Comprehensive Assessment of Coffee Varieties (Coffea arabica L.; Coffea canephora L.) from Coastal, Andean, and Amazonian Regions of Ecuador; A Holistic Evaluation of Metabolism, Antioxidant Capacity and Sensory Attributes
Abstract
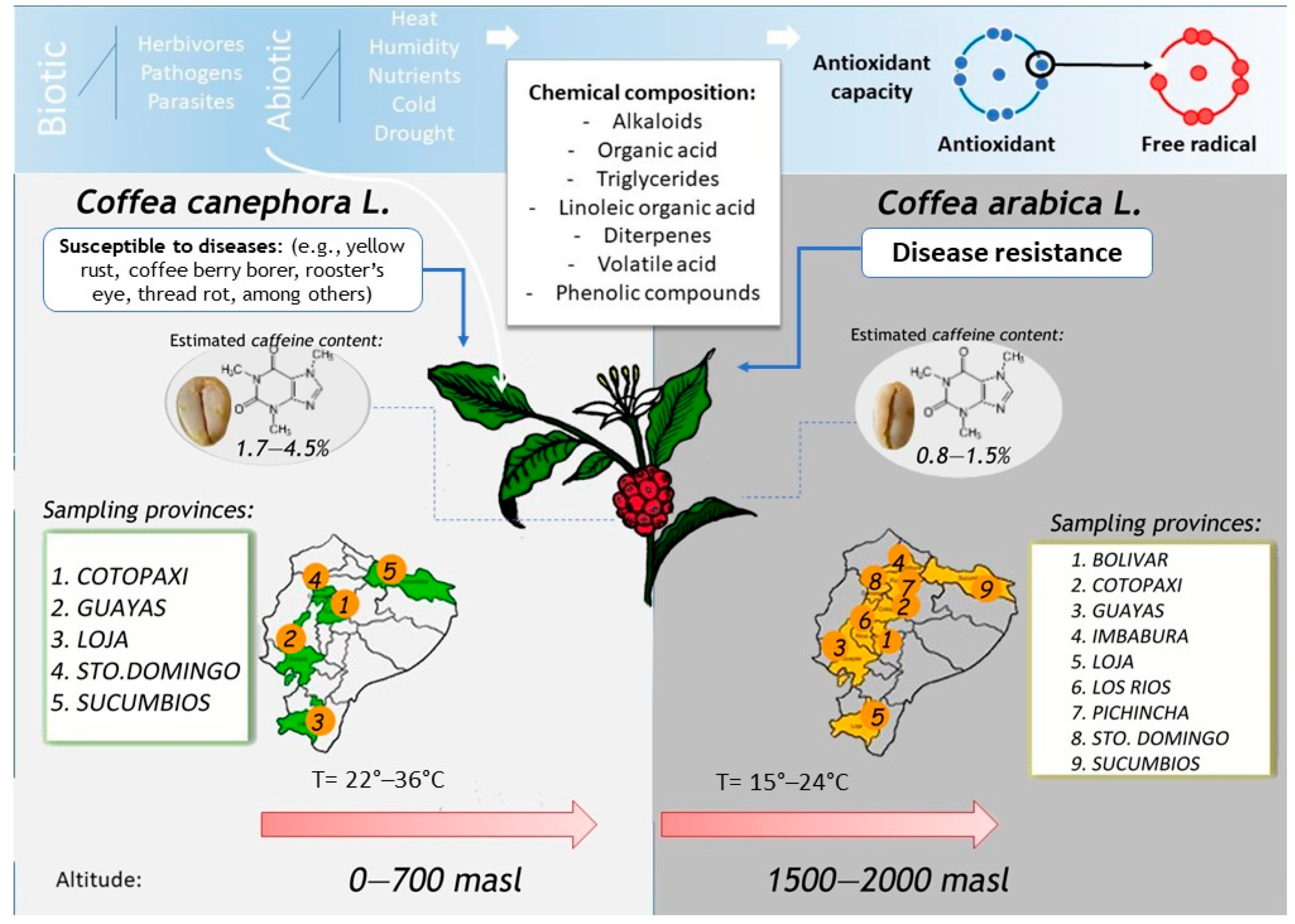
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection and Processing
2.3. Extraction of Active Ingredients
2.4. Determination of Total Phenol Content
2.5. Determination of Flavonoid Content
2.6. HPLC Quantification of Caffeine Content
2.7. Determination of Antioxidant Capacity
2.7.1. Free Radical Scavenging by Use of the DPPH Radical
2.7.2. Free Radical Scavenging through the Use of the ABTS Radical Cation
2.7.3. Ferric Reducing Antioxidant Power Assay FRAP
2.8. Sensory and Organoleptic Evaluation
2.9. Statistical Analysis
3. Results
3.1. Total Phenolic Content and Total Flavonoid Content
3.2. Determination of Caffeine Content
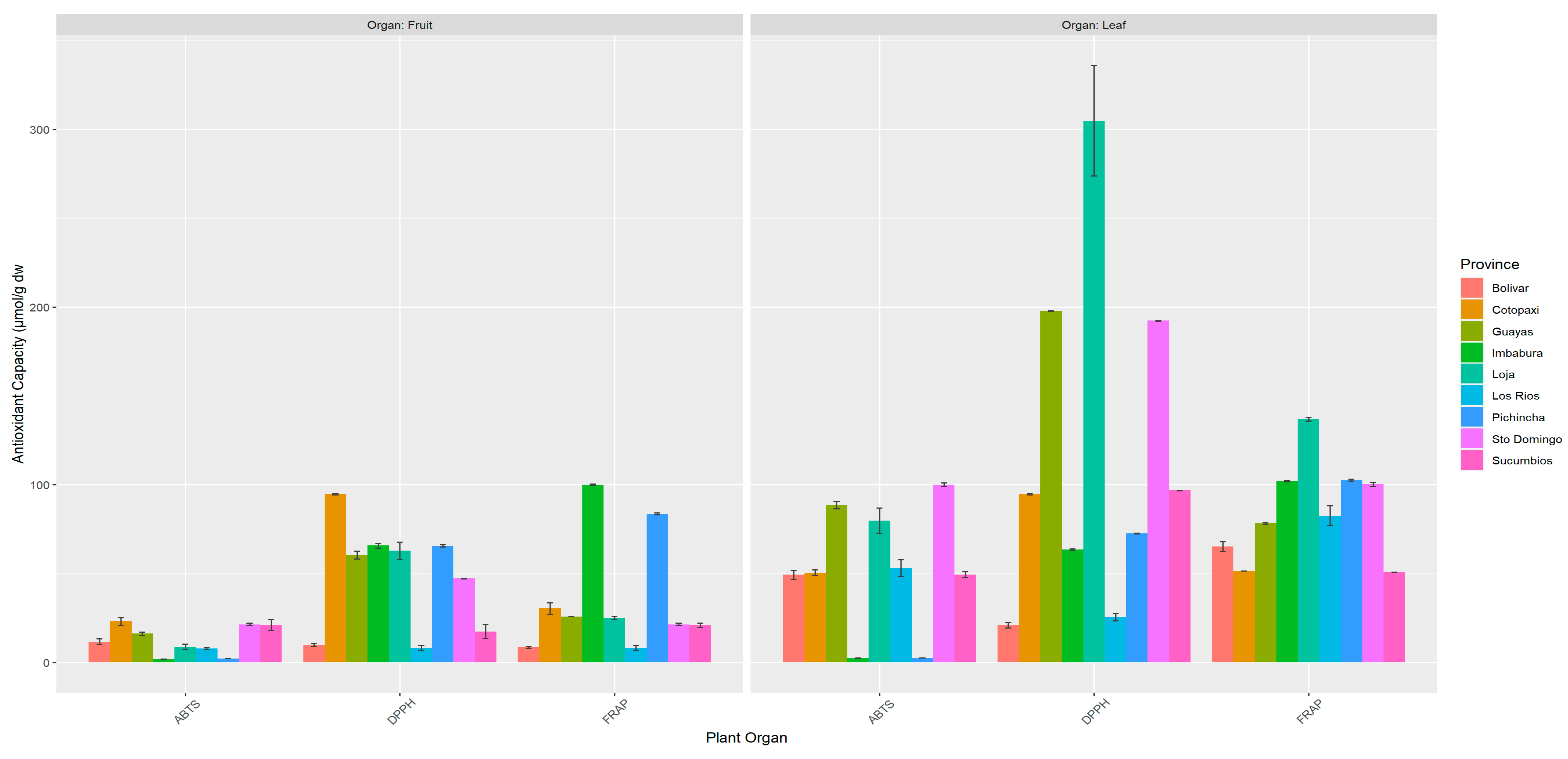
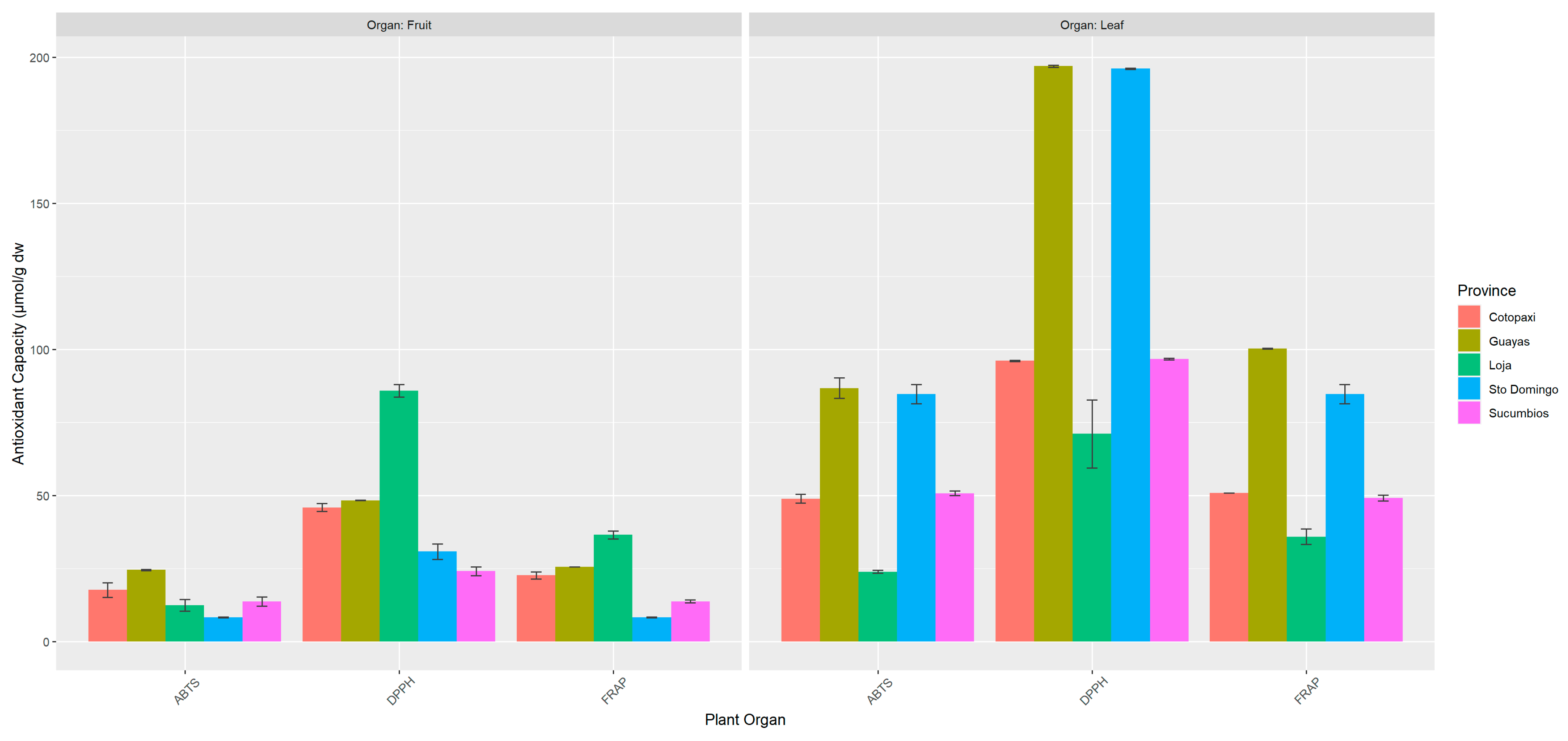
3.3. Antioxidant Capacity
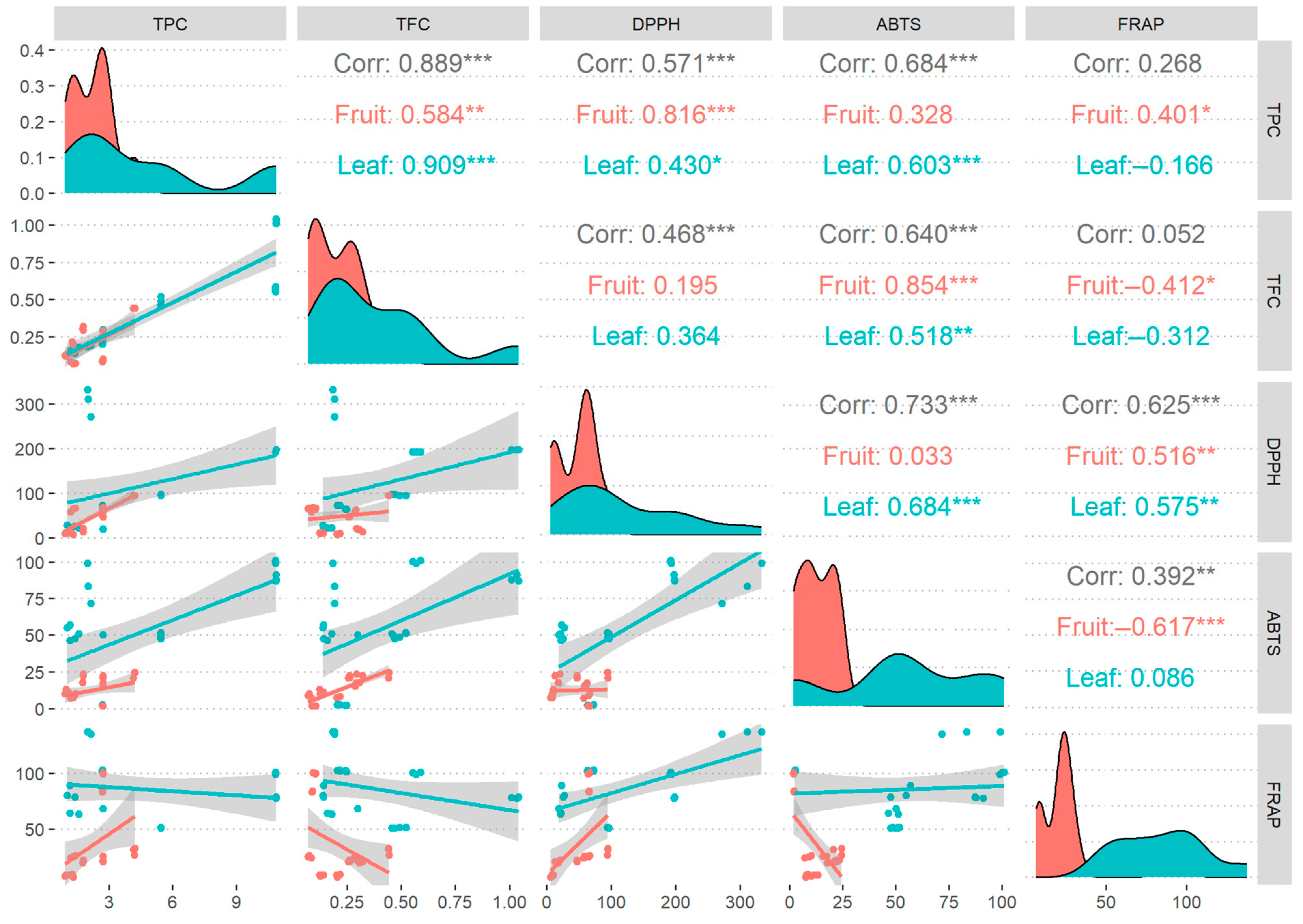
3.4. Correlation
3.5. Sensory and Organoleptic Evaluation of Raw Coffee Beans
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varela, L.A.; Ron, S.R. Geografía y Clima del Ecuador. BIOWEB. Pontificia Universidad Católica del Ecuador. 2018. Available online: https://bioweb.bio/geografiaClima.html/ (accessed on 19 December 2023).
- Gotteland, M.; De Pablo, S. Some truths about coffee. Rev. Chil. Nutr. 2007, 34, 105–115. [Google Scholar] [CrossRef]
- Ponce-Vaca, L.A.; Orellana-Suarez, K.D.; Acuña-Velásquez, I.R.; Alfonso-Alemán, J.L.; Fuentes Figueroa, T. Situación de la caficultura ecuatoriana: Perspectivas. Rev. Estud. Desarro. Soc. Cuba. América Lat. 2018, 6, 307–325. [Google Scholar]
- Guambi, L.A.D.; Cedeño, S.d.R.V.; Talledo, D.S.F. Organoleptic Quality of Arabica Coffees in Relation to the Varieties and Altitudes of the Cultivation Zones, Ecuador. Rev. Iberoam. Tecnol. Postcosecha 2017, 18, 67–77. [Google Scholar]
- Arreaga Ronquillo, E.; Quezada Campoverde, J.; Barrezueta Unda, S.; Cervantes Alava, A.; Prado Carpio, E. Economic impact generated by coffee production in Ecuador in the period 2016–2019. 593 Digit. Publ. CEIT 2021, 6, 83–91. [Google Scholar] [CrossRef]
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef]
- Carvajal Herrera, J.J.; Aristizábal Torres, I.D.; Oliveros Tascón, C.E.; Mejía Montoya, J.W. Colorimetry of the Coffee Fruit (Coffea arabica L.) during its Development and Ripening. Rev. Fac. Nac. Agron. Medellín 2011, 64, 6229–6240. [Google Scholar]
- Farah, A.; Ferreira dos Santos, T. Chapter 1—The Coffee Plant and Beans: An Introduction. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 5–10. [Google Scholar] [CrossRef]
- Beining, A.; Burkhardt, J.; Fetene, M. Water relations of Ethiopian wild coffee populations: Genetic fixation and phenotypic plasticity. In Proceedings of the 22nd International Scientific Colloquium on Coffee, Campinas, International Scientific Association on Coffee, CDROM-A105, Paris, France, 14–19 September 2008. [Google Scholar]
- INIAP. 2017 Guide to Facilitate Learning in the Management of Robusta Coffee (Coffea canephora P.). Learning Guide N° 008. Available online: https://repositorio.iniap.gob.ec/bitstream/41000/4788/7/iniapeecaga008.pdf (accessed on 2 December 2023).
- Vizuete-Montero, M.O.; Figueroa-Saavedra, H.F.; Barbaru-Grajales, A.D.; Zapata- Mayorga, H.A.; Herrera-Ocaña, H.R.; Moya, W. Physio-edaphoclimatic factors show optimal soil suitability for three tropical crops in the Ecuadorian Amazon. Sci. Agric. 2024, 81, e20220214. [Google Scholar] [CrossRef]
- Dethlefsen & Balk. Plant, Varieties and Growing Areas. 2018. Available online: https://www.dethlefsen-balk.de/ESP/10889/Kaffeepflanze.html (accessed on 20 December 2023).
- Clifford, M.N.; Willson, K.C. Coffee: Botany, Biochemistry and Production of Beans and Beverage, 1st ed.; Clifford, M.N., Ed.; Springer: Berlin, Germany; Croom Helm;: London, UK; Sydney, Australia; Westport, CT, USA, 1985; pp. 13–47. [Google Scholar]
- Gómez-Merino, F.; Trejo-Téllez, L.I.; Morales-Ramos, V.; Marín-Garza, T.; Crosby-Galván, M. Nutritional assessment of Robusta coffee (Coffea canephora) beans of different origins processed in Mexico. Agroproductividad 2018, 11, 25–29. [Google Scholar]
- Starovicova, M.; Hartemink, R. Food-Info.net: La planta de café. Food-Info: La Planta de Café. 2021. Available online: https://www.food-info.net/es/products/coffee/plant.htm (accessed on 20 November 2023).
- Cornelissen, P. Pictura Antique Prints: Antique Prints, Art, Maps and Books. Cafe Cofee Tree Coffea Arabica. 2021. Available online: https://pictura-prints.com/product/antique-print-pl-85-cafe-coffee-tree-coffea-arabica-turpin-chaumeton-1814/ (accessed on 20 November 2023).
- Vilaplana, M. Antioxidantes presentes en los alimentos. Vitaminas, minerales y suplementos. Ámbito Farmac. Nutric. 2007, 26, 79–86. Available online: https://www.elsevier.es/es-revista-offarm-4-articulo-antioxidantes-presentes-alimentos-vitaminas-minerales-13112893 (accessed on 20 December 2023).
- Wang, X.; Meng, Q.; Peng, X.; Hu, G.; Qiu, M. Identification of new diterpene esters from green Arabica coffee beans, and their platelet aggregation accelerating activities. Food Chem. 2018, 263, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Good Kitzberger, C.S.; dos Santos Scholz, M.B.; de Toledo Benassi, M. Bioactive compounds content in roasted coffee from traditional and modern Coffea arabica cultivars grown under the same edapho-climatic conditions. Food Res. Int. 2014, 61, 61–66. [Google Scholar] [CrossRef]
- Mitraka, G.-C.; Kontogiannopoulos, K.N.; Batsioula, M.; Banias, G.F.; Assimopoulou, A.N. Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response Surface Methodology Approach. Sustainability 2021, 13, 8818. [Google Scholar] [CrossRef]
- Patay, É.B.; Sali, N.; Kőszegi, T.; Csepregi, R.; Balázs, V.L.; Németh, T.S.; Németh, T.; Papp, N. Antioxidant potential, tannin and polyphenol contents of seed and pericarp of three Coffea species. Asian Pac. J. Trop. Med. 2016, 9, 366–371. [Google Scholar] [CrossRef]
- Buffo, R.A.; Cardelli-Freire, C. Coffee flavor: An overview. Flavour Frag. J. 2004, 19, 99–104. [Google Scholar] [CrossRef]
- Ahmed, S.; Brinkley, S.; Smith, E.; Sela, A.; Theisen, M.; Thibodeau, C.; Warne, T.; Anderson, E.; Van Dusen, N.; Giuliano, P.; et al. Climate Change and Coffee Quality: Systematic Review on the Effects of Environmental and Management Variation on Secondary Metabolites and Sensory Attributes of Coffea arabica and Coffea canephora. Front. Plant Sci. 2021, 12, 708013. [Google Scholar] [CrossRef]
- Buzzo Mori, A.L.; Kalschne, D.L.; Gava Ferrão, M.A.; Almeida da Fonseca, A.F.; Gava Ferrão, R.; de Toledo Benassi, M. Diterpenes in Coffea canephora. J. Food Compos. Anal. 2016, 52, 52–57. [Google Scholar] [CrossRef]
- Kaisangsri, N.; Selammasakul, O.; Sonklin, C. Phenolic Compounds and Biological Activities of Coffee Extract for Cosmetic Product. J. Sci. Engin. 2019, 1, 71–76. Available online: https://www.jstage.jst.go.jp/article/sjse/1/1/1_71/_pdf/-char/ja (accessed on 20 December 2023).
- Madaan, R.; Bansal, G.; Kumar, S.; Sharma, A. Estimation of total phenols and flavonoids in extracts of Actaea spicata roots and antioxidant activity studies. Indian J. Pharm. Sci. 2011, 73, 666–669. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Wanyika, H.N.; Gatebe, E.G.; Gitu, L.M.; Ngumba, E.K.; Maritim, C.W. Determination of caffeine content of tea and instant coffee brands found in the Kenyan market. Afr. J. Food Sci. 2010, 4, 353–358. [Google Scholar] [CrossRef]
- Guo, D.J.; Cheng, H.L.; Chan, S.W.; Yu, P.H.F. Antioxidative activities and the total phenolic contents of tonic Chinese medicinal herbs. Inflammopharmacology 2008, 16, 201–207. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pacetti, D.; Lucci, P.; Núñez, O.; Menichini, F.; Frega, N.G.; Tundis, R. Prunus persica var. platycarpa (Tabacchiera Peach): Bioactive compounds and antioxidant activity of pulp, peel and seed ethanolic extracts. Plant Foods Hum. Nutr. 2015, 70, 331–337. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Garrido-Martín, D.; Calvo, M.; Reverter, F.; Guigó, R. A fast non-parametric test of association for multiple traits. Genome Biol. 2023, 24, 230. [Google Scholar] [CrossRef]
- Osman, M.A.; Mahmoud, G.I.; Shoman, S.S. Correlation between total phenols content, antioxidant power and cytotoxicity. Biointerface Res. Appl. Chem. 2020, 11, 10640–10653. [Google Scholar] [CrossRef]
- Maurage, P.; Heeren, A.; Pesenti, M. Does Chocolate Consumption Really Boost Nobel Award Chances? The Peril of over-Interpreting Correlations in Health Studies. J. Nutr. 2013, 143, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Neto, D.P.D.; Gonot-Schoupinsky, X.P.; Gonot-Schoupinsky, F.N. Coffee as a Naturally Beneficial and Sustainable Ingredient in Personal Care Products: A Systematic Scoping Review of the Evidence. Front. Sustain. 2021, 2, 697092. [Google Scholar] [CrossRef]
- Hudáková, J.; Marcinčáková, D.; Legáth, J. Study of antioxidant effects of selected types of coffee. Folia Vet. 2016, 60, 34–38. [Google Scholar] [CrossRef][Green Version]
- Maxiselly, Y.; Anusornwanit, P.; Rugkong, A.; Chiarawipa, R.; Chanjula, P. Morpho-Physiological Traits, Phytochemical Composition, and Antioxidant Activity of Canephora Coffee Leaves at Various Stages. Int. J. Plant Biol. 2022, 13, 11. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Behne, S.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Chlorogenic and Isochlorogenic Acids in Coffee By-Products. Molecules 2023, 28, 5540. [Google Scholar] [CrossRef] [PubMed]
- Abdulmajid, A.M. Sensory Evaluation of beverage characteristics and biochemical components of Coffee Genotypes. Agric. Food Sci. 2015. Available online: https://api.semanticscholar.org/CorpusID:212469690 (accessed on 20 November 2023).
- Lemos, M.F.; de Andrade Salustriano, N.; de Souza Costa, M.M.; Lirio, K.; da Fonseca, A.F.A.; Pacheco, H.P.; Endringer, D.C.; Fronza, M.; Scherer, R. Chlorogenic acid and caffeine contents and anti-inflammatory and antioxidant activities of green beans of conilon and arabica coffees harvested with different degrees of maturation. J. Saudi Chem. Soc. 2022, 26, 101467. [Google Scholar] [CrossRef]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Phytochemical Profile and Antioxidant Capacity of Coffee Plant Organs Compared to Green and Roasted Coffee Beans. Antioxidants 2020, 9, 93. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Pacheco-Coello, F.; Rabottini-Villamizar, T. Cafeína, Compuestos Fenólicos y Actividad Antioxidante en Granos de café Orgánico (Coffea arabica var. caturra) Sometidos a Tres Tiempos de Tostado, Nirgua, Estado Yaracuy, Venezuela; Saber, Universidad de Oriente: Cumaná, Venezuela, 2022; Volume 34, pp. 20–27. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M.; Chabani, Z.; Farag, M.A.; Domínguez, R. Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications. Molecules 2021, 26, 3880. [Google Scholar] [CrossRef]
- Rajurkar, N.; Gaikwad, K.; Razavi, M.S. Evaluation of Free Radical Scavenging Activity of Justicia Adhatoda: A Gamma. Int. J. Pharm. Pharm. Sci. 2012, 4, 1–4. Available online: https://web.archive.org/web/20180413024655id_/http://ijppsjournal.com/Vol4Suppl4/3734.pdf (accessed on 20 November 2023).
- Tuncay, M.E. Comparison of Spectrophotometric Assays Determining Total Antioxidant Capacity Toplam Antioksidan Kapasiteyi Belirleyen Spektrofotometrik Yöntemlerin Karşılaştırılması. Türk Klin. Biyokim. Derg. 2019, 17, 111–119. Available online: https://tkb.dergisi.org/pdf/pdf_TKB_350.pdf (accessed on 20 November 2023).
- Mendoza Isaza, N.A.; Hoyos-Arbeláez, J.A.; Peláez-Jaramillo, C.A. Antioxidant capacity and total polyphenol content of Stevia rebaudiana stem extracts in several in vitro models. Rev. EIA 2020, 17, 1–9. [Google Scholar] [CrossRef]
- Rivas, D.; Dueñas, A.; Rodriguez, J. Secondary metabolites and antioxidant activity of wild tomatillo (Solanum pimpinellifolium L.). Rev. Ténica La Fac. Ing. 2020, 2, 26–32. [Google Scholar]
- Demianova, A.; Bobkova, A.; Lidiková, J.; Jurcaga, L.; Bobko, M.; Belej, L.; Kolek, E.; Polakova, K.; Iriondo-DeHond, A.; del Castillo, M. Volatiles as chemical markers suitable for identification of the geographical origin of green Coffea arabica L. Food Controñ 2022, 136, 3–7. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Rocha, R.B.; Espindula, M.C.; Ramalho, A.R.; Júnior, J.R.V.; Alves, E.A.; Lunz, A.M.P.; Souza, F.d.F.; Costa, J.N.M.; Fernandes, C.d.F. Amazonian Robustas: New Coffea canephora coffee cultivars for the Western Brazilian Amazon. Crop Breed. Appl. Biotechnol. 2020, 20, e323420318. [Google Scholar] [CrossRef]
- Koshiro, Y.; Zheng, X.-Q.; Wang, M.-L.; Nagai, C.; Ashihara, H. Changes in content and biosynthetic activity of caffeine and trigonelline during growth and ripening of Coffea arabica and Coffea canephora fruits. Plant Sci. 2006, 171, 242–250. [Google Scholar] [CrossRef]
- Perdani, C.G.; Pranowo, D.; Qonitatilah. Total phenols content of green coffee (Coffea arabica and Coffea canephora) in East Java. IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 012093. [Google Scholar] [CrossRef]
- Vega, F.E.; Ziska, L.H.; Simpkins, A.; Infante, F.; Davis, A.P.; Rivera, J.A.; Barnaby, J.Y.; Wolf, J. Early growth phase and caffeine content response to recent and projected increases in atmospheric carbon dioxide in coffee (Coffea arabica and C. canephora). Sci. Rep. 2020, 10, 5875. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.C.; Nicolson, S.W.; Wright, G.A. Plant secondary metabolites in nectar: Impacts on pollinators and ecological functions. Funct. Ecol. 2017, 31, 65–75. [Google Scholar] [CrossRef]
- Cotacallapa, R.E.; Lima-Medina, I.; Cornejo-Condori, G.B. Comparative organoleptic quality of coffee (Coffea arabica L.) in Puno-Peru and La Paz-Bolivia. Rev. Investig. Altoandinas 2019, 21, 283–292. [Google Scholar] [CrossRef]
- Alves, A.L.; Pessoa, M.S.; de Souza, P.E.N.; Partelli, F.L.; Moscon, P.S.; da Silva, E.C.; Guimarães, A.O.; Muniz, E.P.; Pinheiro, P.F.; Borém, F.M.; et al. Influence of Environmental and Microclimate Factors on the Coffee Beans Quality (C. 65 canephora): Correlation between Chemical Analysis and Stable Free Radicals. Agric. Sci. 2018, 9, 9082. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, S.; Banchón, C. Influence of pre- and post-harvest factors on the organoleptic and physicochemical quality of coffee: A short review. J. Food Sci. Technol. 2022, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]


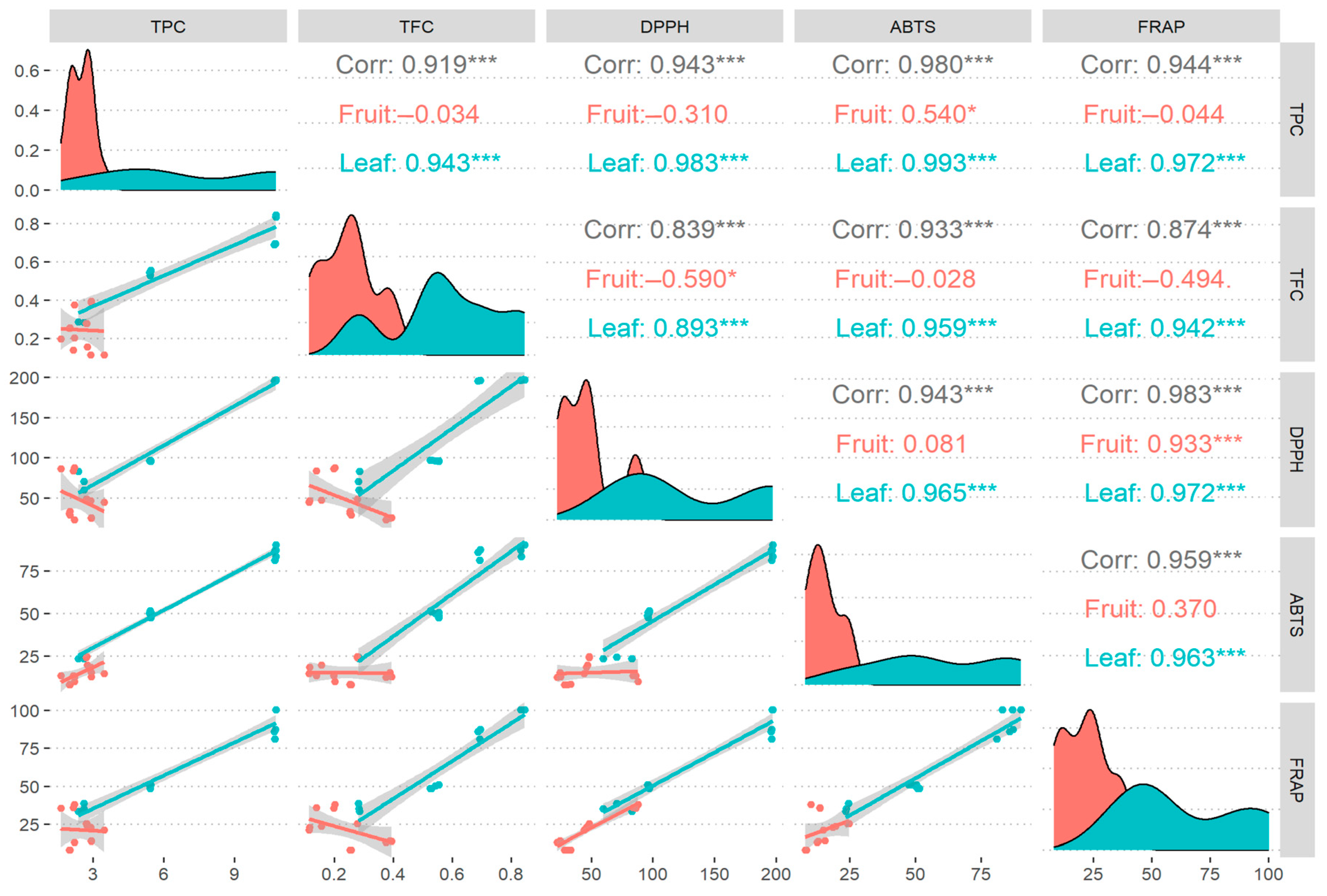
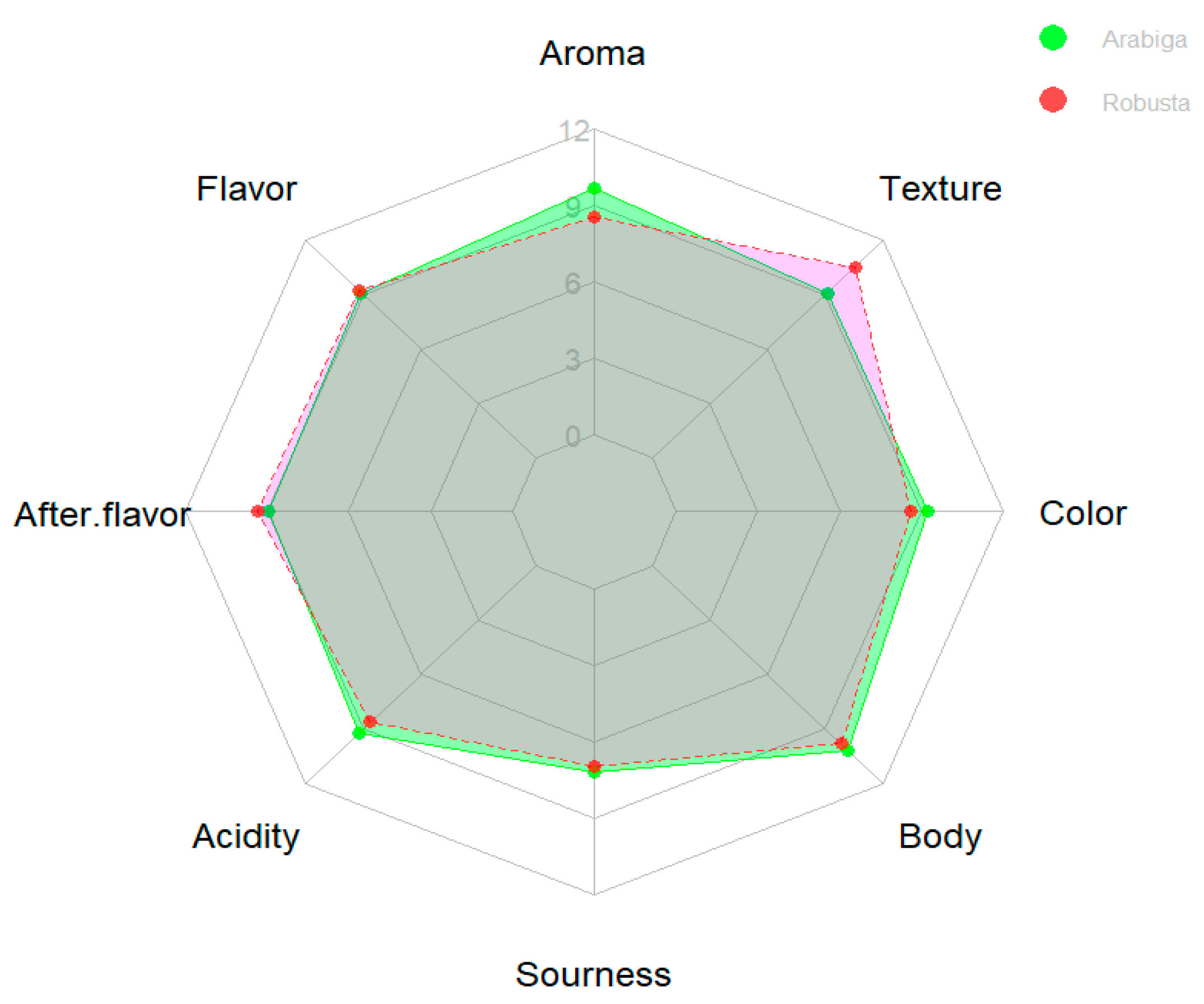
| Species | Province | Caffeine Content (mg/g dw) |
|---|---|---|
| Coffea arabica | Pichincha | 1.76 ± 0.0729 |
| Imbabura | 0.91 ± 0.00629 | |
| Sucumbios | 12.23 ± 0.52208 | |
| Cotopaxi | 13.96 ± 0.27289 | |
| Loja | 9.29 ± 0.03744 | |
| Bolívar | 13.87 ± 0.15794 | |
| Los Ríos | 15.09 ± 0.15803 | |
| Guayas | 13.38 ± 0.23911 | |
| Santo Domingo de los Tsáchilas | 3.26 ± 0.09705 | |
| Coffea canephora | Sucumbios | 10.32 ±0.02242 |
| Cotopaxi | 16.31 ± 0.10919 | |
| Loja | 18.05 ± 0.21232 | |
| Guayas | 11.61 ± 0.12294 | |
| Santo Domingo de los Tsáchilas | 1.97 ± 0.03557 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, R.A.; Ortiz-Pillajo, D.C.; Iturralde-Proaño, K.M.; Vinueza-Pullotasig, M.Y.; Sisa-Tolagasí, L.A.; Villares-Ledesma, M.L.; Melo-Heras, E.J.; Cubi-Insuaste, N.S.; Catana, R.D. Comprehensive Assessment of Coffee Varieties (Coffea arabica L.; Coffea canephora L.) from Coastal, Andean, and Amazonian Regions of Ecuador; A Holistic Evaluation of Metabolism, Antioxidant Capacity and Sensory Attributes. Horticulturae 2024, 10, 200. https://doi.org/10.3390/horticulturae10030200
Mihai RA, Ortiz-Pillajo DC, Iturralde-Proaño KM, Vinueza-Pullotasig MY, Sisa-Tolagasí LA, Villares-Ledesma ML, Melo-Heras EJ, Cubi-Insuaste NS, Catana RD. Comprehensive Assessment of Coffee Varieties (Coffea arabica L.; Coffea canephora L.) from Coastal, Andean, and Amazonian Regions of Ecuador; A Holistic Evaluation of Metabolism, Antioxidant Capacity and Sensory Attributes. Horticulturae. 2024; 10(3):200. https://doi.org/10.3390/horticulturae10030200
Chicago/Turabian StyleMihai, Raluca A., Diana C. Ortiz-Pillajo, Karoline M. Iturralde-Proaño, Mónica Y. Vinueza-Pullotasig, Leonardo A. Sisa-Tolagasí, Mary L. Villares-Ledesma, Erly J. Melo-Heras, Nelson S. Cubi-Insuaste, and Rodica D. Catana. 2024. "Comprehensive Assessment of Coffee Varieties (Coffea arabica L.; Coffea canephora L.) from Coastal, Andean, and Amazonian Regions of Ecuador; A Holistic Evaluation of Metabolism, Antioxidant Capacity and Sensory Attributes" Horticulturae 10, no. 3: 200. https://doi.org/10.3390/horticulturae10030200
APA StyleMihai, R. A., Ortiz-Pillajo, D. C., Iturralde-Proaño, K. M., Vinueza-Pullotasig, M. Y., Sisa-Tolagasí, L. A., Villares-Ledesma, M. L., Melo-Heras, E. J., Cubi-Insuaste, N. S., & Catana, R. D. (2024). Comprehensive Assessment of Coffee Varieties (Coffea arabica L.; Coffea canephora L.) from Coastal, Andean, and Amazonian Regions of Ecuador; A Holistic Evaluation of Metabolism, Antioxidant Capacity and Sensory Attributes. Horticulturae, 10(3), 200. https://doi.org/10.3390/horticulturae10030200








