Abstract
Traditional gladiolus propagation methods are now supplemented with in vitro propagation to meet the demands of modern floriculture in terms of quick production of disease-free, quality planting material. Due to virus infections, vegetative propagation in gladiolus in the field is slow, and is a serious concern in the propagation of gladiolus. In vitro propagation provides an enormous increase in propagation rate and the ability to produce disease-free plant material. Numerous elements, including cultivars, explant type, size of explants, position of explants on medium, plant growth regulators and certain additives, incubation conditions, and sub-culturing time, all have a significant impact on in vitro clonal propagation of gladiolus plants as well as the development of in vitro cormel efficiency. There are certain obstacles and challenges that arise in the in vitro development of plants and the cormels of gladiolus. However, numerous studies and review reports on gladiolus for in vitro propagation have been reported, but very little is known about the factors influencing gladiolus’ in vitro effectiveness. In the present review, we focused on and analyzed research data accumulated over 50 years on diverse strategies for in vitro propagation such as direct, indirect organogenesis, and somatic embryogenesis, as well as various factors such as physical, nutritional, and hormonal influences on in vitro propagation, in vitro cormel formation efficiency, difficulties that arise, and new insights into in vitro development in gladiolus from the available literature worldwide. Future possibilities for further improvement in the in vitro propagation of ornamental gladiolus are also discussed. The current review provides insight into a comprehensive protocol for gladiolus in vitro propagation and emphasizes the importance of continuously advancing tissue culture techniques and factors influencing the in vitro efficiency towards improving in vitro plantlets and cormels in gladiolus (Gladiolus spp.).
1. Introduction
Human life depends on flowers, as is well known; man is born with flowers, grows up with flowers, and eventually passes away with flowers. Flowers are connected to both happiness and sorrow for the individual [1]. Among the flowers, gladiolus (Gladiolus hybridus Hort) ranks first in the world’s bulbous flower trade and eighth in cut flower trade among ornamental flowering plants [2]. It is a beautiful ornamental plant that is used for flower bouquets and outdoor displays. It is indigenous to South Africa and a member of the Iridaceae and Ixiodaceae subfamilies. Because of its magnificent spikes, exquisite and delicate florets in a range of colors, continuous flower opening for an extended length of time, and the excellent keeping quality of the cut spikes, gladiolus is one of the most significant flowers in the world. There are about 260 species in the genus Gladiolus, 250 of which are native to Sub-Saharan Africa and 10 to Eurasia [3]. Some gladiolus species appear to have aided in the evolution of modern gladiolus [4]. Gladiolus species have two major centers of diversification: one in the Mediterranean basin and the other in southern and central Africa [5]. The majority of the species are native to the Mediterranean and tropical regions of South Africa, particularly the Cape of Good Hope region [6].
Gladiolus hybridus Hort., in particular, has a wide spectrum of colors that makes them popular in the cut flower market. The three main nations that grow gladiolus are the United States of America, Germany, and Holland. The main Indian states that produce gladiolus are West Bengal, Sikkim, Himachal Pradesh, Jammu and Kashmir, Punjab, Karnataka, Tamil Nadu, and Maharashtra [7].
2. Gladiolus Cultivation
Seeds, corms, and cormels are the most common ways to propagate gladiolus. Gladiolus seeds, on the other hand, are not appropriate for propagation, and seed-grown plants do not create a viable population of desired plants [8]. Corms and cormels are another common method of propagation and are commonly affected by Fusarium corm rot, bacterial, fungal, and viral diseases; Botrytis blight; and Bacterial leaf rot, among others, resulting in significant losses. Tissue culture has recently enabled the delivery of large quantities of disease-free material that is being used to produce true-type healthy stock in a short period of time [8]. Disease-free cultures can grow a significant number of plants or propagules in a short amount of time. As a result, a large number of studies were conducted using various approaches [9,10,11,12]. However, very little information was discovered in terms of parameters influencing in vitro plantlet and in vitro cormel efficiency.
3. Gladiolus In Vitro Propagation: Advantages and Applications
As far as the author is aware, the first report on the tissue culture of gladiolus that was published used inflorescence discs as an explant and various concentrations of NAA and stable doses of kinetin to induce a callus [13]. They discovered this information by searching research reports on Google, Google Scholar, and other databases. The stem tip of a cormel was then used to induce a callus using various combinations of media and cultural conditions [14]. However, it was found that when different media were used, corms and inflorescence explants produced calluses more effectively than ovarian leaf tissue [15,16,17]. At the moment, several studies on the in vitro propagation of gladiolus under various media and cultural conditions have been conducted [8,11,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43].
Gladiolus in vitro propagation efficiency has been aided by a number of variables (Supplementary Table S1). Gladiolus plant micropropagation, in vitro cormel production, and gladiolus improvement through biotechnological techniques are all dependent on a number of factors, including genotype, culture media, and environmental factors [43]. The type of plant or stage of life of the donor plant determines how the culture is implemented [44]. A range of elements, including light, temperature, humidity, and carbon dioxide, influence the morphology and physiology of mature in vitro plants [45]. Physical elements including room temperature, light, humidity, and dynamic cultures that move to different growth room settings all play a role in the in vitro protocol’s effectiveness. The chemical environment, which includes PGRs, carbohydrates, gelling agents, organic and inorganic compounds, etc., is also significant and has an impact on tissue culture efficiency. These factors actually help the tissue culturist by allowing him to manipulate plants under his control. Plants produced in a lab are more susceptible to environmental restrictions and do not tolerate alteration as well as their ex vitro counterparts. As a result, the compatibility of in vitro plants is essential for proper in vitro plant establishment in the field. When transferred to the field, plants grown in vitro were unable to interact with soil microbes or adjust to their surroundings [46]. It is vital to guarantee that the plants are planted in a moist environment for 2–4 weeks, depending on the rate of new leaf and root creation, in order to prolong life after transplantation to in vivo settings [47,48].
Elegant propagation features of plant tissue culture technologies were reviewed, and discussed the work carried out worldwide on direct and indirect organogenesis and in vitro cormel production [29]. Its assessments omitted to mention the critical aspects that influenced the in vitro conditions that are required for optimal microplant growth and development, as well as cormel production. This review failed to clarify the role of the cell suspension culture via somatic embryogenesis and the factors that affect its efficiency. Little information on gladiolus micropropagation was covered in the review of the use of micropropagation and related biotechnological techniques for ornamental flowering bulbs with a focus on the Iridaceae family [49]. Recently, Datta et al. [50] discussed about ornamental gladiolus breeding. His main areas of interest induced mutation, classical improvement, cytology, characterization, disease management, germplasm collections, and the paucity of knowledge regarding gladiolus production in vitro. In the current analysis, we focus on different methods used for in vitro propagation as well as cormel formation; factors affecting in vitro propagation as well as in vitro cormels’ efficiency; acclimatization of microplantlets and microcormels; and field transfer of microplants as well as cormels. To the author’s knowledge, the present review is unique in the sense of complete micropropagation protocols for microplants as well as in vitro cormels and may also have an impact on how future research is conducted in gladiolus on cell culture.
3.1. Establishment and Regeneration: Shoot Tip Culture, Direct and Indirect Organogenesis
Numerous tissue culturists have looked into the various methods for regenerating microplants in the gladiolus through direct organogenesis indirect organogenesis through a callus, and a cell suspension culture via somatic embryogenesis [10,12,14,30,51,52,53,54,55]. On the other hand, several factors affect how well plantlet regeneration works, including genotypes, PGR types and their concentrations, the kind of explants used, and the makeup of the culture media [8,56,57]. The research carried out by several researchers is presented in (Supplementary Table S1). Figure 1 shows a schematic illustration of in vitro cultivation aiming to improve gladiolus (Gladiolus species).
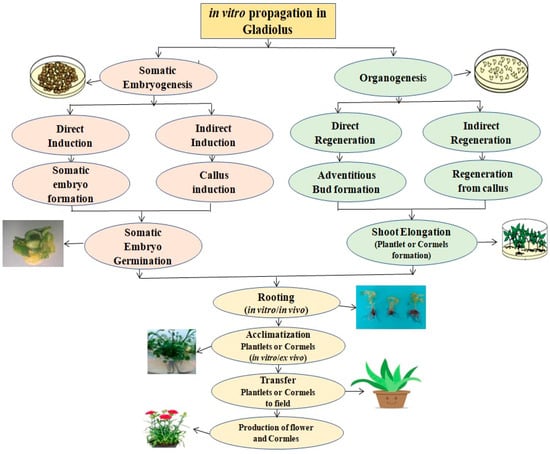
Figure 1.
Diagrammatic illustration of in vitro cultivation aimed at gladiolus (Gladiolus species) improvement.
3.1.1. Organogenesis
It describes how plants develop from aerial components like shoots and leaves, as well as soil-borne roots, bulbs, and corms. Plants can develop directly from the meristem or indirectly through callus induction.
Direct Organogenesis
It is a quicker method of multiplying gladiolus plantlets in large quantities. The frequency of microplants, on the other hand, is largely determined by a number of parameters, including the type of explants employed, genotype, composition of culture medium, and PGR types and concentration (Figure 1 and Supplementary Table S1). Direct organogenesis have demonstrated by researchers utilizing a variety of explants, including corms or inflorescence explants, axillary buds basal leaf tissues, meristem, explants from inflorescence stalk, and various other explants such as shoot tips or cormel sprouts, cormels, and cormel segments [8,9,26,27,28,38,51,58,59,60].
Indirect Organogenesis
By using an artificial medium to induce a callus, indirect organogenesis is accomplished (Supplementary Table S1 and Figure 1). The next step is to induce adventitious organ formation by placing the callus in a culture medium containing plant growth regulators. A few weeks later, a newly planted plantlet is progressively introduced to a novel environment. On the other hand, the rate at which plants regenerate through indirect organogenesis primarily depends on several other factors, such as the quantity and age of the callus, explant type, culture medium composition, type and concentration of plant growth regulators, and type of genotype. The use of explants, growing conditions, and growth regulators in the culture medium affect callus initiation and regeneration [55,61].
Gladiolus is a bulbous ornamental plant that produces calluses from underground parts such as corms, cormlets, and other meristematic tissue parts like embryos, basal meristems, and shoot tips. The initiation of calluses has been recorded in gladiolus from a variable of explants, including portions of the flower stalk [22], corm [39,41], cormel tips [62], slices of corm and basal leaf regions [28,42,63], axillary buds [25] basal leaves and cormel slices [61], apical meristem [19], and cormel sprouts slices [64].
3.2. In Vitro Establishment, Multiplication or Proliferation of Ornamental Gladiolus (Gladiolus Species)
For successful micropropagation, in vitro establishment is one of the most crucial steps. This step is influenced by three key factors: genotype selection, explant selection, and sterilization treatment. Differentiation and development within a specific cultural context and situation can be achieved with the right selection of explants (Table 1 and Table 2 and Supplementary Table S1). Some key elements that affected plantlet in vitro efficiency are shown in Figure 2.

Table 1.
Effect of genotypes on in vitro establishment of ornamental gladiolus (Gladiolus species).

Table 2.
Effect of explants on in vitro establishment efficiency of ornamental gladiolus (Gladiolus species).
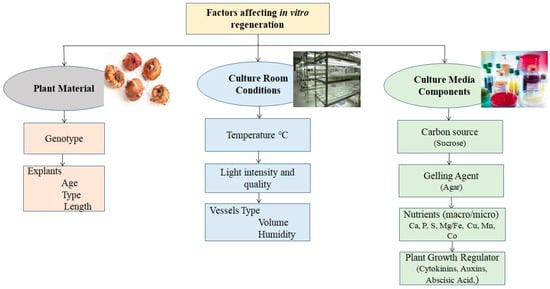
Figure 2.
A schematic representation of some significant variables influencing the gladiolus’s in vitro cormel formation and propagation.
3.2.1. Genotypes/Species/Cultivars
The genotype has a crucial impact on the achievement of any gladiolus in vitro development (Table 1). In a number of studies, the gladiolus genotype showed a considerable impact on callus induction [31,65,68,69,70,71]. In the establishment stage of in vitro propagation of gladiolus with several genotypes, compact calluses formed in Blue Isle and Rosa supreme, while no callus was produced in Jenny Lee [65]. On the other hand, Peter Pears and White Prosperity were found to be superior to other gladiolus cultivars in terms of callus formation on culture media [72], while in another study, 100% callus formation was induced in Rose Supreme, followed by 80% in Peter Pears, and 70% in White Prosperity cultivars [69]. Moreover, the cultivars Priscilla and ChaCha showed superiority in callus induction, but no callus formation was observed in the variety Traderhorn [70]. Similarly, among the three genotypes used, namely White Friendship, Traderhorn, and Peter Pears, White Friendship induced excellent calluses [31].
3.2.2. The Kind and Selection of Explants
The kind of explants used is one of the most important factors in optimizing the tissue culture procedure (Table 2). Due to the fact that the gladiolus plant holds significant commercial value, almost all of its parts are used as explants, resulting in differential reactions [73]. When comparing the feasibility of soil-borne and aerial explants, there was an increase in the contamination of cormel explants compared to aerial portions [26]. On the other hand, donor explants are grown on hydroponic, mineral, or synthetic substrates, so contamination can be avoided [74]. One in vitro protocol study reported the best efficiency with inflorescence explants as compared to other explants such as the apical bud [26].
Various responses have been documented from various explants (Table 2). In a somatic embryogenesis study, Stefaniak [65] observed that young leaf bases produced a small amount of calluses while the basal portion of the leaf induced more calluses. In comparison to the inflorescence axis, cormel segments responded better to callus induction [22] and leaf explants [28], while Beura et al. [71] obtained the best results from an axillary bud. In three types of explants, viz. axillary buds, leaf tissues, and nodal segments [75], they noted axillary buds’ superiority in establishment and proliferation on the culture medium, while the other two did not show any good response. Explants such as leaves, apical buds, flower stalks, and petals could be used to induce calluses, but explants such as floral stems, bracts, and floral spikes did not produce calluses [70]. The cormel-based explants as top slices could not induce calluses in any of the gladiolus varieties among the cormel slices, while the bottom slices exhibited the best results in comparison to the middle slices of the cormel [31].
3.2.3. Mother Plant Sanitation Conditions and Sterilization of Explants
Gladiolus is commonly grown in soil instead, and its corm cormlets are also utilized as explants. As a result, adequate explant sterilization is more important for a successful in vitro process (Supplementary Table S1).
Surface sterilization is the step in the procedure that is most important, as it assures that explants are placed in an environment where microbial contamination is not present [76,77,78]. In most of the in vitro propagation studies, two primary active substances are mercury and chlorine for the sterilization of explants. The surface sterilization of explants was accomplished using a variety of procedures, which differed based on the species of gladiolus and the type of explant. Explants of gladiolus were cleaned with detergents like Teepol, Tween 20, liquid detergent, or savlon, then exposed to sodium hypochlorite (NaOCl) as reported in various works [28,59,79,80]. Numerous research reports also advise applying clorox centramide, dithane M-45, bavistin, antiseptic savlon, dematos with alcohol, and other primary sterilants like HgCl2 and NaOCl [11,23,35,53,64,66,81,82,83]. Gladiolus explants were immersed in 80% ethanol for 2 min before being washed and dried with sterile distilled water, and then disinfected for 3 min before being washed and dried [84]. Moreover, explants treated with 70% ethanol (30 s) followed by 1% NaOCl (10 min) were shown to be the optimum sterilant for axillary bud explants [71]. A combination of 70% ethanol (3 min) and 50% dematos (25 min) was most effective to suppress microflora in gladiolus explants [66]. When corm slices were treated with a combination of 1% bavistin and 0.1% HgCl2 for 10 min, they showed remarkable aseptic culture and established (77.37%) with maximum survival (72.00%), which was more appropriate than other sterilants [64]. Gladiolus explants, namely axillary buds, leaf tissues, and nodal segments sterilized with 70% ethyl alcohol dipping for 30 s followed by 0.2 percent HgCl2 for 7 min, on the other hand, resulted in the greatest culture establishment and number of proliferating cultures [75]. In order to remove any HgCl2 residue, corms used as explants were treated with 70% ethanol for five minutes, then rinsed three times with sterile distilled water. Next, they were surface sterilized for three minutes with mercuric chloride (0.05 percent), and then rinsed three to four times with sterile distilled water as advised [39]. Ca(OCl)2 (10–20%) and bavistin (1%), when combined with HgCl2 (0.1%), were discovered to be optimal for both explant survival and gladiolus aseptic culture [34]. Cormels were cleaned for 30 s with running tap water, followed by sterilization on the surface with 70% ethanol, rinsed three to five times with double-distilled sterile water, and sterilization again with 1% HgCl2 for an additional four to five rinses, as reported [38]. Different sterilization times were found to be effective for the surface sterilization of two different gladiolus varieties. The White Friendship variety treated with 0.1% HgCl2 for 7 to 11 min and the cultivar Fidelio for 7 to 9 min was found to be the most effective [85].
3.2.4. Effect of Plant Growth Regulators
The development of calluses, shoot multiplication, and microplant rooting are all facilitated by plant growth regulators. However, it varies from explant to explant, plant to plant, as well as species to species. The induction of calluses in Gladiolus priorii, G. tristis, and G. virescens was reported by [86] in liquid medium containing BA or 2,4-D. 2,4-D induced a friable callus while BA induced a compact callus. Tripathi et al. [41] used MS media containing 2.0 mg L−1 BAP and 0.5 mg L−1 2,4-D for fine callus initiation. Callus formation and shoot multiplication from the cormel and meristem were observed when the medium was integrated with 1.0 mg L−1 BAP. Friable and capable calluses were induced over the next subculture on either NAA (1, 2 or 4 mg L−1) or 2,4-D (0.5, 1 or 2 mg L−1), while a greater number of shoots regenerated in medium containing 2 mg L−1 BAP from one-month-old calluses [64]. Goo et al. [62] found that medium cultured by NAA (1.0 mg L−1) induced 100% callus formation, while calluses cultured on media integrated with kinetin at 1.0 mg L−1 and NAA at 0.01 mg L−1 produced the maximum number of plantlets. Induced calluses in liquid media with membrane support cultured with 1 mg L−1 NAA and 2 mg L−1 BAP regenerated the most shoots/cluster [59]. Modified MS media containing 1 mg L−1 2–4 D regenerated calluses, although the regeneration of plantlets was better on modified MS media containing 2 mg L−1 BAP and 1 mg L−1 NAA [87]. Good-quality calluses were induced by an optimal dose of 0.1 mg L−1 NAA and 0.2 mg L−1 BAP, whereas the most shoots were produced by media fortified with 0.4 mg L−1 NAA [88]. In one report, Ref. [89] induced calluses in 1/2 MS media without growth regulators. NAA alone in variable concentrations, as well as in combination with other PGRs, has also been reported in some reports. In this context, Ref. [30] induced calluses on media containing 0.2 mg L−1 NAA and elongated in media containing 0.5 mg L−1 NAA. Induction with NAA at 4 mg L−1 resulted in the greatest callus induction; on the other hand, the best proliferation was achieved with basal MS medium supplemented with NAA at 0.5 mg L−1 [31]. A swell-like structure was obtained [90] when explants were cultured on media integrated with 2.0 mg L−1 NAA, while media fortified with 0.2 mg L−1 NAA produced elongated and mature shoot buds. Higher concentrations of NAA have been optimized [54] where they found the maximum callus induction with 7.5 mg L−1 NAA, while good shoots developed on culture media consisting of 0.5 mg L−1 BAP and 0.5 mg L−1 kinetin.
Auxins and cytokinins have been utilized to propagate gladiolus in vitro (Supplementary Table S1). According to a number of reports, cytokinin is required for shoot organogenesis [8,9,28,34,91] and encourages bud/shoot regeneration [53,64,87]. Among the cytokinins, benzyl aminopurine (BAP) or benzyladenine (BA) are important compounds that have been shown to have favorable benefits over other cytokinins [9,51,92,93]. However, variable doses of BAP have been standardized including higher doses of BAP that have been optimized for shoot proliferation, where BAP at 4 mg L−1 resulted in maximum shoot proliferation [9,31,51], while 3.0 mg L−1 BAP was found to be best for shoot proliferation [75], 2–3 mg L−1 BAP was found to have the most potential for gladiolus shoot induction, and a constant dose of 2 mg L−1 BAP has been recommended [93]. Some research reports, however, suggested lower BAP concentrations for shoot regeneration; one such report [94] found that 0.5 mg L-1 BA produced the best results, while [81] obtained better results at concentrations of BAP in the range of 0.5 to 1.0 mg L−1. Furthermore, increasing BAP concentration from 0.5 to 1.0 mg L−1 increased shooting, while further increases resulted in a low rate of shoot development [80]. Several researchers have reported on NAA alone and its positive influence on in vitro growth [31,54,62,63], as well as in combination with other PGRs, as also reported by [28]. 2,4-D, a popular herbicide, has also been utilized to propagate gladiolus in vitro. In this context, Ref. [39] used media containing only 4.52 M 2,4 D and observed maximum callus quantity. On the other hand, Ref. [66] employed a lower dosage of 0.5 mg L−1 BA and 0.5–1.5 mg L−1 2,4-D for shoot induction. For callus induction and maintenance [95], they used 1–10 mg L−1 naphthalene acetic acid (NAA) and 0.5–2.0 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D).
In vitro shoot proliferation necessitates varying amounts of cytokinins and auxins, as well as a lack of PGR [23,96]; thus, organ induction is dependent on modifying auxin and cytokinin availability in the media. Media supplemented with various concentrations of BAP and NAA were also found to be beneficial for shoot proliferation [60,81,88]. A greater number of multiple shoots were recorded with BA (4 mg L−1) and NAA (0.5 mg L−1) [27]. In different culture systems, BAP alone elicited no reaction in three culture systems [59]. However, in the MR system, the highest number of shoots/culture was produced by a combination of NAA (1 mg L−1) and BAP (2 mg L−1), which was followed by DF with NAA (0.5 mg L−1) and BAP (4.0 mg L−1), and AS with NAA (0.2 mg L−1) and BAP (4.0 mg L−1). BAP with IBA has been reported for shoot proliferation. In one report, media containing BAP (18 M) and IBA (36 M) produced the highest numbers of shoots [23].
The majority of the credit for both direct and indirect organogenesis from any explant source goes to the genotypes, PGR types, concentrations, and type of culture media [55,61]. Regarding the in vitro ability of three cultivars, Kaifa, Clara, and Nabila, observed maximum shoots and leaves for cv. Nabila at 2.0 mg L−1 BA, while the other two cultivars required higher concentrations [68]. Similarly, Memon et al. [51] found that in comparison to other varieties, White Prosperity yielded more shoots on medium enriched with BAP (4 mg L−1); Traderhorn and Peter Pears, on the other hand, responded to shoot regeneration in statistically similar ways.
Instead of growth regulators, some authors have used growth retardants in different media for successful in vitro plantlet as well as in vitro cormel formation in gladiolus (Supplementary Table S1). The application of growth retardants under appropriate culture conditions reduced aberrant leaf growth and increased bud development or meristematic clusters with a high proliferation rate without causing hyperhydricity [97]. The addition of paclobutrazol (PAC) to the gladiolus proliferation medium in either bubble or airlift bioreactors induced meristemoid cluster formation [98]. The buds proliferated into nodular proto-corm clusters in the presence of 2.8 mM ancymidol (ANC) or 3.4 mM paclobutrazol (PAC) in the liquid medium, whereas the buds in the control elongated and formed hyperhydrated leaves [99]. In liquid culture, Ref. [100] investigated the role of growth inhibitors on shoot proliferation and morphogenesis. The paclobutrazole produced the most buds per explant. Explants treated with ancymidol and uniconazole generated proto-corm aggregates, with the greatest proto-corm detected in the ancymidol and uniconazole treated explants. The rate of protocorm growth was higher in bubble bioreactor cultures than in shaker cultures.
3.2.5. Shoot Multiplication
The shoot multiplication stage is one of the most crucial phases of gladiolus propagation in vitro. A micropropagation protocol’s success is determined by the rate and mode of shoot multiplication. The in vitro efficiency of plantlets was influenced by a number of parameters, as shown in Figure 2.
Genotypes
When cultivated under similar conditions with different growth regulators, some genotypes showed variation in vitro (Supplementary Table S1). The cultivar White Friendship produced more shoots, while Traderhorn and Peter Pears, on the other hand, responded to shoot regeneration in statistically similar ways [51]. Moreover, in another study, the cultivars Nabila and Kaifa produced the highest rates of shoot and leaf formation [68]. Similar results in three genotypes, viz., Her Majesty, Aldebaran, and Bright Eye, have been observed in another study [22].
Media and Additives
MS, modified MS, LS, and Gamorg media are used in various works for in vitro studies of gladiolus (Supplementary Table S1). At all stages, several researchers have used MS basal medium for shoot multiplication [9,27,28,34,39,51,63,64,68,94]. However, Sutter [101] used LS media with different growth regulators for in vitro regeneration, rooting, and cormel formation in gladiolus. When different genotypes as well as explants were cultured on different media, variable results were observed. In this context, three different basal media, viz. MS, White and B5, were compared; it was discovered that MS basal medium was preferable to White and B5 media. However, in another study, B5 medium produced the most in vitro shoots, and the lowest numbers of shoots per explant were produced on MS media [12,34].
In the micropropagation of gladiolus, solid media, semi-solid media, and liquid media have also been successfully used by various works (Supplementary Table S1). Several researchers have proposed an agar-solidified medium or semi-solid agar (AS) medium for successful in vitro propagation of gladiolus [58,79,86,102]. Shoot bud explants were used to successfully induce meristemoid aggregates and the development of somatic embryos in agitated liquid conditions [72], while faster shoot multiplication in gladiolus using liquid shake culture systems was observed [58]. Scaling up to bioreactor production, which relies on liquid culture, offers the possibility to reduce the cost due to automation and is also less labor-intensive [103], which also offers various advantages over conventionally produced culture [104,105]. Moreover, it was discovered that matrix-supported liquid culture was more efficient in regenerating gladiolus shoots than semi-solid agar and membrane raft culture. Maximum shoot multiplication was achieved in liquid culture with MR, with little to no effects from hyperhydricity [59]. On the other hand, a different study [102] discovered that DF system shoots were more susceptible to hyperhydration than AS and MR system shoots. Moreover, bioreactors have been quite popular among tissue culturists due to their great efficiency [106]. Bioreactor cultures yield two to three times the biomass produced by propagules compared to shaken cultures [97,106]. Modifications in bioreactor design can lower costs and benefit the micropropagator. Very little research has been done on gladiolus micropropagation using bioreactors.
When it comes to gladiolus micropropagation, sucrose is a common carbon source. The optimal concentration for each stage of the process ranges from 2 to 3%, with the exception of cormel formation, where a higher concentration of sucrose is advised (Supplementary Table S1). For better in vitro results, some researchers have adjusted media using sucrose in various percentages, including 2% [35,82,83] and 3% [9,11,27,28,33,34,35,38,39,54,58,59,63,70,81,83,84,86,87,88,89,90,107]. Various researchers have reported varying and higher concentrations of sugar, up to 5% [31], 7% [29], 12% [108], and 15% sucrose [109], as well as different ranges of sucrose, like 2–12% [22], 3–8% [64], 3–9% [67], and 3–12% [110]. In one report, laboratory sucrose produced 92 percent of shoot formation in 7.6 days, whereas table sugar produced 94 percent of shoot induction in 7.4 days [80]. When sucrose was used in different concentrations (25–50–100 mg L−1), Ref. [111] found that 50 mg L−1 of sucrose in the medium increased the height of the shoots in gladiolus.
Various researchers have reported minor differences in gelling agents for in vitro propagation such as 0.2% gelrite [23,55,72], 0.7% agar [34,35,57], and 0.8% agar [34,35,58,59]. Moreover, 0.2% phytogel has also been used as a gelling agent [10]. For somatic embryo generation, Ref. [112] employed agar and phytogel. Agar in the culture medium produced more somatic embryos (81.2%) than phytogel in medium cultures (67.6%). Similarly, Ref. [80] also recommended phytogel in the media for a higher number of shoots per culture vial.
Mineral nutrients, inorganic and organic, are abundant in plant tissue culture media [77]. Doubling or lowering the concentration of each major salt affected the rate of multiplication [113]. It was discovered that for increased shoot multiplication, NH4NO3 was reduced to half strength, KH2PO4 was replaced with 300 mg L−1 of NaH2PO42 H2O, and Kl was completely devoid. More shootlets were observed in the cultivars Peter Pears and Rose Supreme when the medium was supplemented with Eucarbon (EC) [69].
3.2.6. Cultural Conditions
There is a scarcity of accurate information on the part of environmental factors and incubation conditions at play in gladiolus micropropagation. Most authors maintain consistent temperatures, light levels, and photoperiods throughout their work (Supplementary Table S1). According to [113], the rate of shoot multiplication was controlled by propagule size, light, and container volume. The propagules contained 20 buds and demonstrated an 8-fold multiplication on the modified medium grown in the dark. Regarding the impact of pH on gladiolus explant establishment, there is only a single report available, where [85] tried different pHs for establishment. A pH range of 4.8–5.8 was found to be more beneficial for culture media than pH ranges of 6.0 and higher. Some studies have found that temperatures of 25 ± 2 °C are favorable, including [28,35,53,83], while variable temperatures including 25 °C [55,113], 25 ± 0.5 °C [103], 25 ± 0.1 °C [39], 25–27 °C [9,31,51,92], 26 ± 2 °C [54], and 24 ± 2 C [64,88] are also reported. More temperature variation (20–25 °C) was optimized in [16], while 24–26 °C was recommended by [19], and 25 °C during the day and 20 °C at night was suggested by [114].
A very low temperature was also reported by [115], who kept cultures at 15 °C, and had the highest number of shoots per explant in comparison to cultures maintained at 24 °C. The 20 °C temperature was optimized for better culturing in comparison to the cultures kept at 25 °C [22]. When shoots were kept at 15–20 °C, Ref. [22] achieved the best results. The induction of calluses was increased [70,116]. Additionally, higher temperatures of 27 ± 2 °C were advised by [75,80]. In somatic embryogenesis, Ref. [117] achieved successful somatic embryogenesis when cells were cultured under dark conditions at 26 °C. At 23 ± 1 °C to 27 ± 1 °C, Ref. [80] found the maximum number of shoots. However, shoot initiation gradually decreased as the temperature rose from 27 ± 1 °C to 30 ± 1 °C. Darkness produced better results than light [113]. In contrast, Ref. [21] obtained better shoot elongation in light than in darkness. Eight hours a day worked best for the callus subculture [62].
Explants cultured in 250 mL Erlenmeyer flasks produced the most shoot buds per explant and had a higher multiplication rate than the other two containers [96,105], and explants cultured in 350 mL Erlenmeyer flasks produced the most shoot buds and had a higher multiplication rate [58], and they successfully induced somatic embryogenesis by transferring half of the cells in a flask into 30 mL of liquid medium in a 125 mL flask every two weeks [117].
3.2.7. Explants Size
There are only a few papers that emphasize the importance of explant size and placement for successful gladiolus micropropagation. The majority of gladiolus shoot regeneration research has only been documented from homogeneous growth stages and sizes of each explant source, i.e., nodal and flower buds [9,23], whole cormels [9,28], and the shoot tip of cormels [62]. Shoot tips, basal plate explants, and daughter corm explants all produced fewer propagules than longitudinal corm sections [58]. Compared to the lower section, the upper segment of the cormel has a greater potential for effective shoot regeneration [9], while in another study, Memon et al. [92] used transverse sections of cormels (top and bottom) and reported that the cormel top section showed superiority in comparison to the bottom section of the cormel. In one study, transversely sliced corm explants exhibited darkness within the first days of inoculation and died. However, explants (longitudinally sliced corms) exhibited no darkening problem [63]. As explants were cultured horizontally, found that the rate of callus formation increased from the base to the middle and apical explants [77].
A number of researchers have noted a distinct difference in the vitro plantlet capability of diverse explants from various sources and sizes [9,58]. The meristematic tissue within the entire or tip of the cormel was inoculated for micropropagation [28], and it was noted that cormel explants induced up to 90% of shoots, while meristem explants induced up to 40% of shoots. In terms of the sizes and ages of explants, Ref. [9] noted that 0.6 g cormel was the best size as compared to 0.2 and 0.4 g, while for the heading stage of nodal cultures, 12-day-old cormel sprouts and the top segments of cormels were found to be the best stages of explants. As per reference [92], the best stages for a sufficient number of shoots to emerge from each explant were the nodal culture heading stage, the medium size cormels and cormel sprouts, and the top slices of cormels. In addition to having a higher percentage of shoot regeneration from 0.5 mm explants than 1.0 mm explants, the 1.0 mm meristem tip explants also had a higher survival rate [32]. Apical meristems measuring 0.5 mm facilitated later shoot initiation with a 70% survival rate and 40% regeneration potential, while meristems measuring 3 mm contributed to earlier shoot initiation with a 100% survival rate and exceptional regeneration potential [80].
3.2.8. Callus Quality and Age
Callus quality, age, and potential for regeneration all play a role in callogenesis regeneration. Kamo [55] reported that cell suspension from a 4-month-old callus in two cultivars of gladiolus, Jenny Lee and Peter Pears, was decreased when suspension cells had been cultured on liquid medium with a 6-month-old callus. In contradiction to this, Ref. [65] reported that a 2-year-old callus retained its regeneration capacity. Moreover, Ref. [118] regenerated plants from an 8-year-old callus in varieties of gladiolus Jenny Lee. An application of 0.01 mg L−1 Bialaphos stimulated the regeneration capacity of a nine-month-old callus in the cultivar of Jenny Lee and a 4-month-old callus in Florida Flame. Similarly, from a callus that was 1 month old and kept in media containing 0.4 mg L−1 NAA, the greatest number of shoots was generated by [88].
4. Somatic Embryogenesis
Somatic embryogenesis is where undifferentiated somatic cells are transformed into normal embryogenic cells and, eventually, entire plants [119]. The technique starts with the creation and replication of an embryogenic culture, then progresses to the development of somatic embryos and, finally, plant regeneration. The regulation of somatic embryogenesis depends on the callus production stage of the embryo [10]. Nonetheless, the documentation of somatic embryogenesis in gladiolus is not as strong, with limited studies available.
4.1. Factors Affecting Cell Suspension Culture via Somatic Embryogenesis
A lot of factors influence the success or failure of the somatic embryogenesis response, including the explant’s origin, culture media, and in vitro environmental conditions [120]. The next section discusses the common elements that influence cell suspension culture via somatic embryogenesis. Figure 2 depicts some important factors that influenced cell suspension culture via somatic embryogenesis.
4.2. Genotypes/Cultivars/Species
Somatic embryogenesis varies from cultivar to cultivar, and the concentration of growth regulators applied in media for embryogenesis also varies. In one report, the cultivars Blue Isle, Jenny Lee, and Rosa Supreme had the highest embryogenic efficacy, whereas Peter Pears had the lowest [65]. Moreover, some researchers used different plant growth regulators and were able to obtain a somatic embryo from Peter Pears [121].
4.3. Media and Additives
There has been very little study on media for cell suspension culture via somatic embryogenesis [14,63,65,121,122,123]. A gelrite-free modified MS basal medium was used to grow cell suspensions from four gladiolus cultivars [124].
High sucrose levels (12%) had a greater rate of somatic embryogenesis than calluses cultivated at lower sucrose levels (2%). The addition of putrescine to high-sucrose media, on the other hand, boosted somatic embryogenesis [112]. A sugar concentration of 30 g/L was best for callus induction and 20 g L−1 was best for somatic embryogenesis [63], while sucrose at 3% as a carbon source was found to be best for the somatic embryo [65].
The media containing 0.8 percent agar produced more somatic embryos than media containing 0.25 percent phytogel [112]. On the other hand, Ref. [65] recommended 0.2 percent phytogel for somatic embryo development.
4.4. Types of Explants
Stefaniak [65] looked at the efficiency of different explants, where various explants were used for somatic embryogenesis. In the basal part of the leaves, callus formation was higher than in the younger leaves. Corm explants, on the other hand, produced the most embryogenic calluses. Moreover, Ref. [65] discovered a significant frequency of callus development for somatic embryogenesis in the middle leaf.
4.5. Growth Regulators
Gladiolus cell suspensions were initially reported to be made using media enriched with 0.1 mg L−1 NAA [14]. Plantlets were generated from callus and shoot regeneration (neo-organogenesis) as well as cell suspension via primary and secondary somatic embryogenesis by [111]. Auxins (IAA, NAA, and 2,4-D) in the media did not start somatic embryogenesis in callus cultures [112]. Exogenous auxins, on the other hand, have been shown to trigger somatic embryogenesis in callus and gladiolus suspension cultures [65,121,122]. When corm surfaces were on 2,4-D (0.5–1.0 mg L-1), direct somatic embryos were created; however, the embryos did not mature in the induction medium, so additional PGR treatments with GA3 and ABA were used [11]. It was discovered that ABA was less responsive than media containing 0.5–1.0 mg L−1 GA3, with 0.5–1.0 mg L−1 GA3 being the most successful treatment. After the eighth culture, the embryos reached a maximal maturity of 62.15 percent in the 0.5 mg L−1 GA3 given to the medium. The developed embryos transformed into plantlets in BAP-containing medium, and the maximum germination (42.65 percent) was seen when 0.5 mg L−1 BAP was introduced. A 72.66 percent effective callus was seen when 4.52 μM 2,4 D was added to the media [39]. On the other hand, the same media generated 80.15 percent somatic embryo differentiation, with BAP + NAA (8.88 + 1.35 μM) producing 72.87 percent somatic embryos and BAP (2.22 μM) producing the least somatic embryos (16.23 percent efficiency). A 72.66 percent effective callus was seen when 4.52 μM 2,4 D was added to the media [39].
Genotypic differences were observed when well-formed embryogenic callus of cv Peter Pears was fortified with 2,4-D [121,122], but [65] discovered that no callus formed when the same cultivar was cultivated in the same medium with the same hormones. However, with growth regulators such as 2,4-D (2–10 μM), another cultivar, Rosa Supreme, showed good calluses. Using 3.0 mg L−1 TDZ and 0.2 mg L−1 BA that were changed in MS medium without NAA, globular somatic embryos on embryogenic calluses were produced [123]. Remotti [122] fortified the primary media with zeatin or 0.25 M BA, while fortifying the secondary embryo with higher concentrations of zeatin or BA, and at higher concentrations of BA obtained some albino shoots among the regenerated plantlets, indicating that the liquid culture conditions may promote some somaclonal variation.
4.6. Culture Conditions
The temperature being maintained at 23 °C and 16 h of light was best for somatic embryo development [65], while media kept under dark conditions at 24 °C was best for embryonic cell culture [72,122]. For suspension cell maintenance, cells were cultured in the dark at 26 °C on a gyratory shaker at 100 rpm for two weeks [117]. However, Emek and Erdag [63] optimized 24 °C ± 2 °C for somatic embryo development, and 25 ± 1 °C temperatures were effective for callus induction followed by somatic embryogenesis in gladiolus [39]. When the callus was developed at high sucrose levels, Ref. [112] found that a heat shock treatment (50 °C for 1 h) increased somatic embryogenesis by 12 percent.
5. Rooting, Elongation and Microtuberization
5.1. In Vitro Rooting of Microshoots
The acclimatization of microplantlets necessitates the establishment of appropriate roots. Underdeveloped roots are the most common cause of transplanted plant failure [13]. As a result, a large change in the culture environment during the pre-transplant period may enhance root initiation and shoot elongation. The combination of internal and external factors determines in vitro rooting capability (Supplementary Table S1). These are explained as follows:
5.2. Rooting, Elongation, and Microtuberization
Gladiolus plantlets’ in vitro rooting and microtuberization efficiency was influenced by a number of internal and external factors.
5.2.1. Genotypes/Cultivars/Species
In the role of genotype for the induction of rooting, Babu and Chawla [24] reported that the cultivar Yellow Topaz gave 100% root induction compared to 93 percent for American Beauty. A variation in the number of roots in two varieties of gladiolus was observed in [29], where they noticed that the genotype White Friendship had more roots than the cultivar Peter Pears when employed in MS media fortified with IBA (2 mg L−1).
5.2.2. Microshoot Age and Size
The ideal size of shoots was (2.5 to 3.5 cm) for in vitro rooting [27].
5.2.3. Media and Additives
The optimal rooting medium was found to be a basic basal MS medium devoid of plant growth stimulants [62], while in another study, B5 media produced the most roots and the longest roots [12]. Lowered salt concentration of half or one-quarter resulted in excellent roots on MS media [64]. Other additives like activated charcoal can absorb all organic components except sugars and prevent light delivery to the nutritional medium, providing circumstances similar to soil [125]. The addition of activated charcoal to the culture media has been reported to be beneficial for organ formation in cell cultures [126] and improved root growth in gladiolus [18]. The maximum number of roots and growth of roots in terms of length were noticed with 3.0 g L−1 activated charcoal [75]. Vermiculite is another option for ensuring strong root performance for easy transplantation into soil. Horticultural-grade enriched vermiculite in the media revealed substantial root development [19].
Sucrose directly affects the root quality and the induction of rooting in the absence of plant growth regulators, but when replacing the sucrose with mannitol, no improvement in gladiolus rooting was observed [22]. A level of 6% sucrose in rooting media has been recommended by [64], who achieved 100% rooting in cultures in just two weeks. Moreover, Ref. [31] discovered that sucrose concentrations of 3, 5, and 7% inhibited root development.
5.2.4. Growth Regulators
IBA and NAA are commonly employed in the in vitro rooting of plants [75,127]. Increased NAA levels had a significant impact on root length, number of roots, and shape [125]. When media was fortified with IBA 2 mg L−1, the cultivar White Friendship developed the most roots [127], and the same media emerged earlier and with longer roots in the cultivar Peach Blossom [27]. In contrast to the efficiency of two auxins, Ref. [63] found that BA (0.1 mg L−1) caused 20% rooting in Gladiolus anatolicus, while NAA (0.5 or 2 mg L−1) caused no rooting. IBA 0.75 mg L−1 was most effective in earlier root initiation, producing well-developed and more roots as compared to NAA [75]. Using 1.0 mg L−1 IBA, the maximum number and length of roots were seen, while the thickness of the roots was better with 1.0 mg L−1 NAA [75]. On the other hand, Ref. [33] found that increasing IAA and NAA concentrations improved root development. Plantlets treated with 1.0 mg L−1 NAA had more rooting than those treated with IBA [89]. In order to induce roots earlier and produce more roots per shoot, Ref. [93] used MS medium supplemented with 4 mg L−1 IBA.
5.2.5. Cultural Conditions
Plantlets were kept at light intensity (30 or 40 mmol m−2 s−l) by Nhut et al. [58], who observed the highest root induction at 30 mmol m2 s1 and the lowest root induction noted under 40 mmol m−2 s−l light intensity. Moreover, heat shock treatment at various temperatures, viz., 35, 40, 45, and 50 °C, was used to induce rooting in gladiolus [22]. Heat shock at 50 °C was found to be best for rooting in cultivars of Her Majesty and Aldebaran. When cultured in vitro at varying temperatures (15, 20, or 25 °C) of shoots, Ref. [58] observed the highest root formation at 15 °C. However, Ref. [66] found that gladiolus shoots rooted more successfully in vitro at 14 °C, with an 80–100% survival rate.
5.2.6. Corm and Cormel Formation In Vitro
Several researchers have documented gladiolus corm production in vitro (Supplementary Table S1). Various factors influence gladiolus corm and cormlet production in vitro [18,21,57,96,114,115,128,129]. During in vitro corm formation, when explants were sub-cultured on medium, some difficulty may have occurred due to mechanical injury [21,127,128,130].
Several gladiolus varieties have demonstrated in vitro corm development, including Eurovision [128], Friendship [21,127], Pacificia [57], Balady [131], Golden Wave [64], and Green Bay [130]. With the exception of [21], who successfully produced flower spikes under ex vitro conditions after obtaining cormels under in vitro conditions, the corms obtained from these studies did not produce flower spikes under ex vitro conditions. Several factors influenced gladiolus corm/cormel formation in vitro, which is discussed here.
5.2.7. Factors Influencing Cormel Formation In Vitro
In vitro cormel production was affected by anti-gibberellins, high sucrose concentrations, and polyamines [114,129]. The impacts of different parameters on the frequency of cormel formation in gladiolus (Gladiolus species) in vitro are shown in Supplementary Table S1.
Cultivars/Genotypes/Species
Among the three cultivars, cultivar “HM” produced more cormlets per culture flask, whereas cultivar “BE” produced higher mass cormlets per culture flask [129]. Similarly, Ziv [110] obtained maximum cormel formation in Cream White and Friendship in comparison to Bellariana Blue Moon, Her Majesty, and Top Brass. On the other hand, in comparison to Rose Supreme, Peter Pears, and White Prosperity, White Prosperity yielded more cormels [69], and in another study, cultivars of Andrew First Called were found to be superior in terms of microcormel formation than Oscar and Azure Shore [66]. Moreover, May Queen showed superiority in cormel formation in comparison with Adlib Scarlet, Pacific Pink, Sharone, and White Race in different media [67]. In another study, Saha et al. [30] evaluated in vitro cormel frequency in five gladiolus genotypes. The top five included the cultivars Sabnam (99.1%), White Friendship (98.9%), Interpaid (96.4%), Red Ginger (95.3%), and Green Bay (92.5%), while Green Bay produced smaller corms and Sabnam produced larger ones.
Explant Type
Explant types with different PGR combinations produce different numbers of cormels per culture and differ significantly in in vitro cormel induction [9]. There have been reports of using a variety of explants for in vitro cormel production in gladiolus, including nodal buds [9], cormel tips [132], inflorescence stalks [13], axillary buds of corm [20,23,96], and slices of cormel sprouts [64]. Several explants were used by [9] in their investigation of in vitro cormel formation. In terms of mean cormel induction, whole cormels were found to be the best explants among the others.
Media and Additives
MS medium is frequently used for in vitro corms and cormel formation in Gladiolus species (Supplementary Table S1). The MS medium alone, devoid of PGRs, was able to develop cormels [112]. However, several other media have been used for in vitro cormel development, including solid medium [133], agitated liquid media [114,122], and MS agar-solidified media [58]. As compared to semi-solid agar medium, MS liquid media was found superior for the high regeneration frequency of corms [30]. In comparison to liquid shake culture, rapid corm generation was achieved in liquid culture media [114], and larger corm sizes developed on media that were replaced on a regular basis (every three weeks) rather than on media that remained unchanged [90].
Roy found that a greater number of microcorms were present in liquid medium as opposed to agar gelled medium [57]. Similarly, Steinitz et al. [114] obtained corm regeneration in subsequent liquid shake cultures. A liquid medium with an alternative eco-friendly biodegradable matrix such as coir from coconut husk noted the maximum corms developed in the liquid culture compared to those developed in the semi-solid control cultures. As for genotypic differences in media for cormel development, Thun et al. [67] noted that solid medium was only suitable for cormlet production in the May Queen cultivar, whereas liquid medium was preferred for the other four cultivars, namely Adlib Scarlet, May Queen, Pacific Pink, Sharone, and White Race.
As an additive in media, sucrose is a crucial part of the medium and a significant source of carbon for the development of in vitro cormels. According to some publications, the majority of the sucrose is used for the formation of bigger corms [18,26,101]. According to several researchers, the growing proliferation of tuberous organs such as corms and cormels needs a medium with a high sucrose concentration (>50 g L−1) [30,82,110,114,134], and the maximum number of cormels was recorded with 9% sucrose under in vitro conditions of gladiolus [135]. Cormel production was found by Kumar et al. [22] at high sucrose concentrations (>6 percent and up to 12 percent). Contrary to these claims, Grewal et al. [136] found that high sucrose levels of 9–12 percent did not aid in promoting cormlet size, but that sucrose levels of 3 percent resulted in a smaller cormlet. Rooted shoots were cultured on increasing amounts of sucrose; Memon et al. [31] recorded a higher number of cormels with 5% sucrose. Moreover, the 6% sucrose concentration showed maximum cormlet production under in vitro conditions [23]. Sucrose 30 g L−1 was optimized and found to be superior in terms of microbulb formation [35,66]. Cormlet formation was enhanced by higher sucrose concentrations (174–348 M), with 232 M sucrose exhibiting the best cormlet formation [129]. The lower concentration of sucrose, 174 M sucrose, has been optimized for in vitro cormlet formation [96]. Recently, following five months of culture, Ref. [40] found that the treatment with 6% sucrose, 10% coconut dust, and 0.01% activated charcoal produced the highest corm induction percentage (88%).
Sugar concentrations for corm development, however, may differ amongst cultivars within a genus. When it came to cormel weight, the cultivars Bellariana and Cream White performed better when given 60 g L−1 of sucrose as opposed to other cultivars that responded better to 120 g L−1 [110]. Similarly, gladiolus cultivars Her Majesty and American Beauty produced more corms at a sucrose concentration of 10%, whereas Friendship produced corms best at a sucrose concentration of 6% [96]. Sucrose at a level of 6 to 9 percent was superior in in vitro cormel production [115]. However, sucrose replacement with mannitol had no favorable effects on cormel production. In a comparison of two carbon sources used in media for in vitro cormel formation, Ojha et al. [88] observed the highest number of cormel formations in media supplemented with 6% fructose followed by 6% glucose.
Corm growth was inhibited by spermidine (SPD) or spermine (SPM) in the culture medium [129]. On the other hand, the addition of various polyamines (PAs) to the culture medium increased corm size and the number of cormlets produced per culture vessel. The overall concentration of PAs rose during the cormlet development phase and subsequently fell during the cormlet maturation phase [137]. The SPD and SPM demonstrated the significance of these polyamines in the early stages of cormlet development. It has been shown that the induction and development of storage propagules, including those that occur during in vitro propagation, have an impact on a variety of physiological and morphological processes [138,139].
The 1:2 NH4+:NO3-ratio in MS solid medium was shown to be the best for expanding cormlets [67]. Cormels were found in greater numbers on media containing NH4NO3 + KNO3 (14N/l + 100N/1, 28N/1 + 200N/1, and 42N/1 + 300N/1) [33]. Moreover, corm development was reduced by 24% at a higher activated charcoal concentration of 3% [21], and corm size was reduced by 34% at a higher activated charcoal concentration of 3% [21]. When grown with different media, 1.5 g L−1 activated charcoal gave maximum cormels in cultivars of Peter Pears, White Prosperity, and Rose Supreme [69].
Growth Regulators
A range of growth inhibitors have been used to promote cormel formation in gladiolus in vitro (Supplementary Table S1). In vitro, shoot growth was reduced, bud proliferation was increased, and corm development was induced by paclobutrazol and ancymidol when applied to gladiolus [98,128,140]. Explants grown in environments that restrict development, such as those with high osmotic potential in the medium or growth-retarding chemicals present, have been shown to develop tubers [141]. Several growth retardants help to develop in vitro cormel production, including chloromequat [133], paclobutrazol [142,143], daminozide, and ancymidol [98]. Shoot explants cultured in liquid shake culture while being treated with gibberellin biosynthesis inhibitors like paclobutrazol generated corms and bud clusters or meristemoids [142,143,144]. In liquid environments, Steinitz et al. [114] found that 10 mgL−1 paclobutrazol enhanced corm development. However, Ziv [128] examined the efficiency of four growth regulators like B-9, ancymidol, paclobutrazol, and majic in cormlet formation. Paclobutrazol produced the maximum number of cormlets, followed by ancymidol, majic, and B-9, which produced fewer cormlets. Growth inhibitors like daminozide, ancymidol, and paclobutrazol were used to cultivate cormels on agitated liquid media [100]. This resulted in plants lacking leaf bud regeneration, and the buds developed into protocorms. Kumar et al. [129] found that malleic hydrazide inhibited maximum shoot growth as well as cormlet, while CCC showed better performance. When 10.0 M MH was employed in the media, however, shoot growth and cormlet production were practically completely inhibited. Lower concentrations (1.0 and 5.0 M) of Alar (B-9) increased the number of cormlets produced per culture flask. The interaction of paclobutrazol and sucrose was studied by DNagaraju et al. [110], where paclobutrazol combined with sucrose reduced leaf and root size. Similar findings in gladiolus cultivars and the gladiolus hybrid Kinneret were previously reported [18,101,145], where they achieved elongated leaves but small cormels in the presence of high sucrose in the medium. This proves that, contrary to what was previously believed, the development of longer leaves for cormel development is not necessarily correlated with the synthesis of more food through photosynthesis [18]. The greatest corm number and diameter were shown by wild Gladiolus imbricatus and Gladiolus hybridus Hort in media supplemented with 10 M acetic acid and 2 mg L−1 ancymidol [89].
Various works have optimized higher to lower concentrations of IBA for in vitro cormel formation, including 4 mg L−1 IBA [146], 2 mg L−1 [64], and 1 mg L−1 IBA [31,147]. According to [63], cormlets formed in the base of shootlets when grown on media containing 0.1 mg L−1 BA, a very low concentration of IBA. Torabi-Giglou et al. [87] stated that 2 to 4 mg L−1 of BAP, in addition to 1 mg L−1 or 2 mg L−1 NAA, plays an effective role in cormlet formation. On the other hand, when propagules were cultured in vitro on media containing NAA 0.1 to 0.5 mg L−1, the maximum number of cormels per explant with a higher fresh weight was recorded [83]. Additionally, the impact of growth regulators on the in vitro production of gladiolus cormels, such as kinetin, gibberellin, abscisic acid, and naphthalene acetic, was evaluated [141]. Kinetin was responsible for cormel formation, while the other three had no effect. In one report, exogenous cytokinin reduced corm development in gladiolus [21,140], while in the presence of another auxin like ABA, stolon and corm development in the shoots were inhibited [21,141]. In their study, Emek and Erdag [63] used benzyl aminopurine (BAP) at 0.1 mg L−1 for the development of in vitro corms in gladiolus. However, it was reported by Steinitz et al. [140] that BAP has a negative impact on corm formation at the base of the shoot. On the other hand, in a follow-up liquid shake culture, Steinitz [114] observed corm regeneration. When given to the shoots before the signs of corm growth appeared, benzoladenine (BA), at 10.7 M, had a stimulating effect. The size and fresh weight of the corms were reduced in the presence of either of the cytokinins. An integrated application of IAA + NAA (0.5 mg L−1 + 1.0 mg L−1, 0.5 mg L−1 + 0.5 mg L−1), resulted in 9 and 15 cormel formations, respectively [33].
Plant hormone concentrations and fluctuations may differ from genotype to genotype [28], and in vitro cormels were highest in Peter Pears and White Prosperity on media containing 1.0 mg L−1 BAP, while 1.0 mg L−1 BAP + 1.0 mg L−1 Kin was superior in the Rose supreme cultivar [69].
There are just two studies on gibberellins’ function in the in vitro development of corms and cormels. Ginzburg and Ziv [141] reported that gibberellic acid inhibited cormel formation. In contrast, Ref. [82] achieved maximum cormlet yields with 5 or 10 mg L−1 gibberellic acid (GA3).
Cultural Conditions
Cormlet production was influenced by the size of the culture flask; larger flasks produced cormlets with an average weight that was higher [129]. Furthermore, gladiolus propagation in vitro depends heavily on light. Numerous morphogenic responses are regulated by light, which is also necessary for photosynthesis. Furthermore, high irradiance flux may be required for gladiolus corm growth in tissue culture [18]. Photoperiod has a significant impact on gladiolus corm growth and short-day circumstances reduced the size and weight of the corm [148]. No effects of day and dark light have been reported [114]. However, Dantu and Bhojwani [21] discovered an inhibitory effect on corm development under dark conditions. The corms initiated earlier, the frequency of corm formation and corm growth were higher in light as compared to dark. In a study involving varying photoperiod durations, Mokshin et al. [66] discovered that the highest number of microbulb formations occurred during 16 h and 0 h of photoperiod, respectively. The highest corm formation was observed at 30 mMol−2 s−1 light intensity, and the lowest corm formation was achieved at 40 mMol−2 s−1 [58]. In the interaction of light intensity and genotypes, Ref. [67] achieved maximum cormels in May Queen, Sharone, and White Race with light intensity of 68 mMol−2 s−1 PAR, 34 mMol−2 s−1 PAR for Adlib Scarlet, and 135 mMol−2 s−1 PAR for Pacific Pink. An increase in fresh weight in corms was related to an increase in diameter, regardless of different temperatures, light intensity, media, or cultivars.
For in vitro corm formation, most of the studies recommended a 23–25 °C temperature range [13,96,114,125,128,149]. Various works have optimized variable temperatures for in vitro cormel formation, including 26–29 °C [14] and 25–30 °C [129]. In Dharmasena et al. [82], they optimized a constant temperature of 25 ± 1 °C for in vitro cormel formation. When comparing the temperature for the development of in vitro cormels, Ref. [58] noticed maximum numbers of corms at 15 °C and the lowest corms formed at 25 °C.
Some reports suggest that temperature varies from cultivar to cultivar for in vitro cormel formation. In a comparison of three temperatures (24°, 20°, and 15 °C), Ref. [115] discovered that corm formation in the winter-flowering G. tristis requires a lower temperature (15 °C), but corm formation in the summer-flowering G. dalenii requires a higher temperature (24 °C). Gladiolus cultivars Spic and Span grew better at 25 ± 2 °C, while cultivars Pacific Pink, Sharone, and White Race showed the best culture environment for cormlet formation in liquid medium at 20 °C [150]. Similarly, cultivar Sabnam yielded larger in vitro corms at 25 ± 2 °C according to [30], whereas cv. White Prosperity yielded larger corms at 20 ± 2 °C. Among the plantlets kept at 25 °C, the highest fresh weight of cormlets was recorded in cv. Adlib Scarlet, but there was no statistically significant difference in cormlet formation in May Queen [67].
Acclimatization
When plants are moved from in vitro culture to greenhouse conditions, one of the primary causes of in vitro plant mortality is water deficiencies [47]. In this regard, Memon [29] proposed three strategies for the effective acclimatization of plantlets developed in vitro as well as the development of corm and cormel in gladiolus: (i) The ideal shoot-to-root ratio should be present in in vitro regenerated plantlets, but there should be no cormel formation (ii), or cormel formation followed by cormel formation but before their dormancy (iii), when the cormels go dormant and the plant shoot dries up. The first technique, which is widely employed by in vitro propagators, involves putting extra in vitro-regenerated shoots into rooting medium and letting them get used to a high humidity, low light, and cold environment. In order to minimize losses during the hardening process of in vitro-produced plants, they also suggested encouraging plants to develop storage organs, such as cormels in gladiolus. Several studies have demonstrated that adjusting acclimatization conditions prior to or during transplantation can help decrease losses [47,151,152]. However, this process is expensive, and the additional cost has an impact on production. In one in vitro protocol, Lilien-Kipnis and Kochba [125] reported that the rate of plantlet survival after transfer to a growing mix depends on temperature and on the presence of activated charcoal (AC) in the vitro rooting medium. After three weeks at 27 °C, none of the plantlets rooted on 1 M NAA alone and survived. Although 56% of plantlets rooted on activated charcoal (AC) survived at 27 °C, the plants (roots and tops) had not grown and their foliage had become chlorotic. At 17 °C, all the plants survived; both roots and foliage grew; the foliage was dark green and new leaves emerged. Under high irradiance flux, Ref. [18] sub-cultured in vitro plantlets to a pre-transplanting low-mineral and sucrose medium and generated functional roots, which continued to grow after transfer to non-aseptic conditions. Before being transferred to an experimental garden, plantlets were grown in a greenhouse for three months at temperatures ranging from 25 to 27 °C and relative humidity levels ranging from 80 to 85 percent [58]. Plantlets treated with Glomus manihotis exhibited the highest percentage of survival during in vitro hardening, as reported by Pragya et al. [53]. Similar to that of G. manihhotis, the other two AMF strains—Gigaspora gigantean and a mixed strain—were treated similarly. Before being placed inside a glasshouse, plantlets received multiple arbuscular mycorrhiza fungus inoculations. In vitro plantlets have been successfully hardened in a medium containing 2:1 sand and soil mixture [153]. Similar hardening processes were reported in Priyakumari and Sheela [27], where they transplanted the rooted plantlets into plastic pots containing sand and soil (2:1) and observed 100% survival after 15 days. Plantlets without cormlets in one in vitro experiment did not survive in a greenhouse [67]. However, all plantlets with cormels planted on solid medium sprouted 100% of the time and matured into strong plants, whereas cormlets grown in liquid medium sprouted at a rate ranging from 30% to 85% depending on the cultivar. Eighty percent of the plants in the mixture of sand, soil, and peat (1:1:1) survived after 31.8 days of hardening [80]. Moreover, hardened the medium consisting of garden soil, sand, and vermicompost (1:1:1) and found greater survivability (50.98%) of gladiolus plantlets [93].
Ex Vitro Acclimatization
Under ex vivo circumstances, a wide range of variables, such as light intensity, relative humidity, nutrients, temperature, variations in CO2 concentration, etc., cause stress [47]. In vitro-grown plantlets were unable to engage with soil microbes and were also unable to cope with environmental conditions after field transfer [46]. In order to maximize survival after transplantation of plants to in vivo conditions, it is necessary to ensure that plantlets grow in a humid environment over a period of 2–4 weeks, depending on the rates at which new leaves and roots are produced [47,48]. According to one study, Cantor and Tolety [154] gladiolus could withstand temperatures as low as 5 °C and as high as 32 °C. As a result, acclimating outside is not advised. Gladiolus plantlets that are created in vitro and then placed in a greenhouse exhibit superior survival in mesh treatments at chilly temperatures (18 ± 2 °C) [29]. Eighty-four to ninety-two percent of the plantlets survived when kept in the shade at 27–28 °C during the day and 19–21 °C at night [125]. The greatest plantlet survival rate was recorded by González-Pérez et al. [155] using a 75% irradiance mesh. A 50% irradiance mesh was next, and plastic cover had the lowest plantlet survival rate. Furthermore, it is commonly known that plants in controlled environments die mostly when they are transferred to uncontrolled ones [29,87]. In an attempt to determine the reason behind the higher temperature mortality, Ref. [155] discovered that the highest leaf temperature was recorded by plantlets with transparent plastic vessels, and that this temperature was also comparable to the maximum gladiolus growth temperature. This fact resulted in the death of plants in this environment because plantlets in their current state of growth were unable to regulate transpiration via stomata, causing them to dehydrate and die [28]. In comparison to the control (45.17 percent), AMF treatment significantly increased plant survival (60.89 percent). Plantlets treated with triazole at a rate of 4 mg L−1 had a greater survival rate [60]. The plantlets were housed in a shaded setting and misted twice a day after the polyethylene caps were removed after one week. After 30 days, about 80% of the plantlets had started over with new growth [54].
In the greenhouse, Ziv et al. [97] transferred in vitro cormels to pots containing peat, volcanic gravel, and vermiculite. After 12–14 weeks of transplanting, cormels germinated, grew four to five leaves, and developed 18–20 mm corms. After pre-treating with or without 100 mg L−1 GA3 for 24 h, Ref. [82] established the in vitro corms on two different potting mixtures containing sand: leaf mold: brick pieces (1:1:1) or sand: compost: brick pieces (1:1:1) and acclimated the corms on a medium containing soil: sand: brick pieces at a 1:1:1 ratio (1:1:1). Although cormels cultured in MS liquid media and then grown in the field did not produce flowers in the first year due to poor vigor, all plants produced flowers in the second year as if by the mother plants [30], and cormlets treated with 100 mg L−1 GA3 and grown in media containing sand, leaf mold, and pieces of brick (1:1:1) exhibited early sprouting with the highest sprouting percentage (83.3 percent) [64].
Somatic embryogenesis generated cormels grown in the soil. The cormlets successfully germinated, and the next year they produced flowers that were true to type [112]. In vitro, cormlets sprouted at a greater rate than conventionally produced cormels in the field [129]. The only medium that showed promise for cormlet germination of the three cultivars was the one containing AC. Without the need to break dormancy, the majority of the cultivated cormlets proved to germinate successfully [69]. According to some research reports, in vitro corms should be held at a lower temperature for a period of time before being planted in the field. Gladiolus cv. Friendship, Gold Finch, and Her Majesty were produced through in vitro treatment at 5 °C for eight weeks and showed 100% sprouting in the field [21]. In vitro, cormels grew in soil, and Sutter (1986) noted sprouting in cormels, but growth stopped after one month [101]. He suggested that cormlets should be kept at 2–3 °C before being grown and chilled for 4 weeks as suggested earlier by [16]. After being kept at 4 °C for two to three months in vitro, 80–90% of the cormels were sown in the field and 20% of them showed flowering in the first year [88]. Sinha and Roy [64] obtained the cormels after two months from the culture vessels. For two to three months, the cormels were kept at 4 °C. After that, cormels were planted in the field and found to germinate with 80–90% success, with 20% flowering in the first year. Nasir and Riazuddin [124] obtained 85–95% germination of cormels in soil after breaking dormancy at 4 °C for 8 weeks at 4 °C.
6. Difficulty and Challenges
The low rate of multiplication of gladiolus limits its commercial cultivation [156]. Approximately 25 cormels and one or two daughter corms are typically produced by a single mother corm each season [64,147,157,158]. Conversely, within a month of planting, daughter cormels emerged from the mother corm’s axillary buds [159]. It took three to four growing seasons for the flowering spike and daughter corms to reach their typical size. The high rate of corm spoilage during storage and fusarium corm rot has a major effect on the commercial production of corms and cormels [64,160,161]. The local need for planting material is not met by this commercial production of corms and cormels, which ultimately drives up corm prices. Another issue in this regard is the corms’ and cormels’ dormancy [27,161,162]. In vitro methods aid in the propagation of corm-producing species in this scenario, as the majority of hybrid gladiolus cultivars multiply very slowly. On the other hand, in vitro techniques yield material devoid of viruses and other pathogens and accelerate plant growth [39,43,156,163,164,165].
Contamination, delayed subculturing, scorched plantlets, browning, problems with in vitro rooting, somaclonal variations, hyperhydricity, necrosis of the shoot tips, albino plantlets, recalcitrance, and abnormalities in the shoots are some of the challenges associated with in vitro propagation [166]. Among the challenges that occur in the in vitro propagation of plants are low photosynthetic efficiency and insufficient vascular connections between the roots and shoots. It is challenging for in vitro plantlets to survive ex vivo due to their aberrant morphology, anatomy, and physiology [152,166,167,168]. Tissue-cultured plants are unlikely to be actively photosynthesizing, despite the fact that they might appear normal in vitro [169]. Due to the persistence of pathogenic microorganisms, conventional vegetative propagation in bulbous crops like gladiolus can result in phytopathological and physiological deterioration; epigenetic changes are the cause of physiological deterioration [170,171]. Bulbs can be difficult to initiate because of endogenous contamination, inadequate axillary branching, inadequate adventitious regeneration, and subpar growth, which is mostly caused by inadequate medium ingredient translocation [171]. The quality of in vitro plantlets depends on robust, uniform propagules that are disease-free. Over time, seeds serve as barriers in sexual propagation, preventing the spread of microorganisms. If the right procedures are followed, then in vitro propagation results in the production of propagules free of disease. Resetting the epigenetic status through sexual propagation revitalizes the plants. Similar results can be obtained through in vitro propagation, particularly if adventitious regeneration is incorporated [170,171].
Liquid medium has several advantages over semi-solid agar medium because gladiolus also multiplied in liquid medium culture [58,59,128]. Furthermore, inadequate gas exchange when tissues are submerged is the main issue with liquid media [172]. The higher risk of contamination with liquid media is another drawback. Medium agitation can help with suffocation, but shear can harm plant tissues in the process. Mesostematic clusters have been used to propagate nerine and gladiolus in liquid media [173,174,175]. By adding GA synthesis inhibitors, shear damage was lessened, resulting in the formation of only meristematic, nodule-like structures and the inhibition of leaf formation. Strong apical dominance is the cause of poor axillary branching. The control that the shoot apex has over the axillary buds is known as apical dominance. Auxin, which is transported from the shoot apex downward and indirectly inhibits axillary bud outgrowth, and cytokinin, which is transported upward from the roots and functions as a stimulant, are the components of the generally accepted underlying mechanism. On the other hand, one would anticipate maximum growth in vitro under tissue culture conditions, which include plenty of water, an abundance of organic and inorganic nutrients, and an appropriate temperature. However, the growth is only marginally different from field growth [170,176]. The most likely explanation for this is inadequate medium ingredient translocation to the explants’ growing regions. Additionally, water flow in the vascular bundles is necessary for long-distance transport in tissue culture, which occurs from the medium to the growing regions of the shoot, which are typically a few centimeters. Under typical circumstances, transpiration and root pressure propel the flow of water in the xylem. Transpiration should be minimal because the relative humidity in tissue culture containers is nearly 100% [177]. Only a small percentage of the transpiration rate in the field has been measured from the leaves of shoots cultivated in vitro [176,178]. This might not be enough to supply enough nutrients. Furthermore, wound tissue forms soon after the explants are cut, which probably acts as a barrier to xylem flow.
Seeing abnormally micropropagated plants has been common. This is due to a few factors: (1) chimeral segregation; (2) epigenetic modifications (long-lasting variations in the way the information in the genome is expressed); (3) genetic changes (also called somaclonal variation; these are variations in the DNA sequence); and (4) spontaneous elimination of pathogens, specifically viruses [170,171]. Additionally, plants are created through adventitious regeneration, particularly through an intermediary callus phase, and this process frequently results in a higher frequency of somaclonal (genetic) variation [179]. Both adventitious and axillary propagation are followed by epigenetic variation. Bushy plants are a significant issue in certain cultivars [180]. This variation is epigenetic, meaning that it results from the altered expression of the genetic information rather than a change in the genetic information itself. This is most likely connected to DNA methylation and/or histone modifications [171].
Cormel formation in vitro is labor-intensive and time-consuming (often several months) in the latter scenario. The present study’s observation of inhibited shoot growth may be the cause of the reduced cormlet formation, as the low rate of multiplication is a significant hindrance in micropropagation and varies from variety to variety [129]. Just a few studies [63,110,114] describe the production of cormlets in this manner. A low relative growth rate, minimal axillary branching, and poor adventitious regeneration are the main causes of the poor bud generation. The occurrence of off-types, ongoing (endogenous) contamination, and the exorbitant cost of micropropagules as with all other crops are the other major issues.
Plant mortality increases when in vitro plants are hardened [84]. It is best to encourage shoots to form storage organs, like cormels in gladiolus, in order to reduce losses during the hardening process of in vitro-grown plants. When needed, these hardy underground organs under storage can be planted. Nonetheless, whether or not the in vitro plantlets with cormels survive is typically determined by the size of the cormels [181]. Larger bulbs (4–10 mm in diameter) demonstrated higher survival rates than smaller ones (2–4 mm) [181]. Moreover, Paek and Murthy [182] justified that every single in vitro-rooted bulblet measured more than 10 mm in diameter is more appropriate for survival. Typically, cormels experience dormancy, which prevents them from sprouting at planting. Moreover, Hussey [16] concluded that corms must be planted after a four-week cold treatment at a temperature between 2 and 5 °C. Furthermore, plant in vitro propagation is one of the best biotechnological solutions to address a number of global issues, such as salinization, desertification, climate change, and the global water crisis.
Poor genotype propagation, the occurrence of off-types, and the high cost of tissue-cultured propagules due to high labor input are the main drawbacks, which are also shared by other crops. Commercial tissue culture is applied to bulbous crops, particularly lilies and Zantedeschia, but other crops that are bulbous experience minimal or no micropropagation [170].
7. Insights and New Technologies for In Vitro Culture of Gladiolus
One of the most important techniques for growing plants is in vitro propagation because it can quickly multiply a selected plant with minimal starting materials. Because it enables the large-scale propagation of multiple plants—something that is not achievable with conventional vegetative or seed propagation techniques—in vitro culture is widely used in both research and commercial settings. Furthermore, the application of in vitro culture technology can effectively achieve the preservation of plant gene pools and genetic diversity [183]. There are several methods that have been used for multiplication of gladiolus using direct and indirect organogenesis [9,28,38,40,41,60].
Bioreactor technology is necessary for the propagation of gladiolus and other bulbous ornamental plants [106,184]. In order for cultured tissues to grow and develop for plant regeneration via organogenesis or embryogenesis, bioreactors provide the ideal environment for combining cultured tissues with a culture medium and maintaining ideal culture conditions, such as temperature, pH, illumination regimes, and aeration (oxygen, carbon dioxide, and other essential gases) [97,103,106]. Due to the various stages of somatic embryogenesis, different bioreactors are used for embryogenic cultures: immersion-type bioreactors are suitable for cormlet regeneration, and temporary immersion cultures are used for adventitious and axillary shoots. There are many different kinds of bioreactors available today that can be helpful in producing high-quality planting materials [184]. Adopting large-scale cultures for plant type and regeneration mode such as fed-batch cultures for cormlets should be the main focus of future research. A change in the medium’s composition, especially in the area of carbohydrates, can reduce expenses and increase yield. Plant regeneration requires modifications such as enhanced aeration, gas supplementation, and light penetration. Scale-up scope and simplified containers are also necessary for effective production.
Tissue culture has improved gladiolus in many ways, including somaclonal variation and genetic stability [51,185], disease-free planting material selection in vitro [186,187,188], protoplast and somatic hybridization [189,190], synthetic seed technology [191,192], and in vitro mutagenesis [193]. Genetic transformation has been used in a number of reports to create transgenic gladiolus plants [24,194,195,196]. Some studies have shown that the in vitro development of an organogenic callus, shoot, root, somatic embryos, and PLBs involves a range of genes and transcriptions. The transcription of these genes is also impacted by the exogenous administration of different growth regulators [197]. DNA methylation across the genome is altered by tissue culture in the CG, CHG, and CHH contexts (where H represents the A, C, or T). These changes also have an impact on gene expression, which may be a regulating factor for the growth and development of plants grown in vitro. Research has demonstrated that alterations in DNA methylation can affect gene expression in the callus and somatic embryos of Arabidopsis and crop plants [198,199]. Furthermore, a variety of fields, such as gene function discovery, transgenic breeding, and rapid micropropagation, have employed tissue culture for plant regeneration. Numerous methods, including over-expression, gene knockout, and genome editing, which are employed in gene function research, rely on genetic transformation in plants. In most species, the most commonly used genetic transformation receptor is the embryogenic callus. On the other hand, Refs. [194,195,196,200,201] have conducted genetic transformation research and created a number of transgenic gladiolus plants using agrobacterium-based techniques. Gladiolus has not been reported to use the CRISPR-Cas system, despite its importance in the development of desirable plants nowadays. CRISPR/Cas9 has been used to regenerate transgenic modified plants using plant cell and tissue culture systems such as protoplast, calluses, suspension culture, SE, and hairy roots [202]. Protoplasts [203] and shoot culture [204] are two in vitro methods that have been used to modify ornamental petunia using CRISPR Cas9.
RNA technology has a major impact on plant development in vitro and genomic function. The current understanding of the roles that several RNAs play in plant regeneration processes holds promise for reprogramming cell fate. Furthermore, the involvement and effects of small interfering RNAs (siRNAs), long non-coding RNAs (lncRNAs), and microRNAs (miRNAs) showed a significant impact on the cell’s differentiation, proliferation, and de-differentiation regulatory networks [205,206,207]. Plant tissues exhibit a general increase in miRNA levels during the de-differentiation phase [208]. When the callus phase is reached, some miRNAs, such as miR156, miR160, miR166, miR395, miR396, and miR397, exhibit low expression levels; this could be a response to the de-differentiation stimulus [209,210]. In addition to having low levels of miRNA, non-embryogenic calluses have been shown to have up-regulated levels of certain miRNAs, including miR169, miR172, miR390, miR395, and miR408 [211,212]. Compared to less embryogenic tissues, those with high embryogenic potential exhibit higher levels of miRNA; miR156, miR164, and miR394 primarily showed the utility in maize [213]. Since callus initiation repressors miR397, miR160, and miR166 were also discovered to be significant, they influenced the interaction of auxin and cytokinin during organogenesis as well as through controlling response genes and auxin biosynthesis, respectively [214]. Moreover, in the presence or absence of photoperiod, the levels of miR156, miR164, miR168, miR397, miR398, miR408, and miR528 tend to increase after hormone depletion, demonstrating the important influence of hormone on the expression of particular miRNAs during plant regeneration [215]. On the other hand, photoperiod appears to affect certain miRNA targets and the expression levels of miR167a, miR168a, and miR164a, which rise when the callus is cultivated in light [215]. Their stage-specific roles in bud formation are revealed by the increase of those miRNAs [216,217]. In this type of callus or tissue, silencing these miRNAs may improve embryogenic competency and, eventually, its expression. MiR397 continuously accumulates during the phases of plant regeneration, peaking in the adventitious shoots, suggesting a major regulatory role for morphogenesis during the advanced stages of differentiation [217]. Improved control over in vitro regeneration processes like organogenesis and somatic embryogenesis will be possible with a deeper comprehension of the role of plant ncRNAs in somatic cell reprogramming. Since there is no documentation on the application of RNA technology in vitro culture in gladiolus, this technology presents a novel approach to improving gladiolus production.
8. Conclusions and Future Prospects
In this analysis, the success of direct and indirect methods of plantlet regeneration and in vitro cormel formation is discussed from the available literature worldwide. There are various physical and chemical elements that affect in vitro plant development as well as cormels, which are extensively covered in the literature. Based on the currently available literature, gladiolus micropropagation through somatic embryogenesis has been suggested as a feasible technique. Because somatic embryogenesis originates from a single cell, it is one of the most suitable technologies for genetic modification of plants and can lead to plant regeneration. The potential for reducing the time required to obtain seeds is presented by the capacity of cell suspension cultures to generate somatic embryos, particularly in the case of gladiolus. Long-term storage is possible for embryogenic calluses derived from mature gladiolus plants under the correct conditions. Certain studies suggest that the source of gladiolus mass multiplication may be related to the stability of stored embryogenic calluses and their capacity to develop into plants. There are only a few publications on the use of somatic embryogenesis and factors that affect somatic embryogenesis efficiency in gladiolus. As a result, the use of this strategy for gladiolus should be given a lot more emphasis. However, the formation of in vitro corms using in vitro techniques appears to hold promise for future commercial corm production and quality. A little effort has been made with a few genotypes of gladiolus toward the formation of in vitro cormels, followed by the survival of plantlets and flower output. One study found that cormels grown in vitro produced flowers in the field. For the successful establishment of in vitro cormel, field establishment, and eventually generated flowers, more in vitro work on multiple genotypes is required. The use of bioreactors in gladiolus micropropagation is not well understood. In plant propagation, the use of a “bioreactor” can speed up the multiplication and growth of cultures while saving space, energy, and labor [97]. In a bioreactor, the liquid medium allows for close contact with the tissue, which promotes and improves nutrient and PGR uptake, resulting in improved shoot and root growth. Very little study has been done on the application of bioreactors in gladiolus [97,100]. As a result, more research work into the use of bioreactors in gladiolus is required. There is no report available where low-cost in vitro protocols have been recommended for the multiplication of plantlets as well as cormels. Therefore, a low-cost in vitro protocol is urgently needed. Poor in vitro cormel formation, inferior growth, off-type plants, contamination of explants used as cormels, and poor new bud formation are some of the challenges associated with in vitro propagation. Therefore, these points should be considered prior to initiating in vitro work on the gladiolus. Since no research of this kind has been done on gladiolus, modern molecular tools such as CRISPR Cas [202] and RNAi [207] are also used in in vitro culture for genetic improvement in plants. As a result, these methods using in vitro culture are more successful for genetic improvement in gladiolus. However, genetic engineering is a major factor in ornamental gladiolus genetic modification. Suspension cultures, cryopreservation, and synthetic seed technologies have received minimal research in Gladiolus species. As a result, in order to develop an efficient in vitro biotechnological production system for this crop, basic processes for suspension cultures, cryopreservation, and synthetic seed creation are urgently needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10020148/s1, Table S1: Tissue culture studies in gladiolus (Gladiolus species).
Author Contributions
Conceptualization, M.K., V.C. and J.S.; methodology, M.K.Y., S.P., V.K., V.P. and C.C.; resources, A.K., K.K., D.S. and U.S.; writing—original draft preparation, C.C., K.K., D.S., M.K., R.M., S.K., S.M., M.K., M.K.Y. and J.S.; writing—review and editing, M.K., M.K.Y. and J.S.; supervision, M.K., M.K.Y. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
The authors are thankful to anonymous reviewers and the Central Library, Sardar Vallabhbhai Patel University of Agriculture and Technology, Meerut, UP, India for providing access to online articles and the e-resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, M.; Chaudhary, V.; Sirohi, U.; Srivastav, A.L. Economically viable flower drying techniques to sustain flower industry amid COVID-19 pandemic. Environ. Dev. Sustain. 2023, 1–46. [Google Scholar] [CrossRef]
- Pragya; Ranjan, J.K.; Attri, B.; Das, H.K.; Ahmed, N. Performance of gladiolus genotypes for cut flower and corm production under high altitude of Uttarakhand. Indian J. Hortic. 2010, 67, 386–390. [Google Scholar]
- Manning, J.; Goldblatt, P. The Iris Family: Natural History and Classification; Timber Press: Portland, OR, USA, 2008; pp. 138–142. [Google Scholar]
- Anonymous. Gladiolus; Technical Bulletin no. 14; Division of Floriculture and Landscaping, IARI: New Delhi, India, 2002; p. 100. [Google Scholar]
- Delpierre, G.R.; Du Plessis, N.M. The Winter-Growing Gladiolus of South Africa; Tafelberg: Cape Town, South Africa, 1974; p. 71. [Google Scholar]
- Raghava, S.P.S. Genetic Improvement of Gladiolus in India. In Proceedings of the National Conference on Gladiolus, Lucknow, India, 24–25 January 2000; NBRI: Lucknow, India, 2000; p. 1. [Google Scholar]
- Kadam, J.J.; Agale, R.C.; Rite, S.C.; Pandav, S.M. Exploration of fungicides and phyto-extract against Fusarium oxysporum f. sp. gladioli causing corm rot of gladiolus. Discov. Agric. 2014, 2, 61–64. [Google Scholar]
- Hussain, I.; Muhammad, A.; Rashid, H.; Quraishi, A. In vitro multiplication of gladiolus. Plant Tissue Cult. 2001, 11, 121–126. [Google Scholar]
- Memon, N.; Qasim, M.; Jaskani, M.J.; Ahmad, R. In vitro cormel production of gladiolus. Pak. J. Agric. Sci. 2010, 47, 115–123. [Google Scholar]
- Kamo, K.; Rajasekaran, K.; Cary, J. Growth characteristics of micropropagated, regenerated and transgenic gladiolus plants. J. Appl. Hortic. 2014, 16, 193–198. [Google Scholar] [CrossRef]
- Mujib, A.; Ali, M.; Tonk, D.; Zafar, N. Nuclear 2C DNA and genome size analysis in somatic embryo regenerated gladiolus plants using flow cytometry. Adv. Hortic. Sci. 2017, 31, 165–174. [Google Scholar]
- Kumar, A.; Kumar, A.; Sharma, V.; Mishra, A.; Singh, S.; Kumar, P. In vitro regeneration of gladiolus (Gladiolus hybrida L.): Optimization of growth media and assessment of genetic fidelity. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2900–2909. [Google Scholar] [CrossRef]
- Ziv, M.; Halevy, A.H.; Shilo, R. Organs and plantlets regeneration of Gladiolus through tissue culture. Ann. Bot. 1970, 34, 671–676. [Google Scholar] [CrossRef]
- Simonsen, J.; Hildebrandt, A.C. In vitro growth and differentiation of gladiolus plants from callus cultures. Can. J. Bot. 1971, 49, 1817–1819. [Google Scholar] [CrossRef]
- Hussey, G. Totipotency in tissue explants and callus of some members of the Liliaceae, Iridaceae, and Amaryllideceae. J. Exp. Biol. 1975, 26, 253–256. [Google Scholar]
- Hussey, G. In vitro propagation of gladiolus by precocious axillary shoot formation. Sci. Hortic. 1977, 6, 287–296. [Google Scholar] [CrossRef]
- Hussey, G. In vitro propagation of some members of the Liliaceae, Iridaceae and Amaryllideace. Acta Hortic. 1977, 78, 303–309. [Google Scholar] [CrossRef]
- Ziv, M. Transplanting gladiolus plants propagated in vitro. Sci. Hortic. 1979, 11, 257–260. [Google Scholar] [CrossRef]
- Logan, A.E.; Zettler, F.W. Rapid in vitro propagation of virus-indexed gladioli. Acta Hortic. 1985, 164, 169–180. [Google Scholar] [CrossRef]
- Begum, S.; Haddiuzaman, S. In vitro rapid shoot proliferation and corm development in Gladiolus grandiflorus cv. Redbrand. Plant Tissue Cult. 1995, 5, 7–12. [Google Scholar]
- Dantu, P.K.; Bhojwani, S.S. In vitro corm formation and field evaluation of corm-derived plants of gladiolus. Sci. Hortic. 1995, 61, 115–129. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Palni, L.M.S.; Gupta, A.K. In vitro propagation of Gladiolus hybridus Hort.: Synergistic effect of heat shock and sucrose on morphogenesis. Plant Cell Tissue Organ Cult. 1999, 57, 105–112. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, M.S.; Nasir, I.A.; Riazuddin, S. In vitro production of cormlets in gladiolus. Pak. J. Biol. Sci. 2000, 3, 819–821. [Google Scholar] [CrossRef][Green Version]
- Babu, P.; Chawla, H.S. In vitro regeneration and Agrobacterium mediated transformation in gladiolus. J. Hortic. Sci. Biotechnol. 2000, 75, 400–404. [Google Scholar] [CrossRef]
- Boonvanno, K.; Kanchanapoom, K. In vitro propagation of gladiolus. Suranaree J. Sci. Technol. 2000, 7, 25–29. [Google Scholar]
- Ziv, M.; Lilien-Kipnis, H. Bud regeneration from inflorescence explants for rapid propagation of geophytes in vitro. Plant Cell Rep. 2000, 19, 845–885. [Google Scholar] [CrossRef]
- Priyakumari, I.; Sheela, V.L. Micropropagation of gladiolus cv. ‘Peach Blossom’ through enhanced release axillary buds. J. Trop. Agric. 2005, 43, 47–50. [Google Scholar]
- Aftab, F.; Alam, M.; Afrasiab, H.; Afrasiab, H. In vitro shoot multiplication and callus induction in Gladiolus hybridus Hort. Pak. J. Bot. 2008, 40, 517–522. [Google Scholar]
- Memon, N. In vitro propagation of gladiolus plantlets and cormels. J. Hortic. Sci. Ornam. Plants 2012, 4, 280–291. [Google Scholar]
- Saha, B.; Datta, A.K.; Datta, S.; da Silva, J.A.T. In vitro corm development, field evaluation and determination of genetic stability of corm-derived elite Gladiolus germplasm. Floric. Ornam. Biotechnol. 2013, 7, 82–85. [Google Scholar]
- Memon, N.; Jasakni, M.J.; Qasim, M.; Sharif, N. Cormel formation in gladiolus through tissue culture. Pak. J. Agric. Sci. 2014, 51, 475–482. [Google Scholar]
- Nezamabad, P.S.; Habibi, M.K.; Dizadji, A.; Kalantari, S. Elimination of bean yellow mosaic virus through thermotherapy combined with meristem-tip culture in gladiolus corms. J. Crop Prot. 2015, 4, 533–543. [Google Scholar]
- Kundang, N.; Kumar, V.; Kumar, S. Effect of macronutrients and plant growth hormones for the in vitro formation of cormlets of Gladiolus pacifica. CIB-Tech. J. Biotechnol. 2017, 6, 10–16. [Google Scholar]
- Tripathi, M.K.; Malviya, R.K.; Vidhyashankar, M.; Patel, R.P. Effect of plant growth regulators on in vitro morphogenesis in gladiolus (Gladiolus hybridus Hort.) from cultured corm slice. Int. J. Agric. Technol. 2017, 13, 583–599. [Google Scholar]
- Malviya, R.K.; Tripathi, M.; Vidhyashankar, M.; Patel, R.P.; Ahuja, A. Effect of different phytohormones on plant regeneration of gladiolus (Gladiolus hybridus Hort.) from cultured cormel. Asian J. Microbiol. Biotechnol. Environ. Sci. 2018, 19, 155–165. [Google Scholar]
- Devi, P.; Kumar, P.; Sengar, R.S.; Yadav, M.K.; Kumar, M.; Singh, S.K.; Singh, S. In-vitro multiple shoots production from cormel shoot buds in gladiolus (Gladiolus hybrida). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1345–1350. [Google Scholar] [CrossRef]
- Budiarto, K.; Rosario, T.L. Evaluation of culture media for in vitro conservation of gladiolus cultivars. AGRIVITA J. Agric. Sci. 2020, 42, 205–213. [Google Scholar] [CrossRef]
- Deshmukh, V.D.; Kharde, A.V.; Talekar, B.K. Interactive effects of ba and iaa on shoot proliferation of gladiolus (Gladiolus grandiflorus) var. white prosperity. J. Orient. Res. Madras 2021, 90, 12–19. [Google Scholar]
- Isah, T.; Qurratul; Umar, S. Influence of silver nitrate and copper sulphate on somatic embryogenesis, shoot morphogenesis, multiplication and associated physiological biochemical changes in Gladiolus hybridus L. Res. Sq. 2022, 149, 563–587. [Google Scholar]
- Poothong, S.; Duangkon, N.; Sukanthamala, S. The effects of sucrose, activated charcoal, and coconut dust on in vitro corm induction of Gladiolus hybrida under aseptic technique. Health Sci. Sci. Technol. Rev. 2023, 16, 28–39. [Google Scholar]
- Tripathi, M.K.; Tiwari, S.; Tripathi, N.; Malviya, R.K.; Bhatt, D. In vitro morphogenesis in gladiolus (Gladiolus hybridus Hort.) from corm slice. Mol. Biol. Plant Physiol. 2023, 33, 73. [Google Scholar]
- Tripathi, M.K.; Tripathi, N.; Tiwari, S.; Malviya, R.K.; Tiwari, P.N.; Tiwari, S. Plant regeneration from cultured cormel explants in gladiolus (Gladiolus hybridus HORT.). Mol. Biol. Plant Physiol. 2023, 33, 139. [Google Scholar]
- Kumar, M.; Sirohi, U.; Yadav, M.K.; Chaudhary, V. In vitro culture technology and advanced biotechnology tools toward improvement in gladiolus (Gladiolus species): Present scenario and future prospects. Mol. Biotechnol. 2023, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.M.; Daffalla, H.M.; Khalfala, M.M. In Vitro micropropagation of the ornamental plant Dieffenbachia—A review. Univers. J. Plant Sci. 2013, 1, 91–99. [Google Scholar] [CrossRef]
- Azmi, N.S.; Ahmad, R.; Ibrahim, R. Effects of red and blue (RB) LED on the in vitro growth of Rosa kordesii in multiplication phase. In Proceedings of the 2nd International Conference on Agriculture and Biotechnology, Singapore, 12–14 March 2014. [Google Scholar]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef]
- Preece, J.E.; Sutter, E.G. Acclimatization of micropropagated plants to the greenhouse and field. In Micropropagation; Debergh, P.C., Zimmerman, R.H., Eds.; Kluwer Academic Publishers: New York, NY, USA, 1991; pp. 71–93. [Google Scholar]
- Roberts, A.V.; Smith, E.F.; Mottley, J. The preparation of micropropagated plantlets for transfer to soil without acclimatization. In Methods in Molecular Biology; Pollard, J.W., Walker, J.M., Eds.; Springer: New York, NY, USA, 1990; Volume 6, pp. 227–236. [Google Scholar]
- Ascough, G.D.; Erwin, J.E.; Van Staden, J. Micropropagation of Iridaceae—A review. Plant Cell Tissue Organ Cult. 2009, 97, 1–19. [Google Scholar] [CrossRef]
- Datta, S.K. Breeding of Ornamentals: Gladiolus. LS—Int. J. Life Sci. 2020, 9, 115–133. [Google Scholar] [CrossRef]
- Memon, N.; Qasim, M.; Jaskani, M.J.; Awan, F.S.; Khan, A.I.; Sadia, B.; Hussain, Z. Assessment of somaclonal variation in in vitro propagated cormels of gladiolus. Pak. J. Bot. 2012, 44, 769–776. [Google Scholar]
- Bajaj, Y.P.S.; Sidhu, M.M.S.; Gill, A.P.S. Some factors affecting the in vitro propagation of Gladiolus. Sci. Hortic. 1983, 18, 269–275. [Google Scholar] [CrossRef]
- Pragya; Singh, S.K.; Misra, R.L.; Ranjan, J.K. In vitro shoot regeneration from cormel derived callus of gladiolus and bio-hardening of plantlets. Indian J. Biotechnol. 2012, 11, 99–104. [Google Scholar]
- Kabir, M.H.; Mamun, A.N.K.; Yesmin, F.; Subramaniam, S. In vitro propagation of Gladiolus dalenii from the callus through the culture of corm slices. J. Phytol. 2014, 6, 40–45. [Google Scholar]
- Kamo, K. A cultivar comparison of plant regeneration from suspension cells, callus, and cormel slices of gladiolus. In Vitro Cell. Dev. Biol.-Plant 1995, 31, 113–115. [Google Scholar] [CrossRef]
- Pathania, N.S.; Misra, R.L.; Raghava, S.P.S. Precocious shoot proliferation and microcorm production in gladiolus through tissue culture. J. Ornam. Hortic. 2001, 4, 69–73. [Google Scholar]
- Roy, S.K.; Gangopadhyay, G.; Bandyopadhyay, T.; Modak, B.K.; Datta, S.; Mukherjee, K.K. Enhancement of in vitro micro corm production in gladiolus using alternative matrix. Afr. J. Biotechnol. 2006, 5, 1204–1209. [Google Scholar]
- Nhut, D.T.; da Silva, J.A.T.; Huyen, P.X.; Paek, K.Y. The importance of explant source on regeneration and micropropagation of Gladiolus by liquid shake culture. Sci. Hortic. 2004, 102, 407–414. [Google Scholar] [CrossRef]
- Prasad, V.S.S.; Gupta, S.D. In vitro shoot regeneration of gladiolus in semi-solid agar versus liquid cultures with support systems. Plant Cell Tissue Organ Cult. 2006, 87, 263–271. [Google Scholar] [CrossRef]
- Sheena, A.; Sheela, V.L. Effects of the growth retardant triadimefon on the ex vitro establishment of gladiolus (Gladiolus grandiflorus L.) cv. Vinks Glory. Plant Tissue Cult. Biotechnol. 2010, 20, 171–178. [Google Scholar] [CrossRef]
- Kamo, K. Effect of phytohormones on plant regeneration from callus of gladiolus cultivar “Jenny Lee”. In Vitro Cell. Dev. Biol. 1994, 30, 26–31. [Google Scholar] [CrossRef]
- Goo, D.H.; Joung, H.Y.; Kim, K.W. Differentiation of gladiolus plantlets from callus and subsequent flowering. Acta Hort 2003, 620, 339–342. [Google Scholar] [CrossRef]
- Emek, Y.; Erdag, B. In vitro propagation of Gladiolus anatolicus (Boiss.) Stapf. Pak. J. Bot. 2007, 39, 23–30. [Google Scholar]
- Sinha, P.; Roy, S.K. Plant regeneration through in vitro cormel formation from callus culture of Gladiolus primulinus Baker. Plant Tissue Cult. 2002, 12, 139–145. [Google Scholar]
- Stefaniak, B. Somatic embryogenesis and plant regeneration of gladiolus (Gladiolus hort.). Plant Cell Rep. 1994, 13, 386–389. [Google Scholar] [CrossRef]
- Mokshin, E.V.; Lukatkin, A.S.; Teixeira da Silva, J.A. Micropropagation of selected cultivars of lilium and gladiolus. Floric. Ornam. Plant Biotechnol. 2006, 2, 533–539. [Google Scholar]
- Thun, V.; Goo, D.H.; Kim, M.H.; Byun, M.S.; Kim, K.W. Effect of in vitro culture environments and culture methods on cormlet formation of gladiolus. Hortic. Environ. Biotechnol. 2008, 49, 114–120. [Google Scholar]
- Budiarto, K. In vitro regeneration of three gladiolus cultivars using cormel explants. J. ILMU DASAR 2009, 10, 109–113. [Google Scholar]
- El-Kazzaz, A.A.; El-Bagoury, H.; Mahmoud, I.; El Tantawy, A.A.; Al-Ansary, A.A. Production of gladiolus germplasm via in vitro tissue cultures. J. Duhok Univ. 2012, 15, 440–447. [Google Scholar]
- Haouala, F.; Chaieb, E. Effects of explant position and polarity on callus induction and shoot regeneration of gladiolus (Gladiolus hybridus Hort). Floric. Ornam. Biotechnol. 2012, 6, 133–139. [Google Scholar]
- Beura, S.; Singh, R.; Jagadiv, P.N. In vitro cloning of gladiolus cv. American Beauty. J. Ornam. Hortic. 2005, 8, 268–271. [Google Scholar]
- Remotti, P.C.; Löffler, H.J. Callus induction and plant regeneration from gladiolus. Plant Cell Tissue Organ Cult. 1995, 42, 171–178. [Google Scholar] [CrossRef]
- Zaidi, N.; Khan, N.H.; Zafar, F.; Zafar, S.I. Bulbous and cormous monocotyledonous ornamental plants in vitro. Sci. Vis. 2000, 6, 58–73. [Google Scholar]
- Cardoso, J.C.; Teixeira Da Silva, J.A. Micropropagation of gerbera using chlorine dioxide (ClO2) to sterilize the culture medium. In Vitro Cell. Dev. Biol. Plant 2012, 48, 362–368. [Google Scholar] [CrossRef]
- Belanekar, S.B.; Nadkarni, H.R.; Sawant, S.S.; Gokhale, N.B. Micropropagation in gladiolus (Gladiolus grandiflorus L.) var. White Friendship. J. Maharashtra Agric. Univ. 2010, 35, 66–68. [Google Scholar]
- Te-Chato, S.; Naksombut, S.; Boonsiri, J. Effect of variety and explant on callus formation and micropropagation of anthurium. Warasan Songkhla Nakharin J. Sci. Technol. 2002, 24, 569–578. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant tissue culture procedure-background. In Plant Propagation by Tissue Culture; Springer: Dordrecht, The Netherlands, 2008; pp. 1–28. [Google Scholar]
- George, E.F.; Debergh, P.C. Micropropagation: Uses and methods. In Plant Propagation by Tissue Culture; Springer: Dordrecht, The Netherlands, 2008; pp. 29–64. [Google Scholar]
- Gupta, S.D.; Datta, S. Antioxidant enzyme activities during in vitro morphogenesis of gladiolus and the effect of application of antioxidants on plant regeneration. Biol. Plant. 2003, 47, 179–183. [Google Scholar] [CrossRef]
- Mateen, R.M. Development and optimization of micro-propagation, in vitro methodology for gladiolus. BioScientific Rev. 2019, 1, 21–36. [Google Scholar] [CrossRef]
- Choudhary, D.; Agarwal, G.; Singh, V.P.; Arora, A. In vitro micropropagation of Gladiolus grandiflora (var. Snow Princess) flower from cormel explant. Indian J. Plant Physiol. 2010, 15, 90–93. [Google Scholar]
- Dharmasena, P.A.I.U.; Karunananda, D.P.; Eeswara, J.P. Effect of gibberellic acid (GA3) and sugar on in vitro cormlet formation, multiplication and ex vitro sprouting of Gladiolus hybrida variety Princess Lee. Trop. Agric. Res. 2011, 23, 1–10. [Google Scholar] [CrossRef]
- Jala, A. Potential of benzyl adenine, naphthalene acetic acid and sucrose concentration on growth, development, and regeneration of new shoot and cormel on gladiolus. Am. Trans. Eng. Appl. Sci. 2013, 2, 277–285. [Google Scholar]
- González-Pérez, E.; Juárez-Muñoz, J.; Ayala-Garay, O.J.; Yáñez-Morales, M.; De, J. Ex vitro acclimatization of gladiolus plantlets. Propag. Ornam. Plants 2014, 14, 125–132. [Google Scholar]
- Akhare, A.A.; Dhumale, D.B.; Sakhare, S.B.; Ekta, S. Remove from marked records in vitro establishment of gladiolus cv. White Friendship and Fidelio using axillary buds as explant. Asian J. Hortic. 2008, 3, 149–152. [Google Scholar]
- Suzuki, K.; Takatsu, Y.; Gonai, T.; Kasumi, M. Plant regeneration and chromosome doubling of wild Gladiolus species. Acta Hortic. 2005, 673, 175–181. [Google Scholar] [CrossRef]
- Torabi-Giglou, M.; Hajieghrari, B. In vitro study on regeneration of Gladiolus grandiflorus corm calli as affected by plant growth regulators. Pak. J. Biol. Sci. 2008, 11, 1147–1154. [Google Scholar] [CrossRef][Green Version]
- Ojha, A.; Sharma, V.; Sharma, V.N.; Rawat, A. Effect of different carbohydrates sources on the formation of cormlets of economic important plant: Gladiolus pacifica. Int. J. Biotechnol. Biochem. 2010, 6, 485–491. [Google Scholar]
- Rakosy-Tican, E.; Bors, B.; Szatmari, A. In vitro culture and medium-term conservation of the rare wild species Gladiolus imbricatus. Afr. J. Biotechnol. 2012, 11, 14703–14712. [Google Scholar]
- Bera, A.K.; Maity, T.R.; Samanta, A.; Dolai, A.; Saha, B.; Datta, S. Enhancement of in vitro corm production in gladiolus by periodically replacement of liquid media using coir matrix. J. Appl. Hortic. 2015, 17, 222–224. [Google Scholar] [CrossRef]
- Aslam, F.; Habib, S.; Naz, S. Effect of different phytohormones on plant regeneration of Amaryllis hippeastrum. Pak. J. Sci. 2012, 64, 54–58. [Google Scholar]
- Memon, N.; Qasim, M.; Jaskani, M.J.; Khooharo, A.A.; Hussain, Z.; Ahmad, I. Comparison of various explants on the basis of efficient shoot regeneration in gladiolus. Pak. J. Bot. 2013, 45, 877–885. [Google Scholar]
- Devi, K.L.; Maitra, S.; Mandal, M.; Thokchom, R. In-vitro Response of Gladiolus (var. American Beauty) towards Plant Growth Regulators Using Cormel Explants. Int. J. Environ. Clim. Chang. 2023, 13, 388–395. [Google Scholar] [CrossRef]
- ASalih, E.E.; AlSalihy, A.A. Using of UV light type C and growth regulators in propagation and cormels formation in Gladiolus spp. Ann. Rom. Soc. Cell Biol. 2021, 25, 8486–8497. [Google Scholar]
- Kamo, K.; Joung, Y.H. Gladiolus. In Biotechnology in Agriculture and Forestry; Pua, E.C., Davey, M.R., Eds.; Transgenic Crops VI; Springer: Berlin/Heidelberg, Germany, 2007; Volume 61, pp. 289–298. [Google Scholar]
- Dantu, P.K.; Bhojwani, S.S. In vitro propagation and corm formation in gladiolus. Gartenbauwissenschaft 1987, 52, 90–93. [Google Scholar]
- Ziv, M.; Ronen, G.; Raviv, M. Proliferation of meristematic clusters in disposable presterilized plastic bio-reactors for large-scale micropropagation of plants. In Vitro Cell. Dev. Biol. Plant 1998, 34, 152–158. [Google Scholar] [CrossRef]
- Ziv, M. The effect of growth retardants on shoot proliferation and morphologenesis in liquid cultured Gladiolus plants. Acta Hortic. 1990, 280, 207–214. [Google Scholar] [CrossRef]
- Ziv, M. Morphogenic control of plants micropropagated in bioreactor cultures and its possible impact on acclimatization. Acta Hortic. 1992, 319, 119–124. [Google Scholar] [CrossRef]
- Ziv, M.; Lilien-Kipnis, H. Gladiolus. In Handbook of Plant Cell Culture; Ammirato, Ed.; McGraw-Hill: New York, NY, USA, 1990; Volume 5, pp. 461–478. [Google Scholar]
- Sutter, E.G. Micropropagation of Ixia viridifolia and a Gladiolus × Homoglossum hybrid. Sci. Hortic. 1986, 29, 181–189. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Prasad, V.S.S. Shoot multiplication kinetics and hyperhydric status of regenerated shoots of gladiolus in agar-solidified and matrix-supported liquid cultures. Plant Biotechnol. Rep. 2010, 4, 85–94. [Google Scholar] [CrossRef]
- Lipsky, A.K.; Kataeva, N.V.; Butenko, R.G. Comparison of parameters of the gladiolus bud cultures grown in the bio-reactor at different regimes. Acta Hortic. 1997, 447, 671–673. [Google Scholar] [CrossRef]
- Eide, A.K.; Munster, C.; Heyerdahl, P.H.; Lyngved, R.; Olsen, O.A.S. Liquid culture systems for plant propagation. Acta Hortic. 2003, 625, 173–185. [Google Scholar] [CrossRef]
- Rout, G.R.; Mohapatra, A.; Jain, S.M. Tissue culture of ornamental pot plant: A critical review on present scenario and future prospects. Biotechnol. Adv. 2006, 24, 531–560. [Google Scholar] [CrossRef] [PubMed]
- Ziv, M. Simple bio-reactors for mass propagation of plants. Plant Cell Tissue Organ Cult. 2005, 81, 277–285. [Google Scholar] [CrossRef]
- Dogra, N.; Dhatt, K.K. In vitro production of cormels in Gladiolus hybridus through gamma rays. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1308–1318. [Google Scholar] [CrossRef]
- Erdag, B.B.; Calmaz Emek, Y.; Aktas, L.Y. In vitro somatic embryogenesis from cormel derived callus cultures of Gladiolus anatolicus (Boiss.) Stapf. Propag. Ornam. Plants 2009, 9, 176–180. [Google Scholar]
- Rajasekharan, P.E.; Rao, T.M.; Janakiram, T.; Ganeshan, S. Freeze preservation of gladiolus pollen. Euphytica 1994, 80, 105–109. [Google Scholar] [CrossRef]
- DNagaraju, V.; Bhowmik, G.; Parthasarathy, V.A. Effect of paclobutrazol and sucrose on in vitro cormel formation in gladiolus. Acta Bot. Croat. 2002, 61, 27–33. [Google Scholar]
- Ruffoni, B.; Savona, M.; Barberini, S. Biotechnological support for the development of new Gladiolus Hybrids. Floric. Ornam. Biotechnol. 2012, 6, 45–52. [Google Scholar]
- Kumar, A.; Palni, L.M.S.; Sood, A.; Sharma, M.; Palni, U.T.; Gupta, A.K. Heat-shock induced somatic embryogenesis in callus cultures of gladiolus in the presence of high sucrose. J. Hortic. Sci. Biotechnol. 2002, 77, 73–78. [Google Scholar] [CrossRef]
- Dantu, P.K.; Bhojwan, S.S. In vitro propagation of gladiolus: Optimisation of conditions for shoot multiplication. J. Plant Biochem. Biotechnol. 1992, 1, 115–118. [Google Scholar] [CrossRef]
- Steinitz, B.; Cohen, A.; Goldberg, Z.; Kochba, M. Precocious gladiolus corm formation in liquid shake cultures. Plant Cell Tissue Organ Cult. 1991, 26, 63–70. [Google Scholar] [CrossRef]
- De Bruyn, M.H.; Ferreira, D.I. In vitro corm production of Gladiolus dalenii and G. tristis. Plant Cell Tissue Organ Cult. 1992, 31, 123–128. [Google Scholar] [CrossRef]
- Bettaieb, T. Régénération in vitro de Variants Somaclonaux de Glaïeul (Gladiolus grandiflorus Hort.) Tolérants aux Basses Températures. Ph.D. Dissertation, Instituut Voor Tropische Geneeskunde, Antwerpen, Belgium, 2003. [Google Scholar]
- Kamo, K.; Gera, A.; Cohen, J.; Hammond, J.; Blowers, A.; Smith, F.; Van Eck, J. Transgenic gladiolus plants transformed with the bean yellow mosaic virus coat-protein gene in either sense or antisense orientation. Plant Cell Rep. 2005, 23, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Kamo, K.; Van Eck, J. Effect of bialaphos and phosphinothricin on plant regeneration from long and short-term callus cultures of gladiolus. In Vitro Cell. Dev. Biol.-Plant 1997, 33, 180–183. [Google Scholar] [CrossRef]
- Zimmerman, J.L. Somatic embryogenesis: A model for early development in higher plants. Plant Cell 1993, 5, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Nic-Can, G.I.; Galaz-Ávalos, R.M.; De-la-Peña, C.; Alcazar-Magaña, A.; Wrobel, K.; Loyola-Vargas, V.M. Somatic embryogenesis: Identified factors that lead to embryogenic repression. A case of species of the same genus. PLoS ONE 2015, 10, e0126414. [Google Scholar] [CrossRef] [PubMed]
- Kamo, K.; Chen, J.; Lawson, R. The establishment of cell suspension cultures of gladiolus that regenerate plants. In Vitro Cell. Dev. Biol. 1990, 26, 425–430. [Google Scholar] [CrossRef]
- Remotti, P.C. Primary and secondary embryogenesis from cell suspension cultures of gladiolus. Plant Sci. 1995, 107, 205–214. [Google Scholar] [CrossRef]
- Wu, J.; Liu, C.; Seng, S.; Khan, M.A.; Sui, J.; Gong, B.; Liu, C.; Wu, C.; Zhong, X.; He, J.; et al. Somatic embryogenesis and Agrobacterium-mediated transformation of Gladiolus hybridus cv. ‘Advance Red’. Plant Cell Tissue Organ Cult. 2015, 120, 717–728. [Google Scholar] [CrossRef]
- Nasir, I.A.; Riazuddin, S. In vitro selection for Fusarium wilt resistance in gladiolus. J. Integr. Plant Biol. 2008, 50, 601–612. [Google Scholar] [CrossRef]
- Lilien-Kipnis, H.; Kochba, M. Mass propagation of new Gladiolus hybrids. Acta Hortic. 1987, 212, 631–638. [Google Scholar] [CrossRef]
- Fridborg, S.L.; Ericsson, T. Effect of activated charcoal on growth and morphogenesis in cell cultures. Physiol. Plant. 1975, 34, 306–308. [Google Scholar] [CrossRef]
- Hussain, S.C.; Geetha, C.K.; Rajeevan, P.K.; Valsalakumari, P.K. Plant regeneration from root derived callus in gladiolus. J. Ornam. Hortic. 1994, 2, 46–50. [Google Scholar]
- Ziv, M. Enhanced shoot and cormlet proliferation in liquid cultured gladiolus buds by growth retardants. Plant Cell Tissue Organ Cult. 1989, 17, 101–110. [Google Scholar] [CrossRef]
- Kumar, A.; Palni, L.M.S.; Sood, A. Factors affecting in vitro formation of cormlets in Gladiolus hybridus Hort. and their field performance. Acta Physiol. Plant. 2011, 33, 509–515. [Google Scholar] [CrossRef]
- Sen, J.; Sen, S. Two-step bud culture technique for a high frequency regeneration of gladiolus corms. Sci. Hortic. 1995, 64, 133–138. [Google Scholar] [CrossRef]
- Al-Juboory, K.H.; Shibli, R.A.; Skiryn, R. Organogenesis and cormel production from callus culture of gladiolus cv. Balady. Mutah. J. Res. Stud. 1997, 12, 143–160. [Google Scholar] [CrossRef]
- Arora, J.S.; Singh, K.; Grewal, H.S.; Gosal, S.S.; Chanana, Y.R. In vitro cormel production from nodal buds and cormel tips in gladiolus. In Plant Tissue Culture; Islam, A.S., Ed.; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India; Calcutta, India, 1996; pp. 50–53. [Google Scholar]
- Kim, K.W.; Han, S.Y. Cormlet formation in gladiolus shoot base by growth retardants in vitro. J. Korean Soc. Hortic. Sci. 1993, 34, 136–144. [Google Scholar]
- Mares, D.J.; Sowokinos, J.R.; Hawker, J.S. Carbohydrate metabolism in developing potato tubers. In Potato Physiology; Li, P.H., Ed.; Academic Press: Orlando, FL, USA, 1985; pp. 279–327. [Google Scholar]
- Goo, D.H.; Kim, K.W. Influence of sucrose, ABA and daylength on cormlet formation of gladiolus in vitro: Histological observation. J. Korean Soc. Hortic. Sci. 1994, 35, 400–405. [Google Scholar]
- Grewal, H.S.; Gosal, S.S.; Arora, J.S.; Singh, K. Mass propagation of carnation, chrysanthemum and gladiolus through tissue culture. In Proceedings of the International Horticulture Congress (23rd), Florence, Italy, 30 August 1990. [Google Scholar]
- Kumar, A.; Palni, L.M.S. Changes in endogenous polyamines during in vitro cormlet formation in Gladiolus hybridus Hort. Sci. Hortic. 2013, 162, 260–264. [Google Scholar] [CrossRef]
- Martin-Tanguy, J. Metabolism and function of polyamines in plants: Recent development (new approaches). Plant Growth Regul. 2001, 34, 135–148. [Google Scholar] [CrossRef]
- Kaur-Sawhney, R.; Tiburcio, A.F.; Altabella, T.; Galston, A.W. Polyamines in plants: An overview. J. Cell Mol. Biol. 2003, 2, 1–12. [Google Scholar]
- Steinitz, B.; Lilien-Kipnis, H. Control of gladiolus corm and cormel formation in tissue culture. J. Plant Physiol. 1989, 135, 495–500. [Google Scholar] [CrossRef]
- Ginzburg, C.; Ziv, M. Hormonal regulation of cormel formation in gladiolus stolons grown in vitro. Ann. Bot. 1973, 37, 219–224. [Google Scholar] [CrossRef]
- Courduroux, J.C. Etude du mechanisme physiologique de la tuberization chez le topinambour (Helianthus tuberosus L.). Ann. Sci. Nat. Bot. 1967, 8, 215–356. [Google Scholar]
- El-Antalby, H.M.M.; Wareing, P.F.; Hillmarn, J. Some physiological responses to d,1 abscisin (dormin). Planta 1967, 73, 74–90. [Google Scholar] [CrossRef]
- Ziv, M.; Ariel, T. Bud proliferation and plant regeneration in liquid-cultured philodendron treated with ancymidol and paclobutrazol. J. Plant Growth Regul. 1991, 10, 53. [Google Scholar] [CrossRef]
- Steinitz, B.; Yhel, H. In vitro propagation of Narcissus tazetta. HortScience 1982, 17, 333–334. [Google Scholar] [CrossRef]
- Gosal, S.S.; Grewal, H.S. Tissue culture propagation: Problems and potentials, In Horticulture-New Technologies and Applications; Prakash, J., Pierik, R.L.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 197–200. [Google Scholar]
- Memon, N.; Qasim, M.; Jaskani, M.J.; Ahmad, R.; Anwar, R. Effect of various corm sizes on the vegetative, floral and corm yield attributes of gladiolus. Pakastan J. Agric. Sci. 2009, 46, 13–19. [Google Scholar]
- Shillo, R.; Halevy, A.H. The effect of various environmental factors on flowering of gladiolus. IV. Interaction of environmental factors general discussion. Sci. Hortic. 1976, 4, 157–162. [Google Scholar] [CrossRef]
- Bose, T.K.; Jana, B.K.; Mukhopadhyay, T.P. A note on the effect of day length on growth and flowering in Hippeastrum. Indian J. Hortic. 1983, 40, 110–112. [Google Scholar]
- Park, I.S.; Choi, J.D.; Byun, M.S.; Goo, D.H.; Kim, K.W. Effects of liquid shaking culture and growth retardant on cormlet formation of gladiolus ‘Spic & Span’ in vitro. J. Korean Soc. Hortic. Sci. 2001, 42, 215–218. [Google Scholar]
- Huylenbroeck, J.M.V.; Debergh, P.C. Physiological aspects of micropropagated plantlets. Plant Tissue Cult. Biotechnol. 1986, 23, 136–141. [Google Scholar]
- Desjardins, Y. Factors affecting CO2 fixation in striving to optimize photoautotrophy in micropropagated plants. Plant Tissue Cult. Biotechnol. 1995, 1, 13–25. [Google Scholar]
- Jager, A.K.; McAlister, B.G.; Van Staden, J. In vitro culture of Gladiolus carneus. S. Afr. J. Bot. 1998, 64, 146–147. [Google Scholar] [CrossRef][Green Version]
- Cantor, M.; Tolety, J. Gladiolus. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 10, pp. 133–159. [Google Scholar]
- González-Pérez, E.; Aya la-Garay, O.J.; Carrillo-Salazar, J.A.; García de los Santos, G.; Yáñez-Morales, M.J.; Juárez-Muñoz, J. A study of development flower quality and fertilization in gladiolus (Gladiolus grandiflorus Hort.). Rev. Fitotec. Mex. 2011, 34, 277–283. [Google Scholar]
- Memon, N.U.N.; Wahocho, N.A.; Miano, T.F.; Leghari, M.H. Propagation of gladiolus corms and cormels: A review. Afr. J. Biotechnol. 2016, 15, 1699–1710. [Google Scholar]
- Misra, R.L. Propagation of gladiolus through sprouts-An entirely new method in gladiolus propagation. In Floriculture-Technology, Trades and Trends; Prakash, J., Bhandary, K.R., Eds.; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 1994; pp. 67–70. [Google Scholar]
- Kaur, K.; Jhanji, S.; Kaur, G. Assessment of priming of cormels with plant growth substances on vegetative growth and cormel-associated traits in gladiolus. Ann. Plant Soil Res. 2023, 25, 304–309. [Google Scholar]
- Teixeira da Silva, J.A. Thin cell layer technology in ornamental plant micropropagation and biotechnology. Afr. J. Biotechnol. 2003, 2, 683–691. [Google Scholar]
- Riaz, T.; Khan, S.K.; Javaid, A. Screening of gladiolus germplasm for agronomic performance and resistance against corm rot disease. Afr. J. Biotechnol. 2010, 9, 6701–6707. [Google Scholar]
- Tomiozzo, R.; Uhlmann, L.O.; Becker, C.C.; Schwab, N.T.; Streck, N.A.; Balest, D.S. How to produce gladiolus corms? Ornam. Hortic. 2019, 25, 299–306. [Google Scholar] [CrossRef]
- Malik, S.; Kumar, M.; Singh, M.K. Dormancy in gladiolus: The cause and remedy—A review. J. Plant Dev. Sci. 2009, 1, 45–47. [Google Scholar]
- Mangal, M.; Bhardwaj, S.V.; Handa, A.; Jindal, K.K. production of virus tested gladioli through in vitro and in vivo techniques. Acta Hortic. 2003, 624, 511–514. [Google Scholar] [CrossRef]
- Shabbir, A.; Hameed, N.; Ali, A.; Bajwa, R. Effect of different cultural conditions on micropropagation of rose (Rose indica L.). Pak. J. Bot. 2009, 41, 2877–2882. [Google Scholar]
- Madhavan, S.; Balasubramanian, V.; Selvarajan, R. Viruses infecting bulbous ornamental plants and their diagnosis and management. In Virus Diseases of Ornamental Plants; Springer: Singapore, 2021; pp. 277–299. [Google Scholar]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An academic and technical overview on plant micropropagation challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Pospisilova, J.; Solarova, J.; Catsky, J. Photosynthetic responses to stresses during in vitro cultivation. Photosynthetica 1992, 26, 3–18. [Google Scholar]
- Urban, J.; Ingwers, M.W.; McGuire, M.A.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot 2017, 68, 1757–1767. [Google Scholar] [CrossRef]
- Askari, N.; Aliniaeifard, S.; Visser, R.G. Low CO2 levels are detrimental for in vitro plantlets through disturbance of photosynthetic functionality and accumulation of reactive oxygen species. Horticulturae 2022, 8, 44. [Google Scholar] [CrossRef]
- De Klerk, G.J. Micropropagation of bulbous crops: Technology and present state. Floric. Ornam. Biotechnol. 2012, 6, 1–8. [Google Scholar]
- Smulders, M.J.M.; De Klerk, G.J. Epigenetics in plant tissue culture. Plant Growth Regul. 2011, 63, 137–146. [Google Scholar] [CrossRef]
- Jackson, M.B. Ethylene and responses of plants to soil waterlogging and submergence. Annu. Rev. Plant Physiol. 1985, 36, 146–174. [Google Scholar] [CrossRef]
- Lilien-Kipnis, H.; Azizbekova, N.; Ziv, M. Scaled-up proliferation and regeneration of Nerine in liquid cultures Part II. Ontogeny of somatic embryos and bulblet regeneration. Plant Cell Tissue Organ Cult. 1994, 39, 117–123. [Google Scholar] [CrossRef]
- Ziv, M. Morphogenesis of Gladiolus buds in bioreactors for scaled-up propagation of geophytes. In Progress in Plant Cellular and Molecular Biology; Nijkamp, H.J.J., Van Der Plas, L.H.W., Van Aartrijk, J., Eds.; Kluwer: Dordrecht, The Netherlands, 1990; pp. 119–124. [Google Scholar]
- Ziv, M.; Kahany, S.; Lilien-Kipnis, H. Scaled-up proliferation and regeneration of Nerine in liquid cultures. Part I. The induction and maintenance of proliferating meristematic clusters by paclobutrazol in bioreactors. Plant Cell Tissue Organ Cult. 1994, 39, 109–115. [Google Scholar] [CrossRef]
- De Klerk, G.J. Why plants grow in tissue culture? Prophyta Annu. 2010, 2010, 42–44. [Google Scholar]
- Chen, C.C. Humidity in plant tissue culture vessels. Biosyst. Eng. 2004, 88, 231–241. [Google Scholar] [CrossRef]
- Tanaka, K.; Fujiwara, K.; Kozai, T. Effects of relative humidity in the culture vessel on the transpiration and net photosynthetic rates of potato plantlets in vitro. Acta Hortic. 1992, 319, 59–64. [Google Scholar] [CrossRef]
- De Klerk, G.J. How to measure somaclonal variation: A review. Acta Bot. Neerl. 1990, 39, 129–144. [Google Scholar] [CrossRef]
- D’Arth, S.M.; Simpson, S.I.; Seelye, J.F.; Jameson, P.E. Bushiness and cytokinin sensitivity in micropropagated Zantedeschia. Plant Cell Tissue Organ Cult. 2002, 70, 113–118. [Google Scholar] [CrossRef]
- Naik, P.K.; Nayak, S. Different modes of plant regeneration and factors affecting in vitro bulblet production in Ornithogalum virens. Sci. Asia 2005, 31, 409–414. [Google Scholar] [CrossRef]
- Paek, K.Y.; Murthy, H.N. High frequency of bulblet regeneration from bulb scale sections of Fritillaria thunbergii. Plant Cell Tissue Organ Cult 2002, 68, 247–252. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K.; et al. Recent development in micropropagation techniques for rare plant species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Bioreactor systems for micropropagation of plants: Present scenario and future prospects. Front. Plant Sci. 2023, 14, 1159588. [Google Scholar] [CrossRef]
- Cerasela, P.; Giancarla, V.; Camen, D.; Andreea, P.; Eleonora, N.; Alina, S.; Gorinoiu, G. Evaluation of somaclonal variation in Gladiolus grandifloras through molecular markers ISSR. J. Hortic. For. Biotechnol. 2018, 22, 100–104. [Google Scholar]
- Kamo, K.; Jordan, R.; Guaragna, M.A.; Hsu, H.T.; Ueng, P. Resistance to cucumber mosaic virus in gladiolus plants transformed with either a defective replicase or coat protein subgroup II gene from cucumber mosaic virus. Plant Cell Rep. 2010, 29, 695–704. [Google Scholar] [CrossRef]
- Kaur, C.; Kumar, S.; Raj, S.K.; Chauhan, P.S.; Sharma, N. Characterization of a new isolate of bean yellow mosaic virus group-IV associated with mosaic disease of gladiolus in India. J. Plant Pathol. Microbiol. 2015, 6, 10. [Google Scholar] [CrossRef]
- Kaur, C.; Raj, R.; Srivastava, A.; Kumar, S.; Raj, S.K. Sequence analysis of six full-length bean yellow mosaic virus genomes reveals phylogenetic diversity in India strains, suggesting subdivision of phylogenetic group-IV. Arch. Virol. 2018, 163, 235–242. [Google Scholar] [CrossRef]
- Rao, T.M.; Negi, S.S.; Swamy, R.D. Isolation of leaf mesophyll protoplasts in gladiolus. Indian J. Hortic. 1991, 48, 79–82. [Google Scholar]
- Taylor, W.; Bhatti, S.; Long, D.; Sauve, R. In vitro culture of gladiolus. SNA Res. Conf. 2000, 45, 328–330. [Google Scholar]
- Ratilal, P.H. Tissue Culture Plant and Synthetic Seed Production of Gladiolus Variety Amarican Beauty. Master’s Thesis, Department of Floriculture and Landscaping Department, Aspee College of Horticulture and Forestry Gujarat Agriculture University, Navsari, India, 2009. [Google Scholar]
- Sharma, S.; Shahzad, A.; Da Silva, J.A.T. Synseed technology—A complete synthesis. Biotechnol. Adv. 2013, 31, 186–207. [Google Scholar] [CrossRef]
- Pathania, N.S.; Misra, R.L. In vitro mutagenesis studies in gladiolus for induction of resistance to Fusarium oxysporum fsp gladioli. Acta Hortic. 2003, 624, 487–494. [Google Scholar] [CrossRef]
- Kamo, K.; Blowers, A.; Smith, F.; Van Eck, J.; Lawson, R. Stable transformation of gladiolus using suspension cells and callus. J. Am. Soc. Hortic. Sci. 1995, 120, 347–352. [Google Scholar] [CrossRef]
- Kamo, K.; Blowers, A.; Smith, F.; Van Eck, J. Stable transformation of gladiolus by particle gun bombardment of cormels. Plant Sci. 1995, 110, 105–111. [Google Scholar] [CrossRef]
- Lakshman, D.K.; Pandey, R.; Kamo, K.; Bauchan, G.; Mitra, A. Genetic transformation of Fusarium oxysporum f spgladioli with Agrobacterium to study pathogenesis in gladiolus. Eur. J. Plant Pathol. 2012, 133, 729–738. [Google Scholar] [CrossRef]
- Bull, T.; Michelmore, R. Molecular determinants of in vitro plant regeneration: Prospects for enhanced manipulation of lettuce (Lactuca sativa L.). Front. Plant Sci. 2022, 13, 1211. [Google Scholar] [CrossRef]
- Bednarek, P.T.; Orłowska, R. Plant tissue culture environment as a switch-key of (epi)genetic changes. Plant Cell Tissue Organ Cult 2020, 140, 245–257. [Google Scholar] [CrossRef]
- Ghosh, A.; Igamberdiev, A.U.; Debnath, S.C. Tissue culture-induced DNA methylation in crop plants: A review. Mol. Biol. Rep. 2021, 48, 823–841. [Google Scholar] [CrossRef]
- Kamo, K.; Aebig, J.; Guaragna, M.A.; James, C.; Hsu, H.T.; Jordan, R. Gladiolus plants transformed with single-chain variable fragment antibodies to cucumber mosaic virus. Plant Cell Tissue Organ Cult. 2012, 110, 13–21. [Google Scholar] [CrossRef]
- Kamo, K.; Lakshman, D.; Pandey, R.; Guaragna, M.A.; Okubara, P.; Rajasekaran, K.; Cary, J.; Jordan, R. Resistance to Fusarium oxysporum f sp gladioli in transgenic gladiolus plants expressing either a bacterial chloroperoxidase or fungal chitinase genes. Plant Cell Tissue Organ Cult. 2016, 124, 541–553. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Avilez-Montalvo, R.N. Plant Tissue Culture: A Battle Horse in the Genome Editing Using CRISPR/Cas9. Methods Mol. Biol. 2018, 1815, 131–148. [Google Scholar] [PubMed]
- Subburaj, S.; Chung, S.J.; Lee, C.; Ryu, S.M.; Kim, D.H.; Kim, J.S.; Bae, S.; Lee, G.J. Site-directed mutagenesis in Petunia x hybrida protoplast system using direct delivery of purified recombinant Cas9 ribonucleoproteins. Plant Cell Rep. 2016, 35, 1535–1544. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Yang, C.; Li, M.; Guo, Y. Exploiting the CRISPR/Cas9 system for targeted genome mutagenesis in petunia. Sci. Rep. 2016, 6, 20315. [Google Scholar] [CrossRef]
- López-Ruiz, B.A.; Juárez-González, V.T.; Luján-Soto, E.; Dinkova, T.D. The role of small RNAs in plant somatic embryogenesis. In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications; Alvarez-Venegas, R., De-la-Peña, C., Casas-Mollano, J., Eds.; Springer: Cham, Switzerland, 2019; pp. 311–338. [Google Scholar]
- Kanwal, S.; Guo, X.; Ward, C.; Volpe, G.; Qin, B.; Esteban, M.A.; Bao, X. Role of long non-coding RNAs in reprogramming to induced pluripotency. Genom. Proteom. Bioinf. 2020, 18, 16–25. [Google Scholar] [CrossRef]
- Cordeiro, D.; Canhoto, J.; Correia, S. Regulatory non-coding RNAs: Emerging roles during plant cell reprogramming and in vitro regeneration. Front. Plant Sci. 2022, 13, 1049631. [Google Scholar] [CrossRef]
- Luo, Y.C.; Zhou, H.; Li, Y.; Chen, J.Y.; Yang, J.H.; Chen, Y.Q.; Qu, L.H. Rice embryogenic calli express a unique set of microRNAs, suggesting regulatory roles of microRNAs in plant post-embryogenic development. FEBS Lett. 2006, 580, 5111–5116. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D.; Zhang, L.L.; Borthakur, D.; Li, Q.S.; Ye, J.H.; Zheng, X.-Q.; Lu, J.-L. MicroRNAs and their targeted genes associated with phase changes of stem explants during tissue culture of tea plant. Sci. Rep. 2019, 9, 20239. [Google Scholar] [CrossRef]
- Juárez-González, V.T.; López-Ruiz, B.A.; Baldrich, P.; Luján-Soto, E.; Meyers, B.C.; Dinkova, T.D. The explant developmental stage profoundly impacts small RNA-mediated regulation at the dedifferentiation step of maize somatic embryogenesis. Sci. Rep. 2019, 9, 14511. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Han, S.; Yang, W.; Li, W.; Wei, H.; Wei, H.; Li, X.; Qi, L. Four abiotic stress-induced miRNA families differentially regulated in the embryogenic and non-embryogenic callus tissues of Larix leptolepis. Biochem. Biophys. Res. Commun. 2010, 398, 355–360. [Google Scholar] [CrossRef]
- Wu, X.M.; Kou, S.J.; Liu, Y.L.; Fang, Y.N.; Xu, Q.; Guo, W.W. Genomewide analysis of small RNAs in nonembryogenic and embryogenic tissues of citrus: microRNA- and siRNA-mediated transcript cleavage involved in somatic embryogenesis. Plant Biotechnol. J. 2015, 13, 383–394. [Google Scholar] [CrossRef]
- López-Ruiz, B.A.; Juárez-González, V.T.; Sandoval-Zapotitla, E.; Dinkova, T.D. Development-related miRNA expression and target regulation during staggered in vitro plant regeneration of tuxpeño VS-535 maize cultivar. Int. J. Mol. Sci. 2019, 20, 2079. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, X.; Ruan, Y.; Zhang, L.; Cui, Z.; Li, X.; Jia, B. miRNA expression profiling and zeatin dynamic changes in a new model system of in vivo indirect regeneration of tomato. PLoS ONE 2020, 15, e0237690. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Hernández, E.C.; Alejandri-Ramírez, N.D.; Juárez-González, V.T.; Dinkova, T.D. Maize miRNA and target regulation in response to hormone depletion and light exposure during somatic embryogenesis. Front. Plant Sci. 2015, 6, 555. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Liu, M.Y.; Ge, X.X.; Xu, Q.; Guo, W.W. Stage and tissue-specific modulation of ten conserved miRNAs and their targets during somatic embryogenesis of Valencia sweet orange. Planta 2011, 233, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, W.; Hou, L.; Wang, X.; Zheng, F.; Wang, W.; Liang, D.; Yang, H.; Jin, Y.; Xie, X. Analysis of miRNAs and their targets during adventitious shoot organogenesis of Acacia crassicarpa. PLoS ONE 2014, 9, e93438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).