Abstract
Yellowstriped armyworm (YSAW), Spodoptera ornithogalli, is a polyphagous pest that infests various crops, including cotton, cabbage, corn, blackberry, grape, etc. We documented egg clusters, larvae and adults of YSAW in pecan orchards in Georgia. Until now, there have been no reports of YSAW infesting pecan and its suitability as a host. To investigate the survival, development, and reproduction of YSAW on pecan, we used the age-stage, two sex life table. The YSAW successfully completed its lifecycle on pecan with an 82% preadult survival rate. The preadult duration and mean fecundity were 47.84 d and 1212.55 offspring per female. The population parameters, including intrinsic rate of increase (r), finite rate of increase (λ), net reproduction rate (R0), and mean generation time (T) were 0.1184 d−1, 1.1257 d−1, 430.67 offspring/female, and 51.05 d, respectively. This study confirms that pecan, Carya illinoinensis, is a potential host plant of YSAW and perhaps more widespread damage could be observed.
1. Introduction
The genus Spodoptera in the family Noctuidae consists of 31 species, several of which are significant pests of agricultural crops worldwide. Most notably, the recent invasion of fall armyworm, Spodoptera furgiperda, in many countries has renewed interest in research due to their polyphagous nature and potential damage to crops in diverse geographic regions. In North America, ten different species of Spodoptera are found [1], which include S. frugiperda (J. E. Smith), S. exigua (Hubner), S. eridania (Cramer), S. latifascia (Walker), S. praefica (Grote) and S. ornithogalli (Guenee) [2,3]. Species in the genus Spodoptera tend to have a broad host range and typically exhibit gregarious feeding behavior by moving larvae at high density from one food source to another, and therefore recognized by the name armyworm.
Although the members of the genus Spodoptera are known for their wide host range and geographic distribution, surprisingly, some of the species exhibit distinct geographic distributions and host-plant associations [2,4]. For example, several species of agricultural importance are only found in the Eastern Hemisphere, such as S. litura (Fabricius) and S. littoralis (Boisduval), which are not present in the Western Hemisphere [5]. Similarly, the two strains of S. frugiperda, one feeding on corn and another on rice, are well established within the same species [6,7]. Therefore, it is essential to note the host plant and insect association to identify any shifts in their host range for potential emerging pest issues in any given crop.
The host range of an insect species usually represents all the plant species on which individuals can feed and reproduce [8]. Report of host range for a given insect species is somewhat subjective in nature as it depends on the intensity of damage and, most importantly, if the plants are useful to humans, a crop, or ornamental. Often, the insect feeding on wild or uncultivated plants is overlooked or not studied, but new data on established insects are always added to the literature following findings in the field or under controlled conditions [9]. Finally, with the changing climatic conditions, the phenology and habitat of both herbivores and plants could also lead to the expansion of both geographic and host ranges of insect species, and new host reports are expected [10].
One of the less known Spodoptera species in the southeastern US, the yellowstriped armyworm, S. ornithogalli (Guenee) has been primarily reported from cotton, soybean, vegetables, ornamental plants, and a number of weed species [11,12]. The western counterpart of this species, the western yellowstriped armyworm, S. praefica (Grote) is, however, a pest of several crops such as lettuce, lentil, alfalfa, and berries in California, Oregon, and the pacific northwestern region of the US [13]. The distribution range of S. ornithogalli extends from Texas to the eastern US, where several studies have reported this insect feeding on cotton foliage [14]. A new host record for S. ornithogalli is also recently reported from Mexico, where infestation in commercial agave (Agave salmina) resulted in significant damage to the crop [15]. This study reports pecan, Carya illinionensis, a native nut crop to the United States that is commercially cultivated across the southern US, as another new host plant of S. ornithogalli. Pecan has never been reported as a host of S. ornithogalli in the available literature and could potentially be a case of host range expansion of this polyphagous insect. Notably, Georgia is one of the largest cotton-producing states in the nation. Pecan orchards are part of the heterogeneous crop landscape, which may provide opportunities for S. ornithogalli to interact with pecan. Following the natural infestation of pecan trees, the suitability of pecan as a host for this insect was evaluated by a life table study.
2. Materials and Methods
2.1. Insect Culture
A single egg mass (around 200 eggs) of yellowstriped armyworm (YSAW) was collected from a pecan orchard in Chula, Georgia, in April 2023. The larvae were fed on pecan leaves, and the colony was maintained in the laboratory at a room temperature of 23 ± 2 °C and a relative humidity of 60 ± 5%. A 20% sugar solution was provided for adult moths. Fresh pecan leaves (~1–2-month-old) from insecticide-free trees were collected from a pecan orchard (cv. Desirable) at Ponder Farm, Ty Ty, Georgia, for feeding purposes.
2.2. Larval and Pupal Weight
Fresh pecan leaves were continuously provided starting the first instar larvae, and the body weight was measured for the 5th- to 7th-instar larvae (n = 30) and pupae (n = 20) within 24 h of their molting from the previous developmental stage.
2.3. Life Table Study
The development, survival, and reproduction of the YSAW were studied on pecan leaves. A single cluster of 1-day eggs was transferred into a 350 mL plastic container (Fabri-Kal Corp., Piedmont, SC, USA) lined with a moist, unbleached paper towel. Immediately after egg hatching, 45 neonates were transferred individually into small 60 mL portion cups (Rikkel Corp., Brooklyn, NY, USA). Fresh leaves were provided daily to ensure enough food for the larvae and avoid food contamination. Larvae were monitored individually, and their survival and developmental data were recorded. Larval molting was confirmed by the presence of detached head capsule exuviae. After the completion of larval instars, prepupae were placed on a container filled with autoclaved garden soil. Pupae were sexed and put individually into a 540 mL plastic container (Fabri-Kal Corp., Piedmont, SC, USA) filled with autoclaved garden soil. Newly emerged adults were coupled and placed in an insect rearing cage (Megaview Science Co., Taichung, Taiwan). Paper towels were hanged inside the cage for egg laying and eggs were counted at regular intervals.
2.4. Life Table Data Analysis
The life history raw data on the development, survival, and reproduction of YSAW were analyzed as described by Chi [16] using the computer program TWO-SEX-MSChart [17]. The population parameters, including the age-stage survival rate (sxj) (x = age, j = stage), and female age-specific fecundity (fx), were calculated as described by Chi and Liu [18].
2.5. Statistical Analysis
The mean and standard error of population parameters were estimated using the bootstrap method with 100,000 resamplings [19,20]. The mean difference in body weight for male and female pupae was determined using one-way ANOVA in JMP ver. 15 (SAS Institute Inc., Cary, NC, USA). SigmaPlot v.12.5 software was used to generate all the graphs.
3. Results
3.1. Larval and Pupal Weight of Yellowstriped Armyworm
The average body weights for 5th-, 6th-, and 7th-instar larvae were 65.05 mg, 225.23 mg, and 548.15 mg, respectively. The pupal body weight was not significantly different in the male and female pupae. The average body weight for male and female pupae was 441.89 mg and 427 mg, respectively.
3.2. Development, Survival and Reproduction of Yellowstriped Armyworm
Life table parameters, including developmental time, preadult survival rate, and mean fecundity of YSAW reared on pecan are summarized in Table 1. A single egg mass was collected from the colony and eggs hatched on the same day were used for the experiment. The duration for egg hatching was three days. The mean preadult developmental period was 47.84 days and the preadult survival rate was 82%. The longevity for adult females and males was 11.81 and 8.29 days, respectively. The mean fecundity was 1212.56 eggs/female. The adult preoviposition period (APOP), total preoviposition period (TPOP), and oviposition duration were 2.19, 49.12 and 7.38 days, respectively.

Table 1.
Mean (± SE) of stage duration, preadult survival rate, adult longevity, adult preoviposition period (APOP), total preoviposition period (TPOP), and fecundity of yellowstriped armyworm fed on pecan leaves.
3.3. Population Parameters of Yellowstriped Armyworm
The population parameters of YSAW on pecan are provided in Table 2. The values of intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0), gross reproduction rate (GRR) and mean generation time (T) of yellowstriped armyworm on pecan were 0.1184 d−1, 1.1257 d−1, 430.67 offspring/female, 594.86 offspring/female and 51.05 days, respectively.

Table 2.
Population parameter of yellowstriped armyworm fed on pecan leaves.
3.4. Life Table of Yellowstriped Armyworm
The age-stage survival rate (sxj) represents the probability that a newly hatched YSAW egg will survive to age x and stage j on pecan (Figure 1). Overlaps of stage-specific survival curves during the developmental period were observed due to the variable developmental rates among individuals. The probability of newly hatched YSAW eggs surviving to adulthood was 82%. Among the adults, the longer longevity was observed in females (Figure 1).
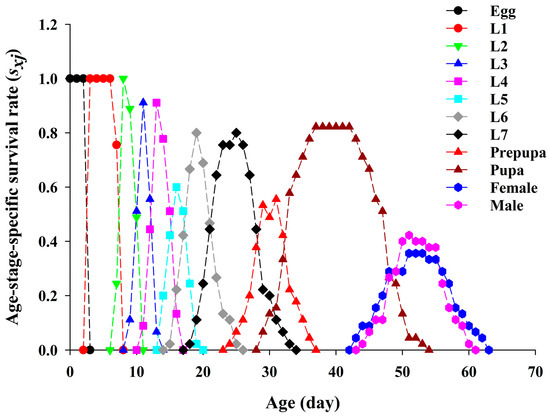
Figure 1.
Age-stage-specific survival rate (sxj) of yellowstriped armyworm fed on pecan leaves.
The age-specific survival rate (lx), the female age-specific fecundity (fx), the age-specific fecundity (mx), and age-specific net maternity (lxmx) of YSAW are shown in Figure 2. The lx curve remained 100% up to first 12 days and decreased up to 82% within the first 28 days. The fx curve is the mean number of fertilized eggs laid by the adult female at age x and the mx curve is the age-specific fecundity at age x. The highest peak of fx, and mx were observed at age 49 days (f49 = 201.92 eggs, m49 = 70.95 offspring). Similarly, the highest value for age-specific net maternity (lxmx) was observed at 49 days, with a value of 58.33 offspring.
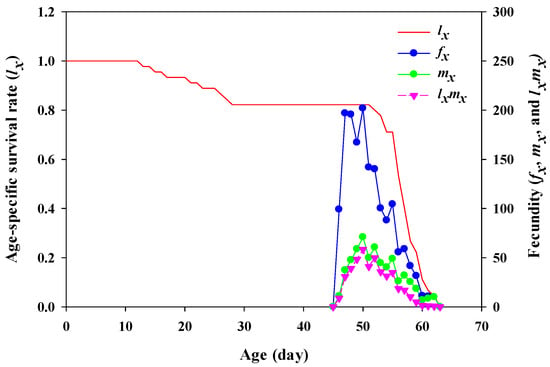
Figure 2.
Age-specific survival rate (lx), female age-specific fecundity (fx), age-specific fecundity (mx), and age-specific net maternity (lxmx) of yellowstriped armyworm fed on pecan leaves.
The age-stage specific life expectancy (exj) of YSAW represents the expected period of time for an individual at age x and stage j is expected to live when fed on pecan leaves (Figure 3). The life expectancy of newly hatched eggs, i.e., e01, was 51.18 d.
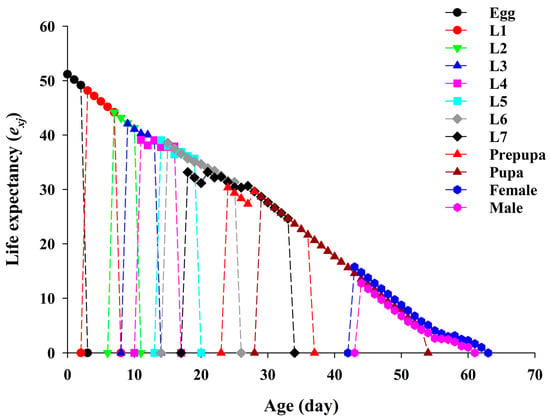
Figure 3.
Age-stage-specific life expectancy (exj) of yellowstriped armyworm fed on pecan leaves.
The age-stage specific reproductive value (vxj) of YSAW represents the contribution of an individual of age x and stage j to the future population (Figure 4). The vxj curve of adult female was sharply increased compared to other developmental stages due to the reproduction start when the adult female emerged. The highest vxj peck was observed for adult female at the age of 46 days with 979.07 eggs.
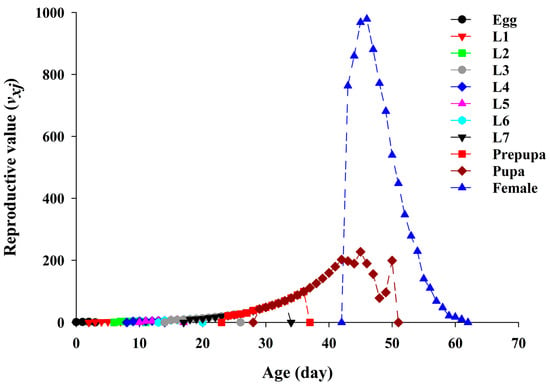
Figure 4.
Age-stage-specific reproductive value (vxj) of yellowstriped armyworm fed on pecan.
4. Discussion
The YSAW is a polyphagous pest reported from more than 200 host plants [21]. There are no reports of YSAW infestation in pecan. This is the first report that showed pecan trees could be a potential host of YSAW. Here, we investigated the biological characteristics of YSAW, including growth, development, survival, and reproduction, during feeding on pecan leaves using an age-stage, two sex life table method.
Life tables are the basis for the population ecology of insects, relative host suitability, and developing efficient pest management strategies. They provide a holistic view of insect population changes and their adaptability in various environments by encompassing crucial life-history parameters like growth, survival, developmental time, and reproduction. This knowledge is critical in developing effective pest management strategies [22].
All these life table parameters can be affected by the host plant species and environmental conditions [23,24]. YSAW successfully completed its lifecycle on pecan and the mean generation time was 51.05 d at 23 ± 2 °C. Fernández et al. [25] reported the average time for YSAW to complete their lifecycle was 33.15 days at 28.99 °C on cotton. They observed six developmental instars for the larvae. However, we observed that all the living larvae were molted to seven instars. Similarly, Bottrell [26] reported up to eight developmental instars in their laboratory population with an artificial diet. These differences in the developmental stages and time could be due to the differences in the host plant species and experimental conditions. Similar results were found in several studies in other Spodoptera species. For example, S. frugiperda has six larval instars in corn, rice and potato [23], but more developmental stages were observed when they fed on banana [27], napier grass, natal grass and sun hemp [28]. In this study, we observed an 82% preadult survival rate of YSAW, which was higher than that previously reported (29%) by Botterell [26]. The adult longevity was higher in females (11.81 days) than in males (8.21 days). Fernández et al. [25] also reported a higher longevity in adult females (13 days) than in males (11 days).
5. Conclusions
Although pecan is not the primary host for the YSAW, they completed their lifecycle in pecan and oviposited eggs on pecan trees in field conditions. These findings indicate that pecan could potentially serve as a host plant of YSAW. Pecan crops may be infested when the insect population is high or in the absence of a primary host. Therefore, it is important to prevent the YSAW from spreading to pecan, consequently reducing the potential for additional sources of outbreaks. Further comparative study on the biology, host preference, and oviposition preference between pecan and major host plant species is needed for a better understanding of YSAW population ecology.
Author Contributions
Conceptualization, R.A. and A.K.B.; methodology, R.A.; software, R.A.; validation, R.A. and A.K.B.; formal analysis, R.A.; investigation, R.A.; resources, R.A. and S.V.; data curation, R.A.; writing—original draft preparation, R.A. and A.K.B.; writing—review and editing, R.A., S.V. and A.K.B.; supervision, A.K.B.; project administration, A.K.B.; funding acquisition, A.K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by USDA-NIFA-SCRI award #2021-51181-35863 and Georgia Commodity Commission for Pecan.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We are thankful for the help and cooperation of pecan growers of the state, and county extension agents of the University of Georgia Cooperative Extension.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lafontaine, J.D.; Schmidt, B.C. Annotated Check List of the Noctuoidea (Insecta, Lepidoptera) of North America North of Mexico. Zookeys 2010, 40, 1–239. [Google Scholar] [CrossRef]
- Todd, E.L.; Poole, R.W. Keys and Illustrations for the Armyworm Moths of the Noctuid Genus Spodoptera Guenee from the Western Hemisphere. Ann. Entomol. Soc. Am. 1980, 73, 722–738. [Google Scholar] [CrossRef]
- Heppner, J.B. Spodoptera Armyworms in Florida (Lepidoptera: Noctuidae); Entomology Circular No. 390; Florida Department Agriculture Consumer Services, Devision of Plant Industry: Tallahassee, FL, USA, 1998.
- Pogue, M.G. A world revision of the genus Spodoptera Guenee (Lepidoptera: Noctuidae). Am. Entomol. Soc. 2002, 43, 1–202. [Google Scholar]
- Kergoat, G.J.; Goldstein, P.Z.; Le Ru, B.; Meagher, R.L.; Zilli, A.; Mitchell, A.; Clamens, A.L.; Gimenez, S.; Barbut, J.; Nègre, N.; et al. A Novel Reference Dated Phylogeny for the Genus Spodoptera Guenée (Lepidoptera: Noctuidae: Noctuinae): New Insights into the Evolution of a Pest-Rich Genus. Mol. Phylogenet. Evol. 2021, 161, 107161. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.P. Host-Associated Genetic Differentiation in Fall Armyworm (Lepidoptera: Noctuidae): A Sibling Species Complex? Ann. Entomol. Soc. Am. 1986, 79, 898–904. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L. The Spodoptera frugiperda Host Strains: What They Are and Why They Matter for Understanding and Controlling This Global Agricultural Pest. J. Econ. Entomol. 2022, 115, 1729–1743. [Google Scholar] [CrossRef]
- Bernays, E.A.; Chapman, R.F. Host-Plant Selection by Phytophagous Insects; Chapman & Hall: New York, NY, USA, 1994; ISBN 0412031116. [Google Scholar]
- Esquivel, J.F.; Mowery, S. V Host Plants of the Tarnished Plant Bug (Heteroptera: Miridae) in Central Texas. Environ. Entomol. 2007, 36, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.K.; Casajus, N.; Hutchinson, R.A.; McFarland, K.P.; Kerr, J.T.; Berteaux, D.; Larrivée, M.; Prudic, K.L. Climate Change and Local Host Availability Drive the Northern Range Boundary in the Rapid Expansion of a Specialist Insect Herbivore, Papilio cresphontes. Front. Ecol. Evol. 2021, 9, 579230. [Google Scholar] [CrossRef]
- King, E.W. Rates of Feeding of Four Lepidopterous Defoliators of Soybeans. J. GA Entomol. Soc. 1981, 16, 283–288. [Google Scholar]
- Capinera, J.L. Yellowstriped Armyworm, Spodoptera ornithogalli (Guenee) (Insecta: Lepidoptera: Noctuidae). University of Florida: Gainesville, FL, USA, 2001; Volume 1. [Google Scholar]
- Halfhill, J.E. Evaluation of Western Yellowstriped Armyworm (Lepidoptera: Noctuidae) as a Pest of Lentils. J. Econ. Entomol. 1982, 75, 733–735. [Google Scholar] [CrossRef]
- Armstrong, J.S.; Gore, J.; Adamczyk, J.J. Efficacy of Single and Dual Gene Cotton Gossypium hirsutum (L.) Events on Yellowstriped Armyworm (Lepidoptera: Noctuidae) in South Texas and the Mississippi Delta. Fla. Entomol. 2011, 94, 594–598. [Google Scholar] [CrossRef]
- Vega-Chávez, J.L.; Hernández, J.M.; Gonzalez-Hernández, H.; Morales-Maldonado, E.R.; Alvarado Cepeda, Y.A. First Report of Spodoptera ornithogalli in Agave Salmiana. Southwest Entomol. 2021, 46, 271–274. [Google Scholar] [CrossRef]
- Chi, H. Life-Table Analysis Incorporating Both Sex and Variable Development Rate among Individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2023; Available online: http://140.120.197.173/Ecology/Download/Twosex-MSChart.zip (accessed on 1 November 2023).
- Chi, H.; Liu, H. Two New Methods for the Study of Insect Population Ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall: New York, NY, USA, 1993. [Google Scholar]
- Huang, Y.B.; Chi, H. Age-Stage, Two-Sex Life Tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a Discussion on the Problem of Applying Female Age-Specific Life Tables to Insect Populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Brito, R.; Specht, A.; Gonçalves, G.L.; Moreira, G.R.P.; Carneiro, E.; Santos, F.L.; Roque-Specht, V.F.; Mielke, O.H.H.; Casagrande, M.M. Spodoptera Marima: A New Synonym of Spodoptera ornithogalli (Lepidoptera: Noctuidae), with Notes on Adult Morphology, Host Plant Use and Genetic Variation Along Its Geographic Range. Neotrop. Entomol. 2019, 48, 433–448. [Google Scholar] [CrossRef]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-Stage, Two-Sex Life Table: An Introduction to Theory, Data Analysis, and Application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Acharya, R.; Malekera, M.J.; Dhungana, S.K.; Sharma, S.R.; Lee, K.Y. Impact of Rice and Potato Host Plants Is Higher on the Reproduction than Growth of Corn Strain Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 256. [Google Scholar] [CrossRef]
- Du Plessis, H.; Schlemmer, M.L.; Van den Berg, J. The Effect of Temperature on the Development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 228. [Google Scholar] [CrossRef]
- Fernández, L.S.; Fernández, C.R.; Mejía, J.E. Life Cycle of Spodoptera ornithogalli (Guenée) in Cotton in the Mid Sinu Valley. Rev. Temas Agrar. 2004, 9, 30–36. [Google Scholar] [CrossRef][Green Version]
- Bottrell, D.G. Rearing and Dynamics of Laboratory Populations of the Yellow-Striped Armyworm, Prodenia ornithogalli Guenee, and Relationship of Its Native Parasites with Sympatric Heliothis spp.; Oklahoma State University: Stillwater, OK, USA, 1968. [Google Scholar]
- Zhou, S.; Qin, Y.; Wang, X.; Zheng, X.; Lu, W. Fitness of the Fall Armyworm Spodoptera frugiperda to a New Host Plant, Banana (Musa nana Lour.). Chem. Biol. Technol. Agric. 2022, 9, 78. [Google Scholar] [CrossRef]
- Chen, W.H.; Itza, B.; Kafle, L.; Chang, T.Y. Life Table Study of Fall Armyworm (Spodoptera frugiperda) (Lepidoptera: Noctuidae) on Three Host Plants under Laboratory Conditions. Insects 2023, 14, 329. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).