Abstract
The continued increase in human populations and use of chemical fertilizers remain a threat to the health and stability of human–ecological systems worldwide. To ameliorate this problem and achieve long-term food security, a variety of ecofriendly technologies have been developed, including the production of cyanobacteria-based biofertilizers. This technology can be optimized through experiments that assess how plant growth is enhanced under different biofertilizer concentrations (g L−1). In this study, the biofertilizer capabilities of various concentrations of sonicated biomass (0, 2.5, 5, 10, 20, and 40 g L−1) derived from the cyanobacteria Arthrospira maxima on the growth of basil (Ocimum basilicum, Lamiaceae) were assessed, comparing their effectiveness with that of a positive control, a commercial biofertilizer (OptiMar Algas Marinas®) administered at 4 mL L−1. Generally, increased concentrations led to enhanced growth parameters; however, discernible differences from the negative control (0 g L−1) were often observed only when concentrations exceeded 5 g L−1. Surprisingly, the negative and positive controls often yielded similar results. A chemical composition analysis of A. maxima revealed high concentrations of the phytohormones, macronutrients, and essential amino acids that likely explain how our A. maxima sample enhanced growth in basil. Further research is required to determine how other crop plants respond to different concentrations of A. maxima. Additionally, assessing the feasibility of creating an economically accessible product with a higher concentration of A. maxima is crucial for practical applications.
1. Introduction
Global human populations continue to rise and are projected to reach 9.8 billion by 2050, a trend that emphasizes the critical need for sustainable solutions to long-term food security [1]. Current large-scale agricultural practices rely on the application of chemical fertilizers to enhance crop productivity, but this boon is inexorably linked with negative impacts on soil biodiversity, human health, and other downstream effects [2]. Recently, biologically based products derived from microalgae have attracted considerable attention as alternatives to chemical fertilization [3] and as sources of a wide variety of bioactive compounds with medical, cosmetic, and nutritional applications [4,5,6]. Limiting the large-scale cultivation of microalgae are the high costs of production, harvesting, and downstream processing [7], which can be ameliorated through complete utilization of biomass [6] and optimization of biofertilizer concentrations upon application. This study contributes towards this optimization through an assay aimed at investigating the impact of escalating concentrations of sonicated cyanobacterial biomass on the growth of basil seedlings (Ocimum basilicum, L.).
Microalgae, which includes prokaryotic cyanobacteria and eukaryotic microorganisms such as green algae, are common inoculants in biofertilizers, along with mycorrhizal fungi, rhizobacteria, and nitrogen-fixing rhizobia [8]. Within this group, cyanobacteria known commercially as Spirulina (Arthrospira maxima and Arthrospira platensis) stand out as the most widely produced microalgae with a long history of use as a food resource in Mesoamerica and Africa [9]. As a result, their potential applications as biofertilizers, biostimulants, and biopesticides are well-documented [10]. Depending on the objective, Spirulina (or other microalgae) is applied as dry, fresh, or liquid biomass to soil or leaves [11]. For example, as living inoculants in soil, Spirulina continually release a variety of signaling molecules that produce quantitative and qualitative changes to crop phytochemical composition, as well as contributing to nutrient cycling through N-fixation [12]. As dry or liquid lysed biomass, the beneficial organic (phytohormones, polyphenols) and inorganic content (macronutrients and microelements) of Spirulina are more immediately available for plant uptake [13]. Among lysing methods, ultrasonication stands out as a procedure with potential for large-scale, sustainable application, as it shears cell walls and membranes in a time-efficient and chemical-free manner [14]. In addition to the choice of lysing method, lysed biomass must be diluted to a cost-effective concentration that may vary in response to the crop to which it is being applied.
While Spirulina has already been documented as an effective biofertilizer and biostimulant in a variety of crop plants [15,16,17], a recent study by [18] showed a lack of response by basil plants to 1 g L−1 of Arthrospira nodosum hydrolysate. The lack of response could be attributed to the low concentration. This argument is supported by a previous study involving active dry yeast as a biofertilizer, where increasing concentrations yielded enhanced growth in basil [19]. Given the economic significance of basil for its culinary and medicinal properties [20], this study aimed to investigate the potential for stimulating basil growth using sonicated biomass of A. maxima and assess its response to varying concentrations of this biomass (2.5, 5, 10, 20, and 40 g L−1). As a positive control, the effects of a commercial biofertilizer derived from a brown alga, Ascophyllum nodosum (L.) biomass at a recommended dose of 4 mL L−1 were tested. A negative control with 0 g L−1 of A. maxima (Setchell and N.L.Gardner) Geitler biomass was also included. It was hypothesized that biometric responses of basil seedlings to biomass concentrations would follow a linear response. To complement this assay, the chemical composition of the A. maxima biomass was provided, determined through elemental, bromatological, and phytohormonal analyses, as well as an aminogram.
2. Materials and Methods
2.1. Preparation of Cyanobacterial Biomass
Arthrospira maxima was cultured in open ponds located and managed by the Universidad de Córdoba in Montería, Colombia. These open ponds (3000 L) were maintained at a pH level ranging between 10 and 11, included a timed air injection system to guarantee air exchange, and contained Zarrouk’s medium, a specialized nutrient solution designed for microalgae cultivation [21]. Cyanobacterial biomass was harvested after 15 days of growth and dewatered using a membrane filter with 40–50 µm pore size. Filtered biomass was sun-dried for 36 h on aluminum trays, which are exposed to an average solar radiation of 4400 wh m−2 and temperatures ranging between 26 and 28 °C [22]. Lastly, using an electric mill (CGoldenwall CNA 679, Cgoldenwall, Fengzhen, China) the dried biomass was pulverized to produce a fine powder of 4–50 µm particle size.
After conducting a preliminary experiment to assess the efficacy of three different cell lysis methods (Table S1; Figures S1–S3), ultrasonication of an aqueous solution of biomass at 40 g L−1 was selected. This method involved homogenizing biomass solutions using an Ultrasonic Homogenizer TF-500N (TEFIC BIOTECH Co., Ltd., Xi’an, China) (400 W) for 15 min with 10 s operating intervals. It is important to note that comparable soluble protein values can be attained within a 10 min timeframe, reducing operational costs.
2.2. Characterization of Cyanobacterial Biomass
Characterization of the chemical composition of A. maxima was analyzed through bromatological, elemental, phytohormonal analyses, and an aminogram.
The obtained powder was analyzed for moisture, protein, ash, and lipid contents according to the Association of Official Analytical Chemists (AOAC) [23]. The protein content was determined by the Kjeldahl method (AOAC 981.10) using a conversion factor of 6.25, whereas the moisture content was evaluated by a gravimetric heating of the sample at 120 °C until constant weight (AOAC 934.06). The ash content was determined by calcination in an oven at 550 °C until reaching constant weight (AOAC 942.05). The lipid content was determined by Soxhlet extraction with ether (AOAC 922.06). The carbohydrate content was calculated from the subtraction of all compounds from 100%. All the analyses were performed in triplicate.
The bromatological analysis included estimating protein, carbohydrate, and total fat content, as well as ash content and humidity. Protein content was estimated following the AOAC 981.10 method, which involves a series of chemical reactions to determine the nitrogen content in a sample as a proxy for proteins [24]. Total fat content was measured according to AOAC 954.02, which involves the use of solvent extraction to isolate fat from the sample. The carbohydrate content was calculated from the subtraction of all compounds from 100%. Ash content was measured according to AOAC 935.42, which involves incinerating the sample, causing all organic components to burn off, leaving behind inorganic minerals (ashes). Lastly, humidity was measured according to AOAC 926.08 by weighing the sample before and after heating the sample until a constant weight was achieved. All the analyses were performed in triplicate. Caloric content was estimated following the Awater general factor system using carbohydrate, protein, and lipid content.
A phytohormonal analysis was conducted using a high-performance liquid chromatography (HPLC) system (Agilent, Santa Clara, USA), composed of a Series 200 UV–Vis detector (Agilent, Santa Clara, USA), binary gradient pump, and vacuum degasser, employing a methanol–water 1:1 mixture method. Types and concentrations of phytohormones in the sample were determined by comparing the retention times and peak patterns of sample components with those of known reference compounds. To precisely identify the peaks, a Hamilton HxSil C18 (Hamilton, Reno, USA) column was employed, and elution was programmed in reverse phase using acetonitrile and a 0.2% phosphoric acid solution as mobile phases [25]. The phytohormones included as standard solutions were as follows: zeatin, gibberellic acid, kinetin, indole-3-acetic acid, 6-benzyladenine, indole butyric acid, 2,4-dichlorophenoxyacetic acid, and 1-naphthaleneacetic acid.
For the total quantification of nitrogen, phosphorus, and potassium present in the ultrasonicated extract of A. maxima, various protocols were implemented. The determination of total nitrogen was carried out using the SM-4500-Norg-B methodology for organic nitrogen and SM-4500-NH3-B for ammoniacal nitrogen. Additionally, the determination of total phosphorus was performed using the ascorbic acid method specified in the SM-4500-PE methodology [26]. Lastly, the determination of potassium was conducted following the AOAC 985.35 methodology.
The amino acid profiling of the sonicated biomass was conducted using liquid chromatography with an Agilent series 1200 HPLC system equipped with a diode array UV/Vis detector (DAD) and an Agilent series 1200 automatic injector, utilizing Chemstation Rev B.04.01 data software. The analysis employed an Analytical Zorbax Eclipse AAA-C18 column (Agilent, Santa Clara, CA, USA) measuring 4.6 × 150 mm with 5 µm particle size, and a precolumn of 4.6 ID 12.5 mm. Column temperature was maintained at 40 °C with a pressure of 132 bar using mobile phase A, 40 mM NaH2PO4 buffer at pH 7.8, and mobile phase B, a mixture of ACN/MeOH/water (45:45:10 v/v/v). Prior to injecting the biomass into the HPLC system, the sample was hydrolyzed with 5 mL of 6N hydrochloric acid and 0.1% phenol to degrade proteins. To detect primary amino acids following liquid chromatography, an injection volume of 25 µL (of which 0.5 µL corresponded to the hydrolyzed solution), a flow rate of 2.0 mL min−1, a runtime of 26 min, and a wavelength of 338 nm were used [27]. Amino acids are reported as µmol of amino acid per gram of dry biomass.
2.3. Shade House Experiment
A total of 128 basil seeds were sown in two 64-hole seedling trays (5.5 × 28 × 45 cm) in a shade house located in CES University in Medellín, Colombia. The tray substrate consisted of a peat-moss-based growing media (PRO-MIX PGX®) (Premier Tech Horticulture, Quebec, QC, Canada) that was sterilized by autoclave (121 °C for 30 min) prior to seeding. Trays were subjected to a 12 h photoperiod at 23–25 °C and relative humidity between 63.5 and 76.4%. An automated irrigation system provided moisture through micro-aspersion at 6 AM, 1 PM, and 3 PM every day for five minutes.
After 14 days, 63 seedlings had germinated (49.2%). These seedlings were then partitioned into seven groups (nine seedlings per group) and assigned experimental treatments. Five groups were assigned to receive concentrations of sonicated, A. maxima biomass (2.5, 5.0, 10.0, 20.0 and 40.0 g L−1). One group was designated the negative control (0 g L−1). The seventh group was designated a positive control, and assigned to receive 4 mL L−1 of OptiMar Algas Marinas® (Vellsam, Tabernas, Spain), a commercially available biofertilizer containing a brown alga, Ascophyllum nodosum, extract. Every 10 days for 50 days, all seedlings received 1 mL of their assigned treatment solution through a micropipette aimed at the leaves closest to the stem to allow the solution to drip into the substrate. Ten days after the last treatment application, all 63 seedlings were harvested to measure biomass metrics.
Stem length (cm) was measured manually immediately post-harvest using an electronic caliper. Prior to measuring root length (cm), roots were rinsed with tap water to remove the attached substrate. Following rinsing, each seedling’s root was photographed using a Canon Powershot 540 HS (Canon, Tokyo, Japan) for the purpose of measuring the longest root in each photograph using the SmartRoot plugin from the ImageJ Software (Version 1.53k ) (Version 1.53k). Fresh seedling mass (g) was obtained by weighing seedlings on a digital analytical balance (Ohaus PX224/E, OHAUS, Latinoamérica, México D.F.). Leaf area (cm2) was estimated using the length and width of leaves. Lastly, the total number of leaves and nodes present on the seedling upon harvest were counted. To obtain dry seedling mass (g) and dry root mass (g), seedlings were dried in an oven (Memmert UN110, Memmert GmbH + Co. KG, Schwabach, Germany) at a temperature of 65 °C for 48 h and weighed again using the same analytical balance.
2.4. Statistical Analysis
All statistical tests were carried out using R [28]. Differences in plant biometrics were compared through analyses of variance (ANOVA) on linear models. Pairwise comparisons were conducted using the Dunn–Šidák correction to adjust for multiple comparisons using the ‘multcomp’ package [29]. An additional set of generalized linear models were created to correct for non-normality of residuals and possible outliers (Poisson log-link for count-based variables and gaussian log-link for measurement-based variables). Differences among linear and generalized models are largely negligible, so, following a parsimonious approach, only the former are presented. Linear models were also created with treatments as numerical rather than factorial variables to estimate the slope of increase by g L−1 of sonicated biomass.
3. Results
3.1. Shade House Experiment Responses
Arthrospira maxima sonicated biomass application stimulated growth in basil seedlings (Figure 1). Fresh seedling mass increased by 0.074 g ± 0.004 (standard error) with each g L−1 of sonicated biomass. Seedlings that received the commercial product at 4.0 mL L−1 did not differ from those that received 5 or 2.5 g L−1 or from the control (t = 2.102, p = 0.563; t = 0.966, p = 0.999; t = −1.036, p = 0.999, respectively), but were lighter than those that received 10 g L−1 or above (p < 0.05). Seedlings that received the highest dose of sonicated biomass (40 g L−1) were 7.5× heavier than the control (t = 15.463, p < 0.001). Similarly, dry seedling mass increased by 0.007 g ± 0.0004 with each additional g L−1 of sonicated biomass, with the highest dose yielding seedlings being 8.4× heavier than the control (t = 13.841, p < 0.001). The commercial product’s effects on dry mass did not differ from a dose of 5 or 2.5 g L−1, or from control (t = 2.071, p = 0.589; t = 1.586, p = 0.926; t = −1.245, p = 0.994, respectively), but were lighter than those that received 10 g L−1 or above (p < 0.05). The responses by stem and root (fresh and dry mass) reflect a similar pattern and are available in Figure S4.
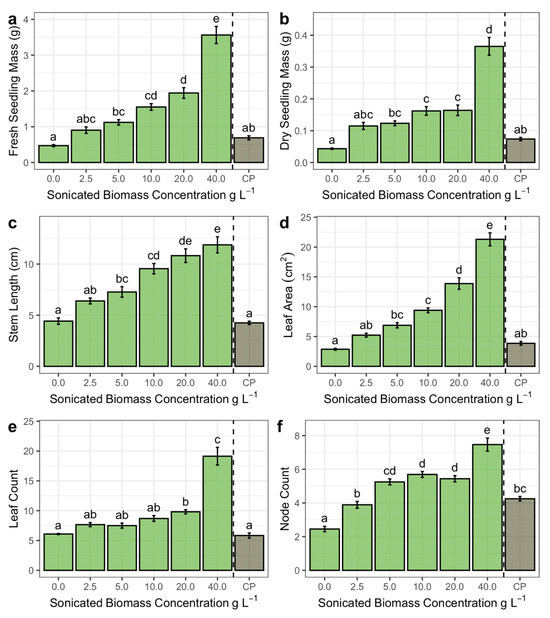
Figure 1.
Mean (a) fresh seedling mass, (b) dry seedling mass, (c) stem length, (d) leaf area, (e) leaf count, and (f) node count in response to A. maxima sonicated biomass concentrations. The commercial product (CP) refers to OptiMar Algas Marinas® at the recommended dose of 4 mL L−1. Error bars represent standard error. Different letters above error bars represent statistically significant differences (p < 0.05).
Stem length increased by 0.18 cm ± 0.019 with each additional g L−1 of sonicated biomass. Stems from seedlings that received the highest dose were 2.7× longer than stems from control seedlings (t = 9.300, p < 0.001). The 20 g L−1 and 40 g L−1 groups did not differ in stem length (t = 1.464, p = 0.965). Stems from seedlings that received the commercial product were similar in length to stems from control seedlings (t = 0.208, p = 1.000) and seedlings that received 2.5 g L−1 (t = 2.406, p = 0.323), but shorter than stems from seedlings that received 5.0 g L−1 (t = 3.665, p = 0.009) or above (p < 0.05). Leaf area increased by 0.455 cm2 ± 0.021 per g L−1 of sonicated biomass, reaching an area that was 7.4× larger in the 40 g L−1 group over the control (t = 18.041, p < 0.001). The leaf area from seedlings that received the commercial product did not differ from seedlings that received 5 or 2.5 g L−1 or from the control (t = 2.872, p = 0.104; t = 1.210, p = 0.996; t = 0. 921, p = 0.999, respectively), but was smaller than those that received 10 g L−1 or more (p < 0.05).
The estimated slope for final leaf count in response to sonicated biomass concentration was 0.32 ± 0.02. However, leaf count did not differ among the control and those that received 2.5, 5, 10 g L−1 or the commercial product (p > 0.05). The group that received 20 g L−1 did differ from the control (t = 3.57, p = 0.013) and commercial product groups (t = 3.913, p = 0.004), with 1.6× more leaves than both. Notably, the 40 g L−1 group differed significantly from all other groups (p < 0.05), with a mean of 19.13 leaves, 3.1× more than the control group. The slope for node count by sonicated biomass concentration was 0.09 ± 0.008. The control group showed the fewest nodes (mean = 2.45), significantly fewer than all other groups (p < 0.05). The commercial product did not differ from the 2.5 or 5 g L−1 groups (t = 0.980, p = 0.999; 2.931, p = 0.088, respectively) but did show fewer nodes than groups that received 10 g L−1 or above (p < 0.05). The 5, 10, and 20 g L−1 groups did not differ in node count (p > 0.05). The 40 g L−1 differed significantly from all other groups (p < 0.05) and showed 3× more nodes than the control group.
3.2. Characterization of A. maxima
In the bromatological analysis of a sonicated biomass sample of A. maxima (40.0 g L−1), mean protein content was 64.46% (standard error = ±0.92). Total carbohydrates accounted for 24.58%, while the mean fat content was minimal at 0.14% (±0.05). The remaining components included mean ash at 5.21% (±0.03) and humidity at 5.61% (±0.08). Total calories in the sample were 357.42 Kcal 100−1 mL−1.
The M Macronutrient composition of the 40 g L−1 sample of sonicated biomass was defined by a nitrogen content of 0.44%, 36.62 mg of potassium, 30.15 mg of phosphorus, and 3.98 mg of magnesium per 100 mL. Macronutrient composition was also analyzed in a dry sample of A. maxima and in the commercial biofertilizer, OptiMar Algas Marinas®, presented in Table 1.

Table 1.
Mean and standard error of macronutrient composition of A. maxima sonicated biomass, dry biomass, and a commercial product, OptiMar Algas Marinas®. Note that the macronutrients in the sonicated biomass represent the contents in a diluted solution of 40 g L−1.
The phytohormone analysis showed the presence of gibberellic acid (0.19 g L−1), 6-benzyladenine (0.37 g L−1), and 1-naphthaleneacetic acid (0.25 g L−1). Notably, this analysis did not detect zeatin, kinetin, indole-acetic acid, indole butyric acid, and 2,4-dichlorophenoxyacetic acid among the tested phytohormones.
The aminogram analysis conducted on the sonicated biomass revealed concentrations of various amino acids summarized in Table 2. Aspartate (17.2%) and arginine (16.6%) were the most abundant amino acids in the biomass sample, followed by glutamate (15.7%) and glutamine (14.2%). Notably, methionine and phenylalanine were not detected in the sample.

Table 2.
Amino acids detected in A. maxima sonicated biomass.
4. Discussion
The biofertilizers, biostimulants, and biopesticides sourced from cyanobacterial biomass offer a sustainable pathway to achieve long-term food security while minimizing environmental impact. To pave this pathway, it is imperative to reduce the costs of production, harvesting, and processing, and perfect the complete utilization of biomass [6,7]. Regarding the latter, understanding the relationship between cyanobacterial biomass concentrations and plant growth can facilitate cost–benefit analyses leading to optimized application methods, ensuring efficient nutrient delivery to crops.
4.1. Shade House Experiment
This study demonstrated that increasing concentrations of A. maxima sonicated biomass, when applied to basil seedlings through foliar application, significantly enhanced their growth. Specifically, higher concentrations of biomass yielded taller seedlings, more plant mass, more leaf area, and higher leaf and node counts. Having a greater leaf area in basil is crucial, as leaves are the most economically important part of this plant [20]. These results stand in contrast to findings by [18], who reported that 1 g L−1 hydrolysate showed negligible effects on basil growth. Together, these studies underscore the significance of examining the relationship between biomass concentration and enhanced plant growth.
Positive and negative controls did not differ in their effects on basil growth, except in node count, where OptiMar yielded more nodes. This limited impact raises questions about the suitability of OptiMar as a comprehensive biofertilizer for basil cultivation. Furthermore, the sonicated biomass only elicited greater growth than the negative control when concentrations were at least 5 g L−1, except for leaf count, where a difference was only observed at 40 g L−1. This suggests that the efficacy of sonicated A. maxima biomass as a biofertilizer for basil is contingent on concentration levels, with a threshold observed at 5 g L−1 for most growth parameters. A similar study using active dry yeast as a biofertilizer on basil also found a positive relationship between growth and concentration, as well as limited differences between the negative control and concentrations below 5 g L−1 [19]. Whether this phenomenon is unique to basil warrants further investigation. A limitation in our study is its duration; basil seedlings were harvested after only 50 days of growth, whereas basil harvest typically occurs 2–3 months after germination. Extending the study’s duration could reveal more noticeable differences among treatments, allowing plants adequate time to grow and exhibit distinct responses to concentration treatments.
4.2. Biomass Characterization
The biofertilizer and biostimulant capacities exhibited by A. maxima in our shade house experiment can be attributed to its high concentrations of essential macronutrients and growth-promoting compounds. Specifically, compounds such as gibberellic acid, 6-benzyladenine, and 1-naphthaleneacetic acid are the phytohormones that are likely responsible for enhancing growth in basil, and may also boost the plant’s defenses [30,31]. The production of phytohormones by Arthrospira spp. has been previously reported [17,32,33]. Notably, our study sample exhibited a unique composition, including gibberellic acid, 6-benzyladenine, and 1-naphthaleneacetic acid—compounds not reported in the aforementioned studies. Gibberellic acid’s role in stimulating stem elongation and cell growth, combined with the cell-division-promoting qualities of 6-benzyladenine, along with the root-promoting effects of 1-naphthaleneacetic acid, likely contribute significantly to the enhanced growth of basil observed in our experiment [34,35,36].
The protein content of dry biomass was 64.46%, which is typical for Arthrospira species [37] and further highlights the potential benefits of a derived biofertilizer. Protein content is characterized by the presence of amino acids that play crucial roles as participants in regulating the metabolic, physiological, and biochemical pathways, as well as serving as intermediates for final metabolites. Some amino acids are biostimulants capable of enhancing plant productivity, especially under abiotic and biotic stress conditions [38,39]. In our A. maxima sample, the amino acids with highest relative composition were aspartate (17.2%), arginine (16.6%), glutamate (15.7%), and glutamine (14.2%) (Table 2), with the former of these three being vital for plant growth [4]. This result stands in some contrast with [40], where the most common amino acids in A. maxima were leucine (10.9%), valine (7.5%), and isoleucine (6.8%). An aminogram on a related species, A. platensis, also reported different composition [41]. These disparities emphasize the variability in amino acid composition within the Arthrospira genus, reflecting species-specific and strain-specific differences in their biochemical makeup.
The high concentrations of nitrogen, phosphorus, and potassium present in the A. maxima dried biomass provide essential nutrients that are vital for robust plant growth, ensuring healthy foliage, strong root development, and overall improved crop yield. These key elements act as fundamental building blocks for various biochemical processes, playing a pivotal role in enhancing plant vitality and resilience. The measured nutrient concentrations of dried biomass fall within the range reported by [42], except for nitrogen, which was measured at a concentration 1.32 times higher than the upper range, at 10.33%. The standards for an organic fertilizer dictate that nitrogen content must match or exceed 1%, and those for nitrogen, together with phosphorus and potassium, must match or exceed 7% [43]. With nitrogen alone exceeding the limit, A. maxima is likely an effective biofertilizer, particularly for nitrogen-poor soils.
4.3. Future Directions
Arthrospira maxima also has potential for the biofortification of crops through soil inoculation. Biofortification is the process of enhancing the nutritional content of food crops by increasing the levels of essential vitamins, minerals, and other nutrients in the plant, usually through plant breeding or genetic engineering [44]. Recent studies have demonstrated that biofortification can also occur through cyanobacterial inoculates that stimulate root growth, solubilize minerals, and enhance nutrient uptake [45,46]. Indeed, some recent studies have demonstrated how soil inoculation with Spirulina increases crop nutrient and vitamin content [47,48]. A systematic exploration and comprehensive documentation of the diverse beneficial properties inherent in Spirulina-derived biological products are essential steps towards enhancing the appeal and accessibility of this technology.
Future research should explore the cost-effectiveness of producing and using different concentrations of A. maxima-sonicated biomass for promoting plant growth and yield in basil, to determine an optimal concentration based on the effects on growth and economic feasibility. Moreover, it is worth investigating whether ultrasonication is a necessary step in the production, as drying biomass is an effective cell-lysing method [49]. Recent research suggests that ultrasonication may be redundant for cell lysis when sun-drying is also part of the protocol (unpublished). Another question that is worth exploring is the impact of culture conditions on the production of secondary metabolites. It is well known that culture conditions impact cyanobacterial growth and secondary metabolite production [30,42,50]. Culture conditions often focus on optimizing the former, but if different culture conditions lead to a higher production of phytohormones, these would be worth exploring.
5. Conclusions
Arthrospira maxima is a species of free-floating filamentous cyanobacteria that holds promising potential as a valuable source of biofertilizers and biostimulants in agriculture, among other biologically based products. In this study, dilutions of sonicated biomass of A. maxima enhanced growth in basil seedlings. Notably, differences from the negative control became evident once the concentration exceeded 5 g L−1. Characterization of the chemical composition of A. maxima revealed the presence and high abundance of phytohormones, essential amino acids, and macronutrients. Biofertilizers offer a more sustainable alternative to synthetic fertilizers, which are currently associated with numerous environmental and health-related concerns. A necessary next step to integrate this technology is a cost–benefit analysis to determine the optimal concentration and application rates for basil and other essential crop species. It is important to emphasize that A. maxima is currently employed in the production of other high-value compounds, generating byproducts that can be harnessed to create biofertilizers. As such, the development and characterization of A. maxima biofertilizers enhances the scope and implementation of sustainability-driven technologies, well beyond the development of effective biofertilizers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10020168/s1, Table S1: Summary of results of cell rupture methods; Figure S1: Effect on soluble protein content: Ultrasound lysis at different powers and operation times; Figure S2: Effect on soluble protein content: Glass beads’ lysis at different bead diameters and operation times; Figure S3: Effect on soluble protein content: Freezing lysis at different operation times for shade-dried and sun-dried biomass; Figure S4: Mean (a) fresh stem mass, (b) fresh root mass, (c) and dry stem mass, and (d) dry root mass in response to A. maxima sonicated biomass concentrations.. The commercial product (CP) refers to Optimar® at recommended dose of 4 mL L−1. Error bars represent standard error. Different letters above error bars, represent statistically significant differences (p < 0.05). Reference [51] is cited in the Supplementary Materials.
Author Contributions
C.A.M.-M. developed the experimental design, conducted the experiment along with J.A.E.-P., curated the data, performed statistical analysis, and wrote the original draft. J.M.D.N. formulated the research goals and aims, supervised along with P.A.Z.O., and administered the project. P.A.Z.O. acquired the necessary funding resources and materials, reviewed the manuscript, and helped to edit the final draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia, Tecnología e Innovación (MINCIENCIAS), Colombia, Project CT 80740-440-2020.
Data Availability Statement
The datasets analyzed during the current study and corresponding code are available in a GitHub repository: https://github.com/camilamarinm/MSBasil (accessed on 6 August 2023).
Acknowledgments
We are grateful to CES University and Universidad de Córdoba for the institutional and logistical support. We also thank CECIF for their services characterizing cyanobacterial samples. A colleague, Luis C. Beltrán, who is proficient in English, provided valuable assistance in writing and editing the final manuscript.
Conflicts of Interest
All authors declare that they have no affiliations with any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Mutale-Joan, C.; Sbabou, L.; Hicham, E.A. Microalgae and Cyanobacteria: How Exploiting These Microbial Resources Can Address the Underlying Challenges Related to Food Sources and Sustainable Agriculture: A Review. J. Plant Growth Regul. 2023, 42, 1–20. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers, Vol 2; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–20. [Google Scholar]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Francés, E.; Escudero-Oñate, C. Cyanobacteria and Microalgae in the Production of Valuable Bioactive Compounds. Microalgal Biotechnol. 2018, 6, 104–128. [Google Scholar] [CrossRef]
- Pathak, J.; Rajneesh; Maurya, P.K.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Cyanobacterial Farming for Environment Friendly Sustainable Agriculture Practices: Innovations and Perspectives. Front. Environ. Sci. 2018, 6, 7. [Google Scholar] [CrossRef]
- Rajneesh; Singh, S.P.; Pathak, J.; Sinha, R.P. Cyanobacterial Factories for the Production of Green Energy and Value-Added Products: An Integrated Approach for Economic Viability. Renew. Sustain. Energy Rev. 2017, 69, 578–595. [Google Scholar] [CrossRef]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef] [PubMed]
- Nowicka-Krawczyk, P.; Mühlsteinová, R.; Hauer, T. Detailed Characterization of the Arthrospira Type Species Separating Commercially Grown Taxa into the New Genus Limnospira (Cyanobacteria). Sci. Rep. 2019, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Freitas, J.; Fernandes, I.; Silva, P. Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth. Sustainability 2023, 15, 12413. [Google Scholar] [CrossRef]
- Vaishampayan, A.; Sinha, R.P.; Hader, D.P.; Dey, T.; Gupta, A.K.; Bhan, U.; Rao, A.L. Cyanobacterial Biofertilizers in Rice Agriculture. Bot. Rev. 2001, 67, 453–516. [Google Scholar] [CrossRef]
- Singh, S. A Review on Possible Elicitor Molecules of Cyanobacteria: Their Role in Improving Plant Growth and Providing Tolerance against Biotic or Abiotic Stress. J. Appl. Microbiol. 2014, 117, 1221–1244. [Google Scholar] [CrossRef]
- Michalak, I.; Górka, B.; Wieczorek, P.P.; Rój, E.; Lipok, J.; Łęska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzyńska-Inger, A.; et al. Supercritical Fluid Extraction of Algae Enhances Levels of Biologically Active Compounds Promoting Plant Growth. Eur. J. Phycol. 2016, 51, 243–252. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Zhou, W.; Yuan, W.; Wang, D. Algal Cell Lysis by Bacteria: A Review and Comparison to Conventional Methods. Algal Res. 2020, 46, 101794. [Google Scholar] [CrossRef]
- Aly, M.S.; Esawy, M.A. Evaluation of Spirulina platensis as Biostimulator for Organic Farming Systems. J. Genet. Eng. Biotechnol. 2008, 6, 1–7. [Google Scholar]
- Dineshkumar, R.; Subramanian, J.; Gopalsamy, J.; Jayasingam, P.; Arumugam, A.; Kannadasan, S.; Sampathkumar, P. The Impact of Using Microalgae as Biofertilizer in Maize (Zea mays L.). Waste Biomass Valorization 2019, 10, 1101–1110. [Google Scholar] [CrossRef]
- Refaay, D.A.; El-Marzoki, E.M.; Abdel-Hamid, M.I.; Haroun, S.A. Effect of Foliar Application with Chlorella vulgaris, Tetradesmus dimorphus, and Arthrospira platensis as Biostimulants for Common Bean. J. Appl. Phycol. 2021, 33, 3807–3815. [Google Scholar] [CrossRef]
- Santini, G.; Rodolfi, L.; Biondi, N.; Sampietro, G.; Tredici, M.R. Effects of Cyanobacterial-Based Biostimulants on Plant Growth and Development: A Case Study on Basil (Ocimum basilicum L.). J. Appl. Phycol. 2022, 34, 2063–2073. [Google Scholar] [CrossRef]
- El-Naggar, A.H.M.; Hassan, M.R.A.; Shaban, E.H.; Mohamed, M.E.A. Effect of Organic and Biofertilizers on Growth, Oil Yield and Chemical Composition of the Essential Oil of Ocimum basillicum L. Plants J. Agric. Res. 2015, 60, 1–16. [Google Scholar]
- Baczek, K.; Kosakowska, O.; Gniewosz, M.; Gientka, I.; Weglarz, Z. Sweet Basil (Ocimum basilicum L.) Productivity and Raw Material Quality from Organic Cultivation. Agronomy 2019, 9, 279. [Google Scholar] [CrossRef]
- Rajasekaran, C.; Ajeesh, C.P.M.; Balaji, S.; Shalini, M.; Siva, R.; Das, R.; Fulzele, D.P.; Kalaivani, T. Effect of Modified Zarrouk’s Medium on Growth of Different Spirulina Strains. Walailak J. Sci. Technol. (WJST) 2016, 13, 67–75. [Google Scholar]
- IDEAM. Irradiación Global Horizontal Medio Diario Anual. Available online: http://bart.ideam.gov.co/cneideam/Galeria_de_mapas/QUIMICA%20DE%20LA%20ATMOSFERA/Irradiación%20Global%20Horizontal%20Medio%20Diario%20Anual.pdf (accessed on 29 May 2023).
- Horwitz, W. AOAC Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Kjeldahl, J. Neue Methode Zur Bestimmung Des Stickstoffs in Organischen Körpern. Fresenius’ Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Yalçın, S.; Şükran Okudan, E.; Karakaş, Ö.; Önem, A.N.; Sözgen Başkan, K. Identification and Quantification of Some Phytohormones in Seaweeds Using UPLC-MS/MS. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 475–484. [Google Scholar] [CrossRef]
- Standard Methods Online—Standard Methods for the Examination of Water and Wastewater. Available online: http://standardmethods.org/ (accessed on 29 April 2023).
- Henderson, J.W.; Ricker, R.D.; Bidlingmeyer, B.A.; Woodward, C. Rapid, Accurate, Sensitive, and Reproducible HPLC Analysis of Amino Acids; Agilent Technical Note: 5980-1193E; Agilent Technologies: Santa Clara, CA, USA, 1999. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2022; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Uniyal, S.; Bhandari, M.; Singh, P.; Singh, R.K.; Tiwari, S.P. Cytokinin Biosynthesis in Cyanobacteria: Insights for Crop Improvement. Front. Genet. 2022, 13, 933226. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Ahmed, M.; Stal, L.; Hasnain, S. Production of Indole-3-Acetic Acid by the Cyanobacterium Arthrospira platensis Strain MMG-9. J. Microbiol. Biotechnol. 2010, 20, 1259–1265. [Google Scholar] [CrossRef]
- Zapata, D.; Arroyave, C.; Cardona, L.; Aristizábal, A.; Poschenrieder, C.; Llugany, M. Phytohormone Production and Morphology of Spirulina Platensis Grown in Dairy Wastewaters. Algal Res. 2021, 59, 102469. [Google Scholar] [CrossRef]
- Bubán, T. The Use of Benzyladenine in Orchard Fruit Growing: A Mini Review. Plant Growth Regul. 2000, 32, 381–390. [Google Scholar] [CrossRef]
- Schwechheimer, C. Understanding Gibberellic Acid Signaling—Are We There Yet? Curr. Opin. Plant Biol. 2008, 11, 9–15. [Google Scholar] [CrossRef]
- Yan, Y.-H.; Li, J.-L.; Zhang, X.-Q.; Yang, W.-Y.; Wan, Y.; Ma, Y.-M.; Zhu, Y.-Q.; Peng, Y.; Huang, L.-K. Effect of Naphthalene Acetic Acid on Adventitious Root Development and Associated Physiological Changes in Stem Cutting of Hemarthria compressa. PLoS ONE 2014, 9, e90700. [Google Scholar] [CrossRef]
- Pan-utai, W.; Poopat, N.; Parakulsuksatid, P.; Pan-utai, W. Photoautotrophic Cultivation of Arthrospira maxima for Protein Accumulation under Minimum Nutrient Availability. Appl. Food Biotechnol. 2020, 7, 225–233. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Liu, Q. Connections Between Amino Acid Metabolisms in Plants: Lysine as an Example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Editorial: Amino Acids in Plants: Regulation and Functions in Development and Stress Defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z. The Chemicals of Spirulina. In Spirulina Platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology; Vonshak, A., Ed.; Taylor & Francis Ltd.: London, UK, 2002; pp. 175–204. ISBN 0-203-48396-0. [Google Scholar]
- Raji, A.A.; Jimoh, W.A.; Bakar, N.H.A.; Taufek, N.H.M.; Muin, H.; Alias, Z.; Milow, P.; Razak, S.A. Dietary Use of Spirulina (Arthrospira) and Chlorella Instead of Fish Meal on Growth and Digestibility of Nutrients, Amino Acids and Fatty Acids by African Catfish. J. Appl. Phycol. 2020, 32, 1763–1770. [Google Scholar] [CrossRef]
- Arahou, F.; Hassikou, R.; Arahou, M.; Rhazi, L.; Wahby, I. Influence of Culture Conditions on Arthrospira platensis Growth and Valorization of Biomass as Input for Sustainable Agriculture. Aquac. Int. 2021, 29, 2009–2020. [Google Scholar] [CrossRef]
- Thuriès, L.; Houot, S.; Viel, M. Cours N°7: Normes et Réglementations, 7.2. Réglamentation: L’example Des Fertilisants Organiques; UVED-CIRAD: Rennes, France, 2010. [Google Scholar]
- Nestel, P.; Bouis, H.E.; Meenakshi, J.; Pfeiffer, W. Biofortification of Staple Food Crops. J. Nutr. 2006, 136, 1064–1067. [Google Scholar] [CrossRef]
- Rana, A.; Joshi, M.; Prasanna, R.; Shivay, Y.S.; Nain, L. Biofortification of Wheat through Inoculation of Plant Growth Promoting Rhizobacteria and Cyanobacteria. Eur. J. Soil. Biol. 2012, 50, 118–126. [Google Scholar] [CrossRef]
- Nishanth, S.; Prasanna, R.; Hossain, F.; Muthusamy, V.; Shivay, Y.S.; Nain, L. Interactions of Microbial Inoculants with Soil and Plant Attributes for Enhancing Fe and Zn Biofortification in Maize Genotypes. Rhizosphere 2021, 19, 100421. [Google Scholar] [CrossRef]
- Parulekar, Y.; Haldankar, P.M.; Dalvi, N.; Salvi, B. Nutraceuticals and Their Biofortification in Vegetable Crops: A Review. Adv. Agric. Res. Technol. J. 2019, III, 219–229. [Google Scholar]
- Kumari, M.; Sharma, D.; Sandeep, S. Biofortification of Vegetable Crops: An Option for Mitigating Hidden Hunger. Int. J. Econ. Plants 2022, 9, 184–193. [Google Scholar]
- Vergel-Suarez, A.H.; García-Martínez, J.B.; López-Barrera, G.L.; Barajas-Solano, A.F.; Zuorro, A. Impact of Biomass Drying Process on the Extraction Efficiency of C-Phycoerythrin. BioTech 2023, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Burja, A.M.; Abou-Mansour, E.; Banaigs, B.; Payri, C.; Burgess, J.G.; Wright, P.C. Culture of the Marine Cyanobacterium, Lyngbya majuscula (Oscillatoriaceae), for Bioprocess Intensified Production of Cyclic and Linear Lipopeptides. J. Microbiol. Methods 2002, 48, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).