Biochemical Profile and Antioxidant Activity of Dried Fruit Produced from Apricot Cultivars Grown in Latvia
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Preparation of Apricot Fruit for Osmotic Dehydration
2.4. Apricot Fruit Osmotic Dehydration
2.5. Apricot Fruit Convective Drying
2.6. Extraction of Free Phenolics and Flavonoids from Prunus armeniaca L. Fruit and Products for Spectrophotometric Analysis
2.7. Extraction of Carotenoids from Prunus armeniaca L. Fruit and Products for Spectrophotometric Studies
2.8. Spectrophotometric Studies
2.8.1. Determination of Phenolic Content
2.8.2. Determination of Flavonoids Content
2.8.3. Determination of Carotenoids Content
2.9. Antiradical Activity of Apricot Fruit and Candied Fruit Derived Extracts
2.9.1. DPPH• Free Radical Scavenging Activity
2.9.2. Ferric Reducing Antioxidant Power (FRAP)
2.10. Physical–Chemical Analysis of Apricot Fruit and Products
2.10.1. Apricot Fruit and Candied Fruit Moisture
2.10.2. Fruit and Candied Fruit Total Soluble Solids
2.10.3. Apricot Fruit and Candied Fruit Titratable Acidity
2.11. The HPLC-RID Conditions for Carbohydrate Analysis
2.12. Determination of Vitamin C Content
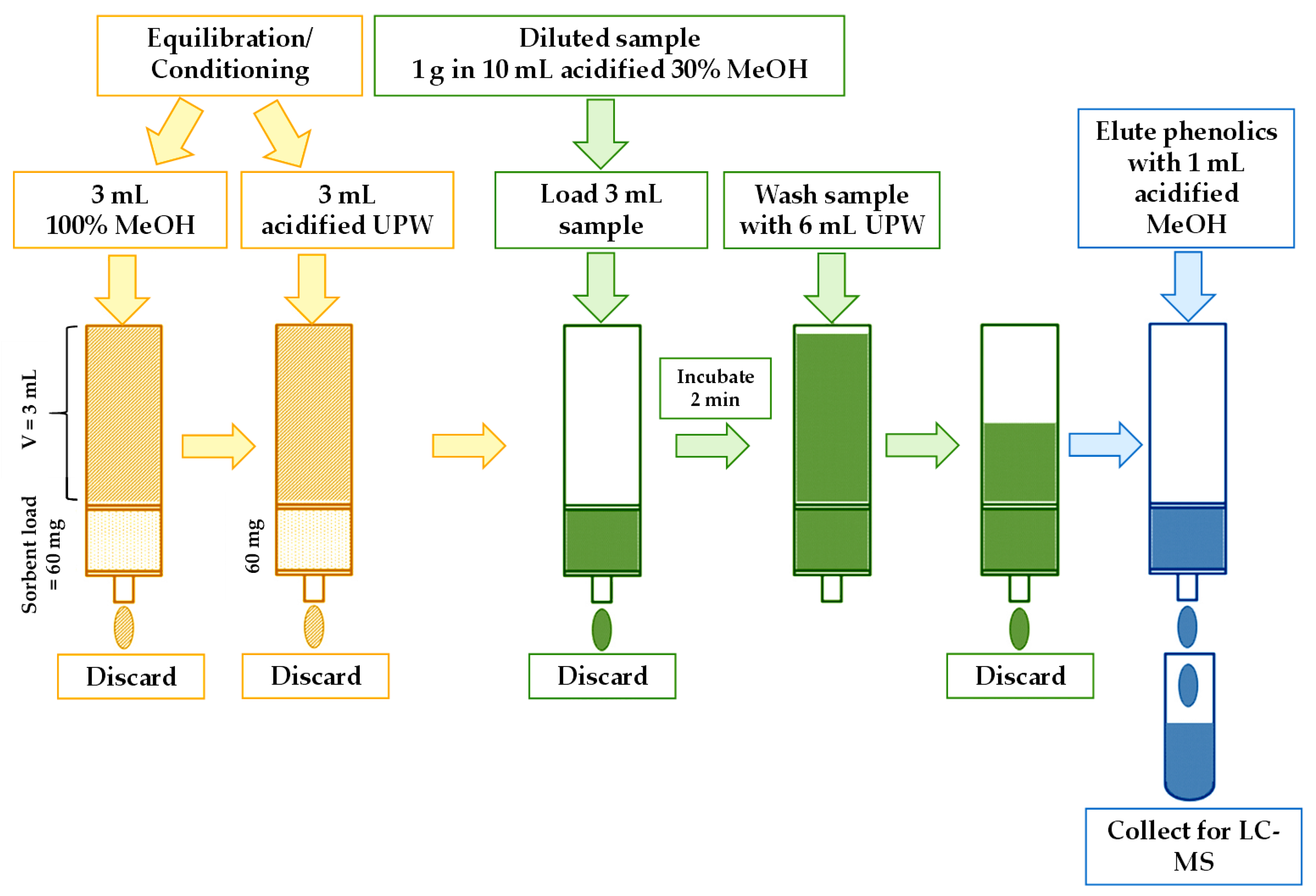
2.13. Solid-Phase Extraction of Free Phenolics from Prunus armeniaca L. Fruit and Products for Analysis by LC-ESI-TQ-MS/MS
2.14. The LC-ESI-TQ-MS/MS Analytical Conditions for Phenolics
2.15. Sensory Evaluation of Candied Apricot Fruit
2.16. Statistical Analysis
3. Results and Discussion
3.1. Physical–Chemical Characteristics of Apricot Fruit and Its Candied Products
3.2. The Composition of Individual Sugars in Apricot Fruit and Its Candied Products
3.3. The Content of Phenolics in Apricot Fruit and Its Candied Products
3.4. The Content of Flavonoids in Apricot Fruit and Its Candied Products
3.5. The Content of Vitamin C in Apricot Fruit and Its Candied Products
3.6. The Content of Carotenoids in Apricot Fruit and Its Candied Products
3.7. The Content of Individual Phenolic Compounds in Apricot Fruit and Its Candied Products
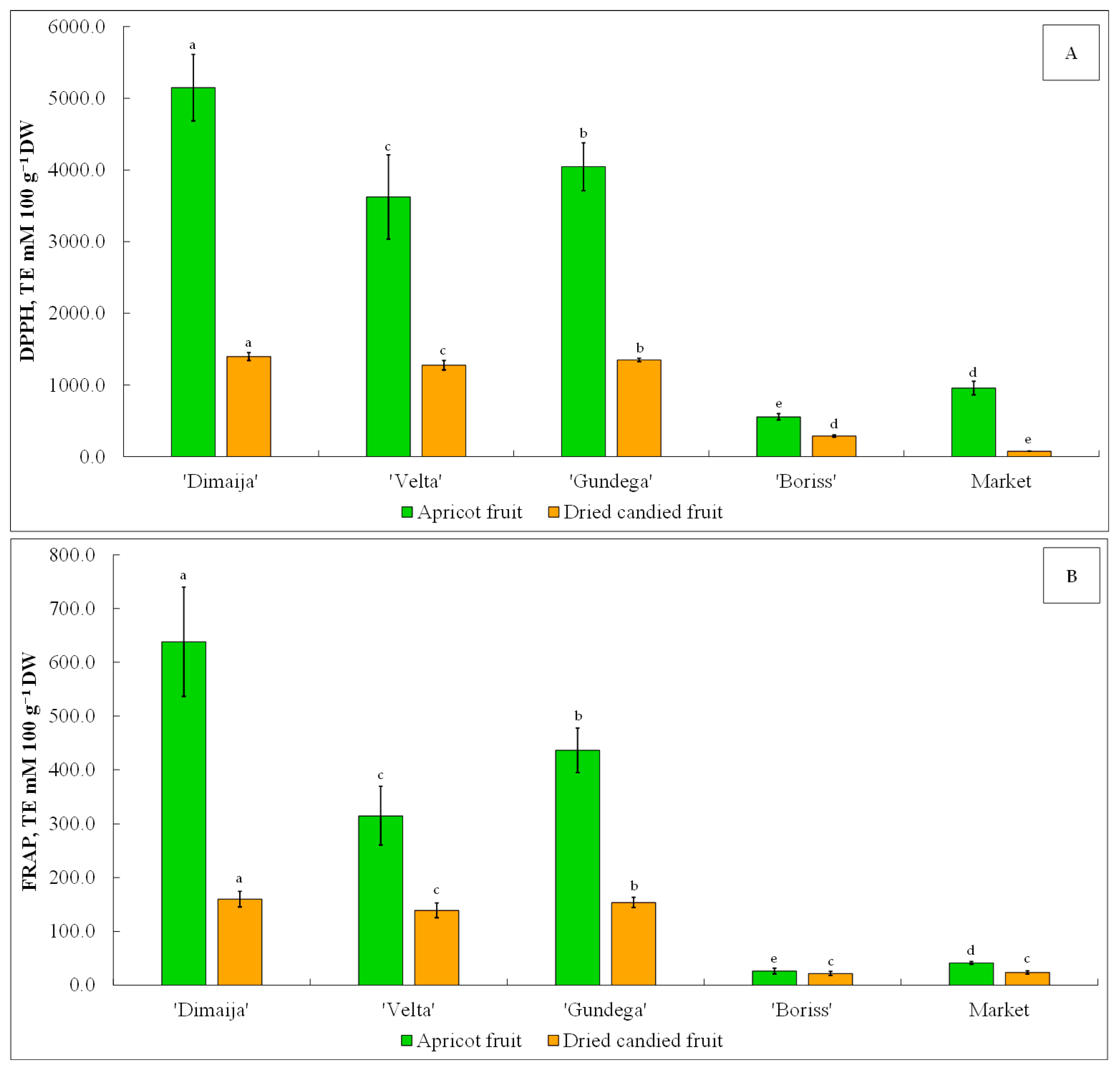
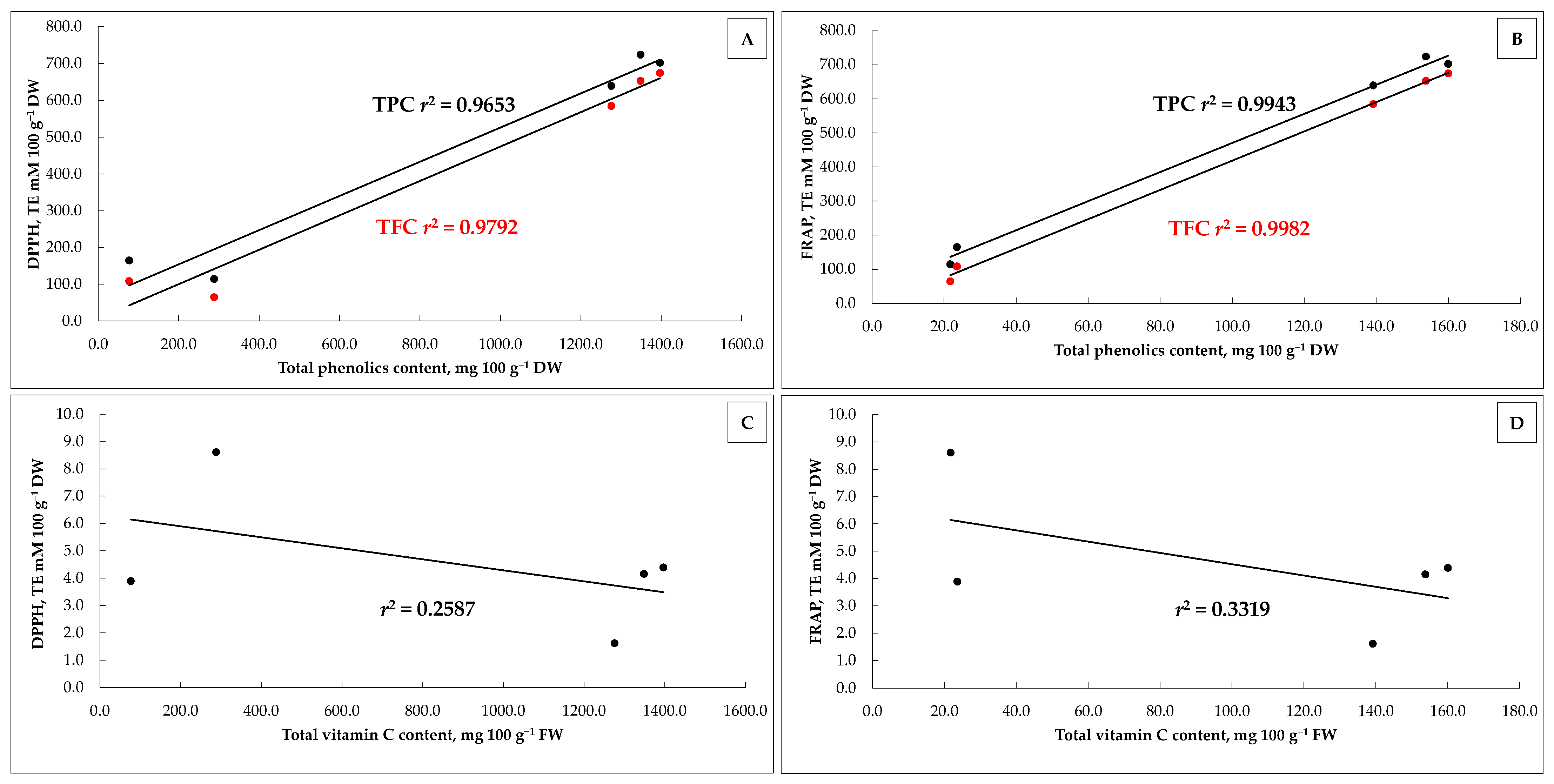
3.8. The Antioxidant Activity of Apricot Fruit and Its Candied Products
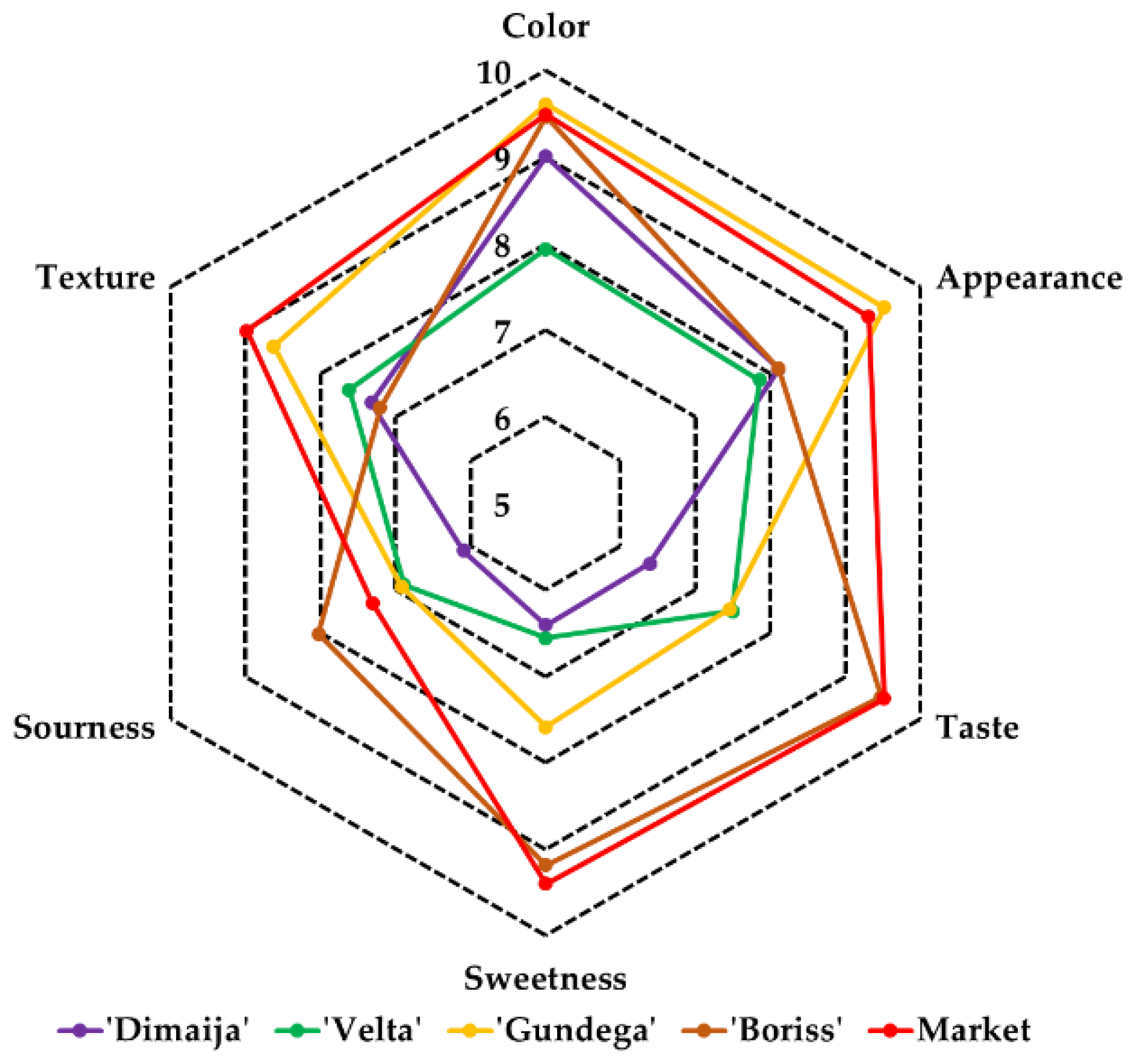
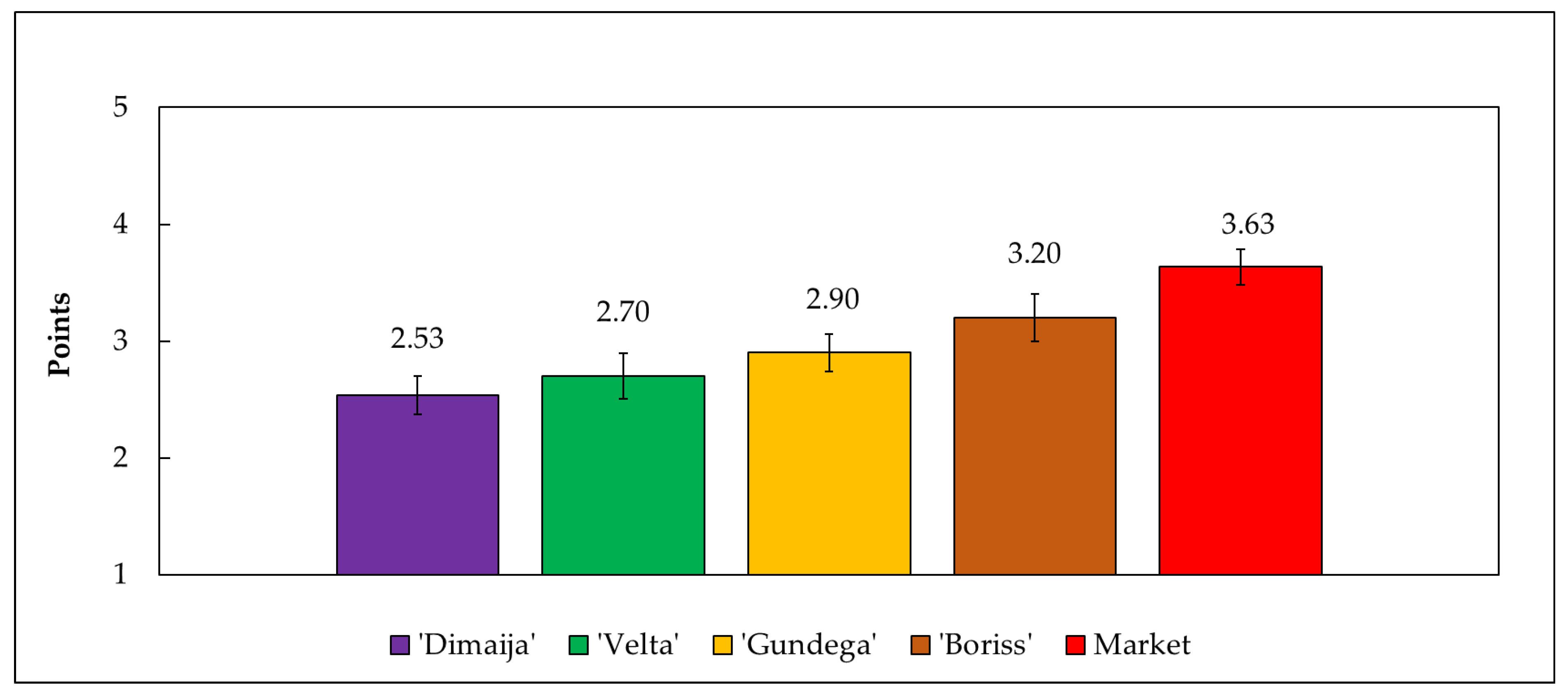
3.9. Sensory Evaluation of Candied Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domínguez, J.M. Drying. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 2, pp. 727–735. ISBN 9780080885049. [Google Scholar]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, A.; Figiel, A. Comparison of traditional and novel drying techniques and its effect on quality of fruits, vegetables and aromatic herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial spoilage of vegetables, fruits and cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Pal, A.; SinghChopra, D. Enzymes. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 335–358. ISBN 9780128132784. [Google Scholar]
- Petikirige, J.; Karim, A.; Millar, G. Effect of drying techniques on quality and sensory properties of tropical fruits. Int. J. Food Sci. Technol. 2022, 57, 6963–6979. [Google Scholar] [CrossRef]
- Mahayothee, B.; Thamsala, T.; Khuwijitjaru, P.; Janjai, S. Effect of drying temperature and drying method on drying rate and bioactive compounds in cassumunar ginger (Zingiber montanum). J. Appl. Res. Med. Aromat. Plants 2020, 18, 100262. [Google Scholar] [CrossRef]
- Veleșcu, I.; Rațu, R.; Arsenoaia, V.-N.; Roșca, R.; Cârlescu, P.; Țenu, I. Research on the process of convective drying of apples and apricots using an original drying installation. Agriculture 2023, 13, 820. [Google Scholar] [CrossRef]
- Krasnova, I.; Seglina, D.; Pole, V. The effect of pre-treatment methods on the quality of dehydrated candied Japanese quince fruits during storage. J. Food Sci. Technol. 2018, 55, 4468–4476. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, S.V. Osmotic dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2014, 51, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Juhnevica, K.; Ruisa, S.; Seglina, D.; Krasnova, I. Evaluation of sour cherry cultivars grown in Latvia for production of candied fruits. In Proceedings of the 6th Baltic Conference on Food Science and Technology: Innovations for Food Science and Production, FOODBALT-2011—Conference Proceedings, Jelgava, Latvia, 5–6 May 2011; pp. 19–22. [Google Scholar]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Sharma, R.R.; Kaushik, R.; Verma, M.K.; Tomar, M.; Kumar, M.; Mekhemar, M. Nutritional and phytochemical traits of apricots (Prunus armeniaca L.) for application in nutraceutical and health industry. Foods 2021, 10, 1344. [Google Scholar] [CrossRef]
- Srednicka-Tober, D.; Kazimierczak, R.; Ponder, A.; Hallmann, E. Biologically active compounds in selected organic and conventionally produced dried fruits. Foods 2020, 9, 1005. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Soltanzadeh, M.; Peressini, D. Properties of dried apricots pretreated by ultrasound-assisted osmotic dehydration and application of active coatings. Food Technol. Biotechnol. 2020, 58, 249–259. [Google Scholar] [CrossRef]
- Jing, Y.; Ma, X.; Jin, P.; Zhu, X. Effects of harvest maturity on chilling injury and storage quality of apricots. J. Food Qual. 2018, 2018, 4954931. [Google Scholar] [CrossRef]
- Mozaffari, M.; Sadeghi, S.; Asefi, N. Prediction of the quality properties and maturity of apricot by laser light backscattering imaging. Postharvest Biol. Technol. 2022, 186, 111842. [Google Scholar] [CrossRef]
- FAOSTAT. Global Crops Production Quantity of Apricots. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 18 January 2024).
- Dried Fruit: Production Estimates for 2022/2023. Available online: https://www.mundus-agri.eu/news/dried-fruit-production-estimates-20222023.n28617.html (accessed on 18 January 2024).
- Kaufmane, E.; Lacis, G. Studies on selection of apricots and peaches with good fruit quality and winterhardiness in Latvia. J. Fruit Ornam. Plant Res. 2004, 12, 321–329. [Google Scholar]
- Howard, L.R.; Braswell, D.D.; Aselage, J. Chemical composition and color of strained carrots as affected by processing. J. Food Sci. 1996, 61, 327–330. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Maluela-Raventos, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Yang, H.; Kim, Y.J.; Shin, Y. Influence of ripening stage and cultivar on physicochemical properties and antioxidant compositions of aronia grown in South Korea. Foods 2019, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Mallek-Ayadi, S.; Bahloul, N.; Baklouti, S.; Kechaou, N. Bioactive compounds from Cucumis melo L. fruits as potential nutraceutical food ingredients and juice processing using membrane technology. Food Sci. Nutr. 2022, 10, 2922–2934. [Google Scholar] [CrossRef]
- Radenkovs, V.; Püssa, T.; Juhnevica-Radenkova, K.; Kviesis, J.; Salar, F.J.; Moreno, D.A.; Drudze, I. Wild apple (Malus spp.) by-products as a source of phenolic compounds and vitamin C for food applications. Food Biosci. 2020, 38, 100744. [Google Scholar] [CrossRef]
- Radenkovs, V.; Kviesis, J.; Juhnevica-Radenkova, K.; Valdovska, A.; Püssa, T.; Klavins, M.; Drudze, I. Valorization of wild apple (Malus spp.) by-products as a source of essential fatty acids, tocopherols and phytosterols with antimicrobial activity. Plants 2018, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.P. Gravimetric Measurements of Water. In Handbook of Food Analytical Chemistry, Water, Proteins, Enzymes, Lipids, and Carbohydrates; Wrolstad, R.E., Acree, T.E., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D., Sporns, P., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2005; pp. 5–33. ISBN 9780471709084. [Google Scholar]
- ISO 2173:2003; Fruit and Vegetable Products, Determination of Soluble Solids, Refractometric Method. International Standardization Organization: Geneva, Switzerland. Available online: https://www.iso.org/standard/35851.html (accessed on 10 January 2024).
- ISO 750:1998; Fruit and Vegetable Products, Determination of Titratable Acidity. International Standardization Organization: Geneva, Switzerland. Available online: https://www.iso.org/standard/22569.html (accessed on 10 January 2024).
- Radenkovs, V.; Juhnevica-Radenkova, K.; Kviesis, J.; Lazdina, D.; Valdovska, A.; Vallejo, F.; Lacis, G. Lignocellulose-degrading enzymes: A biotechnology platform for ferulic acid production from agro-industrial side streams. Foods 2021, 10, 3056. [Google Scholar] [CrossRef] [PubMed]
- EN 14130:2003; Foodstuffs—Determination of Vitamin C by HPLC. European Standard: Brussels, Belgium. Available online: https://standards.iteh.ai/catalog/standards/cen/15d21027-d891-4ab0-aaea-5d87c7cc39b7/en-14130-2003 (accessed on 1 January 2024).
- ISO 13299:2016; Sensory Analysis Methodology, General Guidance for Establishing a Sensory Profile. International Standardization Organization: Geneva, Switzerland. Available online: https://www.iso.org/standard/58042.html (accessed on 10 January 2024).
- Cirillo, A.; De Luca, L.; Izzo, L.; Cepparulo, M.; Graziani, G.; Ritieni, A.; Romano, R.; Di Vaio, C. Biochemical and nutraceutical characterization of different accessions of the apricot (Prunus armeniaca L.). Horticulturae 2023, 9, 546. [Google Scholar] [CrossRef]
- Rampáčková, E.; Mrázová, M.; Čížková, J.; Nečas, T. Pomological traits and genome size of Prunus armeniaca L. Considering to geographical origin. Horticulturae 2022, 8, 199. [Google Scholar] [CrossRef]
- Abu-shama, H.S.; Ahmed, F.A.; Abd El-magied, H.E. Assessment of jelly candy manufactured from prickly pear fruits (Opuntia Spp.). World J. Adv. Res. Rev. 2022, 16, 767–783. [Google Scholar] [CrossRef]
- Kuo, C.H.; Lin, J.; Huang, C.Y.; Hsieh, S.L.; Li, S.; Kuo, J.M.; Shieh, C.J. Predicting sugar content of candied watermelon rind during osmotic dehydration. Food Sci. Technol. 2018, 38, 228–235. [Google Scholar] [CrossRef]
- Melgarejo, P.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Martínez-Nicolás, J.J.; Legua, P.; Martínez, R.; Hernández, F. Antioxidant activity, volatile composition and sensory profile of four new very-early apricots (Prunus armeniaca L.). J. Sci. Food Agric. 2014, 94, 85–94. [Google Scholar] [CrossRef]
- Radenkovs, V.; Kaufmane, E.; Rubauskis, E.; Segliņa, D. Preliminary results on the effect of 1-methylcyclopropene on quality of plums grown in Latvia. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2016, 70, 21–28. [Google Scholar] [CrossRef]
- Leccese, A.; Bartolini, S.; Viti, R. From genotype to apricot fruit quality: The Antioxidant properties contribution. Plant Foods Hum. Nutr. 2012, 67, 317–325. [Google Scholar] [CrossRef]
- Madrau, M.A.; Piscopo, A.; Sanguinetti, A.M.; Del Caro, A.; Poiana, M.; Romeo, F.V.; Piga, A. Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. Eur. Food Res. Technol. 2009, 228, 441–448. [Google Scholar] [CrossRef]
- Kucner, A.; Klewicki, R.; Sójka, M.; Klewicka, E. Osmotic concentration of gooseberry fruits—The influence of temperature, time and pretreatment methods on mass transfer and total polyphenol and organic acid content. Food Technol. Biotechnol. 2014, 52, 411–419. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Vemmos, S.; Pantelidis, G.; Petri, E.; Tzoutzoukou, C.; Karayiannis, I. Physical characters and antioxidant, sugar, and mineral nutrient contents in fruit from 29 apricot (Prunus armeniaca L.) cultivars and hybrids. J. Agric. Food Chem. 2008, 56, 10754–10760. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Molecular basis for optimizing sugar metabolism and transport during fruit development. aBIOTECH 2021, 2, 330–340. [Google Scholar] [CrossRef]
- Zhou, H.; Su, M.; Du, J.; Zhang, X.; Li, X.; Zhang, M.; Hu, Y.; Huan, C.; Ye, Z. Crucial roles of sorbitol metabolism and energy status in the chilling tolerance of yellow peach. Plant Physiol. Biochem. 2023, 204, 108092. [Google Scholar] [CrossRef]
- dos Reis, S.P.; Lima, A.M.; de Souza, C.R.B. Recent molecular advances on downstream plant responses to abiotic stress. Int. J. Mol. Sci. 2012, 13, 8628–8647. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Ramadan, N.S.; Shorbagi, M.; Farag, N.; Gad, H.A. Profiling of primary metabolites and volatiles in apricot (Prunus armeniaca L.) seed kernels and fruits in the context of its different cultivars and soil type as analyzed using chemometric tools. Foods 2022, 11, 1339. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, B.E.; Ruiz, D.; Salazar, J.A.; Rubio, M.; Martínez-García, P.J.; Martínez-Gómez, P. Analysis of metabolites and gene expression changes relative to apricot (Prunus armeniaca L.) fruit quality during development and ripening. Front. Plant Sci. 2020, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Schmitzer, V.; Slatnar, A.; Mikulic-Petkovsek, M.; Veberic, R.; Krska, B.; Stampar, F. Comparative study of primary and secondary metabolites in apricot (Prunus armeniaca L.) cultivars. J. Sci. Food Agric. 2011, 91, 860–866. [Google Scholar] [CrossRef]
- Naryal, A.; Acharya, S.; Kumar Bhardwaj, A.; Kant, A.; Chaurasia, O.P.; Stobdan, T. Altitudinal effect on sugar contents and sugar profiles in dried apricot (Prunus armeniaca L.) fruit. J. Food Compos. Anal. 2019, 76, 27–32. [Google Scholar] [CrossRef]
- Gómez-Martínez, H.; Bermejo, A.; Zuriaga, E.; Badenes, M.L. Polyphenol content in apricot fruits. Sci. Hortic. 2021, 277, 109828. [Google Scholar] [CrossRef]
- Wani, S.M.; Masoodi, F.A.; Haq, E.; Ahmad, M.; Ganai, S.A. Influence of processing methods and storage on phenolic compounds and carotenoids of apricots. Lwt 2020, 132, 109846. [Google Scholar] [CrossRef]
- Yang, C.P.; Fujita, S.; Ashrafuzzaman, M.D.; Nakamura, N.; Hayashi, N. Purification and characterization of polyphenol oxidase from banana (Musa sapientum L.) pulp. J. Agric. Food Chem. 2000, 48, 2732–2735. [Google Scholar] [CrossRef]
- Muñoz-Fariña, O.; López-Casanova, V.; García-Figueroa, O.; Roman-Benn, A.; Ah-Hen, K.; Bastias-Montes, J.M.; Quevedo-León, R.; Ravanal-Espinosa, M.C. Bioaccessibility of phenolic compounds in fresh and dehydrated blueberries (Vaccinium corymbosum L.). Food Chem. Adv. 2023, 2, 100171. [Google Scholar] [CrossRef]
- Bousselma, A.; Abdessemed, D.; Tahraoui, H.; Zedame, F.; Amrane, A. Polyphenols and flavonoids contents of fresh and dried apricots extracted by cold soaking and ultrasound-assisted extraction. Kem. Ind. 2023, 72, 160–168. [Google Scholar] [CrossRef]
- Gómez-Martínez, H.; Gil-Muñoz, F.; Bermejo, A.; Zuriaga, E.; Badenes, M.L. Insights of phenolic pathway in fruits: Transcriptional and metabolic profiling in apricot (Prunus Armeniaca). Int. J. Mol. Sci. 2021, 22, 3411. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.M.; Vulić, J.J.; Ćetković, G.S.; Djilas, S.M.; Šaponjac, V.T.T. Bioactive compounds and antioxidant properties of dried apricot. Acta Period. Technol. 2013, 44, 193–205. [Google Scholar] [CrossRef]
- Miletić, N.; Mitrović, O.; Popović, B.; Nedović, V.; Zlatković, B.; Kandić, M. Polyphenolic content and antioxidant capacity in fruits of plum (Prunus domestica L.) cultivars “Valjevka” and “Mildora” as influenced by air drying. J. Food Qual. 2013, 36, 229–237. [Google Scholar] [CrossRef]
- Patel, P.; Sunkara, R.; Walker, L.T.; Verghese, M. Effect of drying techniques on antioxidant capacity of guava fruit. Food Nutr. Sci. 2016, 7, 544–554. [Google Scholar] [CrossRef][Green Version]
- Ouchemoukh, S.; Hachoud, S.; Boudraham, H.; Mokrani, A.; Louaileche, H. Antioxidant activities of some dried fruits consumed in Algeria. Lwt 2012, 49, 329–332. [Google Scholar] [CrossRef]
- Rasanu, N.; Magearu, V.; Matei, N.; Soceanu, A. Determination of vitamin C in different stages of fruits growing. Chimie 2005, 1–2, 167–172. [Google Scholar]
- Munzuroglu, O.; Karatas, F.; Geckil, H. The vitamin and selenium contents of apricot fruit of different varieties cultivated in different geographical regions. Food Chem. 2003, 83, 205–212. [Google Scholar] [CrossRef]
- Akin, E.B.; Karabulut, I.; Topcu, A. Some compositional properties of main Malatya apricot (Prunus armeniaca L.) varieties. Food Chem. 2008, 107, 939–948. [Google Scholar] [CrossRef]
- Fratianni, F.; D’acierno, A.; Albanese, D.; Di Matteo, M.; Coppola, R.; Nazzaro, F. Biochemical characterization of traditional varieties of apricots (Prunus armeniaca L.) of the Campania region, Southern Italy. Foods 2022, 11, 100. [Google Scholar] [CrossRef]
- Karatas, N. Evaluation of nutritional content in wild apricot fruits for sustainable apricot production. Sustainability 2022, 14, 1063. [Google Scholar] [CrossRef]
- Susanna, B.; Annamaria, L. Quality and antioxidant properties of apricot fruits at ready-to-eat: Influence of the weather conditions under Mediterranean coastal area. J. Food Process Technol. 2016, 7, 538. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Martinez-Monzo, J.; Pasten, A.; Lemus-Mondaca, R. Bioactive compounds and physicochemical characterization of dried apricot (Prunus armeniaca L.) as affected by different drying temperatures. CYTA—J. Food 2019, 17, 297–306. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of osmotic dehydration (with and without sonication) and pectin-based coating pretreatments on functional properties and color of hot-air dried apricot cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.Z.; Xiong, C.H.; Pei, Y.P.; Zhu, Z.Q.; Zheng, X.; Zhang, Y.; Yang, X.H.; Liu, Z.L.; Xiao, H.W. Effects of various storage conditions on total phenolic, carotenoids, antioxidant capacity, and color of dried apricots. Food Control 2022, 136, 108846. [Google Scholar] [CrossRef]
- Ali, S.; Masud, T.; Abbasi, K.S. Physico-chemical characteristics of apricot (Prunus armeniaca L.) grown in Northern areas of Pakistan. Sci. Hortic. 2011, 130, 386–392. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Pintea, A.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Pop, E.A.; Opriță, V.A.; Giuffrida, D.; Cacciola, F.; Bartolomeo, G.; Mondello, L. Carotenoids, fatty acids, and volatile compounds in apricot cultivars from Romania—A chemometric approach. Antioxidants 2020, 9, 562. [Google Scholar] [CrossRef]
- Wani, S.M.; Masoodi, F.A.; Ahmad, M.; Mir, S.A. Processing and storage of apricots: Effect on physicochemical and antioxidant properties. J. Food Sci. Technol. 2018, 55, 4505–4514. [Google Scholar] [CrossRef]
- Souza, C.S.; Daood, H.G.; Duah, S.A.; Vinogradov, S.; Palotás, G.; Neményi, A.; Helyes, L.; Pék, Z. Stability of carotenoids, carotenoid esters, tocopherols and capsaicinoids in new chili pepper hybrids during natural and thermal drying. Lwt 2022, 163, 113520. [Google Scholar] [CrossRef]
- Pénicaud, C.; Achir, N.; Dhuique-Mayer, C.; Dornier, M.; Bohuon, P. Degradation of β-carotene during fruit and vegetable processing or storage: Reaction mechanisms and kinetic aspects: A review. Fruits 2011, 66, 417–440. [Google Scholar] [CrossRef]
- Morais, H.; Rodrigues, P.; Ramos, C.; Forgács, E.; Cserháti, T.; Oliveira, J. Effect of ascorbic acid on the stability of β-carotene and capsanthin in paprika (Capsicum annuum) powder. Nahrung—Food 2002, 46, 308–310. [Google Scholar] [CrossRef]
- Oszmianski, J.; Lee, C.Y. Inhibitory effect of phenolics on carotene bleaching in vegetables. J. Agric. Food Chem. 1990, 38, 688–690. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry behind the Folin–Ciocalteu method for the estimation of (poly)phenol content in food: Total phenolic intake in a Mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.; Xian, W.; Huang, W.; Yang, R. Free and bound phenolic profiles of Rosa roxburghii Tratt leaves and their antioxidant and inhibitory effects on α-clucosidase. Front. Nutr. 2022, 9, 922496. [Google Scholar] [CrossRef]
- Karaçelik, A.A. Phytochemical profiling, antioxidant activities and in vitro/in silico enzyme inhibitory potentials of apricot cultivars grown in Iğdır/Turkey. South African J. Bot. 2023, 156, 257–267. [Google Scholar] [CrossRef]
- Gundogdu, M.; Ercisli, S.; Berk, S.; Kan, T.; Canan, I.; Gecer, M.K. Diversity on color and phenolic compounds in apricot fruits. J. Food Meas. Charact. 2017, 11, 2087–2093. [Google Scholar] [CrossRef]
- Göttingerová, M.; Kumšta, M.; Rampáčková, E.; Kiss, T.; Nečas, T. Analysis of phenolic compounds and some important analytical properties in selected apricot genotypes. HortScience 2021, 56, 1446–1452. [Google Scholar] [CrossRef]
- Tilley, A.; McHenry, M.P.; McHenry, J.A.; Solah, V.; Bayliss, K. Enzymatic browning: The role of substrates in polyphenol oxidase mediated browning: Mechanisms of enzymatic browning. Curr. Res. Food Sci. 2023, 7, 100623. [Google Scholar] [CrossRef]
- Roussos, P.A.; Sefferou, V.; Denaxa, N.K.; Tsantili, E.; Stathis, V. Apricot (Prunus armeniaca L.) fruit quality attributes and phytochemicals under different crop load. Sci. Hortic. 2011, 129, 472–478. [Google Scholar] [CrossRef]
- Jan, N.; Anjum, S.; Wani, S.M.; Mir, S.A.; Malik, A.R.; Wani, S.A.; Hussein, D.S.; Rasheed, R.A.; Gatasheh, M.K. Influence of canning and storage on physicochemical properties, antioxidant properties, and bioactive compounds of apricot (Prunus armeniaca L.) wholes, halves, and pulp. Front. Nutr. 2022, 9, 850730. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jin, T.Z.; Fan, X.; Wu, J. Biochemical degradation and physical migration of polyphenolic compounds in osmotic dehydrated blueberries with pulsed electric field and thermal pretreatments. Food Chem. 2018, 239, 1219–1225. [Google Scholar] [CrossRef]
- Özcan, M.M.; Al Juhaimi, F.; Ahmed, I.A.M.; Uslu, N.; Babiker, E.E.; Ghafoor, K. Effect of microwave and oven drying processes on antioxidant activity, total phenol and phenolic compounds of kiwi and pepino fruits. J. Food Sci. Technol. 2020, 57, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Pashazadeh, H.; Ali Redha, A.; Koca, I. Effect of convective drying on phenolic acid, flavonoid and anthocyanin content, texture and microstructure of black rosehip fruit. J. Food Compos. Anal. 2024, 125, 105738. [Google Scholar] [CrossRef]
- Chen, J.P.; Wang, Y.; Zhang, X.Y.; Sun, P.; Wu, Z.F.; Shang, Y.F.; Yang, S.H.; Ma, Y.L.; Wei, Z.J. Effect of air drying temperature on the phenolics and antioxidant activity of Xuan-Mugua fruit. Food Sci. Technol. 2022, 42, e45322. [Google Scholar] [CrossRef]
- Babiker, E.E.; Mohamed Ahmed, I.A.; Uslu, N.; Özcan, M.M.; Al Juhaimi, F.; Ghafoor, K.; Almusallam, I.A. Influence of drying methods on bioactive properties, fatty acids and phenolic compounds of different parts of ripe and unripe avocado fruits. J. Oleo Sci. 2021, 70, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Zitka, O.; Skutkova, H.; Pavlik, D.; Babula, P.; Krska, B.; Horna, A.; Adam, V.; Provaznik, I.; Kizek, R. Content of phenolic compounds and antioxidant capacity in fruits of apricot genotypes. Molecules 2010, 15, 6285–6305. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; Boekel, M.A.J.S. Van A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2001, 11, 364–373. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-derived by-products—A promising source of bioactive ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef]
- Davarynejad, G.; Khorshidi, S.; Nyéki, J.; Szabó, Z.; Gal-Remennyik, J. Antioxidant capacity, chemical composition and physical properties of some apricot (Prunus armeniaca L.) cultivars. Hortic. Environ. Biotechnol. 2010, 51, 477–482. [Google Scholar]
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D’Ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yeretzian, C. Comparison of nine common coffee extraction methods: Instrumental and sensory analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef]
- Ley, J.P. Masking bitter taste by Molecules. Chemosens. Percept. 2008, 1, 58–77. [Google Scholar] [CrossRef]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different phenolic compounds activate distinct human bitter taste receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef]
- Nagel, C.W.; Herrick, I.W.; Graber, W.R. Is Chlorogenic Acid Bitter? J. Food Sci. 1987, 52, 213. [Google Scholar] [CrossRef]


| Cultivar | Description | ||
|---|---|---|---|
| Fruit | Ripening Time and Productivity | ||
| ‘Dimaija’ |  | The fruit are round, light orange, slightly matte, handsome, large, and uniform. The taste of the fruit is good, and the flesh is yellow, medium firm, fine-grained, and aromatic. The peel is smooth and thin. Stone is medium and separates very well from the flesh. | The ripening time is the end of July—1st or 2nd week of August. The fruit ripen relatively evenly. The cultivar is productive, but since the flower buds are sensitive to severe spring frosts, can be produced irregularly. |
| ‘Velta’ |  | The fruit are large, round, flattened, and have a slight sharpness at the end of the fruit, orange with pronounced redness, with a deep abdominal seam—very attractive. The fruit are aromatic, with a good taste. The flesh is orange, with a medium-firm, fine-grained consistency; the peel is slightly uneven and medium-thick; therefore, the fruit are transportable. Compared to the flesh, the stone is large, elongated in shape, and separates perfectly from the flesh. | The cultivar only produces occasionally but very well in some years. Its ripening time is medium (depending on the year—the last week of July to the second week of August). As the flowers are relatively sensitive to spring frosts, the cultivar does not produce regularly, but very well in some years. |
| ‘Gundega’ |  | The fruit are round and orange, with distinct pinkish, small dots which merge to form a blush, slightly matt, very good-looking, large, uniform. The abdominal suture is deep. The taste of the fruit is delicious; the flesh is yellow, medium firm, fine-grained, and aromatic. Peel is smooth and thin—hence, high taste properties. The stone is small and separates very well from the flesh. | The ripening time is the second half or the end of July (rarely the early beginning of August). The fruit ripen relatively evenly, usually in 2 (rarely 3) picking times. The cultivar is productive and produces regularly and abundantly. |
| ‘Boriss’ |  | The fruit are large, round, yellow–orange, with pronounced redness, shiny, excellent appearance, and uniform. The abdominal suture is deep. The taste of the fruit is delicious; the flesh is yellow, medium firm, fine-grained, and aromatic. Peel is smooth and thin—hence, high taste properties. The stone is small and separates very well from the flesh. | The production time for this cultivar is one of the longest (depending on the year, second–third week of July). The fruit ripen relatively evenly, usually collected in 2 (rarely 3) picking times. The cultivar is productive and produces regularly and abundantly. |
| Indicator | Raw Material | Dried Candied Fruit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Dimaija’ | ‘Velta’ | ‘Gundega’ | ‘Boriss’ | Market (Greece) | ‘Dimaija’ | ‘Velta’ | ‘Gundega’ | ‘Boriss’ | Market (Greece) | |
| TSS, Brix% | 11.3 ± 0.1 c | 11.5 ± 0.1 c | 13.6 ± 0.1 b | 14.8 ± 0.1 a | 11.8 ± 0.2 c | 76.1 ± 0.7 b | 78.6 ± 1.6 a | 76.7 ± 0.1 b | 69.6 ± 0.7 c | 76.4 ± 0.8 b |
| TA, % | 24.0 ± 3.0 b | 35.9 ± 4.5 a | 20.0 ± 2.0 b | 14.5 ± 1.5 c | 13.8 ± 1.6 c | 5.5 ± 0.1 a | 5.9 ± 0.1 a | 5.7 ± 0.1 a | 3.9 ± 0.1 b | 4.2 ± 0.1 b |
| Moisture, % | 89.0 ± 1.3 a | 89.1 ± 1.3 a | 87.4 ± 1.3 b | 87.4 ± 1.3 b | 89.3 ± 1.3 a | 30.2 ± 1.3 b | 25.2 ± 1.3 c | 32.9 ± 0.1 a | 21.8 ± 1.3 d | 25.8 ± 1.3 c |
| MI | 4.7 ± 0.6 | 3.2 ± 0.2 | 6.8 ± 0.6 | 10.2 ± 0.1 | 8.5 ± 0.4 | - | - | - | - | - |
| Saccharide | Raw Material | Dried Candied Fruit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Dimaija’ | ‘Velta’ | ‘Gundega’ | ‘Boriss’ | Market (Greece) | ‘Dimaija’ | ‘Velta’ | ‘Gundega’ | ‘Boriss’ | Market (Greece) | |
| Ribose | BLQ | n.d. | BLQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Fructose | 6.5 ± 0.3 b | 3.4 ± 0.0 d | 8.3 ± 1.0 a | 5.8 ± 0.1 c | BLQ | 23.1 ± 0.3 c | 24.7 ± 0.1 b | 25.5 ± 0.4 a | 12.6 ± 0.6 d | 9.8 ± 0.2 e |
| Glucose | 9.8 ± 0.4 b | 5.4 ± 0.2 c | 9.5 ± 3.0 b | 11.5 ± 0.0 a | 9.7 ± 0.3 b | 22.1 ± 0.3 c | 23.7 ± 1.0 b | 25.8 ± 0.9 a | 13.5 ± 0.9 d | 9.9 ± 0.1 e |
| Sorbitol | 3.3 ± 0.1 d | 4.2 ± 0.4 c | 5.9 ± 0.4 b | 5.2 ± 0.3 b | 14.5 ± 0.3 a | n.d. | n.d. | n.d. | n.d. | 4.1 ± 0.1 |
| Sucrose | 39.3 ± 2.9 c | 46.9 ± 0.0 b | 41.0 ± 0.5 c | 55.0 ± 1.7 a | 23.2 ± 1.2 d | 20.1 ± 0.3 c | 20.1 ± 0.5 c | 18.4 ± 0.9 d | 27.5 ± 1.1 b | 31.4 ± 1.6 a |
| Inositol | n.d. | n.d. | BLQ | n.d. | 23.6 ± 0.9 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Unknown | n.d. | n.d. | n.d. | 1.3 ± 0.0 | BLQ | n.d. | n.d. | n.d. | n.d. | n.d. |
| ∑Sugars | 59.0 ± 3.6 d | 59.9 ± 0.5 d | 64.7 ± 4.9 c | 78.7 ± 2.1 a | 71.1 ± 2.7 b | 65.3 ± 1.0 c | 68.4 ± 1.6 b | 69.7 ± 2.2 a | 53.6 ± 2.7 e | 55.5 ± 2.0 d |
| Compound | Raw Material | Dried Candied Fruit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Dimaija’ | ‘Velta’ | ‘Gundega’ | ‘Boriss’ | Market (Greece) | ‘Dimaija’ | ‘Velta’ | ‘Gundega’ | ‘Boriss’ | Market (Greece) | |
| TPC | 2795.3 ± 285.7 a | 1566.3 ± 237.1 c | 1843.2 ± 156.7 b | 216.4 ± 26.1 e | 582.0 ± 63.1 d | 702.1 ± 35.9 b | 639.2 ± 19.7 c | 723.9 ± 39.7 a | 114.4 ± 10.6 e | 164.8 ± 13.0 d |
| TFC | 2270.5 ± 231.5 a | 1197.4 ± 149.0 c | 1536.0 ± 246.7 b | 122.2 ± 5.7 e | 138.8 ± 22.5 d | 674.6 ± 36.2 a | 584.6 ± 30.2 c | 652.7 ± 26.1 b | 64.7 ± 4.2 e | 108.3 ± 2.9 d |
| Vit C | 148.3 ± 25.7 a | 120.4 ± 13.6 c | 126.4 ± 20.1 b | 75.3 ± 14.1 e | 85.5 ± 9.2 d | 4.4 ± 0.5 b | 1.6 ± 0.0 c | 4.2 ± 0.1 b | 8.6 ± 0.23 a | 3.9 ± 0.2 b |
| Carotenoids | 16.8 ± 1.8 c | 24.4 ± 3.0 b | 13.0 ± 1.3 d | 28.7 ± 1.4 a | 30.4 ± 4.0 a | 1.9 ± 0.2 b | 3.4 ± 0.0 a | 2.1 ± 0.0 b | 3.0 ± 0.4 a | 3.1 ± 0.1 a |
| Phenolic Compound | Raw Material | Dried Candied Fruit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Dimaija’ | ‘Velta’ | ‘Gundega’ | ‘Boriss’ | Market (Greece) | ‘Dimaija’ | ‘Velta’ | ‘Gundega’ | ‘Boriss’ | Market (Greece) | |
| VN | 0.9 ± 0.0 c | 1.9 ± 0.0 b | 0.8 ± 0.0 c | 10.8 ± 0.4 a | 0.9 ± 0.1 c | 1.7 ± 0.4 b | 3.8 ± 0.3 a | 1.1 ± 0.2 bc | 0.8 ± 0.0 c | 1.1 ± 0.2 bc |
| QCT | 2.5 ± 0.3 bc | 1.5 ± 0.0 c | 1.7 ± 0.1 bc | 26.7 ± 1.1 a | 1.4 ± 0.1 c | 6.5 ± 0.0 a | 6.0 ± 0.9 a | 2.4 ± 0.7 b | 2.0 ± 0.2 b | BLQ |
| NCGA | 550.2 ± 8.4 a | 463.9 ± 3.5 b | 84.9 ± 4.1 c | 1.0 ± 0.0 e | 2.1 ± 0.0 d | 136.6 ± 2.7 b | 191.2 ± 10.2 a | 35.1 ± 1.0 c | 5.2 ± 0.2 e | 6.9 ± 0.8 d |
| PCA | 0.4 ± 0.0 a | 0.6 ± 0.1 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.1 ± 0.0 a | 0.7 ± 0.2 a | 0.4 ± 0.0 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a | BLQ |
| CGA | 384.8 ± 7.7 b | 1129.1 ± 10.4 a | 159.8 ± 7.9 c | 21.3 ± 1.3 e | 41.9 ± 3.1 d | 147.1 ± 4.6 b | 587.4 ± 20.6 a | 93.1 ± 1.2 c | 40.4 ± 0.1 d | 24.3 ± 2.5 e |
| CT | 373.3 ± 15.4 a | 10.0 ± 2.5 c | 94.6 ± 14.8 b | 3.5 ± 0.1 e | 6.0 ± 0.4 d | 178.8 ± 4.4 a | 18.9 ± 0.1 c | 102.4 ± 2.9 b | 11.5 ± 0.9 d | 7.3 ± 0.4 e |
| E-CTC | 31.3 ± 3.7 c | 38.8 ± 3.3 b | 170.5 ± 6.5 a | 1.3 ± 0.0 d | 1.9 ± 0. 3 d | 59.2 ± 1.2 c | 86.0 ± 1.0 b | 269.6 ± 2.2 a | 12.0 ± 0.7 d | 6.1 ± 0.3 e |
| CA | 1.3 ± 0.0 | BLQ | BLQ | n.d. | n.d. | 4.0 ± 0.5 a | 4.0 ± 0.3 a | BLQ | 1.4 ± 0.2 b | n.d. |
| SA | n.d. | BLQ | BLQ | BLQ | BLQ | n.d. | BLQ | BLQ | BLQ | BLQ |
| RT | 1249.4 ± 15.0 a | 771.4 ± 7.6 b | 300.5 ± 4.1 c | 89.5 ± 0.8 e | 140.0 ± 4.2 d | 260.3 ± 4.4 a | 184.9 ± 4.8 b | 68.3 ± 0.5 c | 65.8 ± 1.4 d | 55.8 ± 0.0 e |
| t-FA | 1.8 ± 0.4 c | 2.0 ± 0.2 bc | 3.0 ± 0.0 ab | 3.4 ± 0.1 a | 1.4 ± 0.00 c | 3.2 ± 0.1 a | 3.3 ± 0.2 ab | 4.0 ± 0.1 a | 3.9 ± 0.2 ab | 0.9 ± 0.0 c |
| LUT-7G | 0.8 ± 0.0 a | BLQ | 1.0 ± 0.0 a | BLQ | n.d. | n.d. | BLQ | 1.2 ± 0.0 | BLQ | BLQ |
| RHM | n.d. | BLQ | BLQ | n.d. | BLQ | n.d. | BLQ | BLQ | n.d. | BLQ |
| ∑Total | 2596.9 ± 51.0 a | 2419.2 ± 27.6 b | 817.0 ± 37.6 c | 157.9 ± 3.9 e | 195.7 ± 8.2 d | 798.0 ± 18.6 b | 1085.9 ± 38.5 a | 577.2 ± 9.0 c | 143.2 ± 3.9 d | 102.3 ± 4.3 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhnevica-Radenkova, K.; Krasnova, I.; Seglina, D.; Kaufmane, E.; Gravite, I.; Valdovska, A.; Radenkovs, V. Biochemical Profile and Antioxidant Activity of Dried Fruit Produced from Apricot Cultivars Grown in Latvia. Horticulturae 2024, 10, 205. https://doi.org/10.3390/horticulturae10030205
Juhnevica-Radenkova K, Krasnova I, Seglina D, Kaufmane E, Gravite I, Valdovska A, Radenkovs V. Biochemical Profile and Antioxidant Activity of Dried Fruit Produced from Apricot Cultivars Grown in Latvia. Horticulturae. 2024; 10(3):205. https://doi.org/10.3390/horticulturae10030205
Chicago/Turabian StyleJuhnevica-Radenkova, Karina, Inta Krasnova, Dalija Seglina, Edite Kaufmane, Ilze Gravite, Anda Valdovska, and Vitalijs Radenkovs. 2024. "Biochemical Profile and Antioxidant Activity of Dried Fruit Produced from Apricot Cultivars Grown in Latvia" Horticulturae 10, no. 3: 205. https://doi.org/10.3390/horticulturae10030205
APA StyleJuhnevica-Radenkova, K., Krasnova, I., Seglina, D., Kaufmane, E., Gravite, I., Valdovska, A., & Radenkovs, V. (2024). Biochemical Profile and Antioxidant Activity of Dried Fruit Produced from Apricot Cultivars Grown in Latvia. Horticulturae, 10(3), 205. https://doi.org/10.3390/horticulturae10030205








