Developing EST-SSR Markers for Identifying and Evaluating Asparagus Germplasm Resources Based on Transcriptome Sequences
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and DNA Extraction
2.2. Morphological Examination
2.3. Examination of Root Tip Chromosomes
2.4. RNA Extraction and Transcriptome Sequencing
2.5. SSR Marker Development and PCR Primers
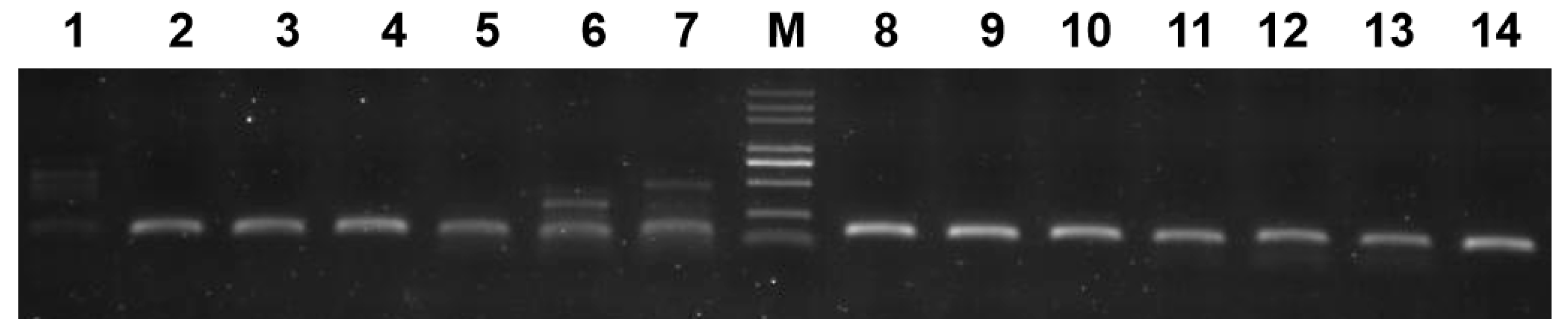
2.6. SSR-PCR and Polymorphism Analysis
2.7. Data Analysis
3. Results
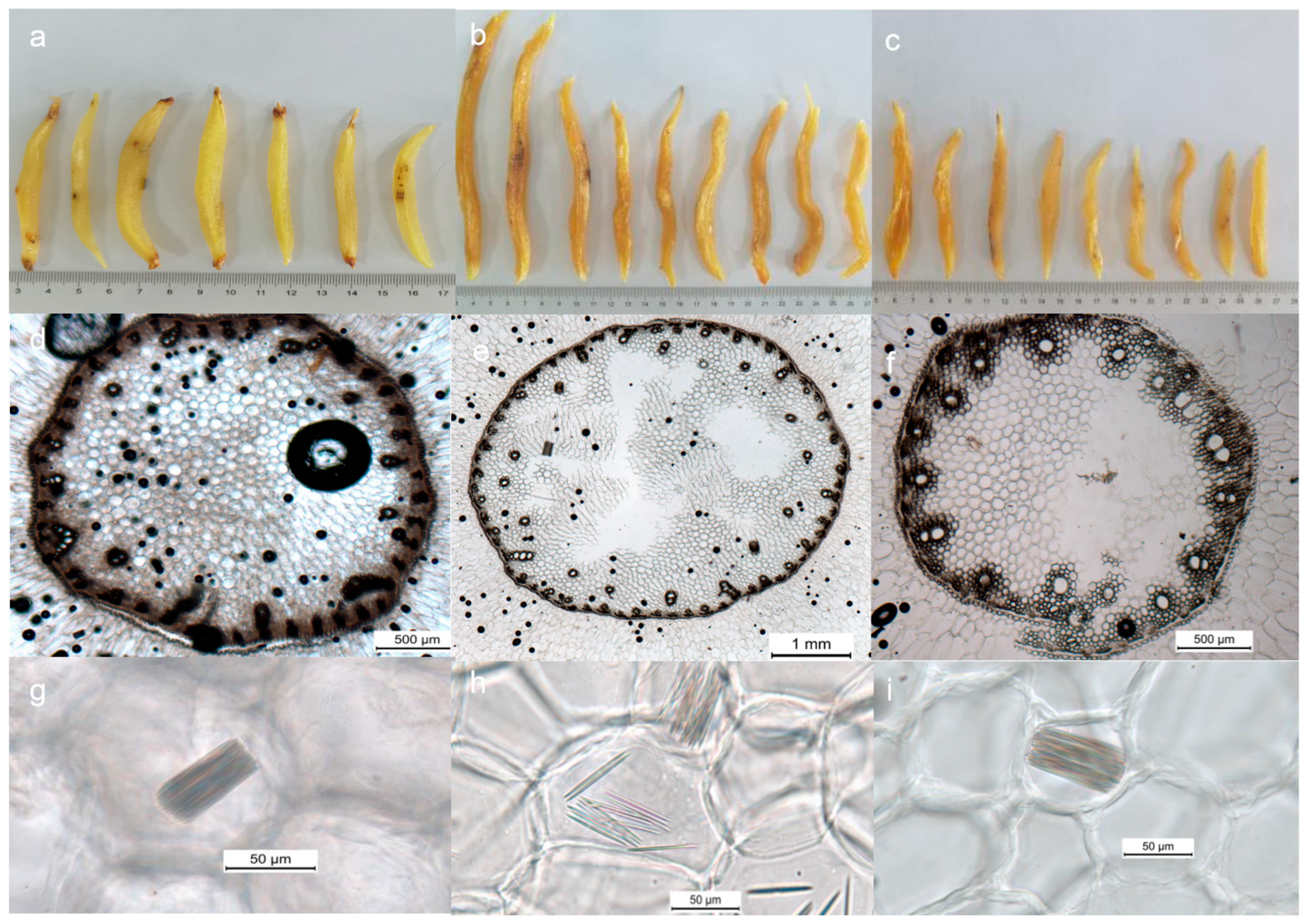
3.1. Plant Morphology
3.2. Microscopic Characteristics of Asparagus Medicinal Materials
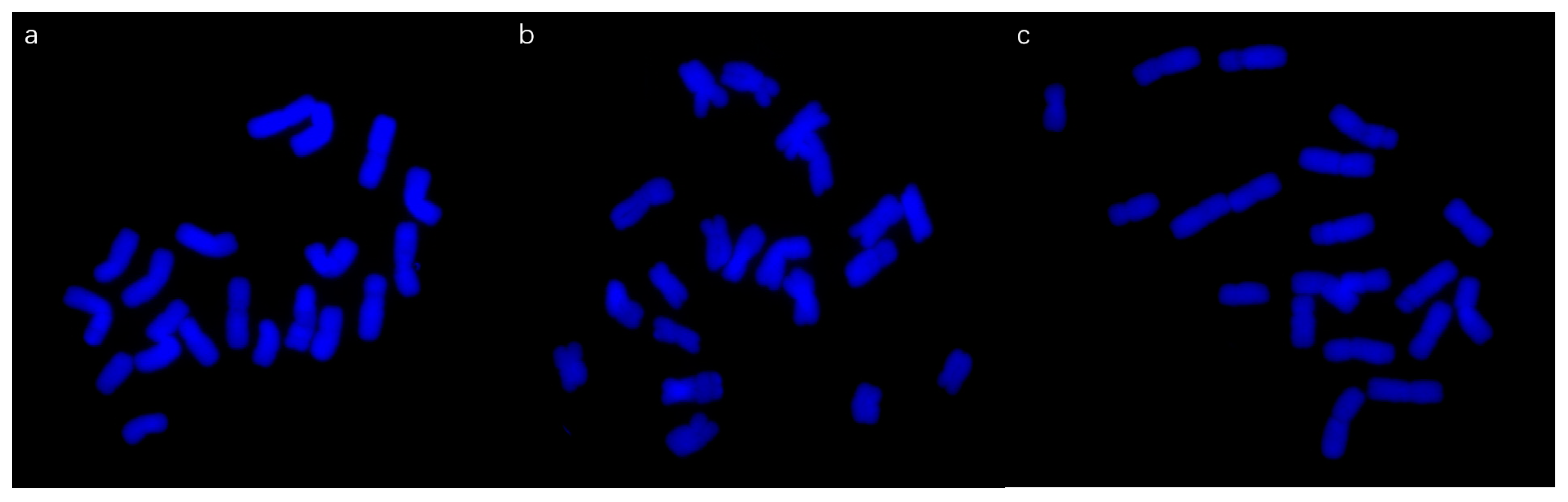
3.3. Root Tip Chromosomes
3.4. Transcriptome and Microsatellite Characteristics of Asparagus
3.5. Development of SSR Primers for Asparagus
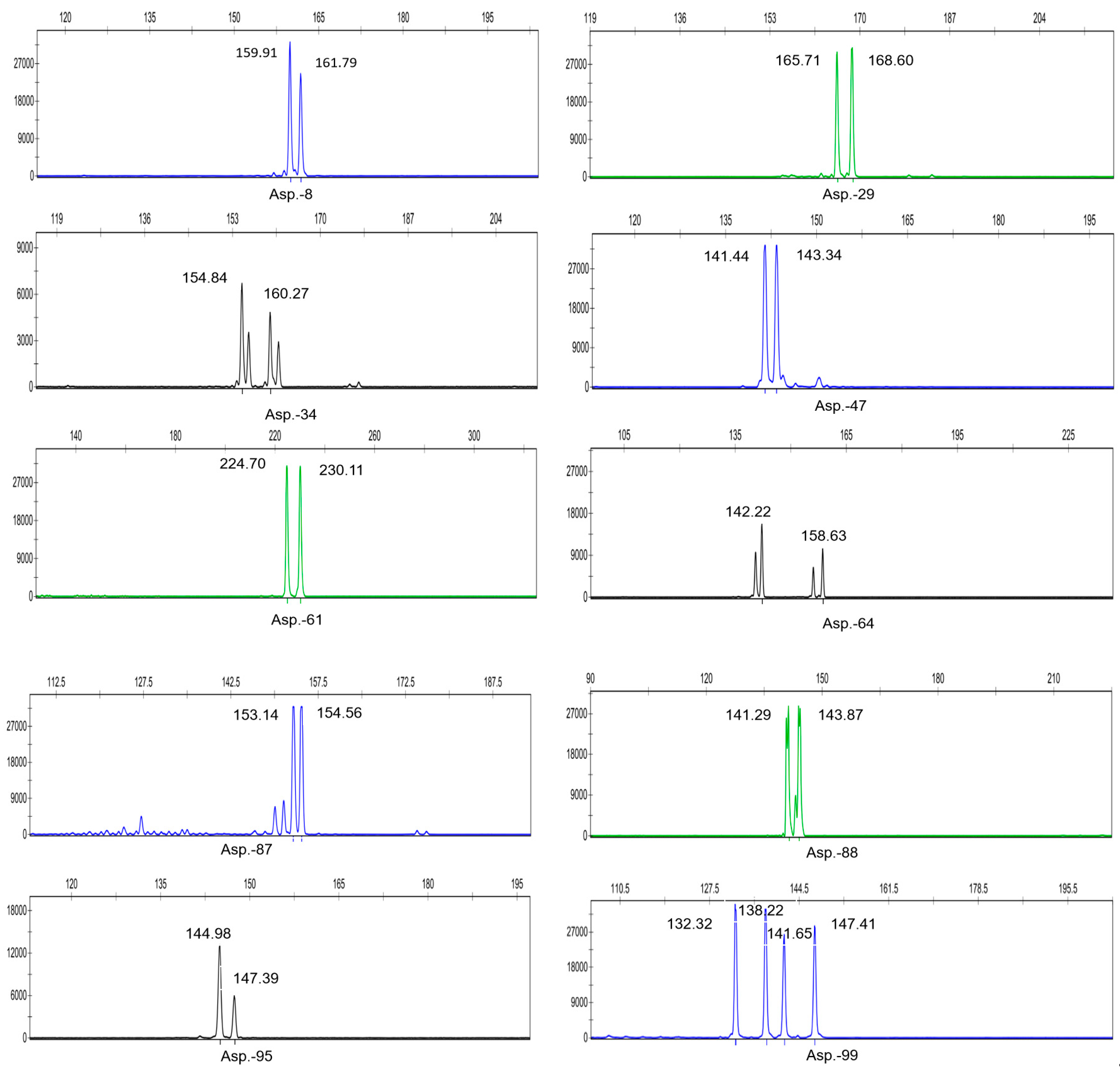
3.6. SSR Primer Diversity
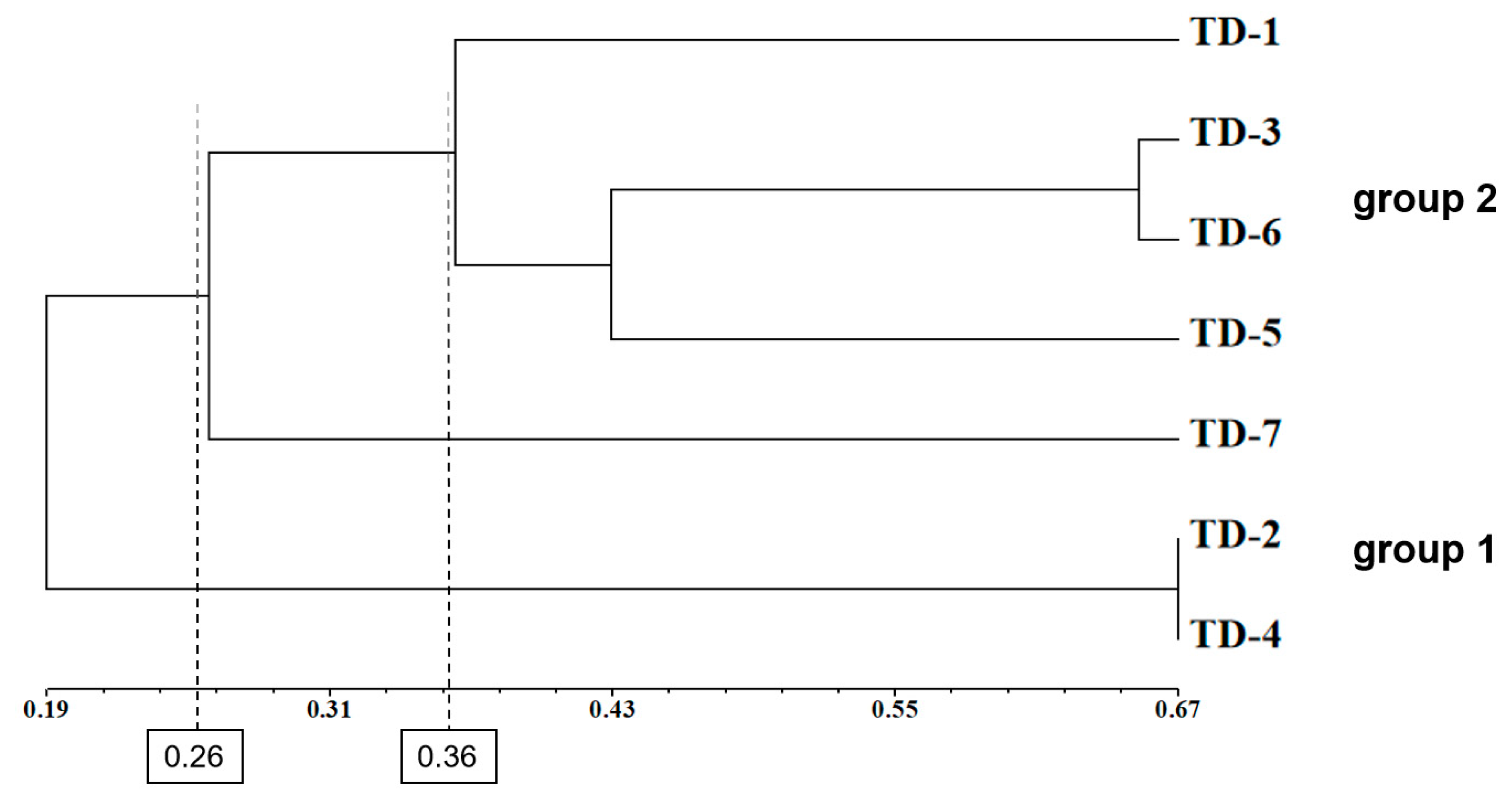
3.7. Genetic Structure of Asparagus Populations
4. Discussion
4.1. Morphological Examination
4.2. Chromosome Examination
4.3. SSR Markers
4.4. Genetic Diversity of SSR in Asparagus
4.5. Genetic Structure of Asparagus Resources
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Wang, S.; Hu, W.; Wang, Z.; Yang, B.; Kuang, H. Asparagus cochinchinensis: A review of its botany, traditional uses, phytochemistry, pharmacology, and applications. Front. Pharmacol. 2022, 13, 1068858. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.L. Study on Chemical Constituents and Cytotoxic Activity of Asparagus cochinchinensis. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2013. [Google Scholar]
- Choi, J.Y.; Kim, S.H.; Kim, J.E.; Park, J.W.; Kang, M.J.; Choi, H.J. Four amino acids as serum biomarkers for anti-asthma effects in the ovalbumininduced asthma mouse model treated with extract of Asparagus cochinchinensis. Lab. Anim. Res. 2019, 35, 32. [Google Scholar] [CrossRef]
- Lei, L.; Ou, L.; Yu, X. The antioxidant effect of Asparagus cochinchinensis (Lour.) Merr. shoot in D-galactose induced mice aging model and in vitro. J. Chin. Med. Assoc. 2016, 79, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.S.; Liu, Y.Y.; Zhu, P.F.; Jin, Q.; Dai, Z.; Luo, X.D. Furostanol saponins from Asparagus cochinchinensis and their cytotoxicity. Nat Prod Bioprospect. 2021, 11, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Novi, S.; Rungkat, F.Z.; Endang, P.; Suwarti; Robert, V.; Dewi, Y.N. Using metabolomics to discover the immunomodulator activity of food plants. Heliyon 2022, 8, 1–14. [Google Scholar]
- Kim, M.; Kim, W.B.; Koo, K.Y.; Kim, B.R.; Kim, D.; Lee, S. Optimal fermentation conditions of hyaluronidase inhibition activity on Asparagus cochinchinensis merrill by Weissella cibaria. J. Microbiol. Biotechnol. 2017, 27, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Kinga, T.; Adam, F.; Agnieszka, F. Functional Food-Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar]
- Pegiou, E.; Mumm, R.; Acharya, P.; Vos, R.; Hall, R.D. Green and white Asparagus (Asparagus offificinalis): A source of developmental, chemical and urinary intrigue. Metabolites 2019, 10, 17. [Google Scholar] [CrossRef]
- Pahwa, P.; Singh, T.; Goel, R.K. Anticonvulsant effect of Asparagus racemosus willd in a mouse model of catamenial epilepsy. Neurochem. Res. 2022, 47, 422–433. [Google Scholar] [CrossRef]
- Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1995; pp. 107–108. [Google Scholar]
- Kantartzi, S.K. Microsatellites Methods and Protocols. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; p. 1006. [Google Scholar]
- Tello, J.; Forneck, A. Use of DNA markers for grape phylloxera population and evolutionary genetics: From RAPDs to SSRs and beyond. Insects 2019, 10, 317. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Cheng, T.; Lin, K.; Zhou, S. Sequencing angiosperm plastid genomes made easy: A complete set of universal primers and a case study on the phylogeny of Saxifragales. Genome Biol. Evol. 2013, 5, 989–997. [Google Scholar] [CrossRef]
- Jiang, C. Establishment of On-Site and Rapid Identification Methods of Traditional Chinese Medicine—Taking Honeysuckle as an Example. Master’s Thesis, China Academy of Chinese Medical Sciences, Beijing, China, 2013. [Google Scholar]
- Li, Q.; Li, B.; Guo, S.X. SSR information in transcriptome of Dendrobium nobile. Chin. J. Trad. Chin. Med. 2017, 42, 63–69. [Google Scholar]
- Hou, Y.Q.; Shi, Y.T.; Zhang, Y.P. Astragali radix and Hedysari radix molecular identification of SSR primers screening and fingerprints code. Chin. J. Mater. Sin. 2016, 41, 1819–1822. [Google Scholar]
- Dai, J.; Shi, X.D.; Gu, Y.X. Development and function analysis of SSR molecular markers in Magnolia officinalis transcriptome. Chin. Herb. Med. 2017, 48, 2726–2732. [Google Scholar]
- Yuan, C.; Peng, F.; Yang, Z.M. EST-SSR markers development of Ligusticum chuanxiong based on transcriptome sequences. China J. Chin Mater. Med. 2017, 17, 3332–3340. [Google Scholar]
- Zeng, G.P.; Pan, Z.P.; Liu, H.C. Genetic diversity of Asparagus cochinchinensis (Lour) Merr. in Guizhou based on ISSR Analysis. Henan J. Agri Sci. 2013, 42, 105–108. [Google Scholar]
- Ou, L.J.; Yan, W.; Liao, Y.X.; Liang, Y.X. Establishment and optimization of ISSR-PCR reaction system in Asparagus Cochinchinensis. Chin. Trad. Herb. Drugs 2011, 42, 353–357. [Google Scholar]
- Chinese Pharmacopoeia National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China: 2020 Edition; China Medical Science &Technology Press: Beijing, China, 2020. [Google Scholar]
- Hao, M.; Luo, J.T.; Yang, M.; Zhang, L.Q.; Yan, Z.H.; Yuan, Z.W.; Zheng, Y.L. Comparison of homoeologous chromosome pairing between hybrids of wheat genotypes Chinese Spring ph1b and Kaixian luohanmai with rye. Genome 2011, 54, 959–964. [Google Scholar] [CrossRef]
- Komuro, S.; Endo, R.; Shikata, K.; Kato, A. Genomic and chromosomal distribution patterns of various repeated DNA sequences in wheat revealed by a fluorescence in situ hybridization procedure. Genome 2013, 56, 131–137. [Google Scholar] [CrossRef]
- Saski, C.A.; Bhattacharjee, R.; Scheffler, B.E.; Asiedu, R. Genomic Resources for Water Yam (Dioscorea alata L.): Analyses of EST-Sequences, De Novo Sequencing and GBS Libraries. PLoS ONE 2015, 10, 134. [Google Scholar] [CrossRef]
- Kavas, M.; Yıldırım, K.; Seçgin, Z.; Gökdemir, G. Discovery of Simple Sequence Repeat (SSR) Markers in Hazelnut (Corylus avellana L.) by Transcriptome Sequencing and SSR-Based Characterization of Hazelnut Cultivars. Scand. J. For. Res. 2020, 35, 227–237. [Google Scholar] [CrossRef]
- Sabreena; Nazir, M.; Mahajan, R.; Hashim, M.J.; Iqbale, J.; Alyemeni, M.N.; Ganai, B.A.; Zargar, S.M. Deciphering Allelic Variability and Population Structure in Buckwheat: An Analogy between the Efficiency of ISSR and SSR markers. Saudi J. Biol. Sci. 2021, 28, 6050–6056. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Teng, G.L.; Liu, S. Progress in clinical application of Asparagus. Chin. Mater. Med. Pharm. Clin. 2012, 4, 61–65. [Google Scholar]
- Luo, X.D.; Xu, G.J.; Xu, L.S. Textual Research on the Materia Medica of Chinese Medicine Asparagus. Chin. J. Chin. Mater. Med. 1996, 10, 578–580. [Google Scholar]
- Zhang, Y.; Zhao, J.S.; Jin, Y. Textual research on traditional Chinese Medicine Asparagi Radix. Mod. Chin. Med. 2020, 22, 1393–1403. [Google Scholar]
- Chen, G.F.; Xu, B. Analysis and B Chromosome Observation of Wild Ornamental Plants Asparagus filicinus in the Yulong Snow Mountain. J. Anhui Agric. Sci. 2019, 47, 178–180. [Google Scholar]
- Wang, J.J.; Nie, Z.L.; Meng, Y. Cytological Advances on Tribe Polygonateae (Asparagaceae). Acta Bot. Occident. Sin. 2016, 36, 834–845. [Google Scholar]
- Chen, G.F.; Sun, W.G.; Hong, D.Y. Systematic significance of cytology in Cyananthus (Campanulaceae) endemic to the Sino-Himalayan region. J. Syst. Evol. 2014, 52, 260–270. [Google Scholar] [CrossRef]
- Fu, C.X.; Zhang, Y. Chromosomal Karyotype Analysis of Three Liliaceae Plants. ACTA J. Zhejiang AF Univ. 1991, 17, 93–98. [Google Scholar]
- Hong, W.; Yue, W.; Ling, Z.X. Development of Simple Sequence REPEAT Markers for Genetic Diversity Analysis Based on the cDNA Sequences of Chinese Yam (Dioscorea spp.). Horticulturae 2022, 8, 1–14. [Google Scholar]
- Kumpatla, S.P.; Mukhopadhyay, S. Mining and Survey of Simple Sequence Repeats in Expressed Sequence Tags of Dicotyledonous Species. Genome 2005, 48, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Li, Y.L.; Zhang, Z.J. Analysis of SSR information in EST resource of Asparagus. Chin. J. Bioinform. 2012, 10, 96–100. [Google Scholar]
- Ma, Z.C. Nutrient Composition and EST-SSR Genetic Diversity Analysis of Asparagus Germplasm Resources. Master’s Thesis, Heilongjiang Bayi Agricultural University, Harbin, China, 2017. [Google Scholar]
- Wang, S.; Wang, X.; He, Q.; Liu, X.X.; Xu, W.L.; Li, L.B.; Gao, J.W.; Wang, F.D. Transcriptome Analysis of the Roots at Early and Late Seedling Stages Using Illumina Paired-End Sequencing and Development of EST-SSR Markers in Radish. Plant Cell. Rep. 2012, 31, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.J.; Yang, C.; Tian, B.; Yang, J.B.; Liu, A.Z. Exploiting EST Databases for the Development and Characterization of EST-SSR Markers in Castor Bean (Ricinus communis L.). BMC Plant Biol. 2010, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.L.; Qi, X.Q.; Wang, L.H.; Zhang, Y.X.; Hua, W.; Li, D.H.; Lyu, H.X.; Zhang, X.R. Characterization of the Sesame (Sesamum indicum L.) Global Transcriptome Using Illumina Paired-End Sequencing and Development of EST-SSR Markers. BMC Genom. 2011, 12, 451. [Google Scholar] [CrossRef]
- Feng, S.; He, R.; Lu, J.; Jiang, M.; Shen, X.; Yan, J.; Wang, Z.; Wang, H. Development of SSR markers and assessment of genetic diversity in medicinal Chrysanthemum morifolium cultivars. Front. Genet. 2016, 7, 113. [Google Scholar] [CrossRef]
- Temnykh, S.; De, C.G.; Lukashova, A.; Lipovich, L.; Cartinhour, S.; McCouch, S. Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): Frequency, length variation, transposon associations, and genetic marker potential. Genome Res. 2001, 11, 1441–1452. [Google Scholar] [CrossRef]
- Jie, G.; Zhimong, L.; Zhao, W. Combined genotype and phenotype analyses reveal patterns of genomic adaptation to local environments in the subtropical oak Quercus acutissima. J. Syst. Evol. 2021, 59, 541–556. [Google Scholar]
- Mei, Y.; Min, Z.; Shouguo, S. Analysis of Genetic Structure of Magnolia sprengeri Populations Based on ISSR Markers. Sci. Silvae Sin. 2014, 50, 76–81. [Google Scholar]
- Ambreen, H.; Kumar, S.; Kumar, A.; Agarwa, M.; Jagannath, A.; Goel, S. Association mapping for important agronomic traits in safflflower (Carthamus tinctorius L.) core collection using microsatellite markers. Front. Plant Sci. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, G.; Yang, W.; Li, Y.; Sun, J.; Zhan, K.; Liu, D.; Zhang, A. Genetic diversity, population structure and marker-trait associations for agronomic and grain traits in wild diploid wheat Triticum urartu. BMC Plant Biol. 2017, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Golkar, P.; Arzani, A.; Rezaei, A.M. Genetic variation in safflflower (Carthamus tinctorious L.) for seed quality-related traits and inter-simple sequence repeat (ISSR) markers. Int. J. Mol. Sci. 2011, 12, 2664–2677. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Zong, S.N.; Li, W.Q. Genetic diversity analysis of natural population of Quercus serrata based on SSR markers. J. Nanjing Univ. Nat. Sci. Ed. 2022, 46, 109–116. [Google Scholar]
- Ylmaz, F.; Shidfar, M.; Hazrati, N.; Kazan, K.; Ergül, A. Genetic analysis of central Anatolian grapevine (Vitis vinifera L.) germplasm by simple sequence repeats. Tree Genet. Genomes 2020, 16, 4. [Google Scholar] [CrossRef]
- Liu, M.H.; Xiao, X.C.; Zhang, C.H. Genetic diversity of Toona sinensis provenance in Sichuan revealed by SSR markers. J. Cent. South Univ. For. Technol. 2022, 42, 60–67. [Google Scholar]
- Yi, R.; Wang, Z.Y.; Liu, L.Y. Genetic diversity analysis of 60 reed fescue based on EST-SSR markers. Acta Agrestia Sin. 2023, 31, 2041–2048. [Google Scholar]
- Hai, L.R.; Zhen, W.W.; Bo, Z. Molecular marker development and genetic diversity exploration in Medicago polymorpha. Peer J. 2023, 11, 14698. [Google Scholar]
- Liao, B.Y.; Wang, F.; Chen, L.J. Genetic diversity of germplasm resources of Melia azedarach revealed by SRAP markers. Sci. Silvae Sin. 2016, 54, 48–58. [Google Scholar]
- Wang, Y.; Yue, D.; Li, X.Z. Genetic diversity of Toona ciliata populations based on SSR markers. J. Resour. Ecol. 2020, 11, 466–474. [Google Scholar]
- Yi, X.G.; Chen, J.; You, L.X. Genetic diversity of Cerasus serrulata assessed by SSR markers. J. Nanjing For. Univ. Nat. Sci. Ed. 2018, 42, 25–31. [Google Scholar]
- Zang, M.Y.; Li, X.; Fang, Y.M. Genetic diversity analysis among natural populations of Quercus fabri based on SSR markers. J. Nanjing For. Univ. Nat. Sci. Ed. 2021, 45, 63–69. [Google Scholar]


| ID | Genotypes | Population | Provenance Location | Sample Size | Longitude (E) | Latitude (N) | Elevation (m) |
|---|---|---|---|---|---|---|---|
| TD-1 | TD1-1 | NJ | Neijiang, Sichuan | 1 | 105°8′ | 29°49′ | 370 |
| TD1-2 | 1 | 105°22′ | 29°49′ | 350 | |||
| TD1-3 | 1 | 105°7′ | 29°47′ | 350 | |||
| TD1-4 | 1 | 105°12′ | 29°49′ | 440 | |||
| TD1-5 | 1 | 105°10′ | 29°49′ | 500 | |||
| TD-2 | TD2-1 | WC | Wuchuan, Guizhou | 1 | 107°55′ | 28°42′ | 1020 |
| TD2-2 | 1 | 108°09′ | 28°58′ | 864 | |||
| TD2-3 | 1 | 108°06′ | 28°41′ | 890 | |||
| TD2-4 | 1 | 108°24′ | 28°42′ | 720 | |||
| TD2-5 | 1 | 108°13 | 28°30′ | 750 | |||
| TD-3 | TD3-1 | YL | Yulin, Guangxi | 1 | 110°1′ | 22°28′ | 80 |
| TD3-2 | 1 | 110°10′ | 22°37 | 75 | |||
| TD3-3 | 1 | 109°59′ | 22°32′ | 80 | |||
| TD3-4 | 1 | 109°58′ | 22°29′ | 80 | |||
| TD3-5 | 1 | 109°56′ | 22°31′ | 85 | |||
| TD-4 | TD4-1 | HH | Honghe, Yunnan | 1 | 103°30′ | 22°51′ | 1380 |
| TD4-2 | 1 | 101°53′ | 24°58′ | 1356 | |||
| TD4-3 | 1 | 102°44′ | 23°27′ | 1983 | |||
| TD4-4 | 1 | 102°32′ | 23°29′ | 1689 | |||
| TD4-5 | 1 | 102°57′ | 25°9′ | 1891 | |||
| TD-5 | TD5-1 | CX | Chuxiong, Yunnan | 1 | 101°53′ | 24°58′ | 1350 |
| TD5-2 | 1 | 101°55′ | 25°1′ | 1324 | |||
| TD5-3 | 1 | 101°43′ | 24°38′ | 1345 | |||
| TD5-4 | 1 | 102°13′ | 24°23′ | 1280 | |||
| TD5-5 | 1 | 103°2′ | 24°56′ | 1320 | |||
| TD-6 | TD6-1 | KY | Kaiyang, Guizhou | 1 | 107°13′ | 26°53′ | 1080 |
| TD6-2 | 1 | 107°11′ | 26°58′ | 1158 | |||
| TD6-3 | 1 | 106°54′ | 26°53′ | 980 | |||
| TD6-4 | 1 | 106°35′ | 27°12′ | 880 | |||
| TD6-5 | 1 | 106°21′ | 27°20′ | 1205 | |||
| TD-7 | TD7-1 | ES | Enshi, Hubei | 1 | 109°29′ | 30°16′ | 780 |
| TD7-2 | 1 | 108°59′ | 29°37′ | 914 | |||
| TD7-3 | 1 | 109°5′ | 29°42′ | 910 | |||
| TD7-4 | 1 | 109°14′ | 29°52′ | 894 | |||
| TD7-5 | 1 | 108°50′ | 29°20′ | 950 |
| Component | Volume |
|---|---|
| 2 × T5 super PCR Mix (PAGE) | 10 μL |
| 10 μM Primer F | 1 μL |
| 10 μM Primer R | 1 μL |
| Template (genomic DNA) | 1 μL |
| ddH2O | 7 μL |
| Total | 20 μL |
| ID | Plant Type | Average Stem Length (cm) | Stem | Number of Leafy Branches | Leafy Branch Morphology | Length of Leafy Branch (mm) | Hard Thorn (mm) | Pedicel Length (mm) | Flower Color | Filiform Adhesiveness | Root Length: Root Thickness | Botanical Origin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TD-1 | Climbing vine | 120 | Smooth stem with longitudinal lines | 3–6 | Sharp triangular shape | 12 | 3 | 2 | Pistachio | Filaments are not attached | 7:1 | A. cochinchinensis |
| TD-2 | Climbing vine | 180 | Stem is smooth and longitudinal lines are not obvious | 6–9 | Sharp triangular shape | 9 | 4 | 2.5 | Yellow | Middle and lower parts of filament are attached | 6:1 | A. taliensis |
| TD-3 | Climbing vine | 140 | Smooth stem with longitudinal lines | 3–5 | Sharp triangular shape | 14 | 3.5 | 2.5 | Pistachio | Filaments are not attached | 6:1 | A. cochinchinensis |
| TD-4 | Climbing vine | 210 | Stem is smooth and longitudinal lines are not obvious | 6–9 | Sharp triangular shape | 8 | 4.5 | 3 | Yellow | Middle and lower parts of filament are attached | 7:1 | A. taliensis |
| TD-5 | Climbing vine | 130 | Smooth stem with longitudinal lines | 3–5 | Sharp triangular shape | 13 | 3.5 | 2 | Pistachio | Filaments are not attached | 6:1 | A. cochinchinensis |
| TD-6 | Climbing vine | 140 | Smooth stem with longitudinal lines | 3–5 | Sharp triangular shape | 12 | 3 | 2.5 | Pistachio | Filaments are not attached | 7:1 | A. cochinchinensis |
| TD-7 | Semi-erect vine | 110 | Stem is smooth and longitudinal lines are not obvious | 2–3 | Sickle shape | 13 | Inconspicuous | No | No | No | 5:1 | A. cochinchinensis |
| ID | Root Shape | Root Length: Thickness | Stone Cell | Calcium Oxalate | Organoleptic |
|---|---|---|---|---|---|
| TD-1 | Spindle | 10:1 | Occasionally seen | Yes | Light yellow, transparent, sweet taste |
| TD-2 | Long spindle | 12:1 | Yes | Yes | Yellow, translucent, bitter |
| TD-3 | Spindle | 10:1 | Yes | Yes | Yellow, transparent, sweet, slightly bitter |
| TD-4 | Long spindle | 13:1 | Yes | Yes | Yellow, translucent, bitter |
| TD-5 | Spindle | 9:1 | Occasionally seen | Yes | Pale yellow, transparent, slightly bitter |
| TD-6 | Spindle | 9:1 | Occasionally seen | Yes | Pale yellow, transparent, sweet, slightly bitter |
| TD-7 | Short spindle | 7:1 | Occasionally seen | Yes | Light yellow, transparent, sweet taste |
| Type of Repeat | Number | Percentage (%) | Number of Predominant Repeat Motifs | Percentage of Predominant Repeat Motifs (%) |
|---|---|---|---|---|
| Dinucleotide | 218 | 20.62 | AG/CT:39 | 17.89 |
| AT/AT:31 | 14.22 | |||
| Trinucleotide | 761 | 71.99 | CGG/CCG:44 | 5.78 |
| CCG/CGG:32 | 4.21 | |||
| Tetranucleotide | 18 | 1.71 | TTTC/GAAA:2 | 11.1 |
| Pentanucleotide | 20 | 1.89 | No | No |
| Hexanucleotide | 40 | 3.78 | No | No |
| Total | 1057 |
| Primer ID | Primer Sequence | Original Repeating Unit | Number of Repeats | Annealing Temperature | Specificity | Polymorphism | PCR Product Size (bp) |
|---|---|---|---|---|---|---|---|
| Asp.-8 | F:AACCTGTACAGCTCGTCGATG | CAC (3 × 5) | 15 | 57 | Yes | Yes | 160 |
| R:TGTTGCAGAACATCGCGAAG | |||||||
| Asp.-29 | F:ATGAGGACGTTGGACCAGTAATC | CGC (3 × 5) | 15 | 59 | Yes | Yes | 158 |
| R:GACAAGCTAGAGAGGTACAGAGC | |||||||
| Asp.-34 | F:TGACGATGATGAGAGGGATGAAG | CCG (3 × 7) | 21 | 57 | Yes | Yes | 158 |
| R:TTCAAAGGGGAAGGGAAAAACTG | |||||||
| Asp.-47 | F:GTCCATGTCTTCCTCCTTCGAC | CGG (3 × 7) | 21 | 60 | Yes | Yes | 157 |
| R:GGACTCCGGCATCGAGAAG | |||||||
| Asp.-61 | F:ACAGATCTCAATCATCCCAGGTT | CAT (3 × 8) | 24 | 57 | Yes | Yes | 155 |
| R:CTCCTTAATCAGAAGGGCTGTGT | |||||||
| Asp.-64 | F:AGCTACTTATCCGCCACTCTTTC | ACC (3 × 7) | 21 | 58 | Yes | Yes | 155 |
| R:TCCCACCTCACTATACAGACCAT | |||||||
| Asp.-87 | F:TTTGAGACTCAAGCAAAAGCACC | TTA (3 × 8) | 24 | 55 | Yes | Yes | 151 |
| R:TGCTTAGGAACTCTAAACACTGT | |||||||
| Asp.-88 | F:TGATCCTGTTCAGGAACGAAGAG | TGC (3 × 7) | 21 | 58 | Yes | Yes | 151 |
| R:TTCCTTTCTCAAGATCCAGAGCC | |||||||
| Asp.-95 | F:CTCGAGTTCACCGTCCAAAAC | CGT (3 × 6) | 18 | 59 | Yes | Yes | 149 |
| R:GAGGAGGACAGGGAGATGCTAT | |||||||
| Asp.-99 | F:GAGTCGCTGAACTTCCATCTGAG | CGC (3 × 8) | 24 | 60 | Yes | Yes | 149 |
| R:GATCCCAACCCGAACCCTACTC |
| Primer ID | No. of Bands | No. of Polymorphic Bands | Proportion (%) | Na Allele Number | Ne Number of Effective Alleles | H Heterozygosity | I Shannon Index |
|---|---|---|---|---|---|---|---|
| Asp.-8 | 14 | 6 | 42.86 | 2.00 | 1.45 | 0.28 | 0.45 |
| Asp.-29 | 14 | 8 | 57.14 | 2.00 | 1.33 | 0.22 | 0.36 |
| Asp.-34 | 9 | 5 | 55.56 | 2.00 | 1.33 | 0.23 | 0.37 |
| Asp.-47 | 12 | 8 | 66.67 | 2.00 | 1.26 | 0.20 | 0.34 |
| Asp.-61 | 21 | 9 | 42.86 | 2.00 | 1.44 | 0.28 | 0.44 |
| Asp.-64 | 14 | 7 | 50.00 | 2.00 | 1.37 | 0.25 | 0.41 |
| Asp.-87 | 13 | 9 | 69.23 | 2.00 | 1.25 | 0.19 | 0.33 |
| Asp.-88 | 16 | 7 | 43.75 | 2.00 | 1.43 | 0.28 | 0.45 |
| Asp.-95 | 9 | 5 | 55.56 | 2.00 | 1.32 | 0.23 | 0.39 |
| Asp.-99 | 22 | 7 | 31.82 | 2.00 | 1.55 | 0.34 | 0.52 |
| Mean | 14 | 7 | 51.54 | 2.00 | 1.37 | 0.25 | 0.40 |
| St. Dev | 0.00 | 0.26 | 0.12 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Yan, F.; Liu, C.; Chen, A.; Wu, J.; Yu, M.; Lyu, X. Developing EST-SSR Markers for Identifying and Evaluating Asparagus Germplasm Resources Based on Transcriptome Sequences. Horticulturae 2024, 10, 121. https://doi.org/10.3390/horticulturae10020121
Liu D, Yan F, Liu C, Chen A, Wu J, Yu M, Lyu X. Developing EST-SSR Markers for Identifying and Evaluating Asparagus Germplasm Resources Based on Transcriptome Sequences. Horticulturae. 2024; 10(2):121. https://doi.org/10.3390/horticulturae10020121
Chicago/Turabian StyleLiu, Dan, Feili Yan, Changmei Liu, Aimeng Chen, Jiahui Wu, Ma Yu, and Xiangyang Lyu. 2024. "Developing EST-SSR Markers for Identifying and Evaluating Asparagus Germplasm Resources Based on Transcriptome Sequences" Horticulturae 10, no. 2: 121. https://doi.org/10.3390/horticulturae10020121
APA StyleLiu, D., Yan, F., Liu, C., Chen, A., Wu, J., Yu, M., & Lyu, X. (2024). Developing EST-SSR Markers for Identifying and Evaluating Asparagus Germplasm Resources Based on Transcriptome Sequences. Horticulturae, 10(2), 121. https://doi.org/10.3390/horticulturae10020121







