Exogenous Sucrose Enhances Growth and Physiological Performance of Tomato Seedlings Under Suboptimal Light Conditions in Passive Greenhouses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions and Plant Material
2.2. Treatments and Experimental Design
2.3. Morphological, Physiological, and Growth Measurements
2.4. Statistical Analysis
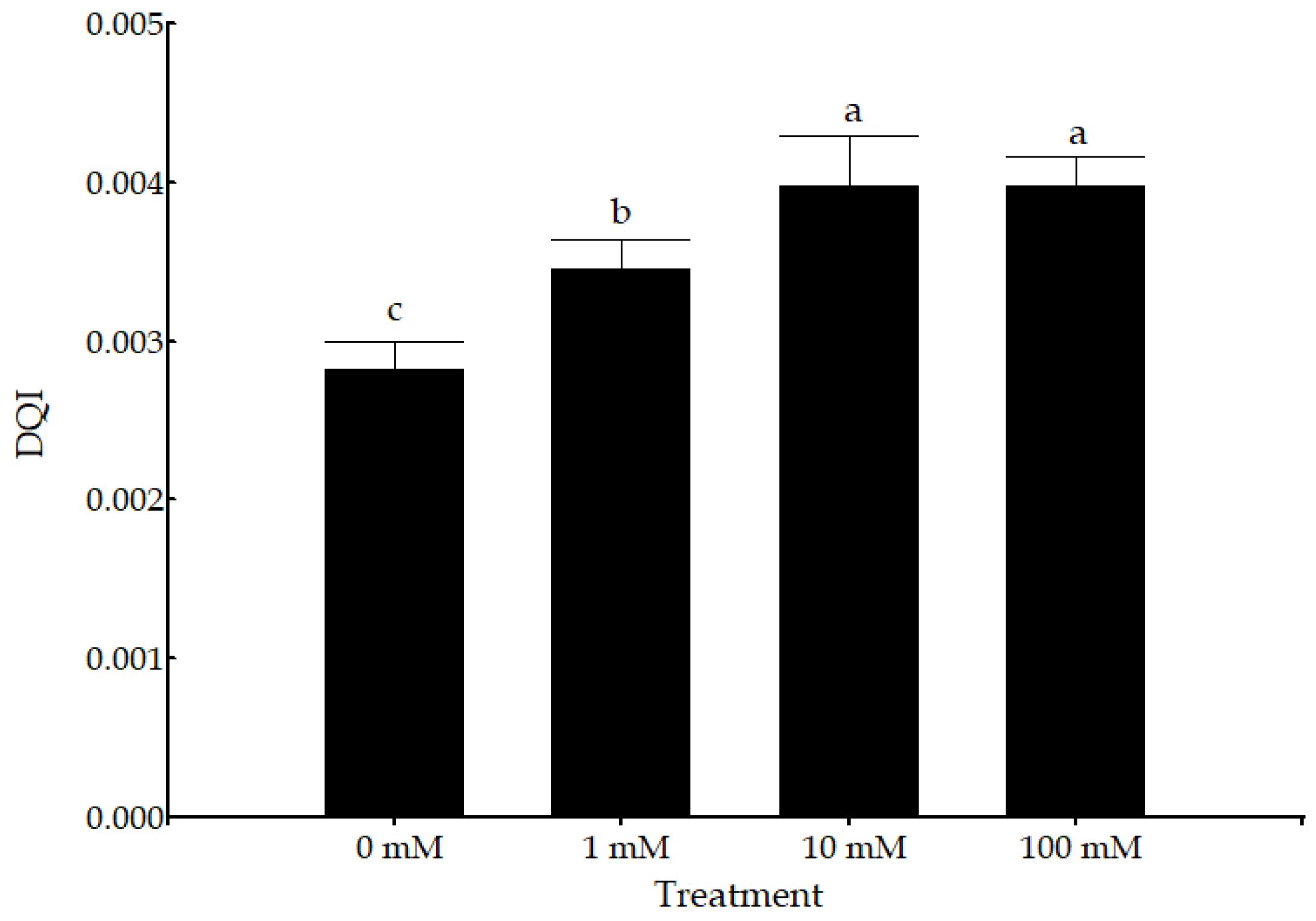
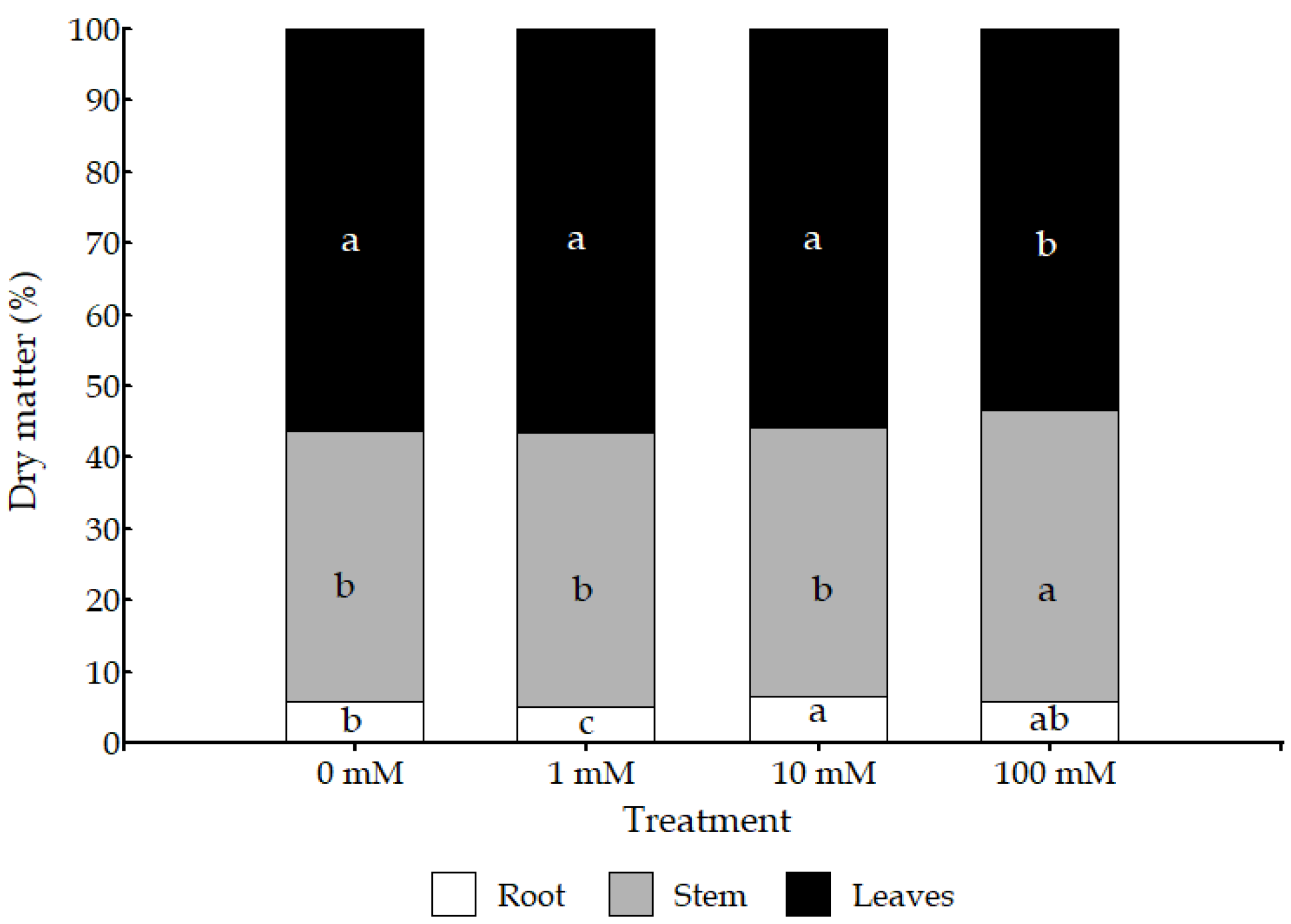
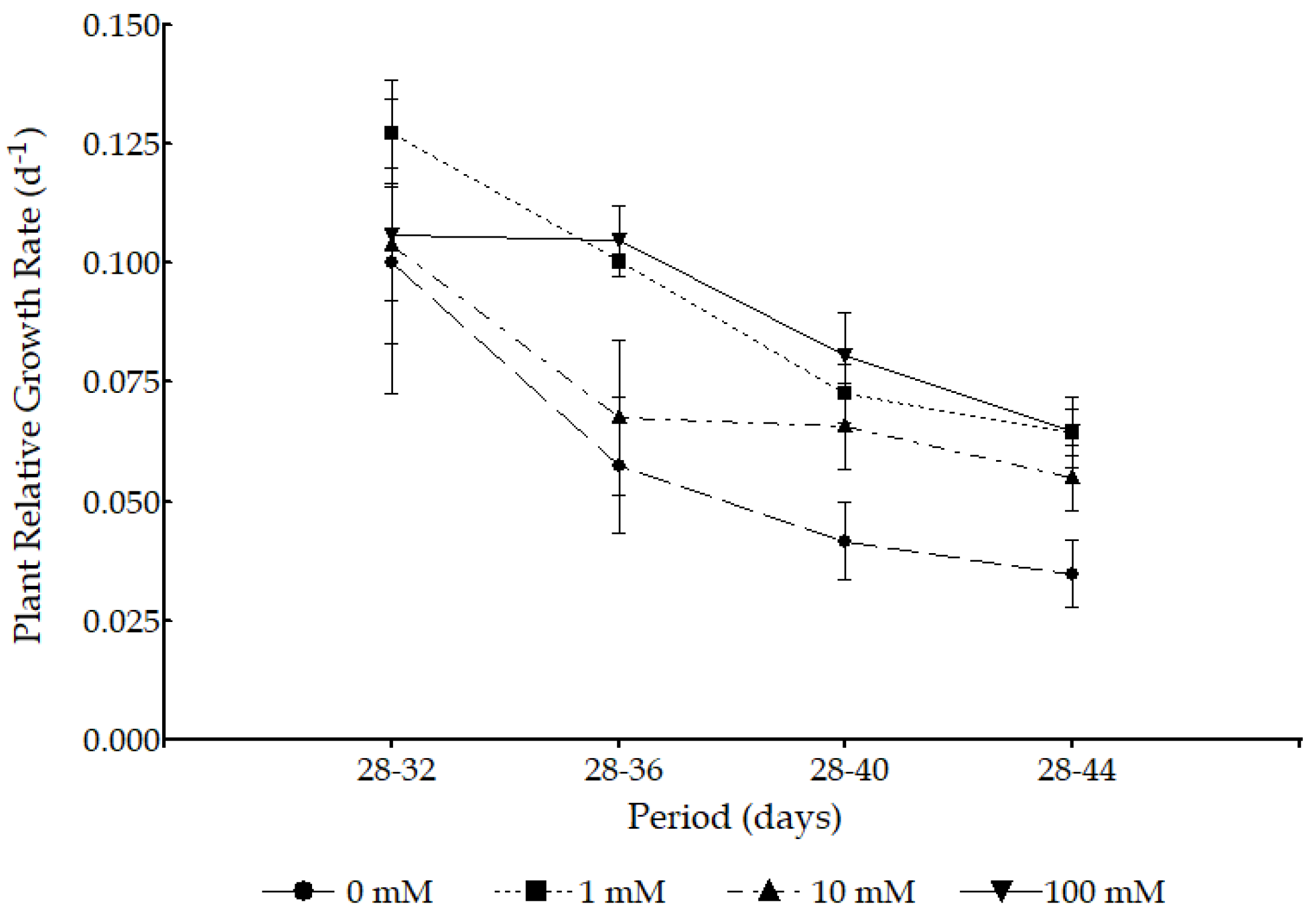
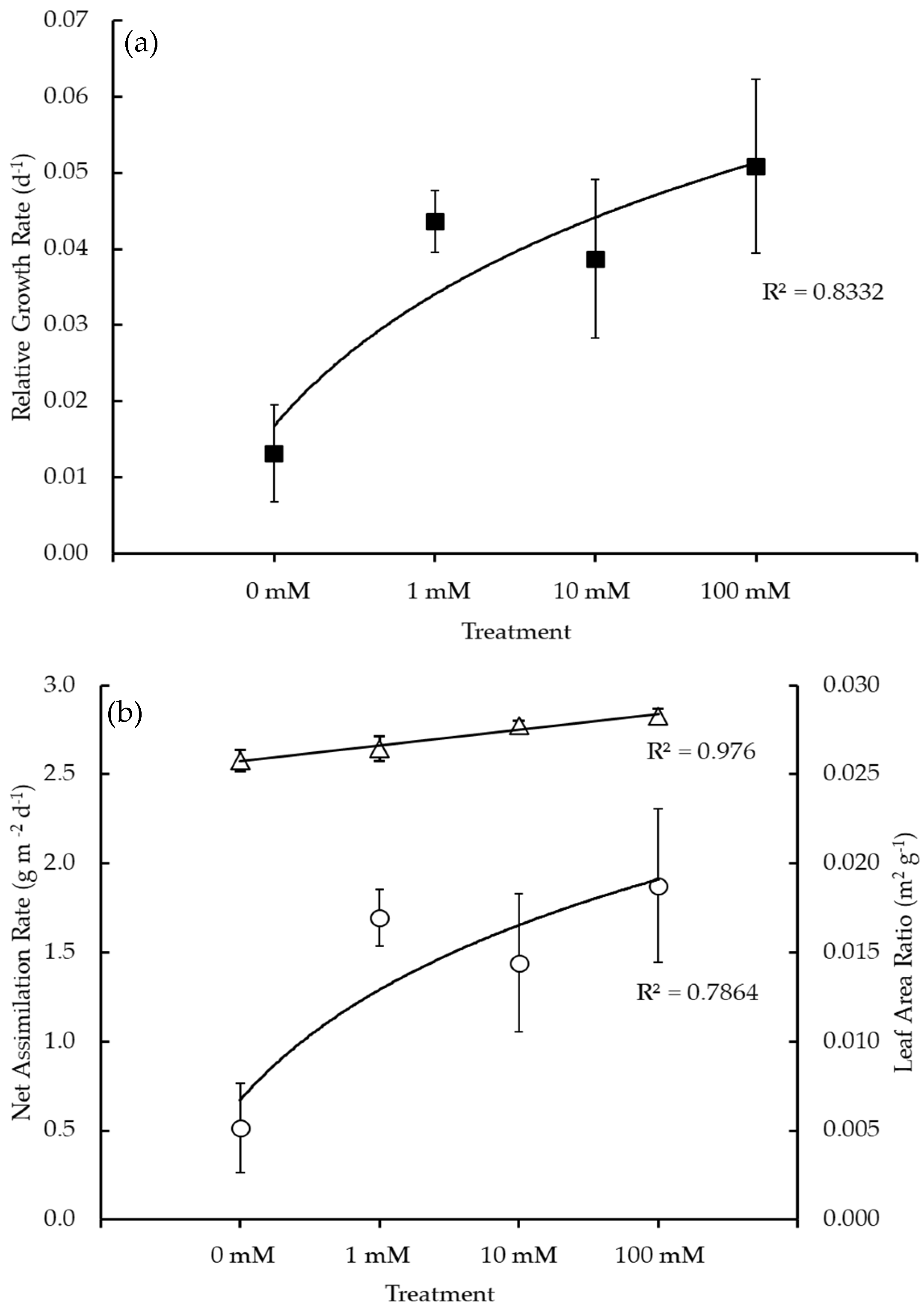
3. Results
3.1. Morphological and Physiological Parameters
3.2. Plant Growth Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 26 October 2024).
- Matsuo, S.; Nanya, K.; Imanishi, S.; Honda, I.; Goto, E. Effects of blue and red lights on gibberellin metabolism in tomato seedlings. Hort. J. 2019, 88, 76–82. [Google Scholar] [CrossRef]
- Zheng, Y.; Zou, J.; Lin, S.; Jin, C.; Shi, M.; Yang, B.; Yang, Y.; Jin, D.; Li, R.; Li, Y.; et al. Effects of different light intensity on the growth of tomato seedlings in a plant factory. PLoS ONE 2023, 18, e0294876. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Huang, Z.W.; Li, J.P.; Su, W.X.; Gan, L.; Xu, Z.G. Effect of different light intensities on the photosynthate distribution in cherry tomato seedlings. J. Hortic. Sci. Biotechnol. 2019, 94, 611–619. [Google Scholar] [CrossRef]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhang, W.; Wang, Z.B.; Ma, L.; Guo, Y.P.; Ren, X.L.; Mei, L.X. Influence of shading on photosynthesis and antioxidative activities of enzymes in apple trees. Photosynthetica 2019, 57, 857–865. [Google Scholar] [CrossRef]
- Sánchez-del Castillo, F.; Portillo-Márquez, L.; Moreno-Pérez, E.D.; Magdaleno-Villar, J.J.; Vázquez-Rodríguez, J.C. Effects of container volume and seedling density on late transplanting and number of flowers in tomato. Rev. Chapingo Ser. Hortic. 2021, 27, 71–84. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.; Yong, H.; Li, L.; Lyu, D. Effects of exogenous sucrose on root physiology and transcriptome of Malus baccata Borkh. under sub-low root-zone temperature. Sci. Hortic. 2024, 337, 113474. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Chai, L.; Lu, T.; Li, Y.; Jiang, W.; Li, Q. Exogenous sucrose confers low light tolerance in tomato plants by increasing carbon partitioning from stems to leaves. J. Agric. Food Chem. 2023, 71, 20625–20642. [Google Scholar] [CrossRef]
- Huang, X.J.; Jian, S.F.; Chen, D.L.; Zhong, C.; Miao, J.H. Concentration-dependent dual effects of exogenous sucrose on nitrogen metabolism in Andrographis paniculata. Sci. Rep. 2022, 12, 4906. [Google Scholar] [CrossRef]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, X.; Yang, S.; Ding, X.; Zou, J. Effect of red and blue light on cucumber seedlings grown in a plant factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Guo, A.; Hu, Y.; Shi, M.; Wang, H.; Wu, Y.; Wang, Y. Effects of iron deficiency and exogenous sucrose on the intermediates of chlorophyll biosynthesis in Malus halliana. PLoS ONE 2020, 15, e0232694. [Google Scholar] [CrossRef]
- Wang, L.H.; Li, G.L.; Wei, S.; Li, L.J.; Zuo, S.Y.; Liu, X.; Gu, W.R.; Li, J. Effects of exogenous glucose and sucrose on photosynthesis in triticale seedlings under salt stress. Photosynthetica 2019, 57, 286–294. [Google Scholar] [CrossRef]
- Gong, H.L.; Chen, Q.Q. Exogenous sucrose protects potato seedlings against heat stress by enhancing the antioxidant defense system. J. Soil. Sci. Plant Nutr. 2021, 21, 1511–1519. [Google Scholar] [CrossRef]
- Qiu, Z.B.; Wang, Y.F.; Zhu, A.J.; Peng, F.L.; Wang, L.S. Exogenous sucrose can enhance tolerance of Arabidopsis thaliana seedlings to salt stress. Biol. Plant 2014, 58, 611–617. [Google Scholar] [CrossRef]
- Hiyane, R.; Hiyane, S.; Tang, A.C.; Boyer, J.S. Sucrose feeding reverses shade-induced kernel losses in Maize. Ann. Bot. 2010, 106, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Gao, Y.; Yang, Y.; Jia, L.; Weng, X. Exogenous sucrose supply regulates the physiological index levels before the flower blooming and fruit abscission stages in the biodiesel tree Sapindus mukorossi Gaertn. HortScience 2022, 57, 1397–1408. [Google Scholar] [CrossRef]
- Berrie, A.M. The Effect of sucrose sprays on the growth of tomato. Physiol. Plant 1960, 13, 9–19. [Google Scholar] [CrossRef]
- Peet, M.M.; Welles, G. Greenhouse tomato production. In Tomatoes, 1st ed.; Heuvelink, E., Ed.; CABI Publishing: Oxfordshire, UK, 2005; pp. 257–304. [Google Scholar]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Sønsteby, A.; Solhaug, K.A.; Heide, O.M. Functional growth analysis of ‘Sonata’ strawberry plants grown under controlled temperature and daylength conditions. Sci. Hortic. 2016, 211, 26–33. [Google Scholar] [CrossRef]
- De Groot, C.C.; Marcelis, L.F.M.; Van Den Boogaard, R.; Lambers, H. Growth and dry-mass partitioning in tomato as affected by phosphorus nutrition and light. Plant Cell Environ. 2001, 24, 1309–1317. [Google Scholar] [CrossRef]
- Ruan, Y.L. Signaling role of sucrose metabolism in development. Mol. Plant 2012, 5, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef] [PubMed]
- Le, V.Q.; Samson, G.; Desjardins, Y. Opposite effects of exogenous sucrose on growth, photosynthesis and carbon metabolism of in vitro plantlets of tomato (L. esculentum Mill.) grown under two levels of irradiances and CO2 concentration. J. Plant Physiol. 2001, 158, 599–605. [Google Scholar] [CrossRef]
- Zinselmeier, C.; Lauer, M.J.; Boyer, J.S. Reversing drought-induced losses in grain yield: Sucrose maintains embryo growth in maize. Crop Sci. 1995, 35, 1390–1400. [Google Scholar] [CrossRef]
- García-González, J.; Lacek, J.; Weckwerth, W.; Retzer, K. Exogenous carbon source supplementation counteracts root and hypocotyl growth limitations under increased cotyledon shading, with glucose and sucrose differentially modulating growth curves. Plant Signal Behav. 2021, 16, e1969818. [Google Scholar] [CrossRef]
- Freixes, S.; Thibaud, M.C.; Tardieu, F.; Muller, B. Root elongation and branching is related to local hexose concentration in Arabidopsis thaliana seedlings. Plant Cell Environ. 2002, 25, 1357–1366. [Google Scholar] [CrossRef]
- Lee-Ho, E.; Walton, L.J.; Reid, D.M.; Yeung, E.C.; Kurepin, L.V. Effects of elevated carbon dioxide and sucrose concentrations on Arabidopsis thaliana root architecture and anatomy. Can. J. Bot. 2007, 85, 324–330. [Google Scholar] [CrossRef]
- MacGregor, D.R.; Deak, K.I.; Ingram, P.A.; Malamy, J.E. Root system architecture in arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. Plant Cell 2008, 20, 2643–2660. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, A.I.; Scalschi, L.; García-Agustín, P.; Camañes, G. Exogenous carbon compounds modulate tomato root development. Plants 2020, 9, 837. [Google Scholar] [CrossRef]
- Malamy, J.E.; Ryan, K.S. Environmental regulation of lateral root initiation in arabidopsis. Plant Physiol. 2001, 127, 899–909. [Google Scholar] [CrossRef]
- Deak, K.I.; Malamy, J. Osmotic regulation of root system architecture. Plant J. 2005, 43, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Baque, M.A.; Elgirban, A.; Lee, E.J.; Paek, K.Y. Sucrose regulated enhanced induction of anthraquinone, phenolics, flavonoids biosynthesis and activities of antioxidant enzymes in adventitious root suspension cultures of Morinda citrifolia (L.). Acta Physiol. Plant 2012, 34, 405–415. [Google Scholar] [CrossRef]
- van der Weele, C.M.; Spollen, W.G.; Sharp, R.E.; Baskin, T.I. Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J. Exp. Bot. 2000, 51, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Frank, A.B.; Black, A.L. Aerial parts of hard red dpring wheat. I. Dry matter distribution by plant development stage. Agron. J. 1987, 79, 845–852. [Google Scholar] [CrossRef]
- Andriolo, J.L.; Streck, N.A.; Buriol, G.A.; Ludke, L.; Duarte, T.S. Growth, development and dry-matter distribution of a tomato crop as affected by environment. J. Hortic. Sci. Biotechnol. 1998, 73, 125–130. [Google Scholar] [CrossRef]
- Vanderlip, R.L.; Arkin, G.F. Simulating accumulation and distribution of dry matter in grain sorghum. Agron. J. 1977, 69, 917–923. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Veierskov, B. Distribution of dry matter in sweet pepper plants (Capsicum annuum L.) during the juvenile and generative growth phases. Sci. Hortic. 1988, 35, 179–187. [Google Scholar] [CrossRef]
- Laby, R.J.; Kincaid, M.S.; Kim, D.; Gibson, S.I. The arabidopsis sugar-insensitive mutants Sis4 and Sis5 are defective in abscisic acid synthesis and response. Plant J. 2000, 23, 587–596. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Wang, W.; Wu, X.; Xiao, Y.; Peng, F. Effects of sucrose on seedling growth and development and SnRK1 activity in Prunus persica. Chin. Bull. Bot. 2019, 54, 744–752. [Google Scholar] [CrossRef]
- Dutra, P.V.; Rossi, P.; Witt, W.W.; Panozzo, L.E. Growth and biomass allocation of Muhlenbergia schreberi. Am. J. Plant Sci. 2014, 5, 2188–2197. [Google Scholar] [CrossRef]
- Bruggink, G.T.; Heuvelink, E. Influence of light on the growth of young tomato, cucumber and sweet pepper plants in the greenhouse: Effects on relative growth rate, net assimilation rate and leaf area ratio. Sci. Hortic. 1987, 31, 161–174. [Google Scholar] [CrossRef]



| Variable | Treatment | |||
|---|---|---|---|---|
| 0 mM | 1 mM | 10 mM | 100 mM | |
| Plant DM (mg) | 61.679 ± 2.344 c | 83.110 ± 3.170 ab | 79.246 ± 3.530 b | 87.500 ± 2.878 a |
| Leaf DM (mg) | 34.688 ± 1.267 b | 47.065 ± 1.703 a | 44.290 ± 2.048 a | 46.725 ± 1.454 a |
| Stem DM (mg) | 23.481 ± 1.064 c | 31.792 ± 1.419 b | 29.827 ± 1.530 b | 35.713 ± 1.327 a |
| Root DM (mg) | 3.510 ± 0.237 c | 4.254 ± 0.254 b | 5.129 ± 0.441 a | 5.063 ± 0.274 a |
| Leaf Area (cm2) | 13.955 ± 0.572 c | 18.089 ± 0.574 b | 17.324 ± 0.766 b | 19.325 ± 0.548 a |
| Stem Diameter (mm) | 1.988 ± 0.023 c | 2.088 ± 0.018 b | 2.005 ± 0.028 c | 2.180 ± 0.022 a |
| Hypocotyl Length (cm) | 5.031 ± 0.068 b | 5.185 ± 0.097 ab | 5.110 ± 0.070 b | 5.281 ± 0.069 a |
| Root Length (cm) | 5.123 ± 0.227 b | 5.956 ± 0.253 a | 5.448 ± 0.298 ab | 5.669 ± 0.237 a |
| Plant Height (cm) | 9.965 ± 0.233 c | 11.165 ± 0.309 b | 10.377 ± 0.258 c | 12.131 ± 0.256 a |
| SLA (m2 g−1) | 0.041 ± 0.001 ab | 0.039 ± 0.001 b | 0.040 ± 0.001 b | 0.042 ± 0.001 a |
| Shoot-to-Root Biomass Ratio | 18.990 ± 1.042 ab | 20.034 ± 0.728 a | 17.688 ± 1.057 c | 17.553 ± 0.686 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Cabezas, M.; España, Á. Exogenous Sucrose Enhances Growth and Physiological Performance of Tomato Seedlings Under Suboptimal Light Conditions in Passive Greenhouses. Horticulturae 2024, 10, 1337. https://doi.org/10.3390/horticulturae10121337
Gómez-Cabezas M, España Á. Exogenous Sucrose Enhances Growth and Physiological Performance of Tomato Seedlings Under Suboptimal Light Conditions in Passive Greenhouses. Horticulturae. 2024; 10(12):1337. https://doi.org/10.3390/horticulturae10121337
Chicago/Turabian StyleGómez-Cabezas, Miguel, and Ángelo España. 2024. "Exogenous Sucrose Enhances Growth and Physiological Performance of Tomato Seedlings Under Suboptimal Light Conditions in Passive Greenhouses" Horticulturae 10, no. 12: 1337. https://doi.org/10.3390/horticulturae10121337
APA StyleGómez-Cabezas, M., & España, Á. (2024). Exogenous Sucrose Enhances Growth and Physiological Performance of Tomato Seedlings Under Suboptimal Light Conditions in Passive Greenhouses. Horticulturae, 10(12), 1337. https://doi.org/10.3390/horticulturae10121337








