, and 4.5 seeds∙cm−2, obtained on the base of the weight of 1000 seeds (2.11 g for rapini, 3.02 g for kale, and 2.05 g cress) and harvested after 11 days from sowing. The experiment was conducted in a growth chamber (Bertagnin model 5-82-3016) at the temperature of 20/20 °C (day/night), relative humidity (RH) ≥60%, photoperiod 18/6 (day/night), PPFD 30 µmol·m−2·s−1. In addition to the common parameters, leaf area (LI-3100C area meter—LI-COR Biosciences, Lincoln, NE, USA) referred to the total leaf area of one tray of microgreens, chlorophyll concentration of cotyledonary leaves (Apogee, MC-100—LI-COR Biosciences, Lincoln, NE, USA), leaf colour of cotyledonary leaves (with Minolta Chroma Meter CR-400; Minolta Camera Co., Ltd., Osaka, Japan) were also measured.
2.3. Second Experiment: Rapini Landraces, Sowing Density and Harvest Time
Based on the significant interest in rapini and the results of the first trial, a second experiment was carried out to investigate further on this species. Two Apulian landraces of rapini named Cima grande and Fasanese were sown at the sowing densities of 3, 4 and 5 seeds∙cm−2, which were obtained based on the weight of 1000 seeds (2.71 g for Cima grande, 2.08 g for Fasanese), and were harvested at two harvest times, after 11 and 14 days from sowing. Two harvest times, or the number of days from sowing to harvest, were chosen to investigate two microgreens’ stages. The experiment was carried out in a Conviron PGW 36 growth chamber (Winnipeg, MB, Canada) at a temperature of 20/15 °C (day/night), a relative humidity percentage (RH) of 70 ± 5%, a photoperiod of 12/12 (day/night), and a photosynthetic photon flux density (PPFD) of 208 µmol·m−2·s−1. Inorganic anions were determined using the procedure reported by [31] and an ion chromatograph Dionex DX120 (Dionex Corporation, Sunnyvale, CA, USA).
2.4. Third Experiment: Kale Landraces and Sowing Density
Based on the results of the first trial and a growing interest in landraces of kale from the Puglia region, a third experiment was carried out to investigate further on this species. Two landraces of kale (Brassica oleracea var. acephala) known as Barese (or “of Bari”) and Altamura (or “of Altamura”) were sown at the seeding densities of 3, 4 and 5 seeds∙cm−2, which were obtained on the basis of the weight of 1000 seeds (3.89 g for both landraces), and harvested after 11 days from sowing, as suggested from preliminary trials. The experiment was carried out in a Conviron PGW 36 growth chamber (Winnipeg, MB, Canada) in the same growing conditions as the second experiment.
3. Results
3.1. First Experiment: Species and Sowing Densities
The species was the prominent cause of differences between kale, cress and rapini microgreens; conversely, the sowing density affected the yield only (Table 1). At the harvest (11 days from sowing), all the species had one true leaf with a length of approximately 1.8 cm.

Table 1.
Yield, biometric and quality parameters of kale, rapini and cress microgreens produced at three sowing densities (3.5, 4.0 and 4.5 seeds·cm−2). Data are means of three replicates (n = 3). Means of each experimental interaction are extensively reported in Table S2 of Supplementary Materials. FW—fresh weight.
The kale reached the highest yield, producing 0.44 and 0.97 kg·m−2 more than rapini and cress, respectively. The highest sowing density (4.5 seeds·cm−2) resulted in a yield 19% higher than the lowest sowing density (3.5 seeds·cm−2) (Table 1).
Neither the choice of the species nor the variation in sowing density had significant effects on the crop developmental stage and true leaf length, coverage or uniformity of the canopy (Table 1). The species differed in dry matter content, biometric parameters, and chlorophyll content. The dry matter content of rapini was 10% higher than that of the other species. Cress microgreens were the smallest in size: the average plant height and the hypocotyl length of rapini and kale microgreens were higher than in cress, both around 28%. Cress reached the smallest leaf area (49 and 61% less than rapini and kale), and kale was the largest (30% more than rapini). Chlorophyll concentration differed between species. Kale showed the highest concentration, which was 27% higher than rapini and almost threefold that of cress (Table 1).
The colour of the cotyledons depended only on the species (Supplementary Materials, Table S1 and Figure S1).
3.2. Second Experiment: Rapini Landraces, Sowing Density and Harvest Time
The higher the sowing density, the higher the yield at the extent of 24% obtained with 5 seeds·cm−2 compared to 3 and 4 seeds·cm−2 (Table 2). Despite this positive result, increasing sowing density from 3 to 5 seeds·cm−2 negatively impacted the length of the true leaf (30% lower at the higher density compared to the other two densities) and partially on the seedling development. In detail, the effect of sowing density on the developmental stage was a function of the harvest time (Figure 4A): at the early harvest date, the density increase had negative impacts on the developmental stage, whereas, at the later harvest date, the parameter was not affected by the density.

Table 2.
Growth, productive and quality parameters of two landraces of rapini (Cima grande and Fasanese), grown as microgreens at three sowing densities (3, 4 and 5 seeds·cm−2) and harvested two times (11 and 14 days after sowing). Data points are the means of three replicates (n = 3). The means of each interaction are extensively reported in Table S3 of Supplementary Materials. FW—fresh weight.
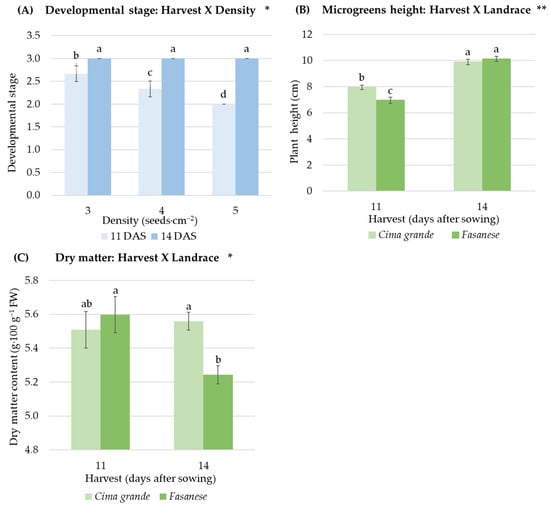
Figure 4.
Visualization of the two-way interactions reported in Table 1. (A) Effect of the interaction between harvest time (11 and 14 days after sowing, DAS) and sowing density (3, 4 and 5 seeds∙cm−2) on the developmental stage of rapini microgreens; (B) effect of the interaction between harvest time (11 and 15 DAS) and landrace (Cima grande e Fasanese) on plant height of rapini microgreens; (C) effect of the interaction between harvest time (11 and 15 DAS) and landrace (Cima grande e Fasanese) on dry matter content of rapini microgreens. FW = fresh weight. Significance: ** and *, significant for p ≤ 0.01 and p ≤ 0.05, respectively; different lowercase letters indicate significant differences (p = 0.05) between groups. Vertical bars represent the standard error of mean values; data points are means of three replicates (n = 3). The means of each interaction are extensively reported in Table S3 of Supplementary Materials.
The delay of three days in the harvest time determined a 30% increase in the average developmental stage of the rapini microgreens (Table 2). The length of the first true leaf was more than double, and the yield grew up to 55% at the later harvest (Table 2). The average plant height increased from 7.5 cm to 10 cm.
At the first harvest date (11 days from sowing), Cima grande was around 14% taller than Fasanese. Conversely, at the second harvest date (14 days from sowing), the differences in plant height between the landraces were not significant anymore (Table 2 and Figure 4B).
The dry matter content of Fasanese microgreens was 7% lower at the later harvest date compared to the first; conversely, in Cima grande, the dry matter was constant between the two harvest times (Figure 4C).
Chlorine concentration in rapini microgreens grew by 21% when increasing the harvest time, and it was 16% higher in Fasanese than in Cima grande (Table 3). Concerning phosphates concentration, an interaction effect was detected between harvest time and landrace: while in Cima grande, the concentration was unaffected by the harvest time, in Fasanese, it decreased with the later harvest (14 days from sowing) (Table 3 and Figure 5).

Table 3.
Concentrations of the main anions (chlorine, Cl−; phosphate, H2PO4−; sulphate, SO42−) expressed on the dry weight (DW) and the concentration of nitrate (NO3−) on fresh weight (FW) as quality parameters of two landraces of rapini (Cima grande and Fasanese), grown as microgreens at three sowing densities (3, 4 and 5 seeds·cm−2) and harvested in two times (11 and 14 days after sowing). Data points are means of three replicates (n = 3). The means of each interaction are extensively reported in Table S4 of Supplementary Materials.
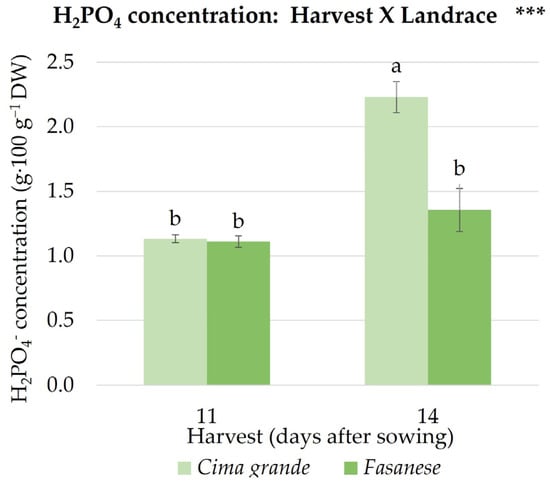
Figure 5.
Visualization of the effect of the two-way interaction between harvest time (11 and 14 DAS) and landrace (Cima grande and Fasanese) on the concentration of phosphate (H2PO4−) in rapini microgreens dry weight (DW). Significance: *** significant for p ≤ 0.001; different lowercase letters indicate significant differences (p = 0.05) between groups. Vertical bars represent the standard error of mean values; data points are means of three replicates (n = 3). The means of each interaction are extensively reported in Table S4 of Supplementary Materials.
Concerning the nitrate content in the fresh biomass, Fasanese microgreens contained 17% more nitrates than Cima grande. The greatest accumulation was found with the lowest sowing density (+18% compared to the higher sowing densities) (Table 3).
3.3. Third Experiment: Kale Landraces and Sowing Density
The sowing density poorly influenced kale microgreens (Table 4). Increasing the sowing density from 3 to 5 seeds seeds∙cm−2, the yield grew by 26%; however, it negatively affected (−41%) the uniformity of the microgreen canopy, which grew irregularly toward the centre (Table 4).

Table 4.
Yield, biometric and quality parameters of two kale landraces (Barese and Altamura), grown as microgreens at three sowing densities (3, 4 and 5 seeds·cm−2) and harvested 14 days after sowing. Data points are means of three replicates (n = 3). The means of each interaction are extensively reported in Table S5 of Supplementary Materials.
The kale landraces Barese and Altamura largely differed for growth and yield parameters (Table 4). At the harvest, Altamura microgreens were at an advanced developmental stage, showing the first true leaf length greater than 5 mm (developmental stage 3). The Barese microgreens had developed a little further from the cotyledonary stage (developmental stage 1). Nevertheless, Barese landrace produced 39% more than Altamura and guaranteed a complete and good coverage of the substrate compared to the poor coverage of Altamura microgreens (+43%) (Table 4).
4. Discussion
4.1. Sowing Density, Yield, and Growth
Although sowing density is known to significantly impact final crop outcomes, such effects are greatly dependent on the species and landrace [32]. A single species/landrace may depend on the growing conditions.
The increase in sowing density increased the yield in all the experiments, and the wider the density range, the larger the result. In fact, the difference in increment from the lowest to the highest density between the first experiment (smaller ranges 3.5, 4.0, 4.5 seeds·cm−2, increment of 20%—Table 1) and the other two experiments (larger ranges 3.0, 4.0, 5.0 seeds·cm−2, increment of 24% for rapini and 26% in kale—Table 2 and Table 4) could be attributed to the different width of the range. Furthermore, the competition for resources determined by sowing density can affect the speed of growth and development. This was found for the rapini landraces. The rapini seedlings grown at the lowest sowing density were more developed and had a larger true leaf than the ones grown at the highest density (Table 2). In kale landraces, the higher the sowing density, the worse the uniformity of the microgreen canopy. These results further confirm that the “correct” density is hard to determine and leads to uncertain answers from seed companies and questions and concerns from growers [33,34]. Moreover, in the case of rapini, the density influenced the final developmental stage when a cycle of 11 days was chosen, whereas seedlings at an equal stage were obtained with a three-day longer cycle. This suggests that the evaluation of sowing density should also take into account the desired seedling stage at harvest and the duration of the growing cycle.
4.2. Sowing Density, Landrace, and Commercial Stage
The great weight of seed cost in the economy of microgreen production means that the choice of species and landrace is truly important. As shown in our three experiments, the yield was influenced by both species and landrace. In a cultivation cycle of 11 days from sowing, kale was the most productive landrace, followed by rapini and lastly cress (Table 1). Despite the result at species level, quite a large difference in yield between the two rapini landraces (Table 2) and the two kale landraces (Table 4) highlights the importance of considering the landrace rather than the species only. Many species [35] offer the potential for microgreen production. Within the species, the cultivation of local varieties and underutilised landraces [35] offers noteworthy possibilities. Among other considerations, local seed sourcing could achieve savings compared to purchasing more recognised commercial varieties from major companies. Growers would then need to be aware of the compromises in choosing sowing density, not only in relation to the variables explored in the current research (developmental stage, height and leaf character at harvest, yields) but also further ones, such as fungal infections [33]. Furthermore, growers intending to use large amounts of seed, whether for the production of microgreens or “near baby leaf”, could therefore economise by choosing such locally available seed landraces rather than the “improved” varieties available from major seed houses at a generally higher cost.
Our results on kale landraces suggest that the growers could choose between landraces of kale given the desired stage of the final product and the cycle duration. At the tested cycle duration (11 days from sowing), Altamura kale could be more appropriate for production closer to the baby leaf category due to the faster development and growth, or it could be harvested earlier than Barese landrace to obtain microgreens. Contrarily, Barese could be better suited to smaller microgreen production since it achieved higher production with plants still at a lesser development stage.
4.3. Harvest Time, Landrace, and Commercial Stage
The final stage of microgreen can be modulated not only by the means of sowing density but also by the harvest time, namely the duration of the growing cycle. From the cycle length (11 and 14 days from sowing) to rapini landraces, we may infer that the growth was increased regardless of the sowing density (Table 2); however, different results were obtained in relation to the tested landrace. In line with the previous research [36,37], the variability in the result was expected and confirmed. It is ascribable to the genotypic peculiarity of different landraces. These differences represent a resource for the growers of microgreens, which can rely on a wide range of choices according to the desired final product. In our case, for rapini microgreens production, choosing a shorter cultivation cycle, Cima grande would be better than Fasanese; on the contrary, if the aim were a final product closer to the baby-leaf stage, the choice of landrace would be indifferent.
The harvest time also had implications for the dry matter content, which can play a favourable role post-harvest since a greater dry weight is reported to extend microgreens’ shelf life over time [38,39]. Rapini showed the highest dry matter content compared to kale and cress after 11 days from sowing (Table 1). However, the dry matter content was differently accumulated by the two landraces (second experiment) between 11 and 14 days from sowing. In line with previous results on leafy Brassicaceae species reported in the literature [23], the dry matter content in Fasanese decreased during the growing cycle from 11 to 14 days from sowing; conversely, it was constant in Cima grande in the same time span (Figure 4C). These results can help to face some technical issues of the production and commercialization of microgreens and similar categories. In detail, the following points need to be considered: the seedlings collected at the later harvest date represent an intermediate category between “microgreens” and the somewhat larger “baby leaf”; microgreens are subject to mechanical damage from technical operations (e.g., washing, drying, etc.) that significantly compromises their shelf-life [40]; the ready-to-eat sector has favoured the baby leaf category. On these bases, the harvest dates could be chosen earlier to produce microgreens or postponed collecting plants more suitable for the ready-to-eat sector.
4.4. Microgreens Quality
The quality of microgreens was investigated in terms of aesthetic, commercial and nutritional characteristics. The choice of different species to diversify in appearance, size and colours of microgreens is a crucial point of microgreens utilization, and the occurrence of species variability is well known [41,42]. The diverse morpho-biometric properties of kale, rapini and cress seedlings, as well as the appearance of the microgreen canopy in a commercial tray (coverage, uniformity, height, true leaf stage and colour), were described in our experiments. In addition, while at the species level, only the genotypic factor influenced these parameters, at the intraspecific level for rapini and kale, sowing density and harvest time also had an impact on microgreens. The results suggest that, after selecting the species for its aesthetical peculiarities, the grower should further tailor the appeal of the microgreens through the adequation of the agronomic techniques to the landrace. In the case of rapini landraces, the microgreen height can be modulated by the comprehensive choice of landrace and harvest time. The choice of the sowing density was particularly important for the optimal coverage of the substrate by the rapini canopy and for the uniform growth of the kale canopy. An optimal substrate coverage is a sign of an adequate sowing density and uniform sowing on the surface, which determine a uniform canopy appearance. In this sense, an optimal sowing density allows the growers to maximise the yield from the area unit while preserving the quality of the microgreens. In both rapini and kale, the lower density was favourable to the improvement of the canopy appearance, possibly due to the lower competition between seedlings. Nevertheless, an intermediate sowing density (4 seeds·cm−2) could be preferred to gain a balance between aesthetics and yield.
The selection of the species should be addressed also in view of their nutritional profile. However, the comparison of the nutritional compound content between species is not always consistent. For example, leafy vegetables are obviously rich in chlorophyll in some species, exceeding concentrations of 1000–2000 mg·kg−1 of fresh weight [43]. It should be noted, however, that chlorophyll values can vary widely even in the same species, making conclusive comparisons between species difficult for this parameter [44]. In the case of mineral nutrients, several studies have demonstrated the variation in nutrient density in relation to the growth stage [35], and this could also lead to choices for varietal selection, cultivation practices, and marketing.
In the present study, the cycle duration influenced the chemical profile of the rapini microgreens. The concentration of chlorine in the dry biomass increased from the earlier (11 days after sowing) to the later harvest (14 days after sowing). The nitrate content in the fresh tissues was measured as an important qualitative parameter for leafy vegetables consumed as fresh and row products, which is the case of microgreens. There were no differences in nitrate accumulation between landraces, but its concentration in plants was lowered by the higher sowing density. Such behaviour is because the nitrogen concentration was the same in the different sowing densities, but there was more competition between plants for the nitrogen source than at higher densities, where every plant had a smaller amount of nitrogen available.
5. Conclusions
The shape, taste, colour, texture and nutritional profile of microgreens make this agrifood product of considerable interest for consumers and therefore for producers. The practicalities and economics of cultivation and marketing, however, require close attention to diverse factors; among the most important are the choices of genotype and sowing density.
To produce rapini microgreens, yield can be increased by choosing a higher sowing density (5 seeds·cm−2) and adopting a longer growing cycle. Higher sowing density also improves crop coverage and could therefore serve in avoiding lodging. The time cycle duration could be planned according to the aims for the final product, whether the earlier or later stage of the microgreens, since the later harvest date (14 days from sowing) determined an advanced developmental stage regardless of the tested sowing densities, whereas at the earlier harvest date (11 days from sowing), the crop developmental stage was reduced by the increase of the sowing density. As for rapini, increasing sowing density improves the yield of kale and cress microgreens without compromising the quality. Despite the crucial role of sowing density, evaluating the landrace and harvest date should be carefully included in the production planning of microgreens due to their comprehensive impact on yield and quality. Finally, the grower would have to consider the potentially important aspect of seed cost, given that density ranges from 30,000 to 50,000 seeds·m−2 (for rapini, corresponding approximatively 69 g and 115 g of seeds, respectively).
The current study provides some pragmatic insights from both the practical and academic points of view regarding some crucial agronomic choices for microgreen production. From a practical point of view, understanding the possible usage of the sowing density was provided with reference to the agronomic and economic side. Furthermore, some indications were provided regarding tailoring the final product according to the landrace, the growing cycle, and the grower goals. From an academic point of view, it adds some useful information regarding the possible exploitation of local agro-biodiversity of vegetables, as very little information is available in the scientific literature. By providing these indications in principle, even if more research is needed in the future, particularly related other species, such an evaluation and the selection of species and landrace would play fundamental roles in the outcomes of agricultural operations and ultimately in marketing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10030274/s1, Figure S1: Diagram of chromaticity in a CIELab space (a*, red/green chromaticity; b*, yellow/blue chromaticity) of cotyledonary leaves of kale, rappini and cress microgreens; Table S1: Color parameters of cotyledonary leaves of kale, rappini and cress microgreens produced at three sowing densities (3.5, 4.0, 4.5 seeds∙cm−2). L, lightness; a*, red/green chromaticity; b*, yellow/blue chromaticity; C, color saturation; h°, hue angle; Table S2: Yield, biometric and quality parameters of kale, rapini and cress microgreens produced at three sowing densities (3.5, 4.0, 4.5 seeds·cm−2). Data are means of three replicates (n = 3). The significance of the main effects and of the interaction effect is reported in Table 1 of the manuscript. FW = fresh weight; Table S3: Growth, productive and quality parameters of two landraces of rapini (Cima grande and Fasanese), grown as microgreens at three sowing densities (3, 4, 5 seeds·cm−2) and harvested in two times (11 and 14 days after sowing). Data points are means of three replicates (n = 3). The significance of the main effects and of the interaction effect is reported in Table 2 and visualised in Figure 4 of the manuscript. FW = fresh weight; Table S4: Concentration of the main anions (chlorine, Cl−; phosphate, H2PO4−; sulphate, SO42−) expressed on the dry weight (DW) and nitrate (NO3−) on fresh weight (FW) as quality parameters of two landraces of rapini (Cima grande and Fasanese), grown as microgreens at three sowing densities (3, 4, 5 seeds·cm−2) and harvested in two times (11 and 14 days after sowing). Data points are means of three replicates (n = 3). The significance of the main effects and of the interaction effect is reported in Table 3 and visualised in Figure 5 of the manuscript; Table S5: Yield, biometric and quality parameters of two landraces of kale (Barese and Altamura), grown as microgreens at three sowing densities (3, 4, 5 seeds·cm−2) and harvested at 14 days after sowing). Data points are means of three replicates (n = 3). The significance of the main effects and of the interaction effect is reported in Table 4 of the manuscript.
Author Contributions
Conceptualisation, P.S.; methodology, P.S.; validation, P.S., A.S. (Angelo Signore), B.L. and A.S. (Annalisa Somma); formal analysis, P.S.; investigation, B.L. and A.S. (Annalisa Somma); resources, P.S., A.S. (Angelo Signore), B.L. and A.S. (Annalisa Somma); data curation, B.L. and A.S. (Annalisa Somma); writing—original draft preparation, A.S. (Angelo Signore), A.S. (Annalisa Somma) and P.S.; writing—review and editing, A.S. (Annalisa Somma), P.S. and A.S. (Angelo Signore); visualisation, A.S. (Annalisa Somma) and P.S.; supervision, P.S.; project administration, P.S.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Rural Development Programme of the Apulia Region (Italy) 2014–2020, Submeasure 16.2 (Support for pilot projects and the development of new products, practices, processes and technologies, and the transfer and the dissemination of the results obtained by the Operational Groups), in the framework of the SOILLESS GO project, project code (CUP) B97H20000990009. Paper no. 19.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Treadwell, D.; Hochmuth, R.; Landrum, L.; Laughlin, W. Microgreens: A New Specialty Crop. Available online: https://journals.flvc.org/edis/article/view/123356 (accessed on 13 March 2023).
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic Constitution, Phytochemical and Macronutrient Content in Three Species of Microgreens as Modulated by Natural Fiber and Synthetic Substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef]
- Bulgari, R.; Baldi, A.; Ferrante, A.; Lenzi, A. Yield and Quality of Basil, Swiss Chard, and Rocket Microgreens Grown in a Hydroponic System. N. Z. J. Crop Hortic. Sci. 2017, 45, 119–129. [Google Scholar] [CrossRef]
- Palmitessa, O.D.; Renna, M.; Crupi, P.; Lovece, A.; Corbo, F.; Santamaria, P. Yield and Quality Characteristics of Brassica Microgreens as Affected by the NH4:NO3 Molar Ratio and Strength of the Nutrient Solution. Foods 2020, 9, 677. [Google Scholar] [CrossRef]
- Lee, J.; Pill, W.; Cobb, B. Seed Treatments to Advance Greenhouse Establishment of Beet and Chard Microgreens. J. Hortic. Sci. Biotechnol. 2004, 79, 565–570. [Google Scholar] [CrossRef]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables BT—Minimally Processed Refrigerated Fruits and Vegetables; Springer: Berlin/Heidelberg, Germany, 2017; pp. 403–432. ISBN 978-1-4939-7018-6. [Google Scholar]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-Scale Vegetable Production and the Rise of Microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 752/2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32014R0752&from=EN (accessed on 15 May 2023).
- Bulgari, R.; Negri, M.; Santoro, P.; Ferrante, A. Quality Evaluation of Indoor-Grown Microgreens Cultivated on Three Different Substrates. Horticulturae 2021, 7, 96. [Google Scholar] [CrossRef]
- Wieth, A.R.; Pinheiro, W.D.; Duarte, T.d.S. Purple Cabbage Microgreens Grown in Different Substrates and Nutritive Solution Concentrations. Rev. Caatinga 2019, 32, 976–985. [Google Scholar] [CrossRef]
- Kamal, K.Y.; Khodaeiaminjan, M.; El-Tantawy, A.A.; Moneim, D.A.; Salam, A.A.; Ash-shormillesy, S.M.A.I.; Attia, A.; Ali, M.A.S.; Herranz, R.; El-Esawi, M.A.; et al. Evaluation of Growth and Nutritional Value of Brassica Microgreens Grown under Red, Blue and Green LEDs Combinations. Physiol. Plant. 2020, 169, 625–638. [Google Scholar] [CrossRef]
- Xiao, Z.; Codling, E.E.; Luo, Y.; Nou, X.; Lester, G.E.; Wang, Q. Microgreens of Brassicaceae: Mineral Composition and Content of 30 Varieties. J. Food Compos. Anal. 2016, 49, 87–93. [Google Scholar] [CrossRef]
- Negri, M.; Bulgari, R.; Santoro, P.; Ferrante, A. Evaluation of Different Growing Substrates for Microgreens Production. In Acta Horticulturae; ISHS (ISHS|International Society for Horticultural Science): Leuven, Belgium, 2021; pp. 109–114. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Growth and Morphology Responses to Narrow-Band Blue Light and Its Co-Action with Low-Level UVB or Green Light: A Comparison with Red Light in Four Microgreen Species. Environ. Exp. Bot. 2020, 178, 104189. [Google Scholar] [CrossRef]
- Kong, Y.; Kamath, D.; Zheng, Y. Blue versus Red Light Can Promote Elongation Growth Independent of Photoperiod: A Study in Four Brassica Microgreens Species. HortScience 2019, 54, 1955–1961. [Google Scholar] [CrossRef]
- Cowden, R.J.; Markussen, B.; Ghaley, B.B.; Henriksen, C.B. The Effects of Light Spectrum and Intensity, Seeding Density, and Fertilization on Biomass, Morphology, and Resource Use Efficiency in Three Species of Brassicaceae Microgreens. Plants 2024, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lalk, G.T.; Bi, G. Fertilization and Pre-Sowing Seed Soaking Affect Yield and Mineral Nutrients of Ten Microgreen Species. Horticulturae 2021, 7, 14. [Google Scholar] [CrossRef]
- Di Gioia, F.; Santamaria, P. MicroGREENS: Nuovi Alimenti Freschi e Funzionali per Esplorare Tutto Il Valore Della Biodiversità; CINECA IRIS: Casalecchio di Reno, Italy, 2015; ISBN 978-88-909289-3-2. [Google Scholar]
- Li, T.; Lalk, G.T.; Arthur, J.D.; Johnson, M.H.; Bi, G. Shoot Production and Mineral Nutrients of Five Microgreens as Affected by Hydroponic Substrate Type and Post-Emergent Fertilization. Horticulturae 2021, 7, 129. [Google Scholar] [CrossRef]
- Moraru, P.I.; Rusu, T.; Mintas, O.S. Trial Protocol for Evaluating Platforms for Growing Microgreens in Hydroponic Conditions. Foods 2022, 11, 1327. [Google Scholar] [CrossRef]
- Signore, A.; Renna, M.; Santamaria, P. Agrobiodiversity of Vegetable Crops: Aspect, Needs, and Future Perspectives. Annu. Plant Rev. Online 2019, 2, 1–24. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Cannata, C.; Basile, F.; Abdelkhalik, A.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.R. The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source. Foods 2022, 11, 423. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient Composition, Oxalate Content and Nutritional Ranking of Ten Culinary Microgreens. J. Food Compos. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Incrocci, L.; Pardossi, A.; Venturi, F.; Taglieri, I.; Ferroni, G.; Guidi, L. Comparison of Three Domestications and Wild-Harvested Plants for Nutraceutical Properties and Sensory Profiles in Five Wild Edible Herbs: Is Domestication Possible? Foods 2020, 9, 1065. [Google Scholar] [CrossRef] [PubMed]
- Anaclerio, M.; Renna, M.; Di Venere, D.; Sergio, L.; Santamaria, P. Smooth Golden Fleece and Prickly Golden Fleece as Potential New Vegetables for the Ready-to-Eat Production Chain. Agriculture 2021, 11, 74. [Google Scholar] [CrossRef]
- Baldi, A.; Bruschi, P.; Campeggi, S.; Egea, T.; Rivera, D.; Obón, C.; Lenzi, A. The Renaissance of Wild Food Plants: Insights from Tuscany (Italy). Foods 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and Microgreens: Trends, Opportunities, and Horizons for Novel Research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- International Seed Testing Association. International Rules for Seed Testing. Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing-1168.html (accessed on 1 September 2022).
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. Available online: https://ia800205.us.archive.org/9/items/watercultureme3450hoag/watercultureme3450hoag.pdf (accessed on 11 February 2024).
- Signore, A.; Serio, F.; Santamaria, P. A Targeted Management of the Nutrient Solution in a Soilless Tomato Crop According to Plant Needs. Front. Plant Sci. 2016, 7, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue Light Dose-Responses of Leaf Photosynthesis, Morphology, and Chemical Composition of Cucumis Sativus Grown under Different Combinations of Red and Blue Light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Nolan, D.A. Effects of Seed Density and Other Factors on the Yield of Microgreens Grown Hydroponically on Burlap; Virginia Tech: Blacksburg, VA, USA, 2018; pp. 1–44. [Google Scholar]
- Johnny Seeds Johnny’s Microgreens Yield Data Trial: Determining Seed Density & Yield per Tray for 29 Varieties. Available online: https://www.johnnyseeds.com/growers-library/vegetables/microgreens/micro-greens-yield-data-trial-summary-discussion.html?q=microgreen (accessed on 13 February 2024).
- Ebert, A.W. Sprouts and Microgreens—Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Khader, V.; Rama, S. Effect of Maturity on Macromineral Content of Selected Leafy Vegetables. Asia Pac. J. Clin. Nutr. 2003, 12, 45–49. [Google Scholar]
- Santos, J.; Oliva-Teles, M.T.; Delerue-Matos, C.; Oliveira, M.B.P.P. Multi-Elemental Analysis of Ready-to-Eat “Baby Leaf” Vegetables Using Microwave Digestion and High-Resolution Continuum Source Atomic Absorption Spectrometry. Food Chem. 2014, 151, 311–316. [Google Scholar] [CrossRef]
- Sánchez, M.-T.; Entrenas, J.-A.; Torres, I.; Vega, M.; Pérez-Marín, D. Monitoring Texture and Other Quality Parameters in Spinach Plants Using NIR Spectroscopy. Comput. Electron. Agric. 2018, 155, 446–452. [Google Scholar] [CrossRef]
- Valverde-Miranda, D.; Díaz-Pérez, M.; Gómez-Galán, M.; Callejón-Ferre, Á.-J. Total Soluble Solids and Dry Matter of Cucumber as Indicators of Shelf Life. Postharvest Biol. Technol. 2021, 180, 111603. [Google Scholar] [CrossRef]
- Kou, L.; Yang, T.; Liu, X.; Luo, Y. Effects of Pre- and Postharvest Calcium Treatments on Shelf Life and Postharvest Quality of Broccoli Microgreens. HortScience 2015, 50, 1801–1808. [Google Scholar] [CrossRef]
- Shilpashree, N.; Devi, S.N.; Manjunathagowda, D.C.; Muddappa, A.; Abdelmohsen, S.A.M.; Tamam, N.; Elansary, H.O.; El-Abedin, T.K.Z.; Abdelbacki, A.M.M.; Janhavi, V. Morphological Characterization, Variability and Diversity among Vegetable Soybean (Glycine max L.) Genotypes. Plants 2021, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.R.; Rhee, J.; Choi, C.S.; Jo, J.S.; Shin, Y.K.; Song, J.W.; Kim, S.-H.; Lee, J.G. Morphological and Biochemical Variation in Carrot Genetic Resources Grown under Open Field Conditions: The Selection of Functional Genotypes for a Breeding Program. Agronomy 2022, 12, 553. [Google Scholar] [CrossRef]
- Giuliani, A.; Cerretani, L.; Cichelli, A. Colors: Properties and Determination of Natural Pigments. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 273–283. [Google Scholar]
- Parry, C.; Blonquist, J.M.; Bugbee, B. In Situ Measurement of Leaf Chlorophyll Concentration: Analysis of the Optical/Absolute Relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).