Abstract
Light is one of the important factors influencing anthocyanin synthesis, and low-light conditions (<2000 Lux) seriously affect the coloration of the peels of purple eggplant. MPK4 is an important protein kinase that regulates a number of physiological processes and is equivalent to the “molecular switch” in the plant. The function of MPK4 has been studied in Arabidopsis, rice, and apple, but its function in eggplant has not been reported. In this study, 16 SmMPK genes were identified using photo-sensitive eggplant ‘LSHX’ as a material. We found that overexpression of SmMPK4.1 could affect the phenotype of eggplant leaves and metabolites of fruits, and knockout of SmMPK4.1 affected the function of synthesizing anthocyanin in eggplant induced by high light (10,000 Lux). Furthermore, we demonstrated that SmMPK4.1 could interact with SmMYB75 in yeast and that SmMPK4.1 negatively regulated the function of anthocyanin synthesis by SmMYB75. The results of this study revealed the function of SmMPK4.1 and provided candidate genes for conducting high-quality breeding of eggplant in production.
1. Introduction
MAPK (Mitogen-activated Protein Kinase), known as MPK, is a conserved serine/threonine protein kinase pivotal in eukaryotic signaling. In Arabidopsis, MAPKs are categorized into A, B, C, and D groups, with MPK4 prominent in group B [1]. MPK4′s multifaceted roles include periplasmic microtubule formation [2], cellular mitosis regulation [3], and modulation of plant innate immunity [4], impacting systemic resistance acquisition [5]. It has been shown that MPK4 deficiency in Arabidopsis caused a severe dwarf phenotype and reduced male fertility [6]. Furthermore, MPK4 plays a crucial role in influencing leaf development and stomatal movement [7]. Recent research highlights MPK4′s role as a vital component of the stomatal CO2/bicarbonate sensing mechanism [8]. Additionally, a correlation exists between MPK4 and plant metabolism, as evidenced by significant alterations in cell wall composition, specifically, an increase in l-arabinose content and a decrease in cellulose content in Arabidopsis mpk4 mutants [9]. Knockout of MPK4 in Arabidopsis results in a reduction in chlorophyll–protein complex (LHC) components [10]. Despite high sequence similarity, deletion mutants of MPK11 do not exhibit significant phenotypic differences compared to MPK4 mutants [6].
MPK4 is responsive to light signals and contributes to the synthesis of certain pigments. In apple ripening, MdMPK4 phosphorylates MdERF17 during light/dark transitions, regulating chlorophyll degradation and fruit greening [11]. In addition, light signaling can also stabilize MYB proteins through the extracellular signaling kinase MPK4 pathway. In apple, MdMPK4 activated by light interacts with MdMYB1, leading to the phosphorylation of MdMYB1 and can stabilize the MdMYB1 protein level and promote the activation of anthocyanin biosynthesis genes, and silencing the expression of MdMPK4 can significantly reduce the content of anthocyanins in apple pericarp under light conditions [12]. In Arabidopsis, light signaling can activate MPK4, and activated MPK4 interacts with the transcription factor MYB75, thereby increasing the stability of MYB75 [13]. Temperature also affects MPK4 expression, and MPK4 can be induced to activate under low-temperature conditions (4 °C) [14]. And H2S can improve the kinase activity of MPK4 to respond to cold stress [15]. Under salt-stress conditions, MPK4 has the capability to interact with and phosphorylate MYB42, leading to an enhancement in the stability and transcriptional activity of the MYB42 protein [16].
The coloration of eggplant (Solanum melongena L.) peel is critical for its commercial and nutritional significance, primarily attributable to its anthocyanin content [17]. MYB is one of the crucial transcription factors in anthocyanin regulation, with numerous studies showcasing its dual roles in both promoting and repressing anthocyanin synthesis in plants [18]. For instance, in Arabidopsis, AtMYB75/AtPAP1 interacts with MPK4, facilitating light-induced anthocyanin accumulation [19]. Conversely, AtMYB4 can inhibit anthocyanin synthesis, serving as a negative regulator [20]. In a tomato, overexpression of MYB genes, such as SlANT1, SlANT1-like, SlANT2/SlMYB75, and SlANT2-like, have all resulted in an increase in anthocyanin content in tomato fruits [21,22]. In an eggplant, overexpression of SmMYB1 enhances anthocyanin accumulation in transgenic seedlings by up-regulating the expression of structural genes involved in anthocyanin synthesis [23]. Furthermore, overexpression of SmMYB35 enhances anthocyanin accumulation in stalks and petals [24], while overexpression of the SmMYB75 gene increases anthocyanin content in callus tissue [25]. On the contrary, overexpression of SmMYB86 suppresses anthocyanin synthesis in eggplant [26]. These findings provide evidence for the significant involvement of MYB genes in anthocyanin synthesis in eggplant.
In addition to the transcription factors mentioned above, anthocyanin regulation is also regulated by environmental factors, with light playing a pivotal role. For light-sensitive crops such as eggplant and apple, shading treatment can entirely inhibit the anthocyanin synthesis pathway in their pericarp. However, exposure to light induces gene expression and protein accumulation of anthocyanin-synthesizing transcription factors, such as SmTT8 and SmMYB1 [27]. In Arabidopsis, light rapidly activates transcription of MYB75 and MYB90, reaching peak transcript levels within 1 and 2 h, respectively. Different light qualities, including blue light, red light, UV-B, and UV-A, can induce the expression of MYB75 [28]. Temperature also influences anthocyanin synthesis primarily through the regulation of structural genes. Specifically, low temperatures (<15 °C) typically induce anthocyanin synthesis [29], whereas high temperatures (>30 °C) inhibit this process [30]. Phytohormones exert diverse effects on anthocyanins, with JA (Jasmonic acid) playing an important role in stress response and promoting anthocyanin synthesis [31]. ABA (Abscisic acid) was found to induce fruit anthocyanin accumulation in grapes; externally applied ABA triggers the expression of structural and regulatory genes for anthocyanin synthesis [32].
The role of MPK4 in light response and its underlying mechanisms remain largely unexplored, particularly in eggplant. This study aims to investigate MPK4′s function in eggplant, particularly its response to light signaling, regulation of anthocyanin synthesis, and exploration of downstream substrates.
2. Materials and Methods
2.1. Plant Material, Strains, and Vectors
Eggplant: The photo-sensitive variety ‘LSHX’ with purple–black peel was utilized [33]. Seeds were pre-treated by soaking in GA (Gibberellic acid) overnight prior to sowing and subsequently incubated in darkness at room temperature for 3–4 days until germination. After germination, seeds were transferred to cavity trays and maintained in an artificial climate chamber set at 25 °C, with a photoperiod of 16 h of light and 8 h of darkness, and 60% humidity. After isolation, all tissues were rapidly frozen in liquid nitrogen and stored at −80 °C.
Tobacco: Nicotiana benthamiana L. was employed. The seeds were sown in pots (nutrient soil: vermiculite = 1:1), interplanted after two leaves had grown, and placed in a climatic chamber for incubation. Tobacco leaves after 1 month of growth were taken for the experiment. The growth conditions were the same as with eggplant.
2.2. Analysis of the SmMPKs Gene Family in Eggplant
To obtain the sequences of all MPKs gene family members in eggplant, the Arabidopsis and eggplant genome sequences were downloaded from the Arabidopsis Information Repository (TAIR: https://www.arabidopsis.org/, accessed on 15 August 2023) and the latest eggplant genome database (http://eggplant-hq.cn/Eggplant/home/index, accessed on 15 August 2023), respectively. The MPK genes were retrieved in the eggplant genome using the bidirectional blast method of TBtools software (Version 2). The retrieved potential eggplant MPKs genes were further validated by NCBI (https://www.ncbi.nlm.nih.gov/, accessed on 15 August 2023) BLASTP. The candidate-specific proteins were subsequently validated for MPK structural domains using the online CDD database (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi, accessed on 15 August 2023), and 16 candidate eggplant MPK genes were finally identified. The number of amino acids, molecular weight, theoretical isoelectric point, instability coefficient, lipolysis index, and total average hydrophilicity of these genes were all predicted using the ExPASy website (https://www.expasy.org/, accessed on 15 August 2023). Prediction of subcellular localization of proteins using the WoLF PSORT website (https://www.genscript.com/wolf-psort.html, accessed on 15 August 2023).
To categorize the gene families of the eggplant MPKs, full-length amino acid sequences of the eggplant and Arabidopsis MPKs were aligned using MUSCLE software (Version 5) under default parameters. A phylogenetic tree was subsequently constructed based on the aligned structures using the proximity method in MEGA11 software, and a bootstrap test was performed (1000 replicates). The obtained phylogenetic trees were then further embellished using iTOL and Evolview. Subfamily classification was performed based on the relationships between Arabidopsis and eggplant MPK genes. The conserved structural domains of MPK genes in eggplant were subsequently obtained by the online CDD search tool of the National Center for Bioinformatics, and the conserved motifs in the eggplant MPK genes were identified using the online software MEME (https://meme-suite.org/meme/, accessed on 15 August 2023).
All potential MPK genes in eggplant were localized and visualized on their chromosomes by TBtools. Sequences 2000 bases upstream of the start codon ATG of eggplant MPK genes were extracted using TBtools software, and cis-acting elements were predicted in the PlantCARE database, followed by visualization using TBtools.
2.3. RNA Extraction and qPCR
The extraction of total RNA from eggplant and tobacco tissues was performed using the Plant RNA Extraction Kit (Accurate Biology, Changsha, China). The purity and concentration of RNA were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Subsequently, 500 ng of RNA underwent first-strand cDNA synthesis employing the PrimeScript First Strand cDNA Synthesis Kit (Takara, Shiga, Japan) following a procedure of 37 °C for 15 min and 85 °C for 5 s. The cDNA was diluted 24 times, and the ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) was used for qPCR analysis. The reaction system was as follows: RNase-free ddH2O 3.2 µL + upstream/downstream quantitative primers 0.4 µL + cDNA 6 µL + ChamQ Universal SYBR qPCR Master Mix 10 µL. The reaction program was a two-step process: 1 cycle at 95 °C for 30 s; 40 cycles at 95 °C for 10 s and 60 °C for 30 s. Signal acquisition was performed at 60 °C, and the dissolution curve was performed using the default parameters of qTOWER3G (Analytik Jena, Jena, Germany). Primer design for qPCR was conducted utilizing Primer Premier 5 software, with the eggplant gene Actin (GU984779.1) employed as the internal reference control. Data analysis was performed utilizing the 2−∆∆Ct method.
2.4. Gene Cloning and Plasmids Construction
Based on the nucleic acid sequence sourced from the latest eggplant genome database, Primer 5 software was utilized to design primers for the cloning of the SmMPK4.1 gene. The cDNA extracted from eggplant ‘LSHX’ peel served as the template during the gene cloning process. The PCR reaction mixture, totaling 25 µL, comprised 6 µL of template, 12.5 µL of 2 × ApexHF FS PCR Master Mix, 0.5 µL each of SmMPK4.1-F and SmMPK4.1-R primers, and 5.5 µL of ddH2O. The amplification program consisted of initial denaturation at 94 °C for 30 s, followed by 35 cycles of denaturation at 98 °C for 30 s, annealing at 55 °C for 15 s, extension at 72 °C for 1 min, and a final extension at 72 °C for 5 min. Purification of PCR products was carried out using the FastPure Gel DNA Extraction Mini Kit (Vazyme, Nanjing, China).
For the construction of the overexpression vector, the coding region of the SmMPK4.1 and SmMYB75 genes were amplified via PCR using SmMPK4.1-YFP F/R and SmMYB75-YFP F/R primers containing vector homology arms. The pHB-YFP vector was then digested with SpeI and BamHI enzymes. The digested PCR product and vector were ligated using DNA ligase (Vazyme, Nanjing, China) and subsequently introduced into Escherichia coli DH5α competent cells following purification. Single clone colonies were selected for PCR validation and sequencing post-transformation, and properly sequenced colonies were cultured via shaking. Gene editing was executed by CRISPR-Cas9 technology using SmU6-1P::sgRNA-Cas9 as a vector [34]. Knockout targets were initially designed according to the gene sequence of SmMPK4.1 at the CRISPR direct website (https://crispr.dbcls.jp, accessed on 15 August 2023). Recombinant primers were designed according to the target site using Primer 5. The sgRNA cloning frame was amplified using the SmU6-1P::sgRNA-Cas9 vector as the template. Subsequently, the SmU6-1P::sgRNA-Cas9 vector underwent double digestion with EcoRI and XbaI. The resulting linearized vector, along with the sgRNA cloning frame, underwent recombination facilitated by DNA ligase (Vazyme, Nanjing, China). The resulting construct was then introduced into Escherichia coli DH5α, yielding the SmMPK4.1 gene editing vector.
2.5. Treatment Conditions for Induced Expression Analysis
Wide-type eggplants exhibiting similar growth conditions were selected for induction treatment after one month of incubation. Light treatment involved initially transferring eggplant seedlings to darkness for 24 h, followed by exposure to UV-B (700 Lux) and blue light (3000 Lux), respectively. Cold treatment was administered by placing eggplants in an artificial culture chamber set to 4 °C. Phytohormone treatments were conducted by root washing and transplantation into 800 mL of MS (Murashige and Skoog Medium) solution for 2 days, followed by the addition of ABA and MeJA at a final concentration of 100 μM. PEG and NaCl treatments involved a similar root washing and transplantation process, followed by the addition of 80 g of PEG and 9.36 g of NaCl (200 mM) to 800 mL of culture solution, respectively. Leaf samples were collected at 0, 1, 4, and 8 h post-treatment, rapidly frozen in liquid nitrogen and stored for subsequent experiments. Each set of treatments included at least three biological replicates. Subsequent qPCR experiments were conducted to analyze SmMPK4.1 expression levels across different samples.
2.6. Tobacco Subcellular Localization
The SmMPK4.1 overexpression plasmid and empty vector were introduced separately into A. tumefaciens strain GV3101 and A. tumefaciens were resuspended in a resuspension solution (MS + 10 mM MES + 10 mM MgCl2 + 200 µM acetosyringone [AS], pH 5.8) to OD600 = 1.0. A. tumefaciens carrying pHB-SmMPK4.1-YFP and pHB-YFP were infiltrated into N. benthamiana leaves. The transformants were cultivated in the dark for 12 h and then moved to the light for 24 h. Yellow fluorescence (505 to 550 nm) was observed using a laser confocal microscope (Leica TCS SP5-II, Leica Microsystems, Deerfield, MA, USA). Yellow fluorescence was detected with a filter set of 514 nm for excitation and 530 nm for emission.
2.7. Eggplant Stabilizes Genetic Transformation
The constructed SmMPK4.1 overexpression and gene editing plasmids were transformed into E. coli DH5α for cloning, sequencing, and plasmid propagation. The verified plasmids were transformed into the eggplant wild-type callus using A. tumefaciens-mediated transformation method described before [33]. To confirm the integration of the target gene fragments into the wild-type genome, the cDNAs of the transformants were used as the templates to amplify via PCR.
2.8. Determination of Anthocyanin Content
Eggplant leaves, both knockout and wild-type, subjected to one week of high-light treatment (10,000 Lux), were weighed (0.3 g–0.5 g), followed by grinding in liquid nitrogen for subsequent anthocyanin content analysis. Similarly, tobacco leaves post-transient expression were weighed using the same method. Anthocyanin determination in eggplant was conducted using the pH difference photometric method [35]. A 96-well plate microplate reader (PowerWave XS, Biotek, Winooski, VT, USA) was used to read the absorption values at 510 and 700 nm.
2.9. Widely-Targeted Metabolomic Analysis of SmMPK4-OE Eggplant Pulp
Pulp from both SmMPK4.1-overexpressing and wild-type eggplants, harvested at identical developmental stages, was immediately preserved in liquid nitrogen and stored under extreme cold conditions (−80 °C). These samples were subsequently transported with dry ice to Wuhan Metware Biotechnology Inc. for comprehensive metabolomic profiling. Metabolomic analysis was performed using an ultra-performance liquid chromatography–electrospray ionization-tandem mass spectrometry (UPLC-ESI-MS/MS) system, as detailed in prior studies [36]. Data interpretation was conducted using Analyst 1.6.1 software (AB SCIEX, Ontario, Canada), with partial least squares discriminant analysis (PLS-DA) applied to highlight significant metabolomic variations between the sample groups. Metabolites were deemed significantly altered based on a variable importance in projection (VIP) score ≥ 1 coupled with a fold change ≥2 or ≤0.5.
2.10. Yeast Two-Hybrid Assays
For Y2H experiments with full-length proteins, the full-length CDS of SmMPK75 were ligated into the vector pGADT7 (AD) to obtain AD-SmMYB75 as preys. The full-length CDS of SmMPK4.1 were then inserted into pGKBT7 (BD) as baits.
The combinations of indicated plasmids were co-transformed into yeast strain AH109, and the transformants were grown on a 2-deficiency selection medium (SD-Trp/-Leu) for 3 d. Following this, 6 monoclonal clones of the combination were selected and inoculated on 2-deficiency, 3-deficiency (SD-Trp/-Leu/-His), and 4-deficiency selection mediums (SD-Trp/-Leu/-His/Ade) simultaneously and cultured for 3 d, and the growth status of yeast cells was photographed. To inhibit the self-activation phenomenon of BD-MPK4, 1 mM of 3-AT (3-amino-1,2,4-triazole) was added to the triple-deficient medium (SD/-Trp-Leu-His), and the bacterial broth was diluted for three concentration gradients.
2.11. Tobacco Transient Conversion
The SmMPK4.1 overexpression, SmMYB75 overexpression vectors, and empty vectors were delivered into tobacco leaf cells by the A. tumefaciens-mediated method. The transformants were cultivated in the dark for 12 h and then moved to the light for 2 d to determine the expression levels of anthocyanin structure genes.
2.12. Dual Luciferase Assay
A dual-luciferase assay was employed to investigate the impact of SmMPK4.1 on the transcriptional activation function of SmMYB75. The assay utilized the SmCHS promoter, which serves as a reporter gene downstream of SmMYB75. The full-length CDS of SmMYB75 and SmMPK4.1 were constructed on pHB-YFP as effector vectors. Promoter sequences of SmCHS were inserted into pGreen0800-LUC as a reporter in previous studies [31]. After the vector was constructed, the reporter and effector were transferred into A. tumefaciens. The reporter and effector A. tumefaciens resuspensions were mixed in a 1:1 volume ratio, and the mixture was kept in the dark for 2 to 3 h before being infiltrated into N. benthamiana leaves. The infested tobacco was treated in the dark for 24 h and then transferred to the light for 24 h. Equal mass samples were taken with a punch for LUC activity assay, and the specific procedure was referred to the instruction manual of the Dual-Luciferase Reporter Assay System kit (Promega, Madison, WI, USA), which was improved according to the actual environment. First, the tobacco leaves were pulverized by freezing in liquid nitrogen. Following this, 200 μL of 1xPassive Lysis Buffer was added quickly and shaken vigorously until there was no precipitate; then, it was centrifuged at 12,000 rpm for 45 s. Next, 4 μL of the supernatant was transferred from the above centrifuge tube to a new RNase-free 1.5 mL centrifuge tube; 20 μL of LAR II Reagent was added to the tube and mixed well, and then the REN value was determined. Subsequently, 20 μL of Stop & Glo Reagent was added to the above centrifuge tube, and the LUC value was determined after mixing. Relative LUC value = LUC/REN.
2.13. Primers
The primers used for gene cloning, vector construction, and qRT-PCR analysis were listed in Supplemental Table S1.
2.14. Statistical Analysis
Data analysis incorporated a minimum of three biological replicates alongside three technical replicates to ensure robustness. Graphical representations were generated using GraphPad Prism 5, with bar heights and error bars depicting the mean values and standard deviations (±SD) of the biological replicates, respectively. Statistical evaluations were conducted using one-way ANOVA coupled with Duncan’s Multiple Range Test (DMRT) and independent t-tests facilitated by SPSS 19.0. Statistical significance was set at p < 0.05, denoted by distinct letters for DMRT outcomes and asterisks for t-test results.
3. Results
3.1. Analysis of the MPK Gene Family in Eggplant
To identify SmMPK genes in eggplant, we performed a bidirectional BLAST query based on 20 AtMPKs in Arabidopsis in the genome database of eggplant and, finally, identified 16 candidate SmMPK genes.
The average size of these proteins was 481 amino acids, ranging from 369 to 619 amino acids. The molecular weight of the proteins ranged from 42,523.68 kDa for Smechr1201521.1 to 70,276.51 kDa for Smechr0702237.1. The theoretical isoelectric points ranged from 5.06 for Smechr1201521.1 to 9.42 for Smechr0702324.1. The predicted instability coefficients varied between 32.35 and 47.56, with a mean value of 40.62. The lipolysis index ranged from as low as 77.62 (Smechr0102309.1) to as high as 94.88 (Smechr1201521.1), and the total average hydrophilicity ranged from −0.542 to −0.26, which suggested that most of the SmMPKs proteins were hydrophilic. Prediction of subcellular localization of all proteins using the WoLF PSORT website showed that four were localized in chloroplasts, eight in the cytosol, and four in the nucleus. The basic information of all genes is listed in Table 1.

Table 1.
Characteristics of 16 SmMPK genes in eggplant.
MPKs can be classified into two categories, TEY and TDY, based on the activation loop. In addition, through the branching structure of the evolutionary tree, MPKs could be further divided into four subgroups, Group A, Group B, Group C, and Group D. Among them, highly homologous to AtMPK4 were SmMPK4.1 (Smechr01014441) and SmMPK4.2 (Smechr05000711), and we finally targeted SmMPK4.1 (Smechr01014441), which is more homologous, for our study based on the results of Blast In NCBI. In order to deeply investigate the structural characteristics of SmMPK proteins in eggplant, we used the online website MEME to predict the conserved motifs, and a total of 10 conserved motifs were identified and predicted. Through comparative analysis, we can find that the more closely related SmMPK protein members show similarity in the distribution of motifs from Figure 1. Multiple sequence comparison and motif analysis showed that the eggplant SmMPK proteins were highly conserved in sequence. Motif1, motif3, and motif4 were located at the N-terminus of all SmMPK proteins, and motif5 was located at the C-terminus of all SmMPK proteins. These results contribute to a more comprehensive understanding of the structural diversity and functional relevance of SmMPK proteins in eggplant.
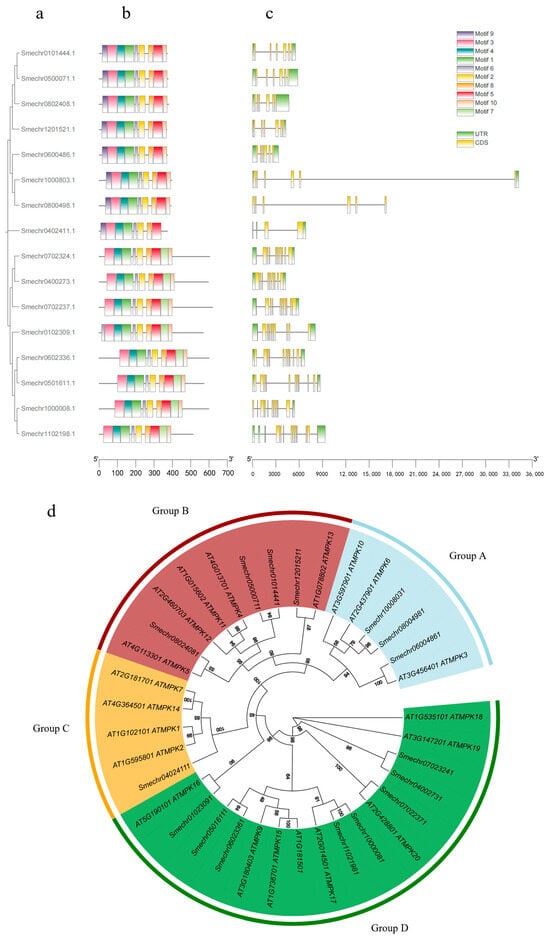
Figure 1.
Structural analysis and phylogenetic tree analysis of eggplant SmMPKs: (a) Evolutionary tree of 16 SmMPK proteins. (b) Composition and distribution of conserved motifs of SmMPK proteins. The number of motifs shown in the legend represents the predicted motif group; the colored boxes represent 10 different motifs, and the bottom scale represents the gene length. (c) The positions of the 3′ non-coding region (UTR), coding region (CDS), and conserved domains are indicated by green, yellow, blue, and pink squares, respectively. (d) Phylogenetic analysis tree of eggplant and Arabidopsis MPKs.
To further study the distribution of SmMPK genes on eggplant chromosomes, we analyzed their chromosomal information using GFF annotation files. From Supplemental Figure S1, we can find that the 16 SmMPK genes are evenly distributed on the nine chromosomes of eggplant, each chromosome containing one to two unequal SmMPK genes, almost throughout the entire genome of eggplant.
Promoter cis-acting elements play a key role in the initiation of gene expression. We identified a total of 24 different classes of cis-acting elements, including light-responsive elements, stress-responsive elements, low temperature-responsive elements, growth factor-responsive elements, and so on. As can be seen in Supplementary Figure S1, light-responsive elements were the most common, and almost all SmMPK genes in eggplant had light-responsive elements, and their total number accounted for half of the number of all elements. SmMPK4.1 contains 10 light-responsive, 4 MeJA-responsive, 5 abscisic acid-responsive, 1 salicylic acid-responsive, 1 auxin-responsive, 1 defense and stress-responsive, and 1 seed-specific regulation element. It was proved that SmMPK4.1 plays an important role in plant light response, hormone regulation, stress resistance, and seed development.
3.2. Subcellular Localization of SmMPK4.1 Protein
Agrobacterium tumefaciens was used to infest tobacco, and the distribution of SmMPK4.1 protein in cells was observed by laser confocal microscopy in the YFP field, bright field, and merged field, respectively. The transient expression of pHB-SmMPK4.1-YFP in tobacco epidermal cells can be seen in Figure 2. The yellow fluorescence was mainly distributed in the cell membrane and nucleus, with a small amount being present in the cytosol, while the control showed that the YFP protein was present in the nucleus, cytosol, and cell membrane. The above results suggest that the SmMPK4.1 protein may function in the nucleus, cytosol, and cell membrane.
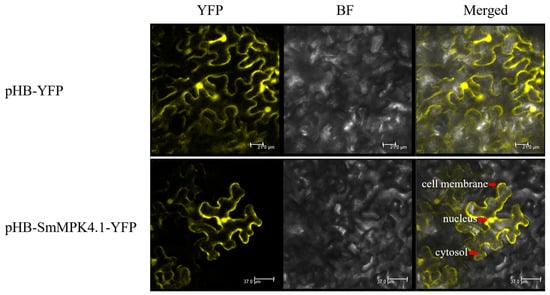
Figure 2.
Subcellular localization in tobacco: YFP (yellow fluorescent protein field); BF (Brighted-field); Merged (a mixed field in which the yellow fluorescent protein field overlaps with the bright field). Scale bars represent 21.0, 37.0 µm in the samples of “pHB-YFP” and “pHB-SmMPK4.1-YFP”, respectively.
3.3. Tissue-Specific Expression of SmMPK4.1
In order to determine the expression of SmMPK4.1 in different tissues of eggplant, we analyzed the expression of SmMPK4.1 in roots, stems, leaves, flowers, peels, flesh, and sepals of ‘LSHX’ using qRT-PCR. As can be seen in Figure 3, SmMPK4.1 was expressed in all tissues of eggplant, with the highest expression level in flowers, followed by flesh, and the lowest expression level in roots. These findings provide a basis for further studies on the function of SmMPK4.1 in eggplant growth and development and response to stress.
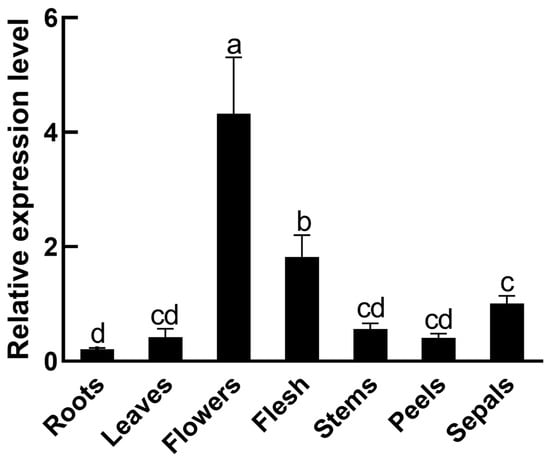
Figure 3.
Expression of SmMPK4.1 in different tissues of eggplant: Setting the SmMPK4.1 gene expression level in sepals to 1. The error line indicates the standard deviation of three biological replicates. Lowercase letters represent the results of One-way ANOVA Duncan test analysis, p < 0.05, indicating significant differences.
3.4. Analysis of SmMPK4.1 Induced Expression
To investigate the function of SmMPK4.1 gene in response to light and environmental stress, we used qRT-PCR to analyze the expression level of SmMPK4.1 under blue light, UV-B, MeJA, ABA, 4 °C, NaCl, and PEG treatment conditions, and the results are shown in Figure 4. Blue light and UV-B significantly activated the up-regulation of SmMPK4.1 expression, and the expression of SmMPK4.1 was elevated three-fold after 8 h of blue light treatment and seven-fold after 8 h of UV-B treatment, which indicated that SmMPK4.1 gene expression was induced by light treatment and might be involved in the light signaling pathway. In terms of hormone treatment, there was no significant change in the expression of SmMPK4.1 after MeJA treatment, indicating that it may not respond to the MeJA signal. ABA slightly induced SmMPK4.1 down-regulation at 1 to 4 h, followed by up-regulation at 8 h. The expression of SmMPK4.1 was elevated two-fold after low-temperature treatment for 8 h. NaCl inhibited the expression of SmMPK4.1, and its expression decreased by 50% after 8 h of treatment. In addition, PEG irregularly affected the expression of SmMPK4.1, which was first decreased, then increased, and then decreased.
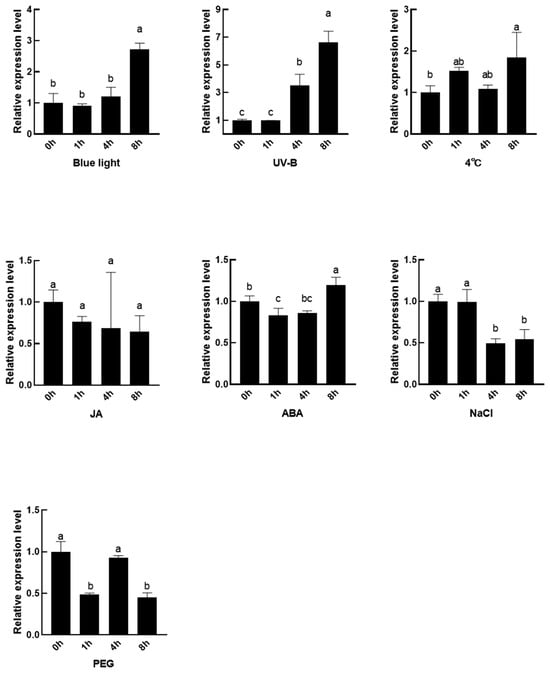
Figure 4.
Expression of SmMPK4.1 induced by different environmental factors. The blue light treatment intensity is 3000 Lux; the UV-B treatment light intensity is 700 Lux; the treatment concentrations of MeJA and ABA are 100 μM; the treatment concentration of NaCl is 200 mM, and the treatment concentration of PEG is 10%. The error line indicates the standard deviation of three biological replicates. Lowercase letters represent the results of One-way ANOVA Duncan test analysis; p < 0.05, indicating significant differences.
3.5. Leaf Phenotype and Gene Expression Characterization of SmMPK4.1 Overexpression Plants
SmMPK4.1 overexpression plants were obtained by stable genetic transformation experiments and were named YFP-9. Positive bands were detected by PCR experiments on extracted DNA of YFP-9 (Figure 5a). Real-time quantitative fluorescence analysis was further performed to detect the expression of SmMPK4.1, and the analysis revealed that the expression level of SmMPK4.1 was significantly higher in the leaves and pulp of SmMPK4.1-OE plant YFP-9 compared to WT (wild-type) plants, which were up-regulated by six-fold and four-fold, respectively (Figure 5b,c). Observations of the overexpression plants at seedling and maturity showed that their leaves showed the phenotypes of brown spots and extreme curling at both seedling and maturity (Figure 5d). The leaf curl phenotype was similarly observed in the Arabidopsis mpk4 mutant. Protein kinases are participants in many processes of cellular physiological activities and are equivalent to “molecular switches” in plants, and the absence or overexpression of protein kinases may cause phenotypic changes in plants.
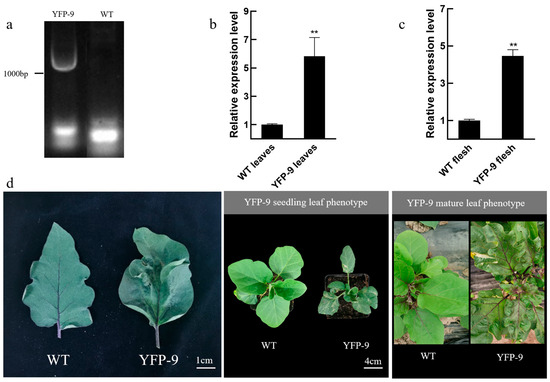
Figure 5.
Characterization and analysis of overexpressing SmMPK4.1 plants: (a) DNA level assay of SmMPK4.1 gene in leaves of wild-type and transgenic eggplants; (b) Relative expression levels of SmMPK4.1 gene detected in wild-type and transgenic eggplant leaves, ** p < 0.01 represents a significant difference; (c) Relative expression levels of SmMPK4.1 gene in wild-type and transgenic eggplant pulp assay; (d) Phenotypes of wild-type ‘LSHX’ and transgenic eggplant leaves. The error line indicates the standard deviation of three biological replicates.
3.6. Widely-Targeted Metabolome Analysis of SmMPK4.1 Overexpression Plants
To gain insight into the functions exercised by SmMPK4.1 in eggplant, we performed a widely-targeted metabolomics analysis using the UPLC-MS platform to identify primary and secondary metabolites in eggplant pulp. As can be seen in Supplemental Table S2, a total of 1797 metabolites were detected.
Differential metabolite profiles between overexpression and wild-type specimens were assessed using clustering heat maps. The clustering analysis, as depicted in Supplemental Figure S2, indicates notable disparities in metabolite composition across the respective groups. Approximately one-third of the alkaloids exhibited significant elevation in overexpression plants. Notably, the phenolic acid content displayed substantial variation between overexpression and wild-type plants, with some displaying heightened levels while others showed decreases, suggesting diverse regulatory mechanisms of SmMPK4.1 on phenolic acid metabolism. Lipid content generally exhibited elevation in overexpression plants. Conversely, most flavonoids, including anthocyanins as secondary metabolites, demonstrated significant reductions, implying a potential impact of SmMPK4.1 overexpression on anthocyanin accumulation in eggplant. Moreover, overexpression plants exhibited higher levels of amino acids, derivatives, and terpenoids compared to their wild-type counterparts.
FC (Fold change and) VIP (Variable Importance in Projection) were further utilized to screen for differential metabolites, and metabolites satisfying both FC > 2 and VIP > 1 were considered as differential metabolites, and the results showed that there were 310 differential metabolites in the comparison between WT and overexpressed SmMPK4 groups, of which 183 metabolites were up-expressed, and 127 metabolites were down-expressed (Figure 6a). As can be seen in Supplemental Figure S3, the overexpressed plants showed an overall significant increase in the content of amino acids and derivatives and a significant decrease in the content of flavonoids. Among the metabolites with the top 20 multiplicity of difference, the phenolic acid substance 4-(6-o-sulfo-beta-d-glucopyranosyloxy)-3-hydroxybenzoic acid (Lahn001701) had the highest up-regulation of 5.18-fold, and the steroids substance dioscin (mws1311) had the highest down-regulation of 4.80-fold (Figure 6b).
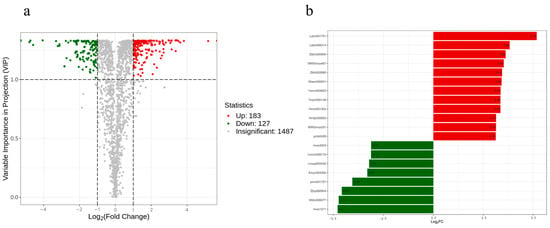
Figure 6.
Differential metabolite analysis: (a) Differential metabolic volcano diagram of transgenic plants; (b) Top 20 metabolites in the multiplicity of difference. The horizontal coordinate is the log2FC of the differential metabolite, and the vertical coordinate is the differential metabolite. Red color represents up-regulation of metabolite content, and green color represents down-regulation of metabolite content.
3.7. SmMPK4.1 Knockout Plants Fail to Respond to High Light-Induced Anthocyanin Synthesis Process
We performed targeted gene editing in ‘LSHX’ eggplant using CRISPR-Cas9. Genomic DNA from T0 generation-positive plants was used as a template for PCR amplification using primers specific for MPK4test-F and MPK4test-R that spanned the target site. Sequencing of the PCR products showed that one plant was a homozygous mutant named Cas9-1 (Figure 7a). In the Cas9-1 mutant, a base insertion occurred at the target site of the SmMPK4.1 gene, resulting in a blockage of the normal translation of the gene. In eggplant, anthocyanin accumulation is regulated by light conditions, and the level of anthocyanin accumulation is positively correlated with the intensity of light. In this study, in order to induce anthocyanin production, we moved eggplant seedlings to be cultured under high-light conditions (10,000 Lux). The results showed that wild-type ‘LSHX’ eggplant leaves could accumulate anthocyanins induced by high-light intensity, but the leaves of eggplant knocked down for SmMPK4.1 did not accumulate anthocyanins under high-light intensity (Figure 7b,c).
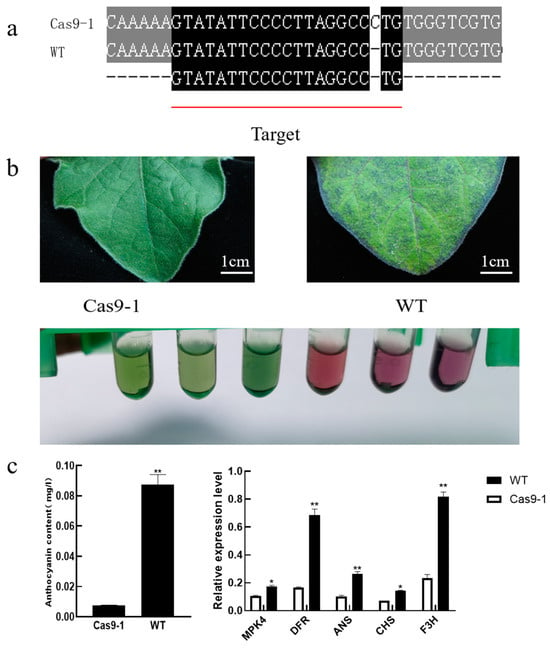
Figure 7.
Identification analysis of gene-edited SmMPK4.1 plants: (a) Sequencing detected mutations in the target gene SmMPK4.1; (b) Phenotypes of wild-type ‘LSHX’ and gene-edited eggplant leaves induced by high light (10,000 Lux); (c) Anthocyanin content and relative expression of synthesized structural genes in wild-type and gene-edited plants under high-light induction. The error line indicates the standard deviation of three biological replicates; * indicates the result of t-test analysis, and * p < 0.05 and ** p < 0.01 represent significant differences.
Meanwhile, we used real-time fluorescence quantitative PCR to assess the expression levels of DFR, ANS, CHS, and F3H, the structural genes for anthocyanin synthesis, in plants. Consistent with the results of the anthocyanin content assay, the expression levels of anthocyanin synthesis genes in the wild type were significantly higher after high-light induction, whereas the expression levels of these four genes in the mutant Cas9-1 were significantly lower than those in the wild type after high-light induction. The above results support that SmMPK4.1 is involved in the anthocyanin accumulation process.
3.8. Yeast Two-Hybrid Demonstration of SmMPK4.1 Interactions with SmMYB75
To verify whether SmMPK4.1 could interact with SmMYB75, SmMYB75 was constructed into pGADT7(AD) vector as prey, and SmMPK4.1 was constructed into pGBKT7(BD) vector as bait and co-transformed into yeast AH109 strain, followed by the defective medium (SD/-Trp-Leu-His) that verified their interactions. As shown in Figure 8, the yeast co-transformed with SmMPK4.1 and SmMYB75 could grow on SD-TLH (SD/-Trp-Leu-His) defective medium, indicating that SmMPK4.1 could interact with SmMYB75 in yeast cells.
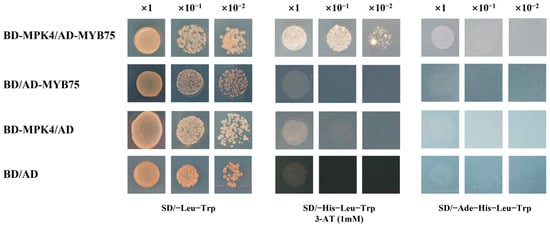
Figure 8.
Yeast two-hybrid experiment to analyze the interaction between SmMPK4.1 and SmMYB75.
3.9. SmMPK4.1 Can Inhibit SmMYB75 to Promote Anthocyanin Synthesis
To further verify the interaction between SmMPK4.1 and SmMYB75, transient overexpression experiments were conducted in tobacco. As can be seen in Figure 9a,b, compared with the control group (YFP), the injection of overexpression of SmMPK4.1 alone did not cause changes in leaf color, while the injection of overexpression of SmMYB75 alone caused the leaves to turn significantly purple, proving the increase in anthocyanin content. When SmMPK4.1 was co-injected with SmMYB75, it was observed that the purple color was weakened, and the anthocyanin content was lower than in the case of SmMYB75 alone. To further demonstrate whether SmMPK4.1 with SmMYB75 regulated anthocyanin content by modulating structural genes related to anthocyanin synthesis, we examined the transcript levels of the NtCHS, NtCHI, NtF3H, NtDFR, and NtANS genes using qRT-PCR. As shown in Figure 9c, when SmMPK4.1 was co-overexpressed with SmMYB75, the expression levels of these five anthocyanin-synthesizing genes in tobacco were significantly down-regulated compared to the overexpression of SmMYB75 alone. It indicates that the presence of SmMPK4.1 inhibited the function of SmMYB75 in promoting anthocyanin synthesis in plants. The above results verified the inhibitory effect of SmMPK4.1 on the anthocyanin synthesis function of SmMYB75 and showed that it reduced anthocyanin content by regulating anthocyanin biosynthesis-related genes.
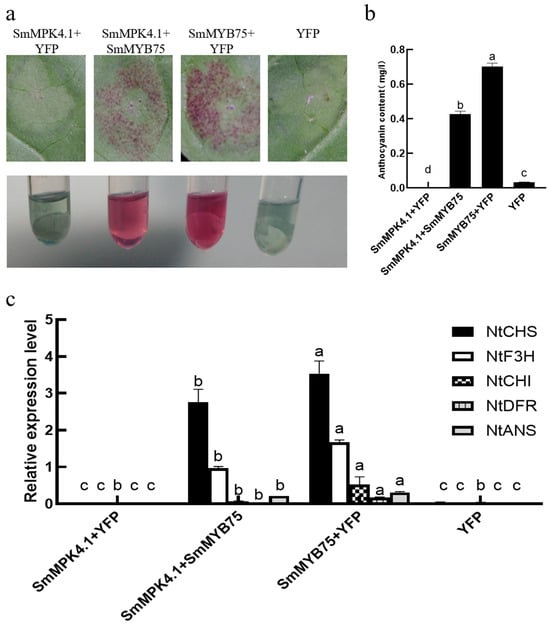
Figure 9.
Transient overexpression detection of SmMYB75 and SmMPK4.1 in tobacco: (a) Phenotypes of tobacco leaves after transient overexpression of SmMYB75 and SmMPK4.1 for 3 days; (b) Anthocyanin content; (c) qRT-PCR analysis of tobacco anthocyanin biosynthetic genes: NtCHS (AF311783), NtCHI (AB213651), NtF3H (AB289450), NtDFR (EF421429), and NtANS (AB723683). Empty vector YFP was used as a control. The error line indicates the standard deviation of three biological replicates. Lowercase letters represent the results of One-way ANOVA Duncan test analysis; p < 0.05, indicating significant differences.
3.10. SmMPK4.1 Negatively Regulates the Transcriptional Activation Function of SmMYB75
On the basis of determining the existence of protein interactions between SmMYB75 and SmMPK4.1, the biological significance of protein interactions needs to be further explored. A dual luciferase assay was used to analyze the effect of SmMPK4.1 on the transcriptional activation function of SmMYB75, and the SmCHS promoter, a downstream target gene of SmMYB75, was used as a reporter gene. The results in Figure 10 showed that SmMPK4.1 could negatively regulate the transcriptional activation ability of SmMYB75 to the SmCHS promoter.
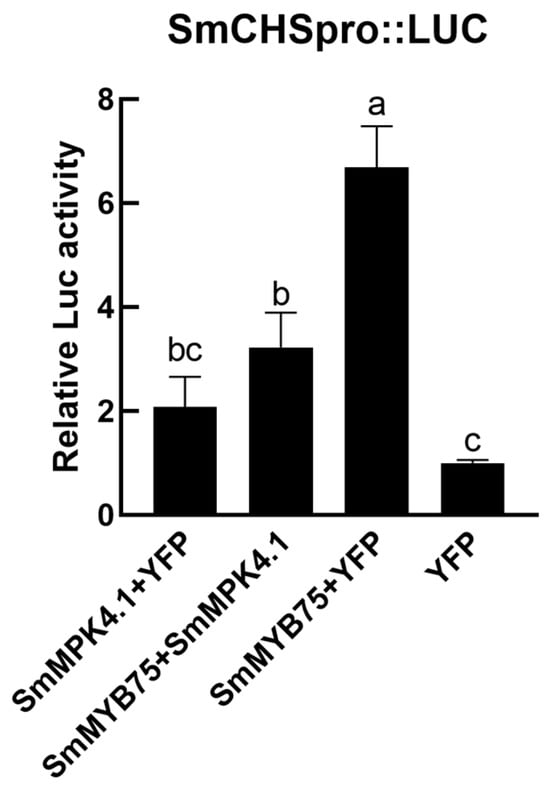
Figure 10.
Dual luciferase experiment of SmMPK4.1 and SmMYB75. The error line indicates the standard deviation of three biological replicates. Lowercase letters represent the results of One-way ANOVA Duncan test analysis; p < 0.05, indicating significant differences.
4. Discussion
Eggplant, a key vegetable crop in China, is renowned for its anthocyanin-rich peel, which not only shields the plant from ultraviolet radiation damage but also offers significant health benefits [17,37]. Anthocyanin biosynthesis in eggplant is predominantly governed by light exposure; in photoreceptive cultivars, shading entirely inhibits this pathway [27]. MYB transcription factors are pivotal in anthocyanin biosynthesis, with MAPK pathways shown to modulate MYB activity through diverse interactions [12,16,38]. The distinct substrates of MAPK signaling underscore its specificity [39], with the identification of these substrates enhancing our understanding of MAPK’s regulatory roles [40]. Recent evidence underscores the critical role of MAPK cascades in mediating plant responses to light stimuli. Light exposure activates Arabidopsis MPK3, MPK4, and MPK6 [41], with the MKK3–MPK6 cascade facilitating blue light-induced hypocotyl growth through MYC2 phosphorylation [42]. Light activation of MdMPK4 in apples prompts its interaction with MdMYB1, resulting in the phosphorylation of MdMYB1 [12]. In this study, the accumulation of anthocyanins induced by strong light was significantly reduced in the mpk4 mutant, indicating that MPK4 plays a crucial role in regulating anthocyanin accumulation. These findings align with observations in Arabidopsis [13] and are further supported by analogous reductions in anthocyanin levels in apple pericarp upon MPK4 silencing [12]. Notably, the responsiveness of SmMPK4.1 to light and low temperatures underscores the gene’s potential involvement in eggplant’s adaptive responses to environmental cues, mirroring findings in other model plants [13,15].
Contrary to expectations, overexpression of SmMPK4.1 inhibits the synthesis of anthocyanins in eggplant. SmMYB75 can promote the accumulation of anthocyanins [25]. In this study, transient overexpression of tobacco experiments and dual-luciferase experiments demonstrated that SmMPK4.1 inhibited the function of SmMYB75 in promoting plant anthocyanin synthesis. Metabolomic profiling revealed that SmMPK4.1 overexpression leads to a decrease in eggplant flavonoid levels. This is contrary to the previous results obtained in Arabidopsis [13] and apple [12], which show that MPK4 can stabilize MYB protein and promote anthocyanin synthesis. We speculate that this may be due to the different expression patterns of genes in different crops, or it may be that another SmMPK4.2 in eggplant that is homologous to AtMPK4 has the function of stabilizing SmMYB75.
Beyond its roles in light signal transduction and anthocyanin biosynthesis, our findings reveal a critical function of SmMPK4.1 in regulating plant metabolism and leaf morphogenesis. Overexpression of SmMPK4.1 in eggplant induced phenotypic alterations, including leaf curling and the development of brown spots across various growth stages. Metabolomic profiling revealed substantial disparities in key metabolites such as flavonoids, lipids, alkaloids, and phenolic acids between transgenic and wild-type plants. MPK4 serves as a critical “molecular switch” in plants, orchestrating various physiological processes, including plant immunity and morphogenesis [3,4,5]. In Arabidopsis, mpk4 mutants display a leaf curl-like phenotype [6], while recent studies in hybrid aspen further underscore MPK4′s impact on leaf development [7]. In soybean, GmMPK4 kinase activity correlates with cell death, potentially contributing to the formation of brown spots on leaves [43]. Overexpression of OsMPK4 in rice results in late growth retardation and increased chlorophyll content [44]. Overexpression of BnMPK4 in kale-type oilseed rape enhances its stomatal closure and pathogen resistance [45]. A previous study conducted in Arabidopsis showed that deletion of MPK4 severely affects the expression of lipid-localized gene [46]; in our study, the overexpression of SmMPK4.1 eggplant showed an overall increase in the content of lipids in eggplant plants, indicating that MPK4 played an important role in the lipid synthesis pathway. These findings collectively highlight the multifaceted involvement of MPK4 in plant morphological structures and metabolic processes, underscoring its significance in shaping plant physiology and development.
In this investigation, we expanded the understanding of the MPK gene family in eggplant by identifying 16 SmMPK genes, aligning with the diversity observed in other species such as Arabidopsis [47], rice [48], and pear [49]. SmMPK4.1 interacts with SmMYB75 in yeast and tobacco cells, indicating that SmMYB75 may be a new substrate of SmMPK4.1. However, whether they interact in eggplant and the specific mechanism of interaction remain unclear. Future research directions may include exploring whether other light-responsive transcription factors are also regulated by MAPK to gain a deeper understanding of the function of MAPK in plant light signaling pathways. These investigations will contribute to the refinement of the regulatory network governing MAPK signaling and anthocyanin synthesis. The successful generation of plants with gene-edited knockout of SmMPK4.1 presents a robust platform for in-depth exploration of MPK4 function. By establishing gene-edited SmMPK4.1 plants, we lay the groundwork for creating double or triple-mutant plants in conjunction with other genes. This approach offers a novel research avenue to unravel the intricate regulatory network of MPK4 in plant growth, development, and stress. This advancement holds paramount importance in enhancing our comprehension of MPK4′s role in plant molecular biology.
5. Conclusions
There are 16 members of the MPK gene family in eggplant. SmMPK4.1 was localized in the nucleus, cytosol, and cell membrane and was widely expressed in the tissues of eggplant. UV-B, blue light, and low temperature up-regulated SmMPK4.1 expression, and NaCl down-regulated SmMPK4.1 expression. Plants overexpressing SmMPK4.1 exhibited brown spots and extreme curling in both seedling and mature stages. Metabolomic analysis revealed significant differences in metabolites between plants overexpressing SmMPK4.1 and wild-type plants, with 310 differential metabolites. In overexpressed plants, most amino acids and derivatives rose, and most flavonoids fell. Compared to the wild type, leaves of eggplants with SmMPK4.1 knocked out were unable to accumulate anthocyanins under high light intensity. Yeast two-hybrid results indicated that SmMPK4.1 interacted with SmMYB75. Additionally, when SmMPK4.1 and SmMYB75 were co-transiently overexpressed in tobacco, the anthocyanin content was lower than when SmMYB75 was transiently overexpressed alone. Dual-luciferase assay confirmed that SmMPK4.1 significantly inhibited the SmCHS promoter downstream of SmMYB75.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10030239/s1.
Author Contributions
J.L. and Y.D. designed this project; J.L. and Y.D. performed the experiments and prepared the manuscript; J.L., Y.D., Z.H., J.H., S.L. and N.Z. participated in data analysis and revision of this manuscript; H.C., the corresponding author, supervised the entire experimental process. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32272721 and 32172563).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rasmussen, M.W.; Roux, M.; Petersen, M.; Mundy, J. MAP kinase cascades in Arabidopsis innate immunity. Front. Plant Sci. 2012, 3, 169. [Google Scholar] [CrossRef]
- Beck, M.; Komis, G.; Mueller, J.; Menzel, D.; Samaj, J. Arabidopsis homologs of nucleus- and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization. Plant Cell 2010, 22, 755–771. [Google Scholar] [CrossRef]
- Beck, M.; Komis, G.; Ziemann, A.; Menzel, D.; Samaj, J. Mitogen-activated protein kinase 4 is involved in the regulation of mitotic and cytokinetic microtubule transitions in Arabidopsis thaliana. N. Phytol. 2011, 189, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, J.; Bi, D.; Zhang, Z.; Cheng, F.; Chen, S.; Zhang, Y. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 2008, 18, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, M.J.; Parker, J.E.; et al. Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 2000, 103, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Chen, J.-G.; Ellis, B.E. AtMPK4 is required for male-specific meiotic cytokinesis in Arabidopsis. Plant J. 2011, 67, 895–906. [Google Scholar] [CrossRef]
- Witon, D.; Sujkowska-Rybkowska, M.; Dabrowska-Bronk, J.; Czarnocka, W.; Bernacki, M.; Szechynska-Hebda, M.; Karpinski, S. Mitogen-Activated Protein Kinase 4 impacts leaf development, temperature, and stomatal movement in hybrid aspen. Plant Physiol. 2021, 186, 2190–2204. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Bosmans, K.C.; Hsu, P.K.; Paul, K.; Seitz, C.; Yeh, C.Y.; Wang, Y.S.; Yarmolinsky, D.; Sierla, M.; Vahisalu, T.; et al. Stomatal CO2/bicarbonate sensor consists of two interacting protein kinases, Raf-like HT1 and non-kinase-activity activity requiring MPK12/MPK4. Sci. Adv. 2022, 8, eabq6161. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y. MPK4 negatively regulates the l-arabinose synthesis of cell wall in Arabidopsis. Biochem. Biophys. Res. Commun. 2022, 613, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lott, A.A.; Zhu, W.; Dufresne, C.P.; Chen, S. Mitogen-Activated Protein Kinase 4-Regulated Metabolic Networks. Int. J. Mol. Sci. 2022, 23, 880. [Google Scholar] [CrossRef]
- Wang, S.; Wang, T.; Li, Q.; Xu, C.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X.; Han, Z.; Wu, T. Phosphorylation of MdERF17 by MdMPK4 promotes apple fruit peel degreening during light/dark transitions. Plant Cell 2022, 34, 1980–2000. [Google Scholar] [CrossRef]
- Yang, T.; Ma, H.Y.; Li, Y.; Zhang, Y.; Zhang, J.; Wu, T.; Song, T.T.; Yao, Y.C.; Tian, J. Apple MPK4 mediates phosphorylation of MYB1 to enhance light-induced anthocyanin accumulation. Plant J. 2021, 106, 1728–1745. [Google Scholar] [CrossRef]
- Li, S.; Wenyi, W.; Gao, J.; Yin, K.; Wang, R.; Wang, C.; Petersen, M.; Mundy, J.; Qiu, J.-L. MYB75 Phosphorylation by MPK4 Is Required for Light-Induced Anthocyanin Accumulation in Arabidopsis. Plant Cell 2016, 28, 2866–2883. [Google Scholar] [CrossRef]
- Furuya, T.; Matsuoka, D.; Nanmori, T. Membrane rigidification functions upstream of the MEKK1-MKK2-MPK4 cascade during cold acclimation in Arabidopsis thaliana. FEBS Lett. 2014, 588, 2025–2030. [Google Scholar] [CrossRef]
- Du, X.Z.; Jin, Z.P.; Liu, Z.Q.; Liu, D.M.; Zhang, L.P.; Ma, X.L.; Yang, G.D.; Liu, S.; Guo, Y.R.; Pei, Y.X. H2S Persulfidated and Increased Kinase Activity of MPK4 to Response Cold Stress in Arabidopsis. Front. Mol. Biosci. 2021, 8, 635470. [Google Scholar] [CrossRef]
- Sun, Y.H.; Zhao, J.; Li, X.Y.; Li, Y.Z. E2 conjugases UBC1 and UBC2 regulate MYB42-mediated SOS pathway in response to salt stress in Arabidopsis. N. Phytol. 2020, 227, 455–472. [Google Scholar] [CrossRef]
- Takahama, U. Oxidation of vacuolar and apoplastic phenolic substrates by peroxidase: Physiological significance of the oxidation reactions. Phytochem. Rev. 2004, 3, 207–219. [Google Scholar] [CrossRef]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Hemm, M.R.; Herrmann, K.M.; Chapple, C. AtMYB4: A transcription factor general in the battle against UV. Trends Plant Sci. 2001, 6, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Kiferle, C.; Fantini, E.; Bassolino, L.; Povero, G.; Spelt, C.; Buti, S.; Giuliano, G.; Quattrocchio, F.; Koes, R.; Perata, P.; et al. Tomato R2R3-MYB Proteins SlANT1 and SlAN2: Same Protein Activity, Different Roles. PLoS ONE 2015, 10, e0136365. [Google Scholar] [CrossRef]
- Jian, W.; Cao, H.; Yuan, S.; Liu, Y.; Lu, J.; Lu, W.; Li, N.; Wang, J.; Zou, J.; Tang, N.; et al. SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic. Res. 2019, 6, 22. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Chu, G.; Huang, C.; Tian, S.; Zhao, Z.; Chen, G. Anthocyanin Accumulation and Molecular Analysis of Anthocyanin Biosynthesis-Associated Genes in Eggplant (Solanum melongena L.). J. Agric. Food Chem. 2014, 62, 2906–2912. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Ge, H.; Shi, S.; Li, D.; Liu, Y.; Chen, H. A light-responsive transcription factor SmMYB35 enhances anthocyanin biosynthesis in eggplant (Solanum melongena L.). Planta 2022, 255, 12. [Google Scholar] [CrossRef]
- Shi, S.; Liu, Y.; He, Y.; Li, L.; Li, D.; Chen, H. R2R3-MYB transcription factor SmMYB75 promotes anthocyanin biosynthesis in eggplant (Solanum melongena L.). Sci. Hortic. 2021, 282, 110020. [Google Scholar] [CrossRef]
- Li, L.; He, Y.; Ge, H.; Liu, Y.; Chen, H. Functional characterization of SmMYB86, a negative regulator of anthocyanin biosynthesis in eggplant (Solanum melongena L.). Plant Sci. 2021, 302, 110696. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, Y.-J.; Zhou, L.; Liu, Y.; Jiang, M.; Ren, L.; Chen, H. Transcriptome profiling of genes related to light-induced anthocyanin biosynthesis in eggplant (Solanum melongena L.) before purple color becomes evident. BMC Genom. 2018, 19. [Google Scholar] [CrossRef]
- Cominelli, E.; Gusmaroli, G.; Allegra, D.; Galbiati, M.; Wade, H.K.; Jenkins, G.I.; Tonelli, C. Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Leyva, A.; Jarillo, J.A.; Salinas, J.; Martinez-Zapater, J.M. Low temperature induces the accumulation of phenylalanine ammonia-lyase and chalcone synthase mRNAs of Arabidopsis thaliana in a light-dependent manner. Plant Physiol. 1995, 108, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Jalaluddin, M.; Garner, J.O.; Yoshimoto, M.; Yamakawa, O. Artificial shading and temperature influence on anthocyanin compositions in sweetpotato leaves. Hortscience 2005, 40, 176–180. [Google Scholar] [CrossRef]
- An, X.-H.; Tian, Y.; Chen, K.-Q.; Liu, X.-J.; Liu, D.-D.; Xie, X.-B.; Cheng, C.-G.; Cong, P.-H.; Hao, Y.-J. MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol. 2015, 56, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Gagné, S.; Cluzet, S.; Mérillon, J.-M.; Gény, L. ABA Initiates Anthocyanin Production in Grape Cell Cultures. J. Plant Growth Regul. 2011, 30, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhou, L.; Sheng, L.; Chen, H. Establishment of Agrobacterium tumefaciens-mediated genetic transformation system for eggplant cotyledons. J. Shanghai Jiao Tong Univ. Agric. Sci. 2018, 36, 1–6. [Google Scholar] [CrossRef]
- Wang, D.; Wang, M.; Liu, J.; Zhou, X.H.; Liu, S.Y.; Yang, Y.; Zhuang, Y. Cloning of U6 Promoters and Establishment of CRISPR/Cas9 Mediated Gene Editing System in Eggplant. Hortic. Plant J. 2022, 49, 791–800. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wen, L.; Chen, Z.; Zhang, Z.; Pang, X.; Deng, Z.; Liu, T.; Guo, Y. Study on metabolic variation in whole grains of four proso millet varieties reveals metabolites important for antioxidant properties and quality traits. Food Chem. 2021, 357, 129791. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.K.; Kim, C.Y.; Hong, Y.J.; Choe, C.M.; You, T.W.; Seong, G.J. Purified high-dose anthocyanoside oligomer administration improves nocturnal vision and clinical symptoms in myopia subjects. Br. J. Nutr. 2005, 93, 895–899. [Google Scholar] [CrossRef]
- Nguyen, X.C.; Hoang, M.H.T.; Kim, H.S.; Lee, K.; Liu, X.-M.; Kim, S.H.; Bahk, S.; Park, H.C.; Chung, W.S. Phosphorylation of the transcriptional regulator MYB44 by mitogen activated protein kinase regulates Arabidopsis seed germination. Biochem. Biophys. Res. Commun. 2012, 423, 703–708. [Google Scholar] [CrossRef]
- Mao, G.; Meng, X.; Liu, Y.; Zheng, Z.; Chen, Z.; Zhang, S. Phosphorylation of a WRKY Transcription Factor by Two Pathogen-Responsive MAPKs Drives Phytoalexin Biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1639–1653. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK Cascades in Plant Disease Resistance Signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Lee, H. Mitogen-Activated Protein Kinase Kinase 3 Is Required for Regulation during Dark-Light Transition. Mol. Cells 2015, 38, 651–656. [Google Scholar] [CrossRef]
- Sethi, V.; Raghuram, B.; Sinha, A.K.; Chattopadhyay, S. A Mitogen-Activated Protein Kinase Cascade Module, MKK3-MPK6 and MYC2, Is Involved in Blue Light-Mediated Seedling Development in Arabidopsis. Plant Cell 2014, 26, 3343–3357. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-Y.; Zhang, C.; Li, Z.-C.; Wang, Z.-R.; Jiang, X.-X.; Shi, Y.-F.; Tian, S.-N.; Braun, E.; Mei, Y.; Qiu, W.-L.; et al. The MAPK Kinase Kinase GmMEKK1 Regulates Cell Death and Defense Responses. Plant Physiol. 2018, 178, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Xu, L.; Wang, Q.; Lou, Y. Expressing OsMPK4 Impairs Plant Growth but Enhances the Resistance of Rice to the Striped Stem Borer Chilo suppressalis. Int. J. Mol. Sci. 2018, 19, 1182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Schneider, J.D.; Lin, C.; Geng, S.; Ma, T.; Lawrence, S.R.; Dufresne, C.P.; Harmon, A.C.; Chen, S. MPK4 Phosphorylation Dynamics and Interacting Proteins in Plant Immunity. J. Proteome Res. 2019, 18, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, B.; Xin, X.; Ren, D. Protein Kinases in Shaping Plant Architecture. Curr. Protein Pept. Sci. 2018, 19, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Hamel, L.-P.; Nicole, M.-C.; Sritubtim, S.; Morency, M.-J.; Ellis, M.; Ehlting, J.; Beaudoin, N.; Barbazuk, B.; Klessig, D.; Lee, J.; et al. Ancient signals: Comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci. 2006, 11, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.P.; Richa, T.; Kumar, K.; Raghuram, B.; Sinha, A.K. In Silico Analysis Reveals 75 Members of Mitogen-Activated Protein Kinase Kinase Kinase Gene Family in Rice. DNA Res. 2010, 17, 139–153. [Google Scholar] [CrossRef]
- Jiao, J.; Liu, X.; Wu, J.; Xu, G.; Zhang, S. Characterization of the MAPK Gene Family and PbrMAPK13 Response to Hormone and Temperature Stresses via Different Expression Pattern in Pyrus × bretschneideri Pollen. J. Am. Soc. Hortic. Sci. 2017, 142, 163–174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).