1. Introduction
Climate change has caused a notable escalation in average global temperatures, with them rising by approximately 1.1 °C since the pre-industrial era [
1]. Furthermore, heat waves have become increasingly intense, frequent, and prolonged worldwide [
2]. Almost all of the hottest years on record, except one, have occurred since 2000 [
3]. From 1950 to 2017, most regions worldwide experienced at least one additional heat wave per decade [
4]. Projections indicate that the upward trajectory of rising temperatures and the occurrence of more frequent, longer, and more intense extreme heat events will persist in the future [
5]. By the end of this century, an estimated 1.2 billion individuals could be impacted by heat stress, which is four times the current number affected, and by the year 2045, around 75% of the world’s existing food production is expected to encounter severe risks due to elevated temperatures [
6].
Elevated temperatures induce various morpho-anatomical alterations in plants, impacting seed germination, plant growth, flower shedding, pollen viability, gametic fertilization, fruit set, fruit weight, and quality [
7]. Variations in the daily mean maximum and minimum temperatures negatively impact vegetable farming, as most of the physiological, biochemical, and metabolic processes in plants are influenced by temperature [
8]. Heat stress can induce a range of irreversible damages to plant metabolism and development [
9,
10], impeding various physiological and biochemical processes [
11]. These include the inhibition of photosynthesis [
12], stomatal conductance [
13], and reduction in leaf chlorophyll content [
14], leading to the production of malondialdehyde [
15].
In Egypt, tomatoes (
Solanum lycoperscon L.) were ranked as the most popular vegetable crop in terms of cultivated area and total production. They cover approximately 160,000 hectares, accounting for 28% of the overall vegetable area. In 2019, Egypt produced 6.75 million tons of tomatoes, and the nation ranks fifth in tomato production globally, following China, India, Turkey, and the USA [
16,
17,
18]. Tomato fruits have health benefits due to their rich mineral content, including potassium and antioxidants such as vitamins A, C, lycopene, and tocopherol [
19].
Tomatoes grow perfectly at temperatures between 21 and 25 °C and will be negatively impacted at temperatures above 27 °C or below 16 °C. Metabolic processes in tomato plants are negatively impacted by excessive temperature [
20], as the elevated temperature causes significant yield losses in productivity and quality. Exposing plants to high temperatures leads to bud drops, abnormal flower development, viability reduction, and carbohydrate shortage [
21]. Symptoms of heat stress in tomatoes include sunburn, disruption of lycopene synthesis, the appearance of yellow areas in the affected tissues, poor fruit sets, ripening delay, yellow-shouldered fruit, white cores, and blossom-end rot [
22]. The optimum temperature for developing the lycopene pigment in tomato fruit is between 16 and 26 °C [
23]. Lycopene degradation begins above 27 °C and breaks down at 40 °C [
23]. Similarly, temperatures above 25 °C significantly impact pollination and fruit set [
24]. In addition, heat stress inhibits ripening by mitigating the accumulation of ripening-related mRNAs, thereby inhibiting continuous protein synthesis, including ethylene production, lycopene accumulation, and cell-wall dissolution [
25].
Hormonal imbalances are responsible for the weak metabolic performance of tomato plants under heat stress [
26]. The application of exogenous plant growth regulators has been studied as a potential method to alleviate the adverse impacts of heat stress, as these substances actively participate in plant responses and mechanisms that provide physiological protection against high temperatures [
27,
28]. Cytokinins play a role in mediating plant responses to abiotic stress [
29], as they delay senescence, maintain chloroplast activity, decline chlorophyll degradation, and enhance protein and nucleic acid synthesis. Notably, 6-Benzylaminopurine (BAP) is a first-generation synthetic cytokinin that elicits plant growth and development responses, setting blossoms to increase fruit quality and inhibit the respiratory kinase in plants [
30,
31]. The application of cytokinins in tomato plants has been reported to improve plant height, dry matter content, chlorophylls content, carotenoid content, vitamin C content, total organic acid content, catalase enzyme activity and peroxidase enzyme activity of the fruit [
32]. It also improved plant resistance to bacterial and fungal pathogens [
33,
34].
In consequence, the main objective of this investigation is to study the effect of the foliar applications of BAP (6-Benzylaminopurine) concentrations on tomato crops cultivated in the most heated months in Egypt. We hypothesize that BAP will enhance tomato growth by protecting the physiological and metabolic processes, increasing yield productivity and fruit quality.
2. Materials and Methods
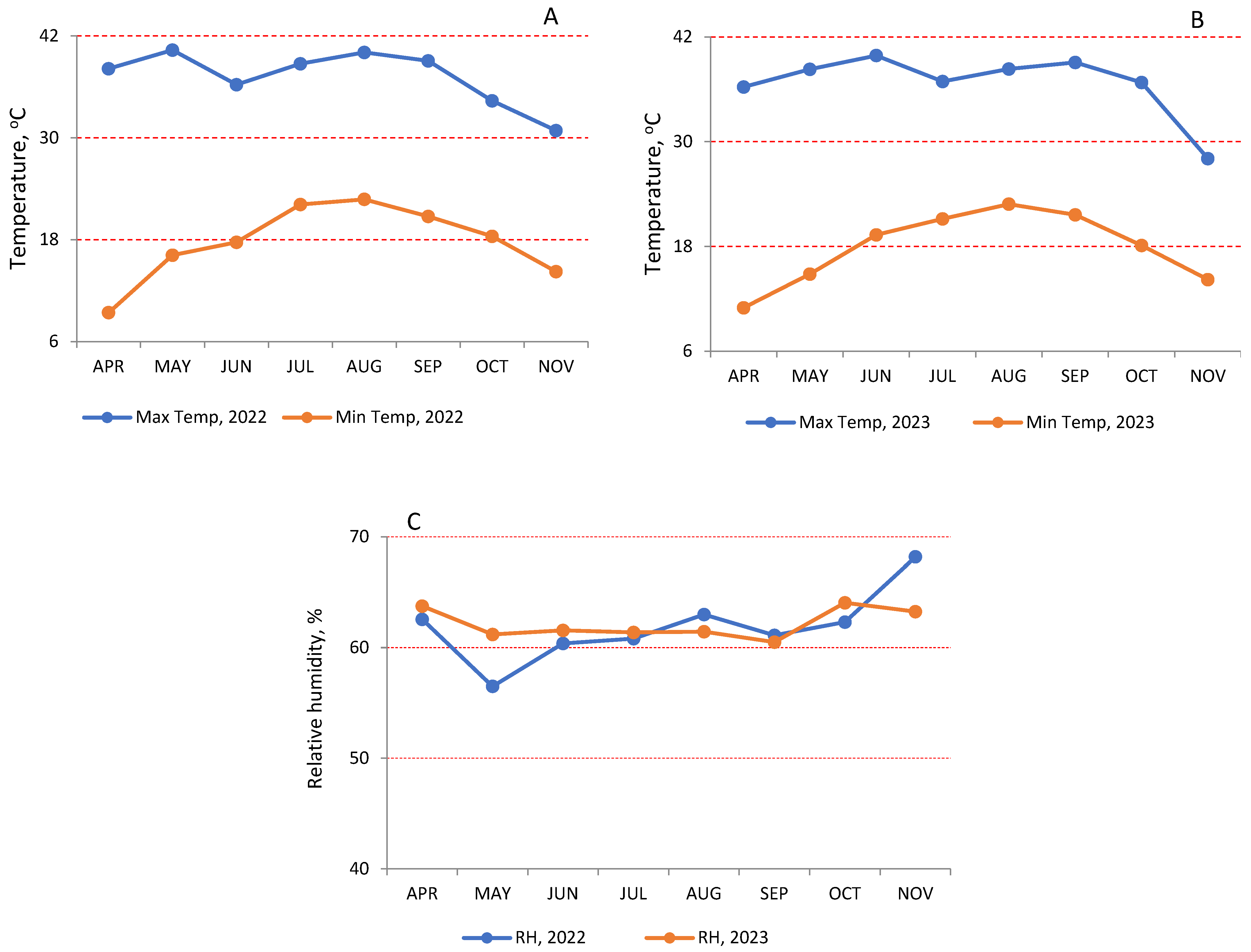
The meteorological data: Based on data compiled by The World Bank for Climate Change knowledge, Egypt experiences a predominantly arid climate characterized by dryness and high temperatures (
Figure 1). The summer season (May–September) is sweltering and dry. Inland desert regions of Egypt witness significant temperature fluctuations, with daytime temperatures reaching as high as 43 °C during summer and receiving very little annual precipitation [
35]. In both seasons (2022 and 2023), the average air temperature during the growing season (June–September) exceeded more than 35 (
Figure 1A,B). In August 2022 and June 2023, the temperature reached more than 40 °C, while in September (both seasons), the temperature settled at 39 °C. The minimum temperatures were above 18 °C during the period of experimentation (June–October) in both seasons (
Figure 1A,B). This period recorded no precipitation, as Egypt is in arid zone conditions. The relative humidity (RH) registered less than 65% during the whole period of the experiment in both seasons (
Figure 1C).
The experiment site and design: The experiment was conducted in open fields during the late summer seasons of 2022 and 2023 at a research farm affiliated with the National Research Center, Nubareyah area, Beheira Governorate, Egypt. Firstly, tomato seeds were sown in trays containing a mixture of peat moss and vermiculite (1:1) enriched with macro and micronutrient elements. Trays were kept in the greenhouse, and standard agricultural practices for tomato seedling production were conducted. Seedlings at 35 days old were transplanted in the open field on 7 June 2022 and 9 June 2023. Irrigation and pest management were applied as recommended by the Egyptian Ministry of Agriculture [
36]. Plants were fertilized with N at 230, P
2O
5 at 45, and K
2O at 70 kg/feddan (0.42 ha). A drip irrigation system was used, and the flow rate of the drip irrigation drippers was four liters/hour. Each treatment had a separate sub-main line with a control valve so that it could be irrigated separately.
The experimental design involved a split plot. The main plots were the three tomato cultivars (Castlerock, GS 12-F1, and Fayrouz F1), while the subplots consisted of three concentrations of BAP (0, 300, 600 ppm) (2% 6-Benzylaminopurine). The BAP solution was applied four times throughout the season to the cultivated plants using a hand-pressure atomizer 30 days after transplanting (DAT) at intervals of 15 days. Each replicate consisted of three rows that were 15 m long and 1.5 wide. The seedlings were transplanted in two lines on the rows and 50 cm between plants within the row, and the distance between the lateral irrigation lines was 1.5 m.
2.1. Growth and Yield Components
Samples of nine plants from each plot were randomly taken at 30, 60, 90, and 120 days after transplanting (DAT), and the following characteristics were recorded: plant height (cm), number of the lateral branches/plant (Pcs), and number of leaves/plant (Pcs). For measuring flowering and fruiting, three plants were randomly labelled from each plot to record the following parameters: number of flowers/plant (Pcs), number of clusters/plant (Pcs), number of fruits/plant (Pcs), average fruit weight (g), fruit yield/plant (kg), total yield (ton/ha), and marketable yield percentage (%).
2.2. Physio-Biochemical Attributes
The following parameters were measured at 90 DAT: chlorophyll content (chlorometer), acidity, total soluble solids (refractometer), and ascorbic acid content (vitamin C). Ascorbic acid was measured via methods described by the Association of Analytical Communities, particularly the 2,6-dichlorophenol indophenol titration method, and expressed as mg·100 g
−1 FW [
37]. To measure titratable acidity (TA), tomato fruits were homogenized with distilled water and then titrated with 0.1 N NaOH. The results, expressed as citric acid content, have been calculated by the following formula:
where N is the normality of NaOH, 0.0064 is the conversion factor for citric acid, V is the volume of NaOH (mL), and m is the sample weight (g). The maturity index (sugar-to-acid ratio) was calculated by dividing the total soluble solid by the titratable acidity of the given samples under analysis, as described by Abdelkader [
37]. The taste index was calculated using the formula [
38]:
2.3. Antioxidant Enzyme Assays
Proline: 2 mL of proline extract, 2 mL of acid ninhydrin, and 2 mL of glacial acetic acid were added and incubated for one hour in a boiling water bath followed by an ice bath. The absorbance was measured at 520 nm using Shimadzu 240 UV/VIS, Tokyo, Japan. A standard curve was obtained using a known concentration of authentic proline [
39].
Total phenolics: The phenolic contents were determined using a spectrophotometric method [
40]. Phenolics were extracted by ethanol 80% and estimated by adding 1 mL of sample and 70 mL distilled water, followed by a Folin–Ciocalteau reagent and 15 mL of saturated sodium carbonate solution, incubated at room temperature for 30 min and measured at 765 nm in a spectrophotometer. Gallic acid was used to make the calibration curve.
Total antioxidant activity: The stock solution was prepared by dissolving 24 mg 1,1-diphenyl-2-picrylhydrazyl (DPPH) with 100 mL methanol and then stored at 20 °C until needed. The solution was obtained by mixing 10 mL stock solution with 45 mL methanol to absorb 1.1 ± 0.02 units at 515 nm. Extracts (750 μL) were allowed to react with 1500 μL of the DPPH solution for 5 min in the dark. Then, the absorbance was taken at 515 nm. The standard curve was linear between 25 and 800 μmol Trolox [
41].
Enzyme extraction and assay: Antioxidant enzymes were extracted from fresh leaf material (0.5 g) using a mortar and pestle in ice-cold K-phosphate buffer (pH = 7) containing 0.1 mM EDTA. The homogenate was centrifuged at 45,000× g for 15 min in the cooled centrifuge (0 °C), and the supernatant was used to determine the antioxidant enzyme activities with a spectrophotometer (Shimadzu 240 UV/VIS, Japan).
Peroxidase assay (PO): The assay mixture was performed with a pyrogallol mixture prepared according to the Sigma Manual, and it contained 0.32 mL of pyrogallol, 0.16 mL of 147 mM-H
2O
2, phosphate buffer pH 6.5, 2.1 mL H
2O, and 0.1 mL of enzyme extract. The change in absorbance was continuously monitored at 420 nm. One unit of enzyme activity was calculated as a 0.001/min change in absorbance [
42].
Polyphenol oxidase assay (PPO): The assay mixture was performed with 2.8 mL from a 100 mM potassium phosphate buffer (pH 6.0) containing 1.3 mM pyrogallol and 100 μL of crude extract at room temperature. The increase in absorbance was monitored continuously for 3 min at 430 nm. One unit was determined as the amount of the enzyme that caused a change of 0.001/min in absorbance [
42].
Superoxide dismutase assay (SOD): Enzyme activity was determined by measuring the inhibition in photo reduction of nitroblue tetrazolium (NBT) by a SOD enzyme [
42]. The reaction mixture contained 50 mM sodium phosphate buffer (pH 7.6), 0.1 mM EDTA, 50 mM sodium carbonate, 12 mM L-methionine, 50 μM NBT, 10 μM riboflavin, and 100 μL of crude extract in a final volume of 3.0 mL. The control reaction was performed without crude extract. The SOD reaction was conducted by exposing the reaction mixture to white light for 15 min at room temperature. After 60 min incubation, absorbance was recorded at 560 nm using a spectrophotometer. One unit (U) of SOD activity was defined as the amount of enzyme causing 50% inhibition of photochemical reduction of NBT under assay conditions [
42].
Statistical analysis: The recorded data were statistically processed using the analysis of variance (ANOVA). The means were compared using Duncan’s multiple range test and the Least Significant Difference (LSD) at the level of probability
p ≤ 0.05% [
43].
4. Discussion
Hormones naturally exist in plant tissues, while plant growth regulators (PGRs) are considered exogenous substances applied to control the outcome of plant growth and develop or mitigate the impact of environmental stresses on cultivated crops [
44,
45]. Cytokinin (CKs) are vital in promoting plant growth and stabilizing photosynthetic processes during stress. Exogenous application of CKs can mitigate plant disorders caused by various abiotic stresses [
46,
47]. Regarding this matter, benzylaminopurine (BAP) refers to a synthetic PGR known as a cytokinin, which is frequently utilized in agricultural practices [
48] for managing the growth and development of plants cultivated under abiotic stress conditions such as excessive temperature [
49].
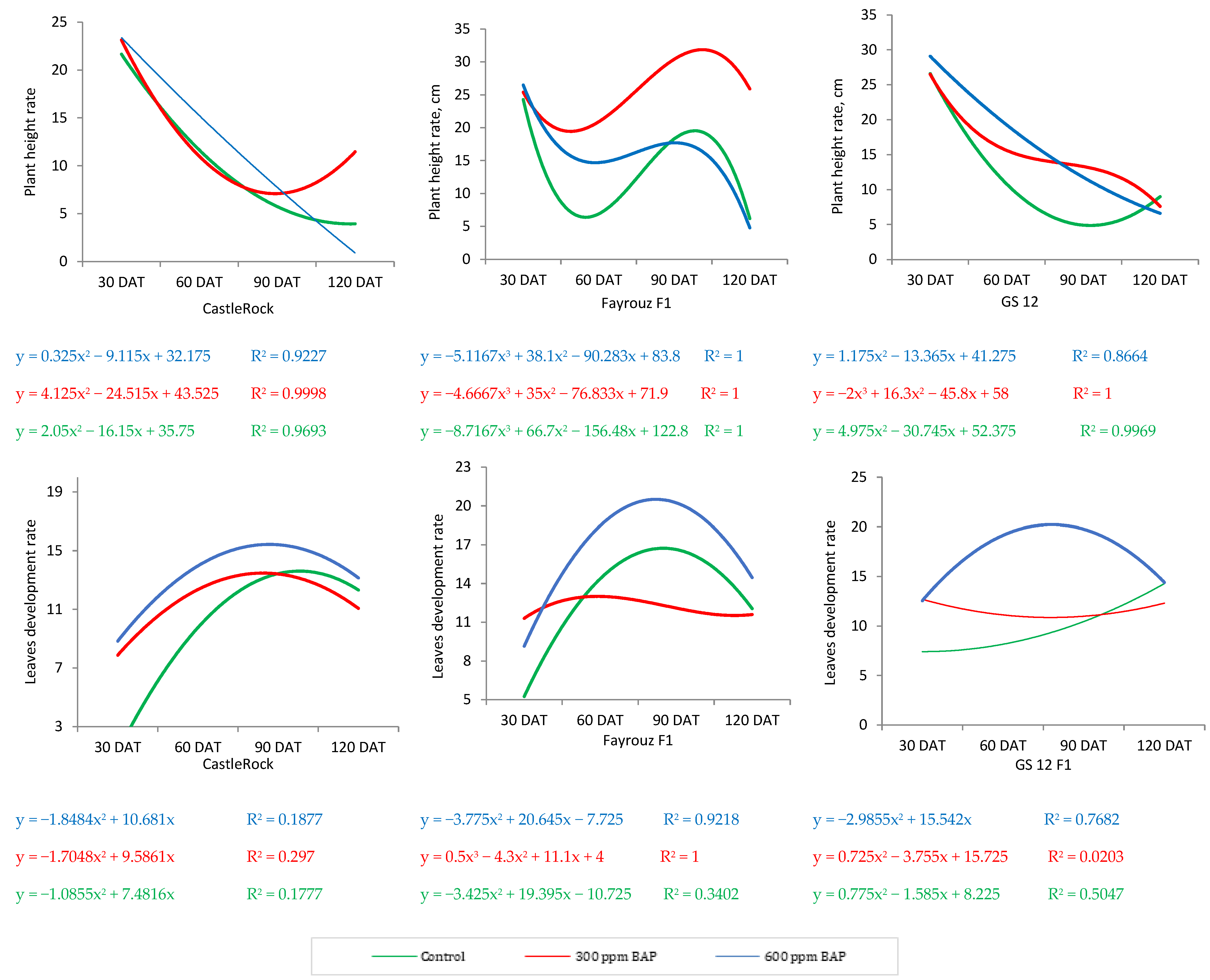
In the present investigation, the BAP application improved the growth and development of tomato plants by accumulating metabolites and inducing the antioxidant activities of the cultivated tomato genotypes. As the primary growth, spraying BAP (600 ppm) on vegetative parts of tomato genotypes increased growth rates such as plant height and NLP (
Figure 6), which indicates biological improvements compared to non-treated plants. According to our results, BAP induced growth improvement was dependent on tomato genotype. For instance, compared to the Castlerock and Fayrouz genotypes, the GS 12 hybrid showed higher biomass accumulation (plant height, number of leaves, and stem diameters). In this regard, various research studies showed variations in growth and development among different genotypes [
50,
51]. PGRs (BAP) probably enhanced the overall capability of the cells to produce essential organic compounds through biosynthesis [
52,
53].
Furthermore, promoting leaf pigment contents (chlorophyll) in tomato leaves exposed to BAP explains the dynamic growth and development metrics. Here, the BAP application enhanced the total chlorophyll and carotenoids as evidence of the photosynthesis process efficiency under heat stress conditions. Similar to our findings, cytokinin application improved photosynthetic pigments [
50,
54]
PGRs enhance flowering, ageing, and fruit development. Cks may play vital roles in regulating tomato fruit growth and development. Exogenous application of the synthetic CK (Forchlorfenuron) promotes fruit set percentage and fruit development [
55,
56]. These findings are in line with our study outputs. We can observe that applying 600 ppm of cytokinin (Benzylaminopurine) enhanced fruit development and weight, reflecting the total tomato yield per hectare. Through combining with the maximum dose of BAP, the GS 12 genotype registered the highest yield component parameters, except the marketable yield percentage found in the Castlerock genotype, compared to the other Fayrouz F1 and GS 12 F1 genotypes. The high levels of cytokinin in tomato fruits are involved in delaying the ripening process, while the lower levels of cytokinin increase the ripening rate [
57,
58].
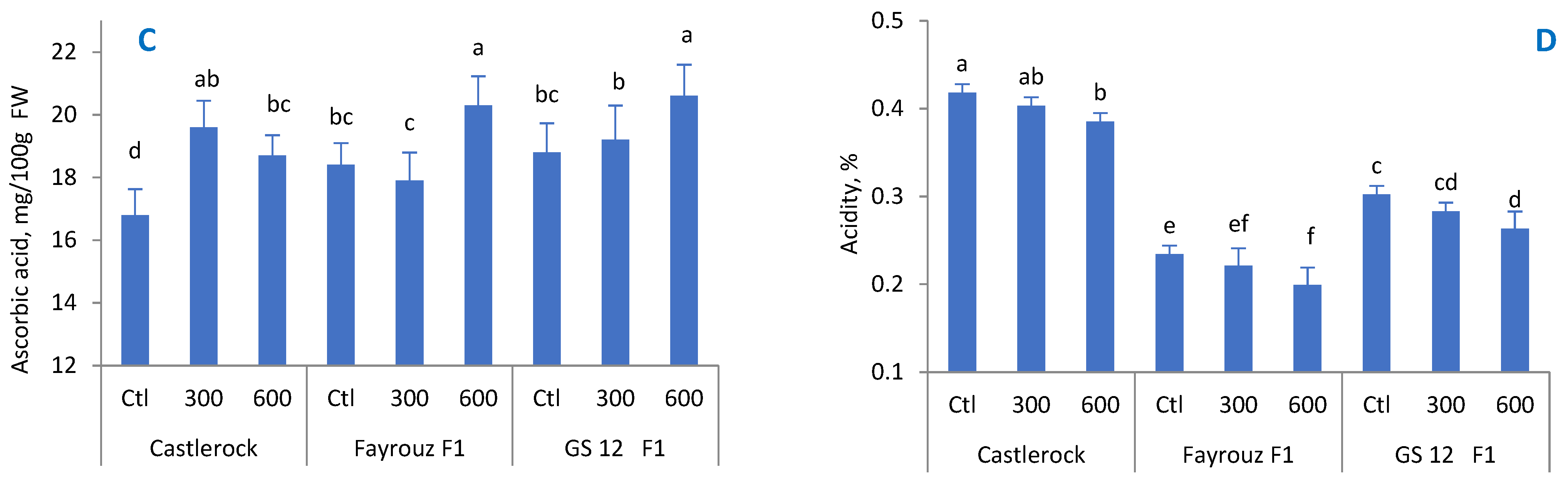
Abiotic stresses affect the metabolic processes in tomato fruit [
59]. For example, water deficit promotes metabolites, while vitamin C and carotenoids are significantly reduced when tomato plants are exposed to heat stress during fruit formation stages [
60,
61]. In this regard, our results indicate that tomato fruit decreased along without BAP application under heat stress, while application of 600 ppm BAP altered the impact of exceeded temperatures. The results are similar to those reported by other researchers who showed a variation in fruit quality and quantity depending on the cultivated variety and environmental conditions [
62,
63,
64,
65]. High temperature conditions could result in an increase in the TSS content in tomato fruits [
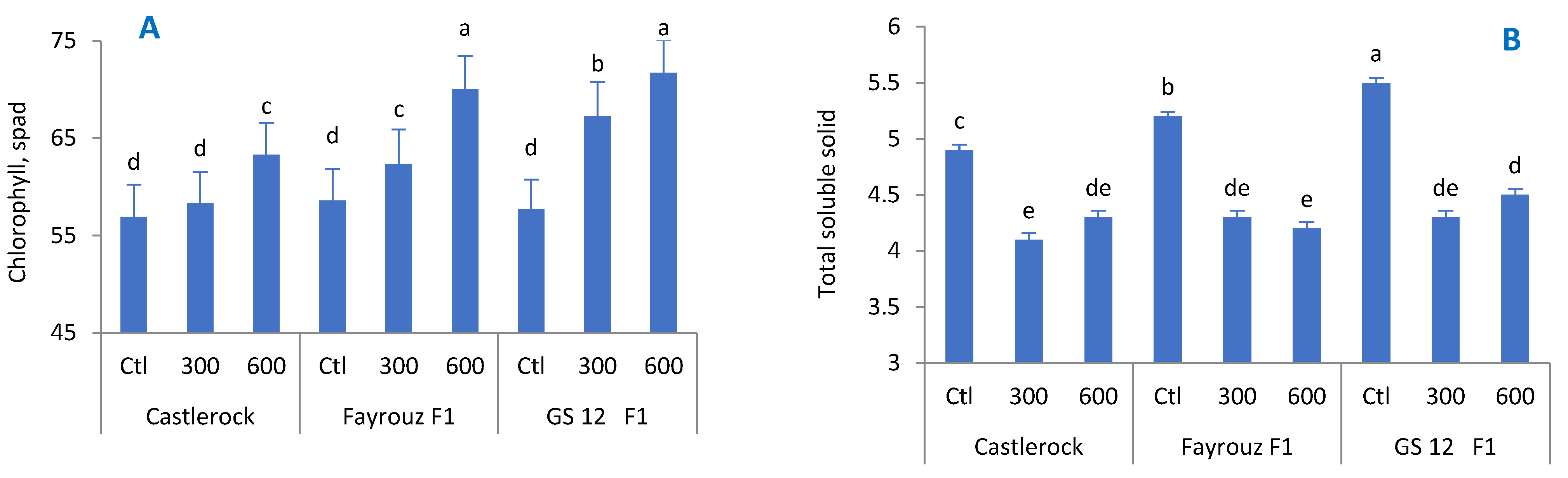
66]. The reduction in the TSS of treated tomato fruits compared to the control group could be a result of slowing down respiration and metabolic activity [
67]. High temperatures increase the loss of fruit moisture through transpiration, which, in turn, increases the TSS percentage content [
68,
69]. BAP treatment enhanced the ability of tomato plants to withstand high temperatures and preserve fruit moisture, which led to lower TSS percentages compared to the untreated control.
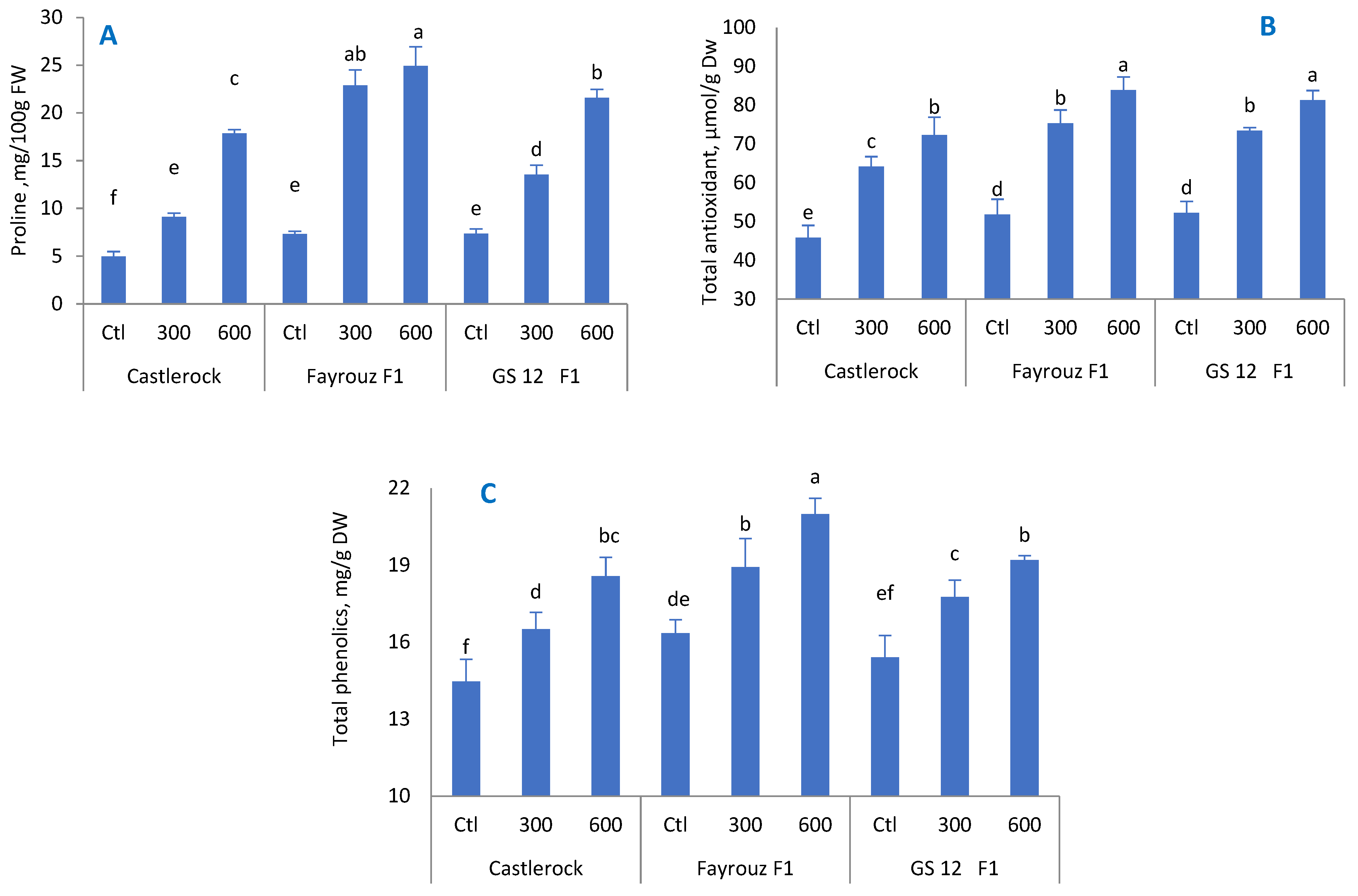
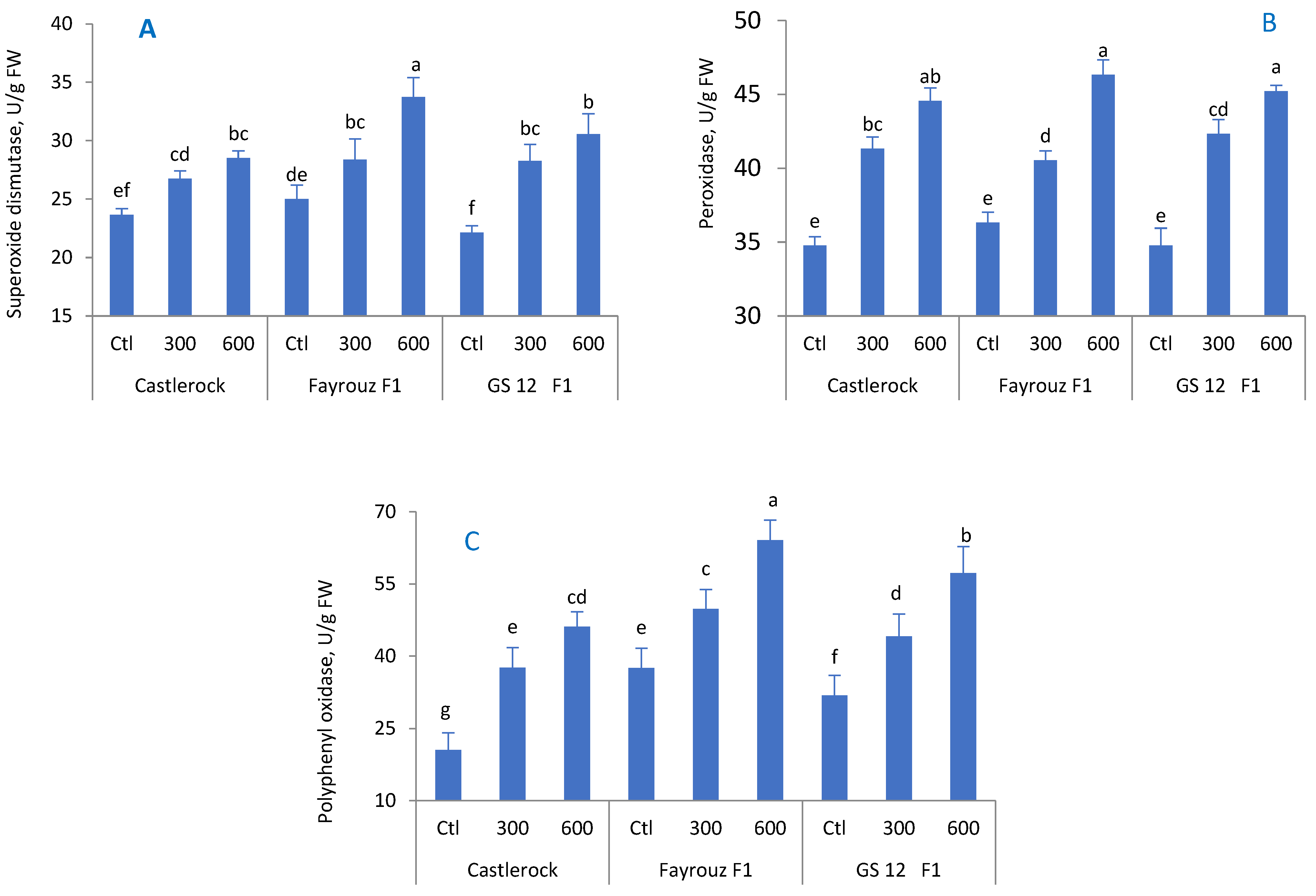
The positive effect of BAP treatment on total phenolic content, total antioxidant activity, and total proline content was also reported in tomatoes [
70], eggplants [
71], and cherry tomatoes [
72]. Exogenous BAP has been found to enhance plant tolerance to stress [
73]. Phenolic compounds are secondary plant metabolites that work as antioxidants and protect cell components during stressful conditions [
74,
75,
76]. When plants are exposed to high temperatures, they actively accumulate organic or inorganic substances, such as proline, to reduce the cellular osmotic potential and maintain the structural stability of cell membranes to counteract the harmful effects of heat stress on plants [
72]. Increasing proline content helps plants regulate the cells’ osmotic potential, improving water absorbance and translocation under stress [
77]. Similar findings reported that treating plants with BAP at ten μM L
−1 significantly enhanced the enzymatic antioxidant activities, such as ascorbate peroxidase, superoxide dismutase, catalase, and peroxidase, in tomatoes and eggplants [
70,
71,
78]. Plants have defensive mechanisms that consist of antioxidants with enzymatic or non-enzymatic activities to cope with oxidative damage and reduce abiotic stress damage [
79]. PPO, POD, and SOD scavenge superoxides, which in turn produces H
2O
2 and O
2, resulting in decreasing oxidative damage [
70,
80].