The Effect of Salinity and Drought on the Essential Oil Yield and Quality of Various Plant Species of the Lamiaceae Family (Mentha spicata L., Origanum dictamnus L., Origanum onites L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
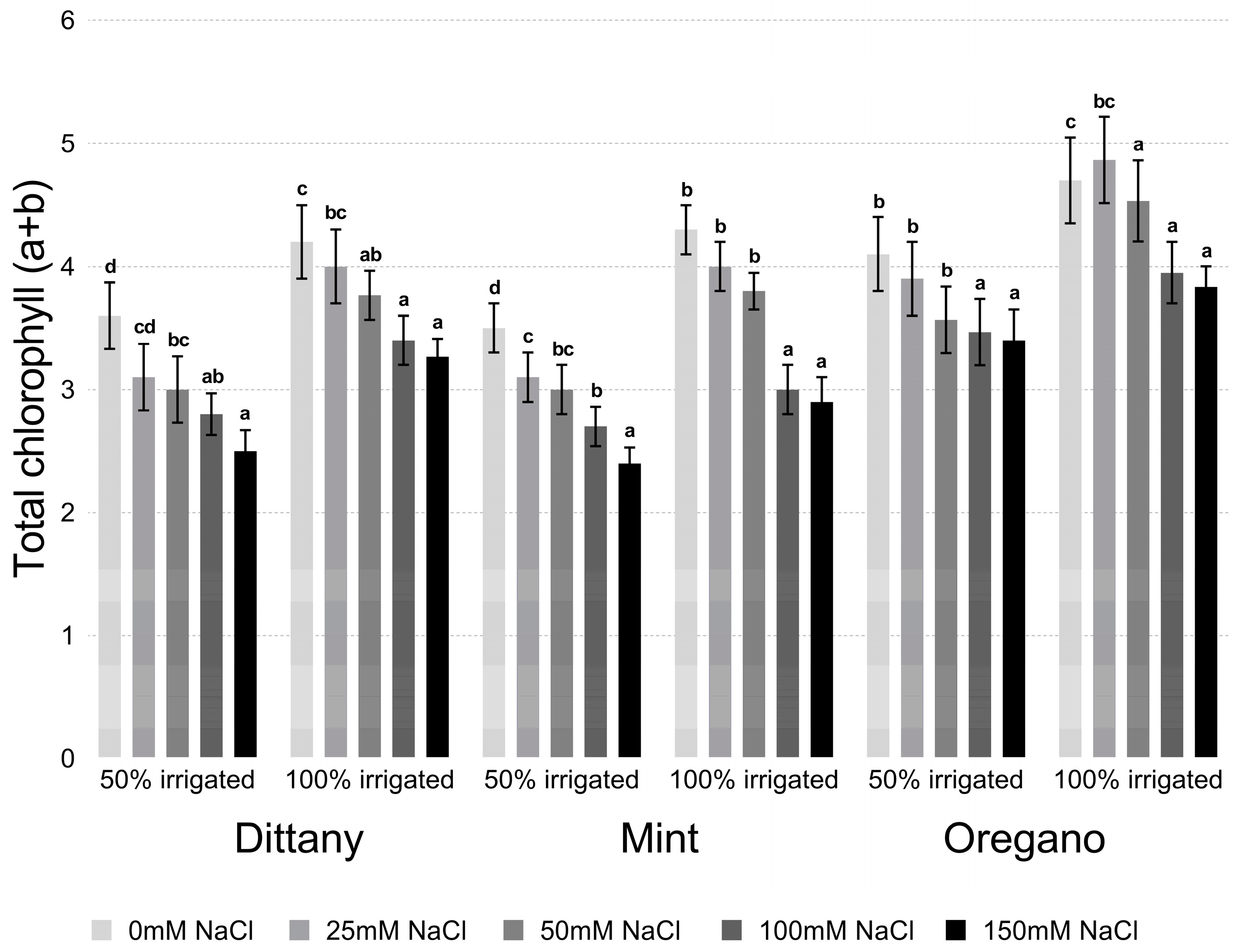
2.2. Chlorophyll Estimation
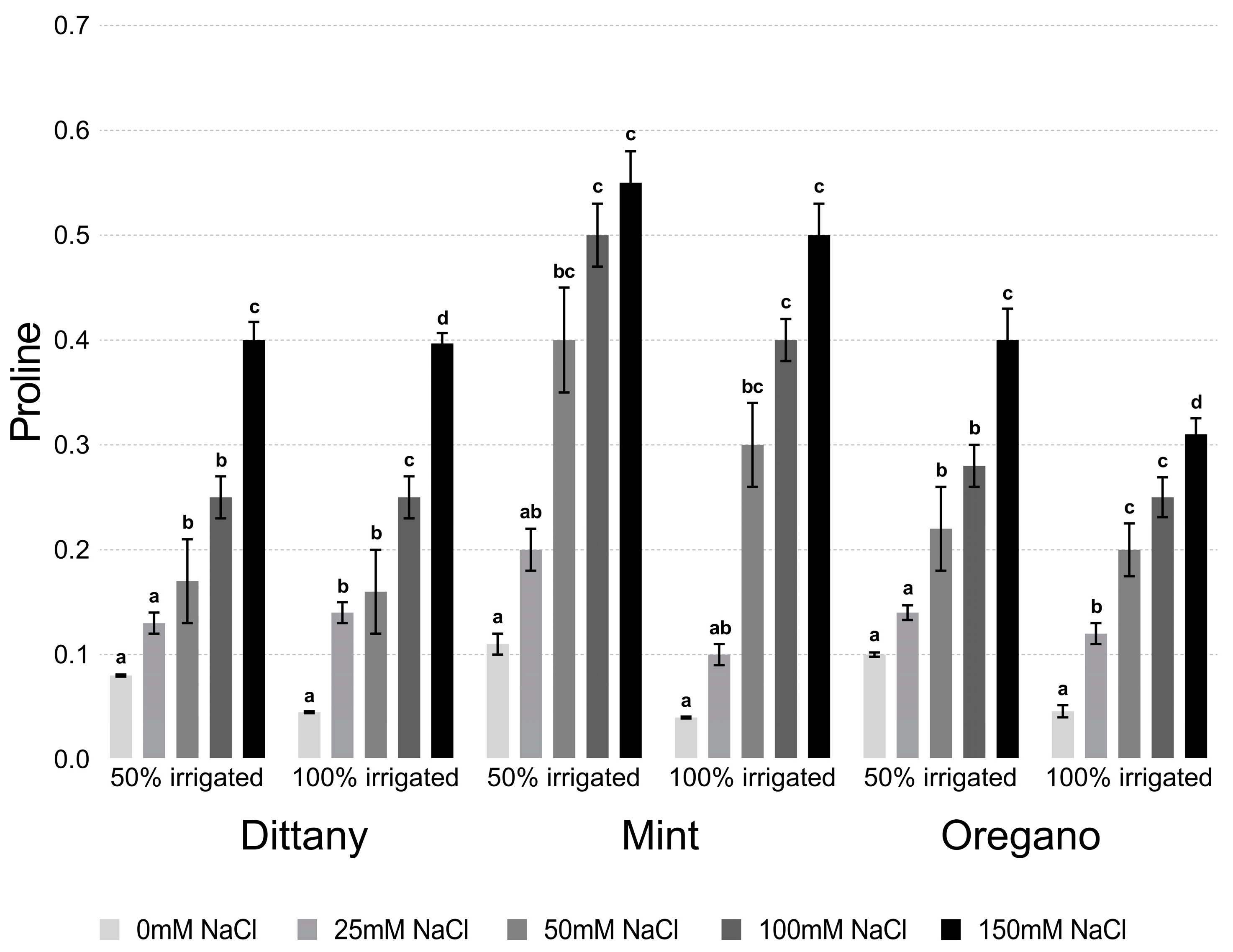
2.3. Determination of Proline
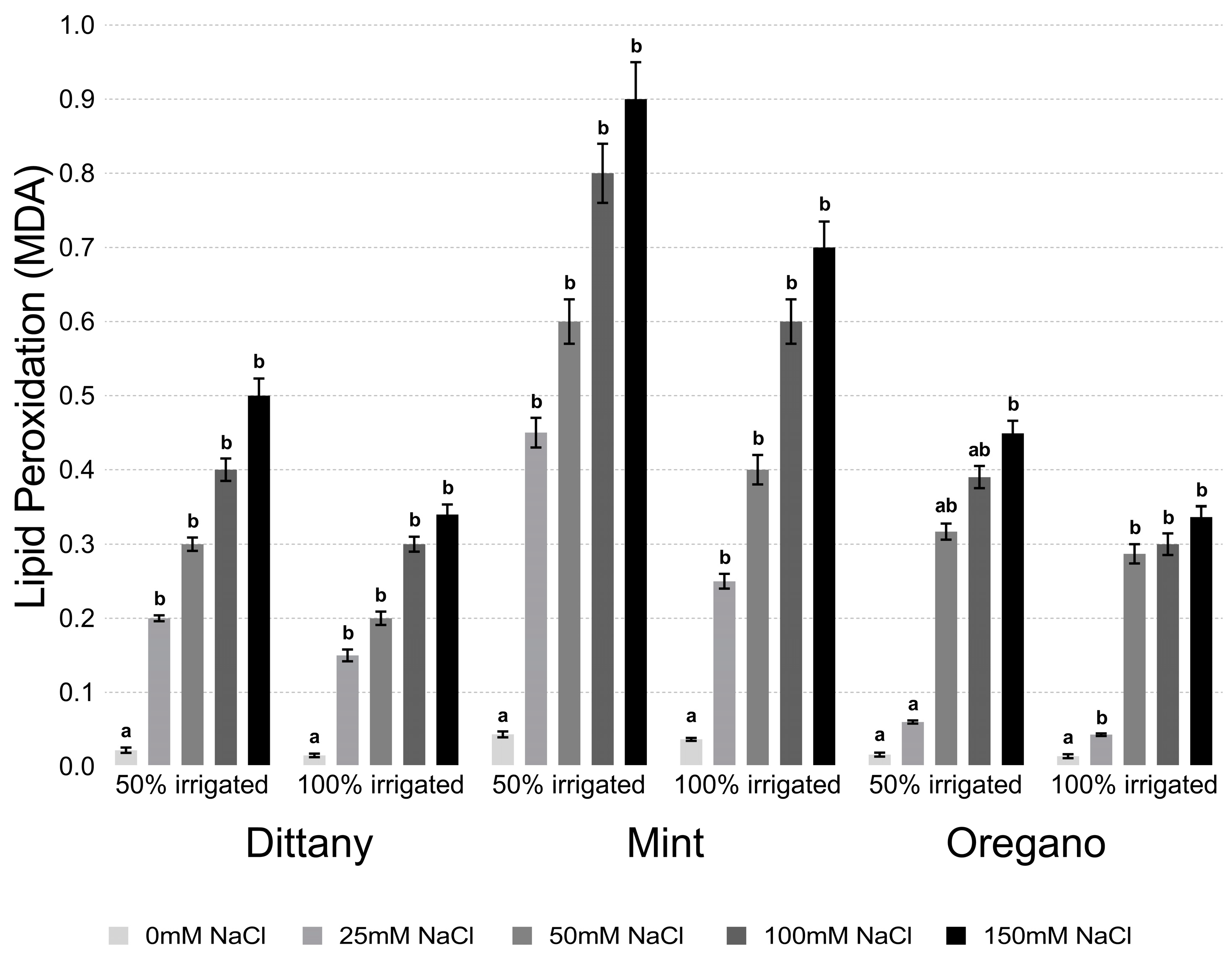
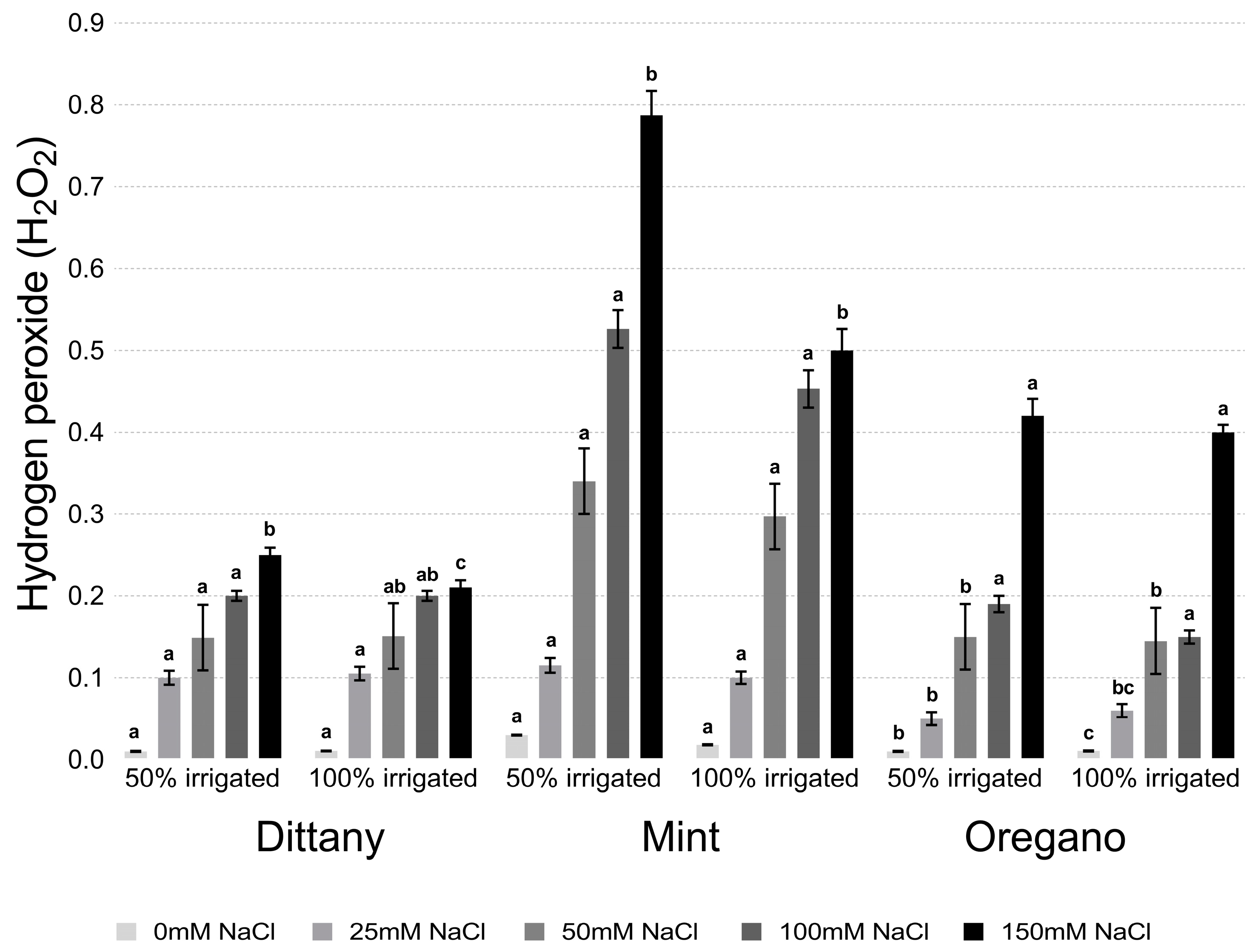
2.4. Estimation of Lipid Peroxidation and Hydrogen Hyperoxide
2.5. Isolation of Essential Oils
2.6. GC-MS Analysis of Essential Oils
2.7. Identification and Quantification of Essential Oils Components
2.8. Statistical Analysis
3. Results
3.1. Plant Growth
3.2. Secondary Metabolites
3.2.1. Proline and Chlorophyll Content
3.2.2. Lipid Peroxidation and Hydrogen Peroxide Assays
3.3. Essential Oil Content and Main Constituents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stefanakis, M.K.; Papaioannou, C.; Lianopoulou, V.; Philotheou-Panou, E.; Giannakoula, A.E.; Lazari, D.M. Seasonal Variation of Aromatic Plants under Cultivation Conditions. Plants 2022, 11, 2083. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular plants of Greece: An annotated checklist. Supplement. Willdenowia 2016, 46, 301–347. [Google Scholar] [CrossRef]
- Karaca, M.; Ince, A.G.; Aydin, A.; Ay, S.T. Cross-genera transferable e-microsatellite markers for 12 genera of the Lamiaceae family. J. Sci. Food Agric. 2013, 93, 1869–1879. [Google Scholar] [CrossRef]
- Tibaldi, G.; Fontana, E.; Nicola, S. Growing conditions and postharvest management can affect the essential oil of Origanum vulgare L. ssp. hirtum (link) Ietswaart. Ind. Crops Prod. 2011, 34, 1516–1522. [Google Scholar] [CrossRef]
- Tonk, F.; Yüce, S.; Bayram, E.; Akçali Giachino, R.R.; Sönmez, Ç.; Telci, İ.; Furan, M. Chemical and genetic variability of selected Turkish oregano (Origanum onites L.) clones. Plant Syst. Evol. 2010, 288, 157–165. [Google Scholar] [CrossRef]
- Stefanakis, M.K.; Touloupakis, E.; Anastasopoulos, E.; Ghanotakis, D.; Katerinopoulos, H.E.; Makridis, P. Antibacterial activity of essential oils from plants of the genus Origanum. Food Control 2013, 34, 539–546. [Google Scholar] [CrossRef]
- Papaioannou, C.; Stefanakis, M.K.; Batargias, C.; Kilias, G.; Anastasopoulos, E.; Katerinopoulos, H.E.; Papasotiropoulos, V. Genetic Profiling and Volatile Oil Content of Oregano Genotypes from Greece. Rev. Bras. Farmacogn. 2020, 30, 295–300. [Google Scholar] [CrossRef]
- Stefanakis, M.K.; Anastasopoulos, E.; Katerinopoulos, H.E.; Makridis, P. Use of essential oils extracted from three Origanum species for disinfection of cultured rotifers (Brachionus plicatilis). Aquac. Res. 2014, 45, 1861–1866. [Google Scholar] [CrossRef]
- Stefanaki, A.; van Andel, T. Mediterranean aromatic herbs and their culinary use. In Aromatic Herbs in Food. Bio-Active Compounds, Processing and Applications; Academic Press: Cambridge, MA, USA, 2021; pp. 93–121. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Bakchiche, B.; AL Salamat, H.A.; Rezzoug, M.; Gherib, A.; Flamini, G. Chemical composition. antioxidant antimicrobial and antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complement. Med. Ther. 2018, 18, 201. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Mentha spicata L. essential oil. phytochemistry and its effectiveness in flatulence. J. Tradit. Complement. Med. 2018, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Mulugeta, S.M.; Radácsi, P. Influence of Drought Stress on Growth and Essential Oil Yield of Ocimum Species. Horticulturae 2022, 8, 175. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Zhao, C.X. Water-deficit stress-induced anatomical changes in higher plants. Comptes Rendus Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ahl, H.A.; Omer, E.A. Medicinal and aromatic plants production under salt stress. A review. Herba Pol. 2011, 57, 72–87. [Google Scholar]
- Khorsandi, O.; Hassani, A.; Sefidkon, F.; Shirzad, H.; Khorsandi, A. Effect of salinity (NaCl) on growth, yield, essential oil content and composition of Agastache foeniculum kuntz. IJMAPR 2010, 26, 438–451. [Google Scholar] [CrossRef]
- Dehghani Bidgoli, R.; Azarnezhad, N.; Akhbari, M.; Ghorbani, M. Salinity stress and PGPR effects on essential oil changes in Rosmarinus officinalis L. Agric. Food Secur. 2019, 8, 2. [Google Scholar] [CrossRef]
- Sarmoum, R.; Haid, S.; Biche, M.; Djazouli, Z.; Zebib, B.; Merah, O. Effect of Salinity and Water Stress on the Essential Oil Components of Rosemary (Rosmarinus officinalis L.). Agronomy 2019, 9, 214. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Romero, M.J.; Llanderal, A.; Cermeño, P.; Lao, M.T.; Segura, M.L. Effects of Drought Stress on Biomass, essential oil content, nutritional parameters and costs of production in six Lamiaceae Species. Water 2019, 11, 573. [Google Scholar] [CrossRef]
- Benitez, L.; Vighi, I.; Nogueira do Amaral, M.; Auler Moraes, G.; Rodrigues, L.; Braga, A. Correlation of proline content and gene expression involved in the metabolism of this amino acid under abiotic stress. Acta Physiol. Plant. 2016, 38, 267. [Google Scholar] [CrossRef]
- Kren, S.; Sönmez, Ç.; Özçakal, E.; Kurttas, Y.S.K.; Bayram, E.; Gürgülü, H. The effect of different irrigation water levels on yield and quality characteristics of purple basil (Ocimum basilicum L.). Agric. Water Manag. 2012, 109, 155–161. [Google Scholar] [CrossRef]
- Szabó, K.; Zubay, P.; Zámboriné, N. What shapes our knowledge of the relationship between water deficiency stress and plant volatiles? Acta Physiol. Plant. 2020, 42, 130. [Google Scholar] [CrossRef]
- Wintermans, J.F.; De Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of Lead and Copper on Photosynthetic Apparatus in Citrus (Citrus aurantium L.) Plants. The Role of Antioxidants in Oxidative Damage as a Response to Heavy Metal Stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Binott, J.J.; Owuoche, J.O.; Bartels, D. Physiological and molecular characterization of Kenyan barley (Hordeum vulgare L.) seedlings for salinity and drought tolerance. Euphytica 2017, 213, 139. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.T.; Edreva, A.M. Oxidative stress and some antioxidant systems in acid rain-treated bean plants Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2005; pp. 217, 2567–2570. [Google Scholar]
- National Institute of Standards and Technology (NIST). 2017. Available online: http://webbook.nist.gov/chemistry/name-ser.html (accessed on 21 December 2021).
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed as-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Al-Fraihat, A.H.; Al-Dalain, S.Y.; Zatimeh, A.A.; Haddad, M.A. Enhancing Rosemary (Rosmarinus officinalis, L.) Growth and Volatile Oil Constituents Grown under Soil Salinity Stress by Some Amino Acids. Horticulturae 2023, 9, 252. [Google Scholar] [CrossRef]
- Avasiloaiei, D.I.; Calara, M.; Brezeanu, P.M.; Murariu, O.C.; Brezeanu, C. On the Future Perspectives of Some Medicinal Plants within Lamiaceae Botanic Family Regarding Their Comprehensive Properties and Resistance against Biotic and Abiotic Stresses. Genes 2023, 14, 955. [Google Scholar] [CrossRef] [PubMed]
- Estaji, A.; Roosta, H.; Rezaei, S.; Hosseini, S.; Niknam, F. Morphological, physiological and phytochemical response of different Satureja hortensis L. accessions to salinity in a greenhouse experiment. J. Appl. Res. Med. Aromat. Plants 2018, 10, 25–33. [Google Scholar] [CrossRef]
- Olfati, J.; Moqbeli, E.; Fathollahi, S.; Estaji, A. Salinity stress effects changed during Aloe vera L. vegetative growth. J. Stress Physiol. Biochem. 2012, 8, 152–158. [Google Scholar]
- Iqbal, H.; Yaning, C.; Waqas, M.; Shareef, M.; Raza, S.T. Differential response of quinoa genotypes to drought and foliage-applied H2O2 in relation to oxidative damage, osmotic adjustment and antioxidant capacity. Ecotoxicol. Environ. Saf. 2018, 164, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S. The Fermentation Analogy: A Point of View for Understanding the Intriguing Role of Proline Accumulation in Stressed Plants. Front. Plant Sci. 2016, 7, 1339. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanli, M.; Gafarabad, M.; Amirkian, T.; Mamaghani, B.A. Investigation of proline, total protein, Chlorophyll, ascorbate and dehydroascorbate changes under drought stress in Akria and Mobil tomato cultivars. Plant Sci. 2013, 45, 241–249. [Google Scholar]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Differential responses of phenolic compounds of Brassica napus under drought stress. Iran. J. Plant Physiol. 2018, 8, 2417–2422. [Google Scholar]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2009, 15, 1360–1385. [Google Scholar] [CrossRef]
- Hien, D.; Jacobs, M.; Angenon, G.; Hermans, C.; Thanh, T.; Le Van Son, T.; Roosens, N. Proline accumulation and D 1-pyrroline-5-carboxylate synthetase gene properties in three rice cultivars differing in salinity and drought tolerance. Plant Sci. 2003, 165, 1059–1068. [Google Scholar] [CrossRef]
- Witzel, K.; Matrosa, A.; Strickert, M.; Kaspar, S.; Peukert, M.; Mühlinge, K.H.; Börnera, A.; Mock, H.P. Salinity Stress in Roots of Contrasting Barley Genotypes Reveals Time-Distinct and Genotype-Specific Patterns for Defined Proteins. Mol. Plant 2014, 7, 336–355. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Ismail, M.R.; Othman, R.; Rahim, A.A. Relative salinity tolerance of warm season turfgrass species. J. Environ. Biol. 2011, 32, 309–312. [Google Scholar]
- Abdali, R.; Rahimi, A.; Siavash Moghaddam, S.; Heydarzadeh, S.; Arena, C.; Vitale, E.; Zamanian, M. The Role of Stress Modifier Biostimulants on Adaptive Strategy of Oregano Plant for Increasing Productivity under Water Shortage. Plants 2023, 12, 4117. [Google Scholar] [CrossRef]
- Krause, S.T.; Liao, P.; Crocoll, C.; Boachon, B.; Förster, C.; Leidecker, F.; Wiese, N.; Zhao, D.; Wood, J.C.; Buell, C.R.; et al. The biosynthesis of thymol, Carvacrol, and thymohydroquinone in Lamiaceae proceeds via cytochrome P450s and a short-chain dehydrogenase. Proc. Natl. Acad. Sci. USA 2021, 118, e2110092118. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.d.R.; Palermo, T.B.; Giordano, W.; Banchio, E. Influence of Drought Stress and PGPR Inoculation on Essential Oil Yield and Volatile Organic Compound Emissions in Mentha piperita. Horticulturae 2022, 8, 1120. [Google Scholar] [CrossRef]
- Allel, D.; Ben-Amar, A.; Abdelly, C. Leaf photosynthesis, chlorophyll fluorescence and ion content of barley (Hordeum vulgare) in response to salinity. J. Plant Nutr. 2018, 41, 497–508. [Google Scholar] [CrossRef]
- Sun, S.Q.; He, M.; Cao, T.; Yusuyin, Y.; Han, W. Antioxidative responses related to H2O2 depletion in Hypnum plumaeforma under the combined stress induced by Pb and Ni. Environ. Monit. Assess. 2010, 163, 303–312. [Google Scholar] [CrossRef]
- Singh, S.; Eapen, S.; D’Souza, S. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 2006, 62, 233–246. [Google Scholar] [CrossRef]
- Ounoki, R.; Ágh, F.; Hembrom, R.; Ünnep, R.; Szögi-Tatár, B.; Böszörményi, A.; Solymosi, K. Salt Stress Affects Plastid Ultrastructure and Photosynthetic Activity but Not the Essential Oil Composition in Spearspearmint (Mentha spicata L. var. crispa “Moroccan”). Front. Plant Sci. 2021, 12, 739467. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Bae, L.D.; Yong, J.K.; Ashraf, M.W.; Chun, L.S.; Shik, R.E. Effect of salt (NaCl) stress on germination and early seedling growth of four vegetables species. J. Cent. Eur. Agric. 2006, 7, 273–282. [Google Scholar]
- Aziz, E.A.; Hendawi, S.T.; Azza, E.E.D.; Omer, E.A. Effect of soil type and irrigation intervals on plant growth, essential oil and constituents of Thymus vulgaris plant. Am.-Eurasian J. Agric. Environ. Sci. 2008, 4, 443–450. [Google Scholar]
- Misra, A.; Srivastava, N.K. Influence of water stress on Japanese mint. J. Herbs Spices Med. Plants 2000, 7, 51–58. [Google Scholar] [CrossRef]
- Danesh-Shahraki, H.; Pirbalouti, A.G.; Rajabzadeh, F.; Kachouei, M.A. Water Deficit Stress Mitigation by the Foliar Spraying of Salicylic Acid and Proline on the Volatile Oils and Growth Features of Hyssop (Hyssopus officinalis L.). J. Essent. Oil Bear. Plants. 2023, 26, 115–129. [Google Scholar] [CrossRef]
- Singh, M.; Ramesh, S. Effect of irrigation and nitrogen on herbage, oil yield and water-use efficiency in rosemary grown under semi-arid tropical conditions. J. Med. Aromat. Plant Sci. 2000, 22, 659–662. [Google Scholar]
- Govahi, M.; Ghalavand, A.; Nadjafi, F.; Sorooshzadeh, A. Comparing different soil fertility systems in Sage (Salvia officinalis) under water deficiency. Ind. Crops Prod. 2015, 74, 20–27. [Google Scholar] [CrossRef]
- Rahimi, A.; Mohammadi, M.M.; Siavash Moghaddam, S.; Heydarzadeh, S.; Gitari, H. Effects of Stress Modifier Biostimulants on Vegetative Growth, Nutrients, and Antioxidants Contents of Garden Thyme (Thymus vulgaris L.) Under Water Deficit Conditions. J. Plant Growth Regul. 2022, 41, 2059–2072. [Google Scholar] [CrossRef]
| Treatnents | First Month of Treatment 100% Irrigation | At the End of Blooming 100% Irrigation | First Month of Treatment 50% Irrigation | At the End of Blooming 50% Irrigation | DW/FW 100% Irrigation | DW/FW 50% Irrigation |
|---|---|---|---|---|---|---|
| 0 mM NaCl | ||||||
| Spearmint | 16.27 ± 1.72 | 32.00 ± 2.45 | 16.00 ± 1.30 | 31.00 ± 2.30 | 0.68 ± 0.07 | 0.64 ± 0.07 |
| Dittany | 17.20 ± 2.39 | 23.60 ± 1.14 | 17.00 ± 2.10 | 23.00 ± 1.40 | 0.17 ± 0.05 | 0.16 ± 0.02 |
| Oregano | 14.60 ± 1.82 | 25.10 ± 0.90 | 14.50 ± 1.82 | 24.60 ± 0.89 | 0.63 ± 0.03 | 0.70 ± 0.06 |
| 25 mM NaCl | ||||||
| Spearmint | 16.17 ± 1.47 | 33.67 ± 1.86 | 16.00 ± 1.40 | 33.00 ± 1.60 | 0.67 ± 0.02 | 0.60 ± 0.03 |
| Dittany | 15.20 ± 1.31 | 26.60 ± 3.91 | 15.00 ± 1.10 | 26.00 ± 2.10 | 0.21 ± 0.03 | 0.20 ± 0.04 |
| Oregano | 13.00 ± 0.71 | 27.80 ± 3.96 | 12.60 ± 0.60 | 26.90 ± 2.40 | 0.64 ± 0.06 | 0.67 ± 0.07 |
| 50 mM NaCl | ||||||
| Spearmint | 15.40 ± 1.79 | 31.67 ± 3.37 | 15.00 ± 1.50 | 32.50 ± 3.20 | 0.72 ± 0.07 | 0.67 ± 0.06 |
| Dittany | 17.20 ± 2.77 | 26.20 ± 1.92 | 17.00 ± 1.60 | 26.00 ± 1.92 | 0.22 ± 0.02 | 0.20 ± 0.03 |
| Oregano | 14.60 ± 2.30 | 27.60 ± 2.07 | 14.00 ± 1.30 | 26.40 ± 1.87 | 0.61 ± 0.09 | 0.57 ± 0.08 |
| 100 mM NaCl | ||||||
| Spearmint | 15.17 ± 0.75 | 31.17 ± 1.94 | 15.00 ± 0.50 | 31.50 ± 1.40 | 0.70 ± 0.11 | 0.68 ± 0.09 |
| Dittany | 19.60 ± 2.68 | 26.00 ± 2.60 | 19.00 ± 1.80 | 25.40 ± 2.30 | 0.23 ± 0.04 | 0.21 ± 0.03 |
| Oregano | 16.40 ± 2.05 | 27.60 ± 2.80 | 16.20 ± 2.50 | 26.60 ± 2.10 | 0.69 ± 0.02 | 0.60 ± 0.04 |
| 150 mM NaCl | ||||||
| Spearmint | 15.67 ± 1.21 | 32.17 ± 2.9 | 15.50 ± 1.10 | 32.00 ± 2.20 | 0.68 ± 0.17 | 0.66 ± 0.10 |
| Dittany | 17.80 ± 2.68 | 27.80 ± 2.24 | 16.00 ± 2.68 | 27.00 ± 2.35 | 0.20 ± 0.01 | 0.18 ± 0.02 |
| Oregano | 15.20 ± 2.05 | 28.80 ± 2.78 | 14.70 ± 1.50 | 27.40 ± 2.60 | 0.70 ± 0.05 | 0.63 ± 0.08 |
| Treatments | M. spicata | O. dictamnus | O. onites |
|---|---|---|---|
| 0 mM NaCl | 3.24 ± 0.08 b | 3.61 ± 0.18 d | 4.86 ± 0.09 b |
| 25 mM NaCl | 3.60 ± 0.05 c | 2.00 ± 0.06 a | 4.53 ± 0.08 a |
| 50 mM NaCl | 2.94 ± 0.07 a | 2.11 ± 0.03 a | 5.54 ± 0.15 c |
| 100 mM NaCl | 3.06 ± 0.13 ab | 2.60 ± 0.12 b | 6.57 ± 0.11 e |
| 150 mM NaCl | 3.15 ± 0.11 ab | 2.93 ± 0.13 c | 6.07 ± 0.16 d |
| p-value | <0.001 | <0.001 | <0.001 |
| Compounds | tR 1 | RI 2 | RI 3 | 0 mM NaCl | 25 mM NaCl | 50 mM NaCl | 100 mM NaCl | 150 mM NaCl | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Limonene | 8.402 | 1026 | 1029 | 21.69 ± 0.01 a | 23.22 ± 0.05 b | 21.81 ± 0.23 a | 23.16 ± 0.10 b | 22.88 ± 0.25 b | <0.001 |
| 1,8-Cineole | 8.463 | 1029 | 1031 | 9.81 ± 0.03 a | 9.54 ± 0.05 a | 11.46 ± 0.18 b | 10.40 ± 0.08 b | 10.34 ± 0.11 b | <0.001 |
| Carvone | 11.912 | 1242 | 1243 | 53.11 ± 0.12 a | 51.32 ± 0.02 a | 52.70 ± 0.07 a | 52.60 ± 0.18 a | 50.91 ± 0.15 a | >0.05 |
| β-Caryophyllene | 14.661 | 1418 | 1419 | 1.81 ± 0.02 e | 1.36 0.01 c | 0.87 ± 0.01 a | 1.11 ± 0.04 b | 1.61 ± 0.04 d | <0.001 |
| % Total | 85.80 | 85.44 | 86.84 | 87.26 | 85.29 |
| Compounds | tR 1 | RI 2 | RI 3 | 0 mM NaCl | 25 mM NaCl | 50 mM NaCl | 100 mM NaCl | 150 mM NaCl | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| α-Thujene | 6.493 | 930 | 932 | 1.24 ± 0.02 e | 1.05 ± 0.01 b | 0.77 ± 0.01 a | 1.14 ± 0.00 c | 1.18 ± 0.01 d | <0.001 |
| α-Terpinene | 8.185 | 1012 | 1014 | 2.05 ± 0.00 b | 1.91 ± 0.01 a | 1.91 ± 0.03 a | 2.05 ± 0.01 b | 2.07 ± 0.00 b | <0.001 |
| p-Cymene | 8.314 | 1022 | 1024 | 34.81 ± 0.07 b | 43.25 ± 0.04 c | 33.31 ± 0.62 a | 35.16 ± 0.10 b | 33.57 ± 0.29 a | <0.001 |
| γ-Terpinene | 8.924 | 1052 | 1054 | 13.54 ± 0.07 c | 10.16 ± 0.03 a | 13.37 ± 0.10 c | 12.41 ± 0.04 b | 12.50 ± 0.12 b | <0.001 |
| Linalool | 9.602 | 1092 | 1095 | 2.02 ± 0.04 ab | 3.05 ± 0.03 d | 1.95 ± 0.05 a | 2.07 ± 0.01 b | 2.34 ± 0.02 c | <0.001 |
| Thymol | 12.514 | 1290 | 1290 | 0.12 ± 0.01 a | 0.15 ± 0.01 b | 0.16 ± 0.01 b | 0.12 ± 0.00 a | 0.16 ± 0.00 b | <0.001 |
| Carvacrol | 12.669 | 1299 | 1298 | 34.77 ± 0.05 b | 25.25 ± 0.27 a | 36.13 ± 0.37 c | 34.41 ± 0.20 b | 35.84 ± 0.15 c | <0.001 |
| % Total | 88.55 | 84.82 | 87.60 | 87.36 | 87.65 |
| Compounds | tR 1 | RI 2 | RI 3 | 0 mM NaCl | 25 mM NaCl | 50 mM NaCl | 100 mM NaCl | 150 mM NaCl | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| α-Thujene | 6.493 | 930 | 932 | 1.20 ± 0.01 b | 1.72 ± 0.02 d | 2.45 ± 0.03 e | 1.00 ± 0.01 a | 1.29 ± 0.02 c | <0.001 |
| α-Pinene | 6.621 | 937 | 939 | 0.78 ± 0.01 b | 1.16 ± 0.00 d | 1.62 ± 0.02 e | 0.67 ± 0.00 a | 0.85 ± 0.00 c | <0.001 |
| Myrcene | 7.695 | 986 | 990 | 1.28 ± 0.00 b | 1.72 ± 0.01 c | 2.47 ± 0.05 d | 1.16 ± 0.01 a | 1.34 ± 0.00 b | <0.001 |
| α-Terpinene | 8.176 | 1012 | 1014 | 3.18 ± 0.02 a | 4.80 ± 0.02 c | 5.24 ± 0.02 d | 3.43 ± 0.01 b | 3.41 ± 0.02 b | <0.001 |
| p-Cymene | 8.314 | 1022 | 1024 | 3.45 ± 0.01 a | 4.73 ± 0.01 d | 5.88 ± 0.01 e | 3.51 ± 0.01 b | 3.66 ± 0.01 c | <0.001 |
| Limonene | 8.400 | 1026 | 1029 | 1.12 ± 0.00 a | 1.50 ± 0.00 d | 1.96 ± 0.02 e | 1.16 ± 0.01 b | 1.25 ± 0.02 c | <0.001 |
| γ-Terpinene | 8.924 | 1052 | 1054 | 8.65 ± 0.01 a | 12.55 ± 0.08 d | 13.96 ± 0.03 e | 9.59 ± 0.00 c | 8.95 ± 0.02 b | <0.001 |
| cis-Linalool oxide | 9.439 | 1172 | 1174 | 0.78 ± 0.00 a | 1.11 ± 0.01 d | 1.03 ± 0.02 c | 0.82 ± 0.01 b | 0.82 ± 0.01 b | <0.001 |
| Linalool | 9.602 | 1092 | 1095 | 11.71 ± 0.02 d | 10.77 ± 0.07 a | 11.40 ± 0.07 c | 10.96 ± 0.08 b | 11.25 ± 0.03 c | <0.001 |
| Borneol | 10.728 | 1162 | 1165 | 1.52 ± 0.00 | 1.36 ± 0.00 | 1.08 ± 0.00 | 1.59 ± 0.00 | 1.45 ± 0.00 | – |
| Terpinen-4-ol | 10.891 | 1172 | 1174 | 6.52 ± 0.00 b | 8.53 ± 0.53 d | 4.56 ± 0.01 a | 7.62 ± 0.01 c | 6.81 ± 0.02 b | <0.001 |
| α-Terpineol | 11.097 | 1188 | 1188 | 3.93 ± 0.01 b | 3.91 ± 0.02 b | 2.97 ± 0.00 a | 4.16 ± 0.02 c | 3.95 ± 0.05 b | <0.001 |
| Thymol | 12.514 | 1290 | 1290 | 0.32 ± 0.03 c | 0.20 ± 0.01 a | 0.17 ± 0.00 a | 0.27 ± 0.01 bc | 0.26 ± 0.03 b | <0.001 |
| Carvacrol | 12.669 | 1299 | 1298 | 45.41 ± 0.15 d | 36.46 ± 0.35 b | 35.44 ± 0.24 a | 45.56 ± 0.10 d | 44.50 ± 0.03 c | <0.001 |
| % Total | 89.85 | 90.50 | 90.24 | 91.49 | 89.80 | ||||
| Ratio of carvacrol to precursors | 1.03 | 0.68 | 0.65 | 0.99 | 0.98 | ||||
| Monoterpene Hydrocarbons | 19.66 | 28.18 | 33.58 | 20.52 | 20.75 | ||||
| Sum of precursors | 44.12 | 53.86 | 54.62 | 45.67 | 45.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanakis, M.K.; Giannakoula, A.E.; Ouzounidou, G.; Papaioannou, C.; Lianopoulou, V.; Philotheou-Panou, E. The Effect of Salinity and Drought on the Essential Oil Yield and Quality of Various Plant Species of the Lamiaceae Family (Mentha spicata L., Origanum dictamnus L., Origanum onites L.). Horticulturae 2024, 10, 265. https://doi.org/10.3390/horticulturae10030265
Stefanakis MK, Giannakoula AE, Ouzounidou G, Papaioannou C, Lianopoulou V, Philotheou-Panou E. The Effect of Salinity and Drought on the Essential Oil Yield and Quality of Various Plant Species of the Lamiaceae Family (Mentha spicata L., Origanum dictamnus L., Origanum onites L.). Horticulturae. 2024; 10(3):265. https://doi.org/10.3390/horticulturae10030265
Chicago/Turabian StyleStefanakis, Michalis K., Anastasia E. Giannakoula, Georgia Ouzounidou, Charikleia Papaioannou, Vaia Lianopoulou, and Eleni Philotheou-Panou. 2024. "The Effect of Salinity and Drought on the Essential Oil Yield and Quality of Various Plant Species of the Lamiaceae Family (Mentha spicata L., Origanum dictamnus L., Origanum onites L.)" Horticulturae 10, no. 3: 265. https://doi.org/10.3390/horticulturae10030265
APA StyleStefanakis, M. K., Giannakoula, A. E., Ouzounidou, G., Papaioannou, C., Lianopoulou, V., & Philotheou-Panou, E. (2024). The Effect of Salinity and Drought on the Essential Oil Yield and Quality of Various Plant Species of the Lamiaceae Family (Mentha spicata L., Origanum dictamnus L., Origanum onites L.). Horticulturae, 10(3), 265. https://doi.org/10.3390/horticulturae10030265







