The Metacaspase Gene PoMCA1 Enhances the Mycelial Heat Stress Tolerance and Regulates the Fruiting Body Development of Pleurotus ostreatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Gene Structure and Protein Properties Analysis
2.3. Phylogenetic Analysis and Multiple Sequence Alignment
2.4. DNA/RNA Extraction and cDNA Synthesis
2.5. Gene Cloning and Plasmids Construction
2.6. A. tumefaciens Mediated Transformation (ATMT) into P. ostreatus Mycelia
2.7. Quantitative Real-Time Reverse-Transcription PCR (qRT-PCR)
2.8. Determination of Mycelial Growth Rate (MGR)
2.9. Heat Stress Treatment
2.10. Fruiting Body Cultivation and Phenotypic Analysis of Transformants
2.11. Statistical Analysis
3. Results
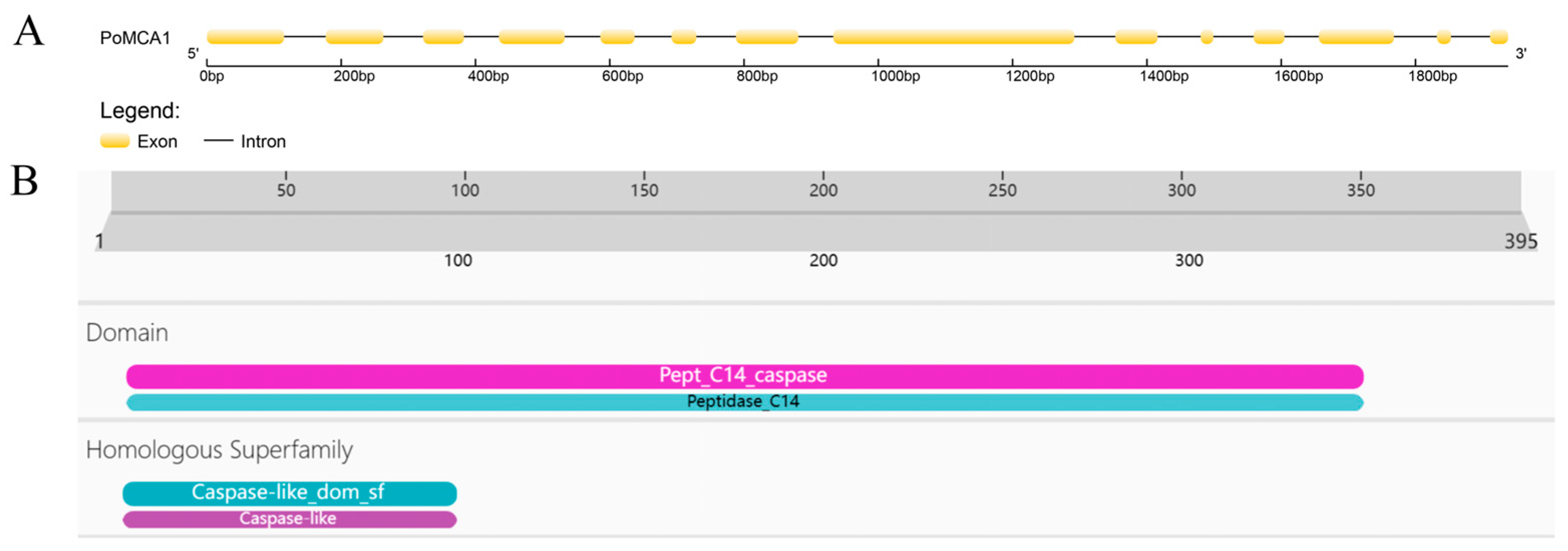
3.1. Identification of PoMCA1 from P. ostreatus
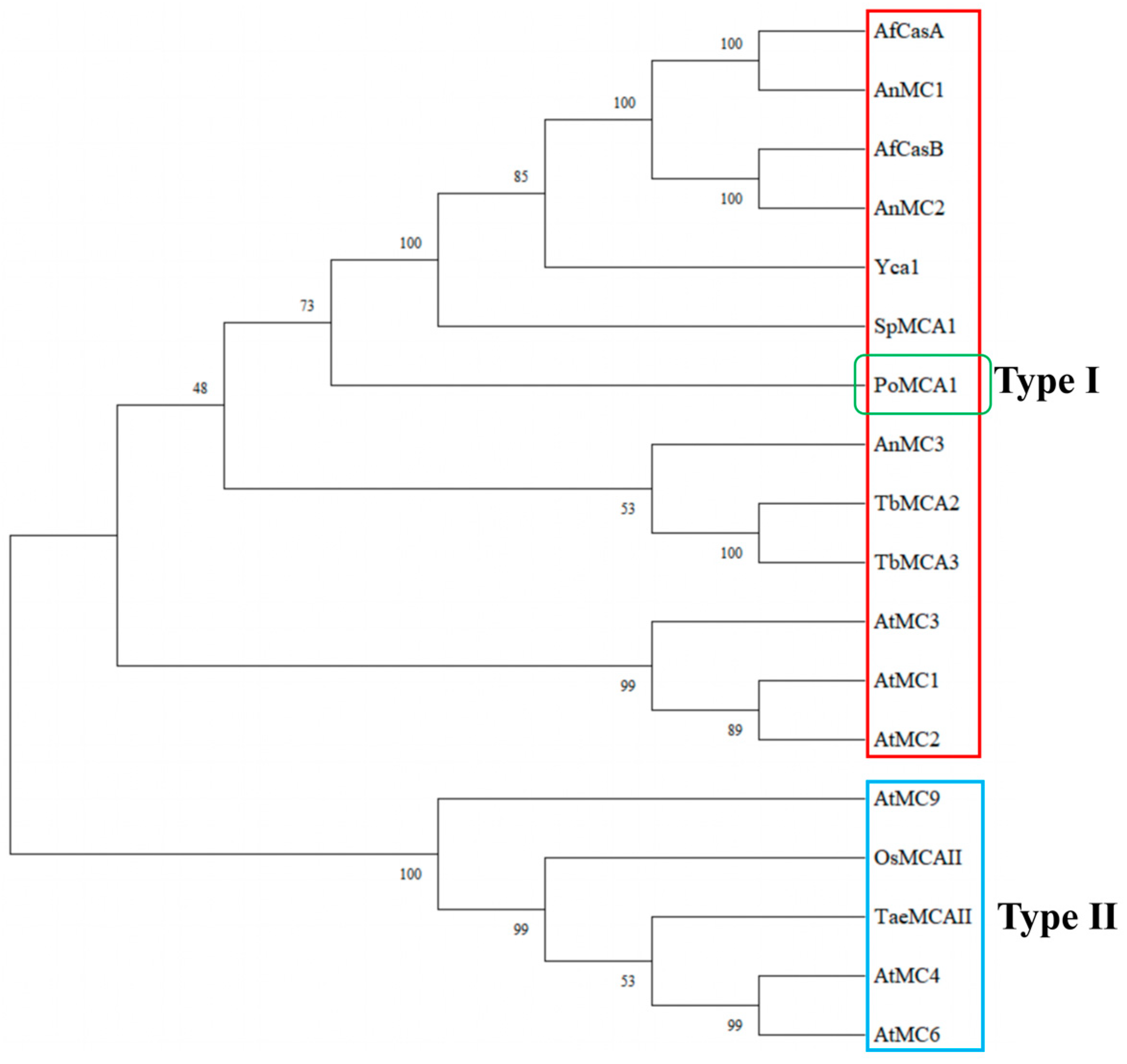
3.2. Multiple Sequence Alignment
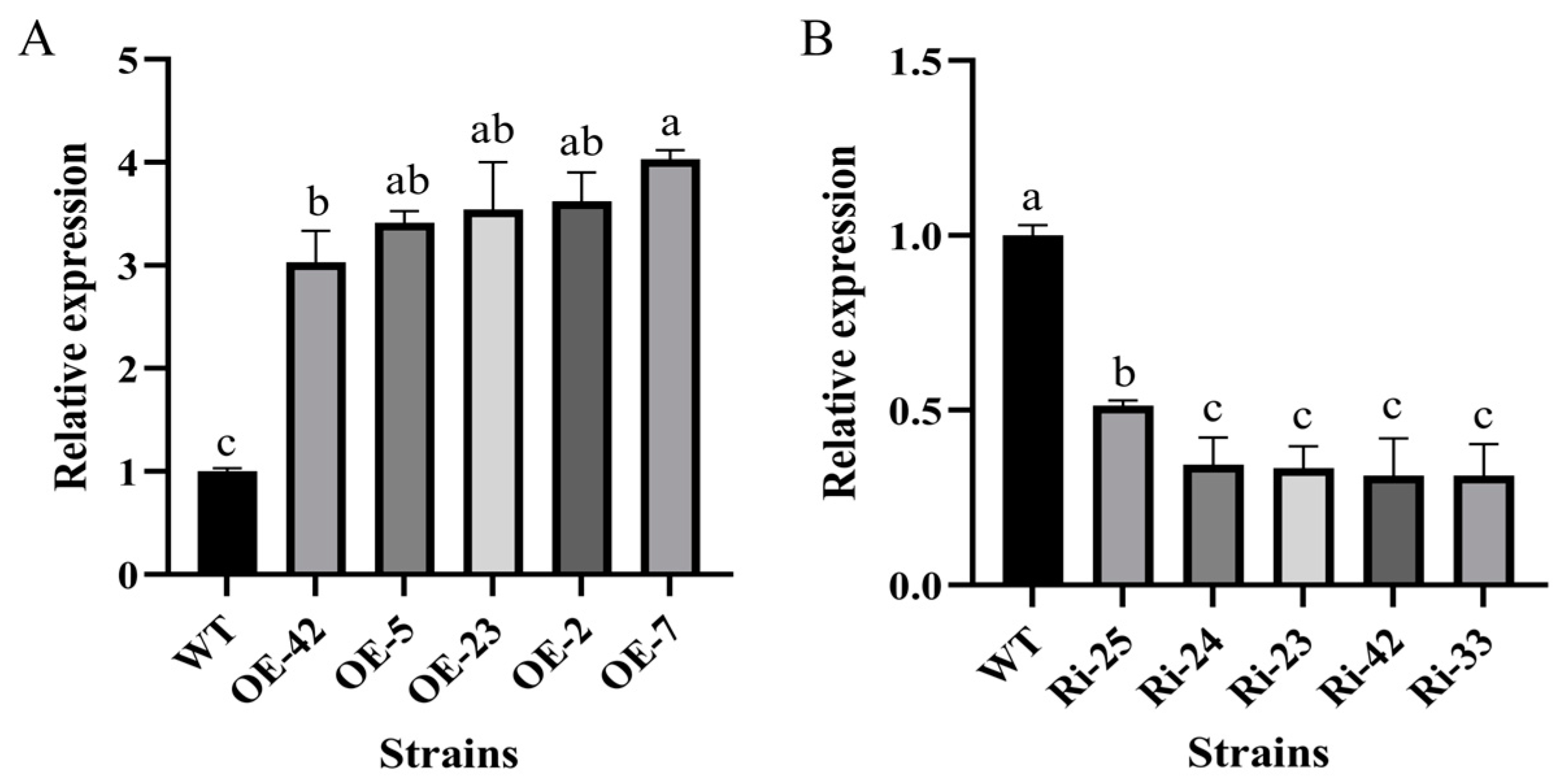
3.3. Generation of Overexpression and Interference Transformants
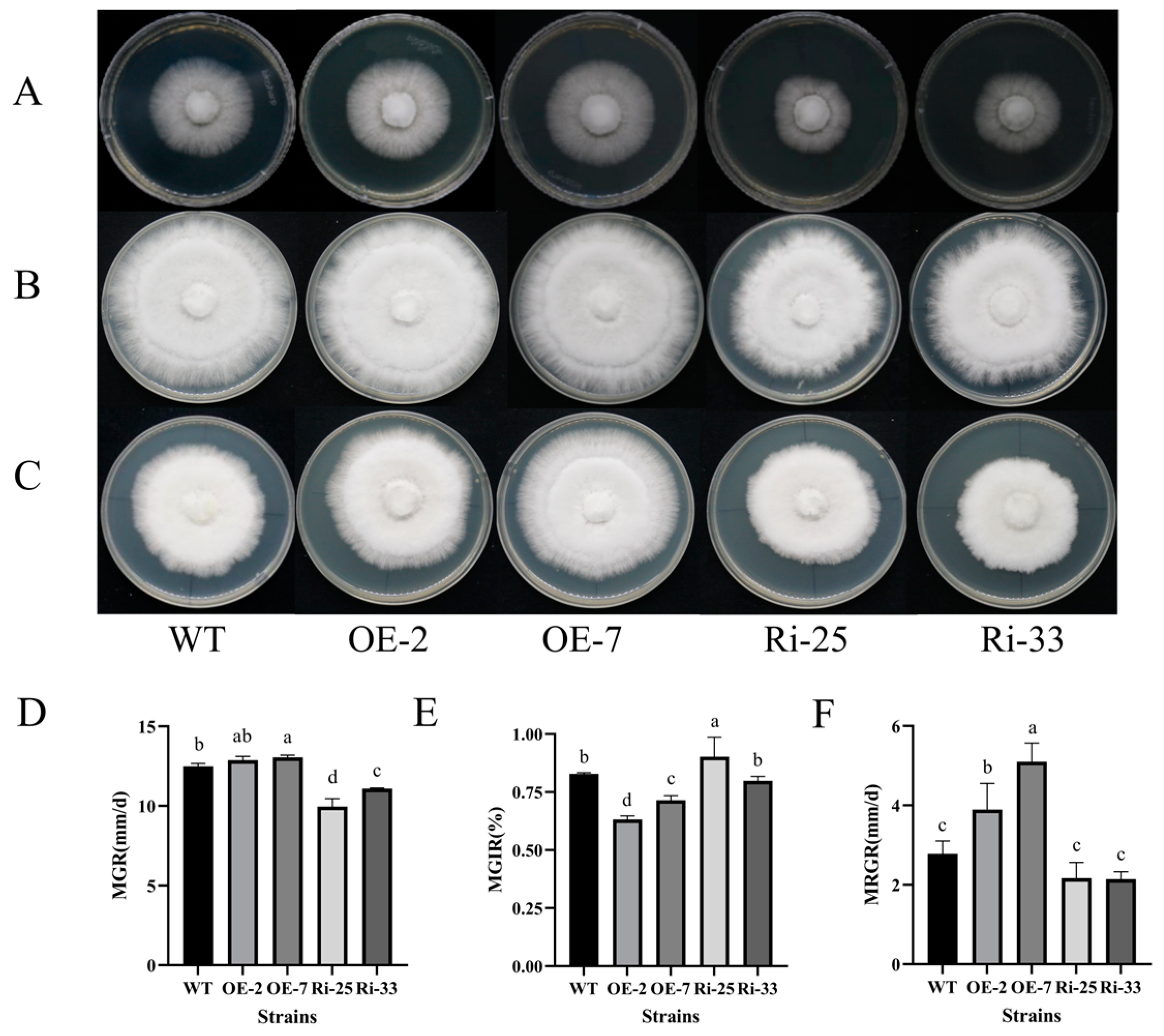
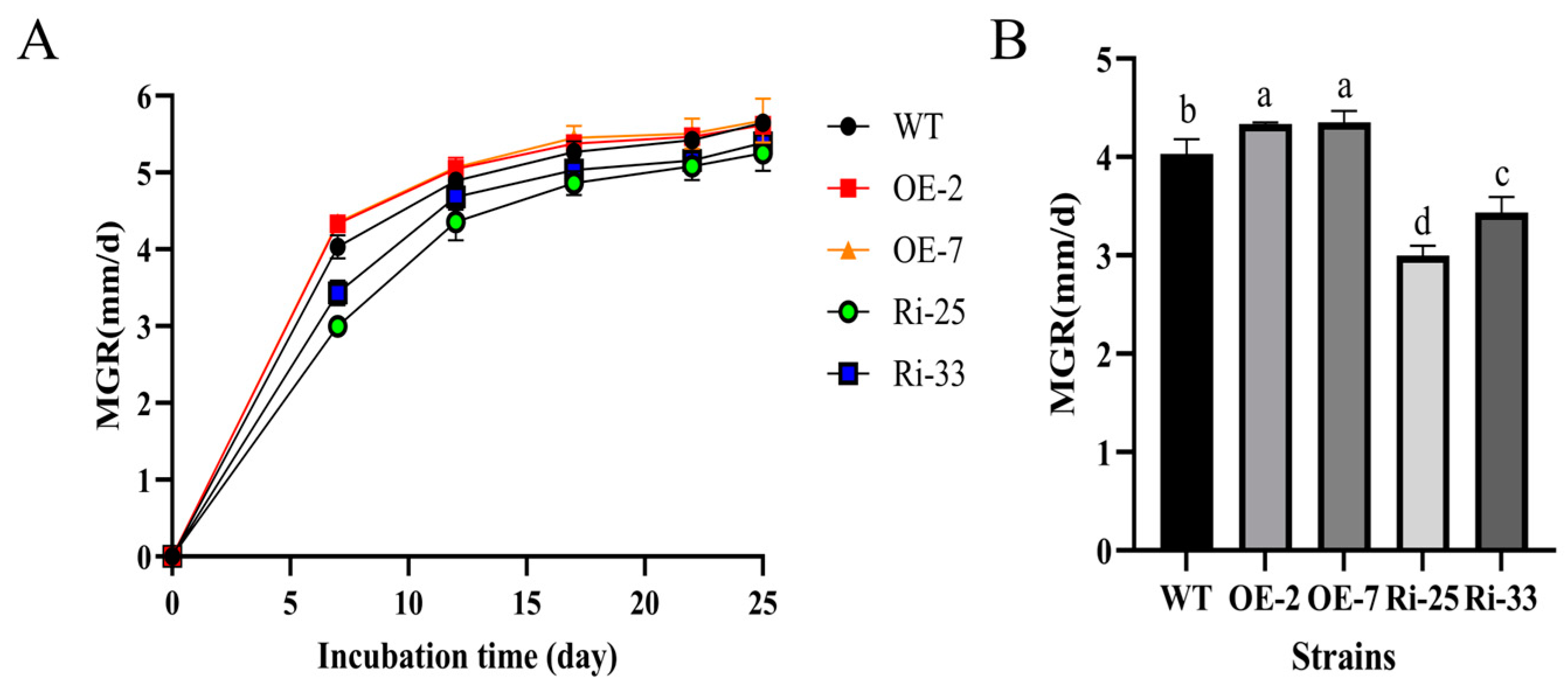
3.4. PoMCA1 Promoted Mycelial Growth and Heat Resistance on PDA
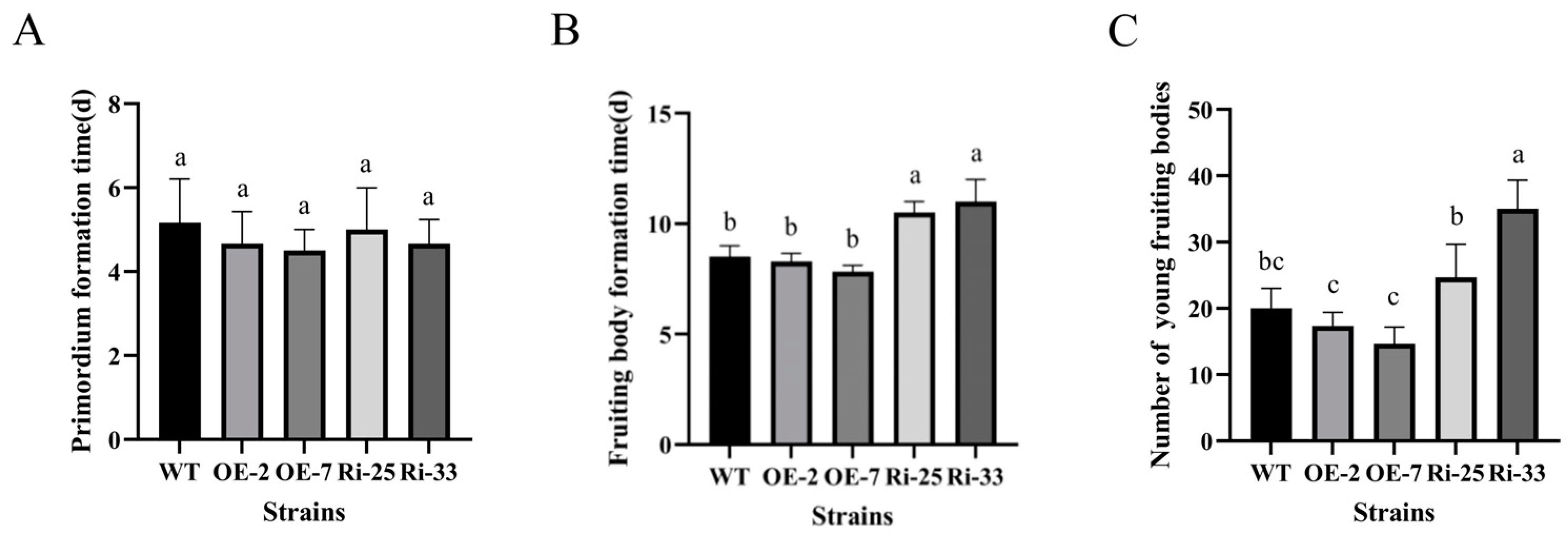
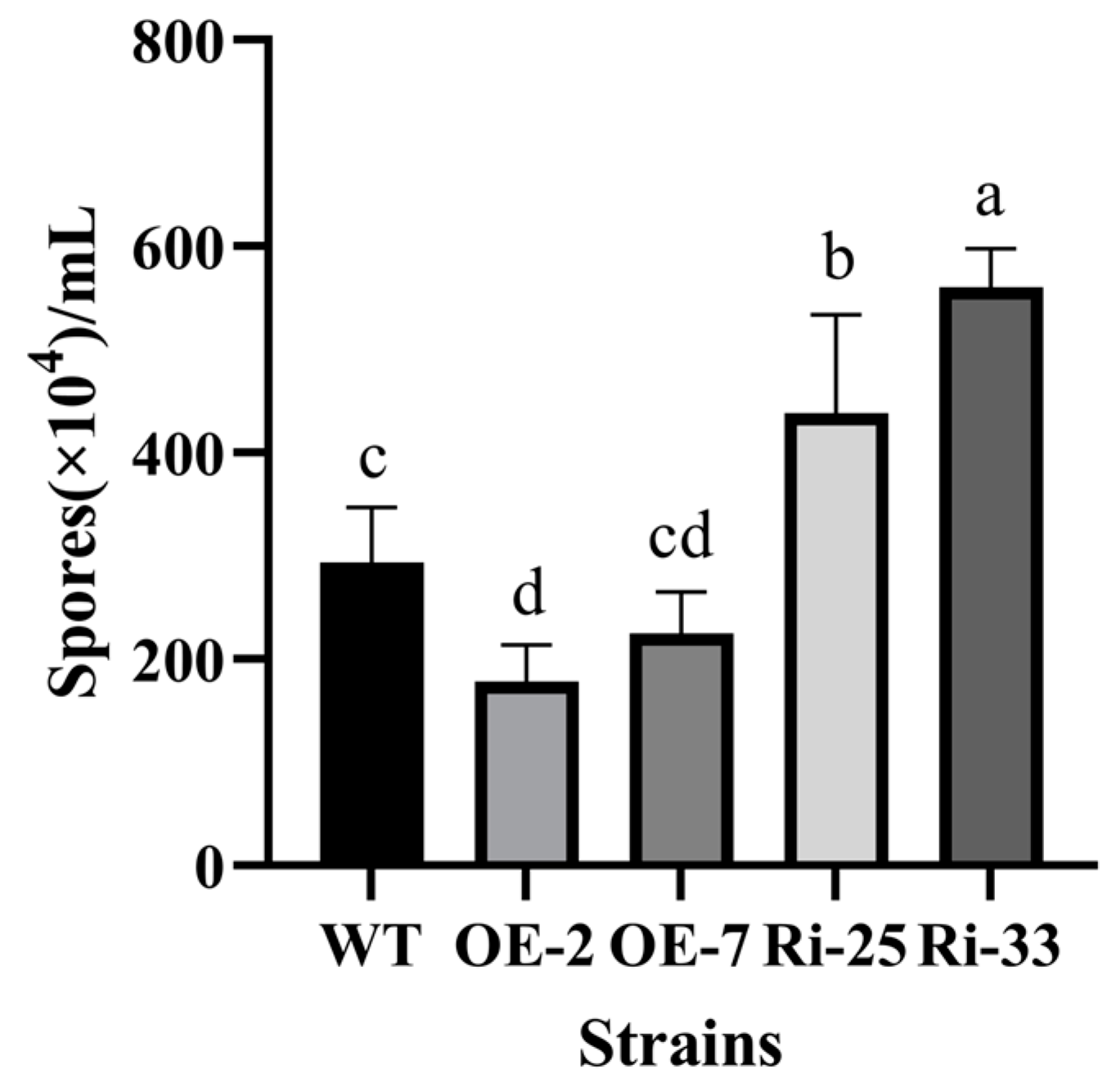
3.5. PoMCA1 Regulated the Fruiting Body Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, J.; Liu, C.; Zhang, Q.; Li, J.; Qi, Y.; Qiu, L.; Gao, Y.; Wen, Q. Effect of five different cultivation substrates on nutrients in fruiting body of Pleurotus ostreatus. J. Henan Agric. Sci. 2016, 45, 103–106. [Google Scholar]
- Wen, Q.; Zhu, L.; Yu, H.; Chen, H.; Li, J.; Shen, J. Effects of spent mushroom substrate of factory-cultivated Flammulina velutipes on the sterilized raw material cultivation and nutritional component of Pleurotus ostreatus. North. Hortic. 2021, 02, 124–130. [Google Scholar]
- Lei, M.; Wu, X.; Zhang, J.; Wang, H.; Huang, C. Establishment of an efficient transformation system for Pleurotus ostreatus. World J. Microbiol. Biotechnol. 2017, 33, 214. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, L.; Wu, X.; Gao, W.; Zhang, J.; Huang, C. Expression patterns of two pal genes of Pleurotus ostreatus across developmental stages and under heat stress. BMC Microbiol. 2019, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Liu, Z.; Yan, K.; Xu, L.; Chang, M.; Meng, J. Mnsod1 promotes the development of Pleurotus ostreatus and enhances the tolerance of mycelia to heat stress. Microb. Cell Fact. 2022, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Characterization and Function Analysis of Catalase Genes in Pleurotus ostreatus. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. [Google Scholar]
- Qi, Y.; Chen, H.; Zhang, M.; Wen, Q.; Qiu, L.; Shen, J. Identification and expression analysis of Pofst3 suggests a role during Pleurotus ostreatus primordia formation. Fungal Biol. 2019, 123, 200–208. [Google Scholar] [CrossRef]
- Aram, L.; Arama, E. Sporoptosis: Sowing the seeds of nuclear destruction. Dev. Cell 2012, 23, 5–6. [Google Scholar] [CrossRef]
- Lee, K.P.; Kim, C.; Landgraf, F.; Apel, K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 10270–10275. [Google Scholar] [CrossRef]
- Mottram, J.C.; Helms, M.J.; Coombs, G.H.; Sajid, M. Clan CD cysteine peptidases of parasitic protozoa. Trends Parasitol. 2003, 19, 182–187. [Google Scholar] [CrossRef]
- Szallies, A.; Kubata, B.K.; Duszenko, M. A metacaspase of Trypanosoma brucei causes loss of respiration competence and clonal death in the yeast Saccharomyces cerevisiae. FEBS Lett. 2002, 517, 144–150. [Google Scholar] [CrossRef]
- Madeo, F.; Herker, E.; Maldener, C.; Wissing, S.; Lachelt, S.; Herlan, M.; Fehr, M.; Lauber, K.; Sigrist, S.J.; Wesselborg, S.; et al. A caspase-related protease regulates apoptosis in yeast. Mol. Cell 2002, 9, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Vercammen, D.; van de Cotte, B.; De Jaeger, G.; Eeckhout, D.; Casteels, P.; Vandepoele, K.; Vandenberghe, I.; Van Beeumen, J.; Inze, D.; Van Breusegem, F. Type II metacaspases Atmc4 and Atmc9 of Arabidopsis thaliana cleave substrates after arginine and lysine. J. Biol. Chem. 2004, 279, 45329–45336. [Google Scholar] [CrossRef] [PubMed]
- Uren, A.G.; O’Rourke, K.; Aravind, L.; Pisabarro, M.T.; Seshagiri, S.; Koonin, E.V.; Dixit, V.M. Identification of paracaspases and metacaspases: Two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol. Cell 2000, 6, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, D.; Bohn, B.; Cabreira, C.; Leipelt, F.; Dias, N.; Bodanese-Zanettini, M.H.; Cagliari, A. Caspases in plants: Metacaspase gene family in plant stress responses. Funct. Integr. Genom. 2015, 15, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Klemencic, M.; Funk, C. Type III metacaspases: Calcium-dependent activity proposes new function for the p10 domain. New Phytol. 2018, 218, 1179–1191. [Google Scholar] [CrossRef]
- Tsiatsiani, L.; Van Breusegem, F.; Gallois, P.; Zavialov, A.; Lam, E.; Bozhkov, P.V. Metacaspases. Cell Death Differ. 2011, 18, 1279–1288. [Google Scholar] [CrossRef]
- Hamann, A.; Brust, D.; Osiewacz, H.D. Deletion of putative apoptosis factors leads to lifespan extension in the fungal ageing model Podospora anserina. Mol. Microbiol. 2007, 65, 948–958. [Google Scholar] [CrossRef]
- Wang, C.C.; Lü, P.T.; Zhong, S.L.; Chen, H.B.; Zhou, B.Y. LcMCII-1 is involved in the ROS-dependent senescence of the rudimentary leaves of Litchi chinensis. Plant Cell Rep. 2017, 36, 89–102. [Google Scholar] [CrossRef]
- Kosec, G.; Alvarez, V.E.; Aguero, F.; Sanchez, D.; Dolinar, M.; Turk, B.; Turk, V.; Cazzulo, J.J. Metacaspases of Trypanosoma cruzi: Possible candidates for programmed cell death mediators. Mol. Biochem. Parasitol. 2006, 145, 18–28. [Google Scholar] [CrossRef]
- Zalila, H.; González, I.J.; El-Fadili, A.K.; Delgado, M.B.; Desponds, C.; Schaff, C.; Fasel, N. Processing of metacaspase into a cytoplasmic catalytic domain mediating cell death in Leishmania major. Mol. Microbiol. 2011, 79, 222–239. [Google Scholar] [CrossRef]
- Richie, D.L.; Miley, M.D.; Bhabhra, R.; Robson, G.D.; Rhodes, J.C.; Askew, D.S. The Aspergillus fumigatus metacaspases CasA and CasB facilitate growth under conditions of endoplasmic reticulum stress. Mol. Microbiol. 2007, 63, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Minina, E.A.; Filonova, L.H.; Fukada, K.; Savenkov, E.I.; Gogvadze, V.; Clapham, D.; Sanchez-Vera, V.; Suarez, M.F.; Zhivotovsky, B.; Daniel, G.; et al. Autophagy and metacaspase determine the mode of cell death in plants. J. Cell Biol. 2013, 203, 917–927. [Google Scholar] [CrossRef]
- Kwon, S.I.; Hwang, D.J. Expression analysis of the metacaspase gene family in Arabidopsis. J. Plant Biol. 2013, 56, 391–398. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, P.; Wei, R.; Li, S.; Zhang, X.; Yu, Y.; Wang, Y. The metacaspase gene family of Vitis vinifera L.: Characterization and differential expression during ovule abortion in stenospermocarpic seedless grapes. Gene 2013, 528, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, H.; Hong, Y.; Liu, S.; Li, D.; Song, F. Stress-responsive expression, subcellular localization and protein-protein interactions of the rice metacaspase family. Int. J. Mol. Sci. 2015, 16, 16216–16241. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, Z.; Chen, J.; Wang, S.; Hao, L.; Li, D. Genome-wide identification and expression analysis of the metacaspase gene family in Hevea brasiliensis. Plant Physiol. Biochem. 2016, 105, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, J.; Wei, Y. Identification and analysis of the metacaspase gene family in tomato. Biochem. Biophys. Res. Commun. 2016, 479, 523–529. [Google Scholar] [CrossRef]
- Dubey, N.; Trivedi, M.; Varsani, S.; Vyas, V.; Farsodia, M.; Singh, S.K. Genome-wide characterization, molecular evolution and expression profiling of the metacaspases in potato (Solanum tuberosum L.). Heliyon 2019, 5, e01162. [Google Scholar] [CrossRef]
- Lim, H.W.; Kim, S.J.; Park, E.H.; Lim, C.J. Overexpression of a metacaspase gene stimulates cell growth and stress response in Schizosaccharomyces pombe. Can. J. Microbiol. 2007, 53, 1016–1023. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Zhao, M.R.; Wu, X.L.; Zhang, J.X. Metabolic response of Pleurotus ostreatus to continuous heat stress. Front. Microbiol. 2020, 10, 3148. [Google Scholar] [CrossRef]
- Watanabe, N.; Lam, E. Arabidopsis metacaspase 2d is a positive mediator of cell death induced during biotic and abiotic stresses. Plant J. 2011, 66, 969–982. [Google Scholar] [CrossRef]
- Kim, S.M.; Bae, C.; Oh, S.K.; Choi, D. A pepper (Capsicum annuum L.) metacaspase 9 (Camc9) plays a role in pathogen-induced cell death in plants. Mol. Plant Pathol. 2013, 14, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.H.; Van Griensven, L.J.L.D. Morphogenetic cell death in developing primordia of Agaricus bisporus. Mycologia 1997, 89, 274–277. [Google Scholar] [CrossRef]
- Georgiou, C.D. Lipid peroxidation in Sclerotium rolfsii: A new look into the mechanism of sclerotial biogenesis in fungi. Mycol. Res. 1997, 101, 460–464. [Google Scholar] [CrossRef]
- Fernandez, J.; Lopez, V.; Kinch, L.; Pfeifer, M.A.; Gray, H.; Garcia, N.; Grishin, N.V.; Khang, C.H.; Orth, K. Role of two metacaspases in development and pathogenicity of the rice blast fungus Magnaporthe oryzae. mBio 2021, 12, e03471-20. [Google Scholar] [CrossRef]
- Chen, L.L.; Ma, Y.M.; Peng, M.Y.; Chen, W.B.; Xia, H.Q.; Zhao, J.Y.; Zhang, Y.K.; Fan, Z.; Xing, X.P.; Li, H.L. Analysis of apoptosis-related genes reveals that apoptosis functions in conidiation and pathogenesis of Fusarium pseudograminearum. mSphere 2021, 6, e01140-20. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, J.; Zhao, M.; Zhang, L.; Wu, X. The Metacaspase Gene PoMCA1 Enhances the Mycelial Heat Stress Tolerance and Regulates the Fruiting Body Development of Pleurotus ostreatus. Horticulturae 2024, 10, 116. https://doi.org/10.3390/horticulturae10020116
Pei J, Zhao M, Zhang L, Wu X. The Metacaspase Gene PoMCA1 Enhances the Mycelial Heat Stress Tolerance and Regulates the Fruiting Body Development of Pleurotus ostreatus. Horticulturae. 2024; 10(2):116. https://doi.org/10.3390/horticulturae10020116
Chicago/Turabian StylePei, Jingqi, Mengran Zhao, Lijiao Zhang, and Xiangli Wu. 2024. "The Metacaspase Gene PoMCA1 Enhances the Mycelial Heat Stress Tolerance and Regulates the Fruiting Body Development of Pleurotus ostreatus" Horticulturae 10, no. 2: 116. https://doi.org/10.3390/horticulturae10020116
APA StylePei, J., Zhao, M., Zhang, L., & Wu, X. (2024). The Metacaspase Gene PoMCA1 Enhances the Mycelial Heat Stress Tolerance and Regulates the Fruiting Body Development of Pleurotus ostreatus. Horticulturae, 10(2), 116. https://doi.org/10.3390/horticulturae10020116




