Physiological Behavior and Antioxidant Responses of Abelmoschus esculentus (L.) Exposed to Different Concentrations of Aluminum and Barium
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Culture
2.2. Growth
2.2.1. Relative Growth Rate
2.2.2. Growth Percentage
2.3. TME Contents
2.4. Malondialdehyde (MDA)
2.5. Hydrogen Peroxide (H2O2)
2.6. Antioxidant Enzyme Activity
2.6.1. Catalase CAT (EC 1.11.1.6)
2.6.2. Superoxide Dismutase SOD (EC 1.15.1.1)
2.6.3. Glutathione Reductase GR (EC 1.6.4.2)
2.7. Determination of Reduced Glutathione (GSH) and Phytochelatin (PC) Concentrations
2.8. Statistical Analyses
3. Results
3.1. Growth
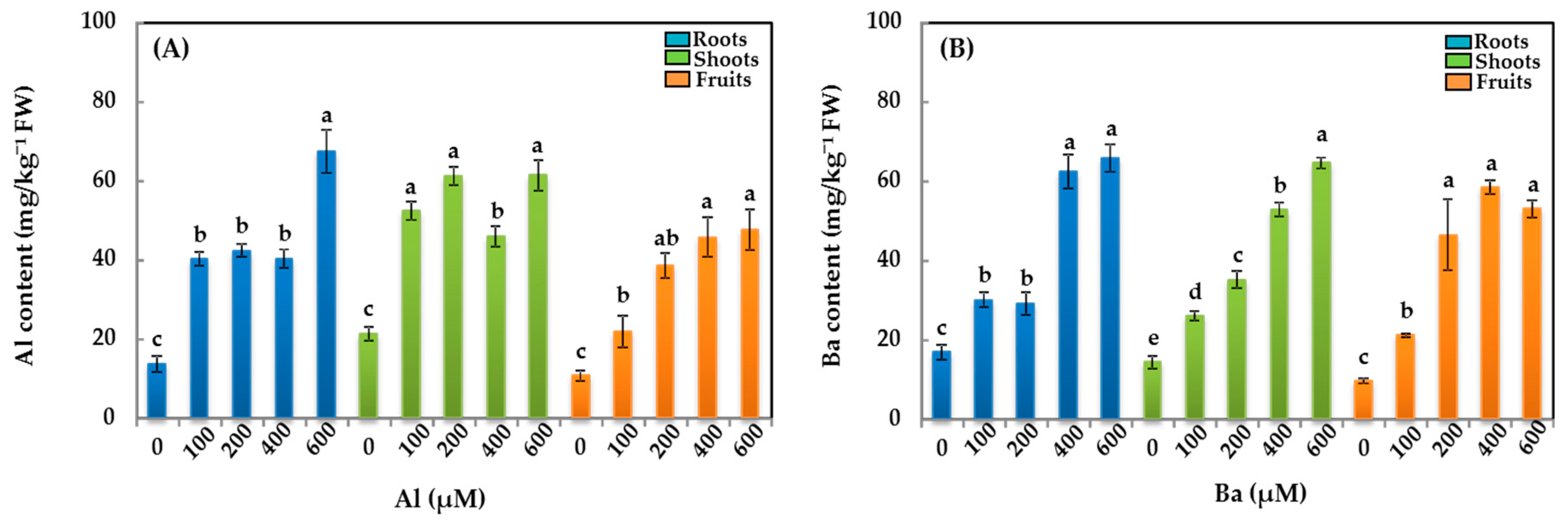
3.2. TME Contents
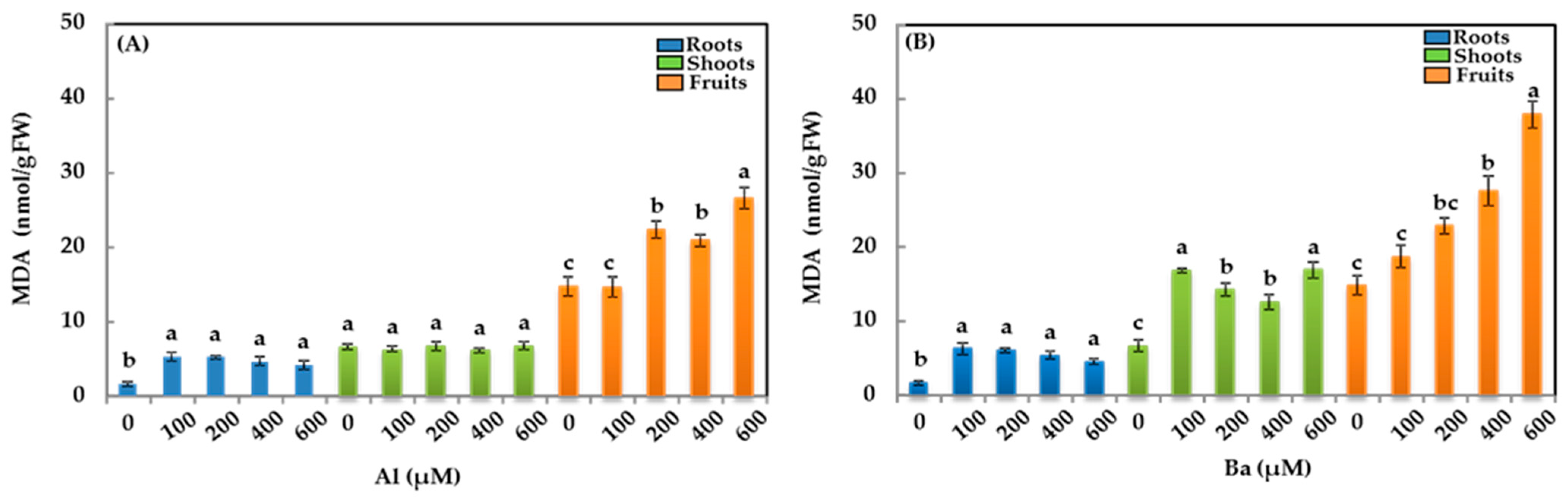
3.3. Malondialdehyde (MDA)
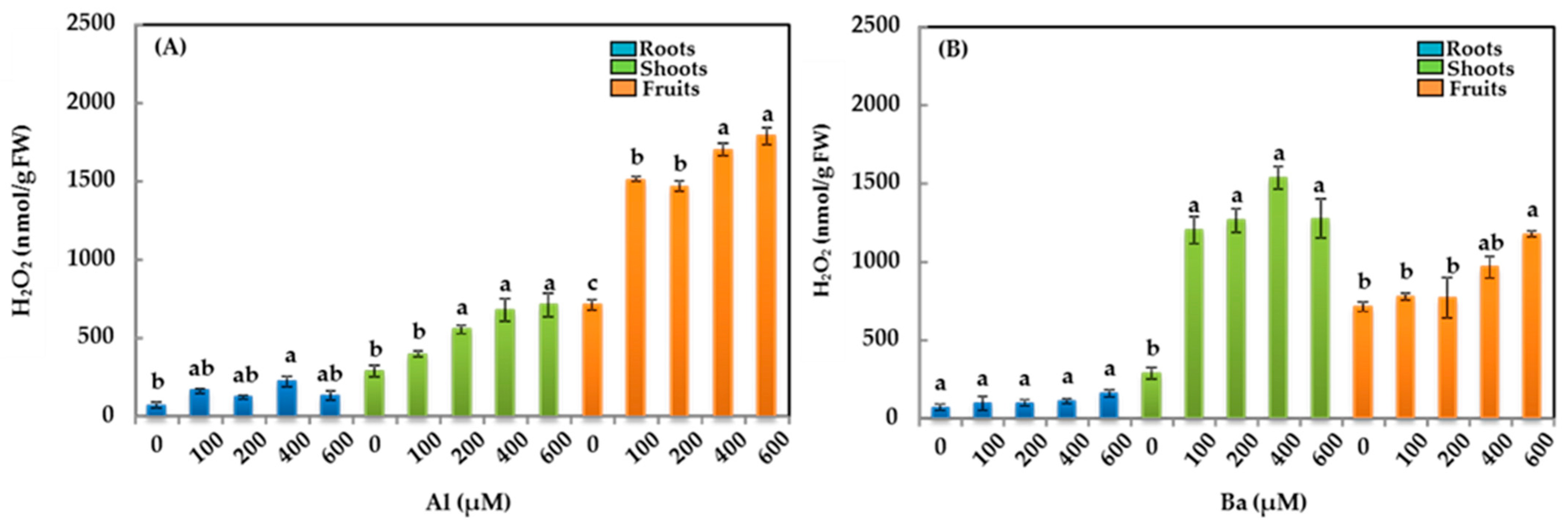
3.4. Hydrogen Peroxide (H2O2)
3.5. Enzymatic Activities
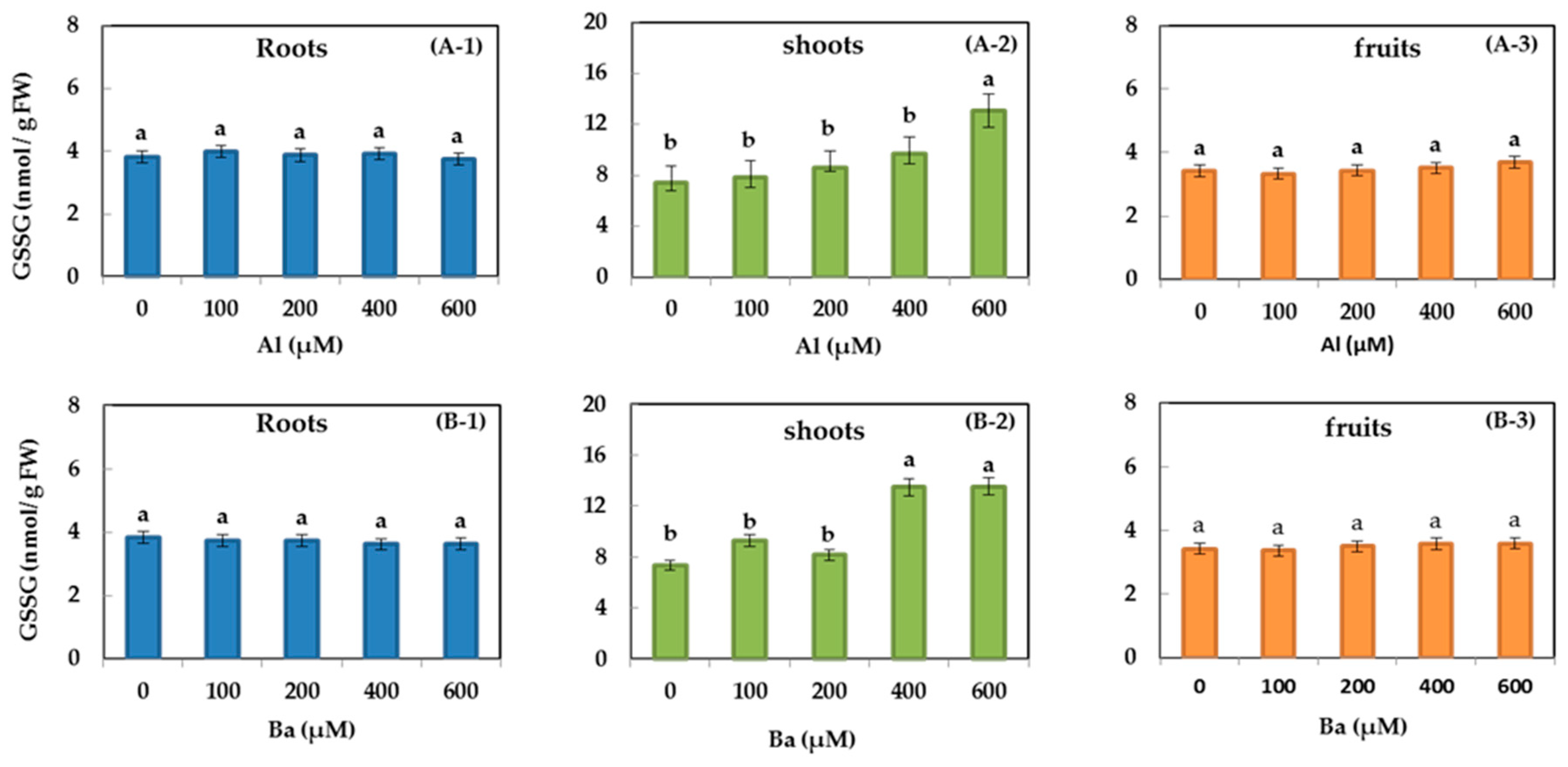
3.6. GSH and GSSG
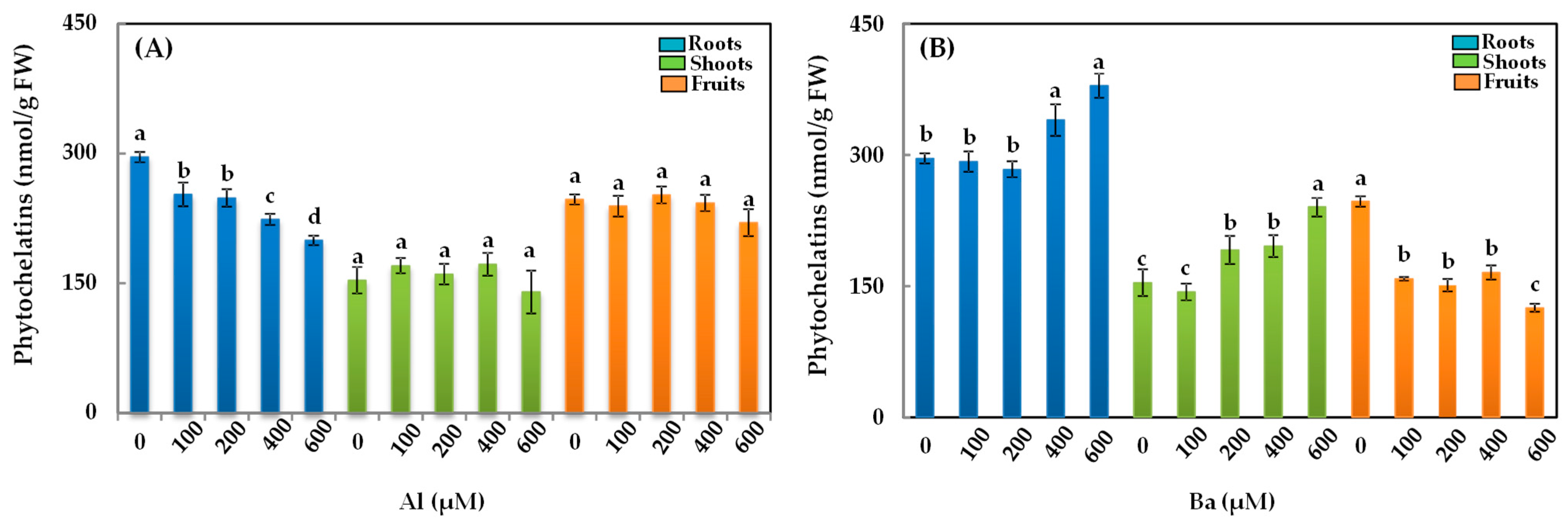
3.7. Phytochelatins (PCs)
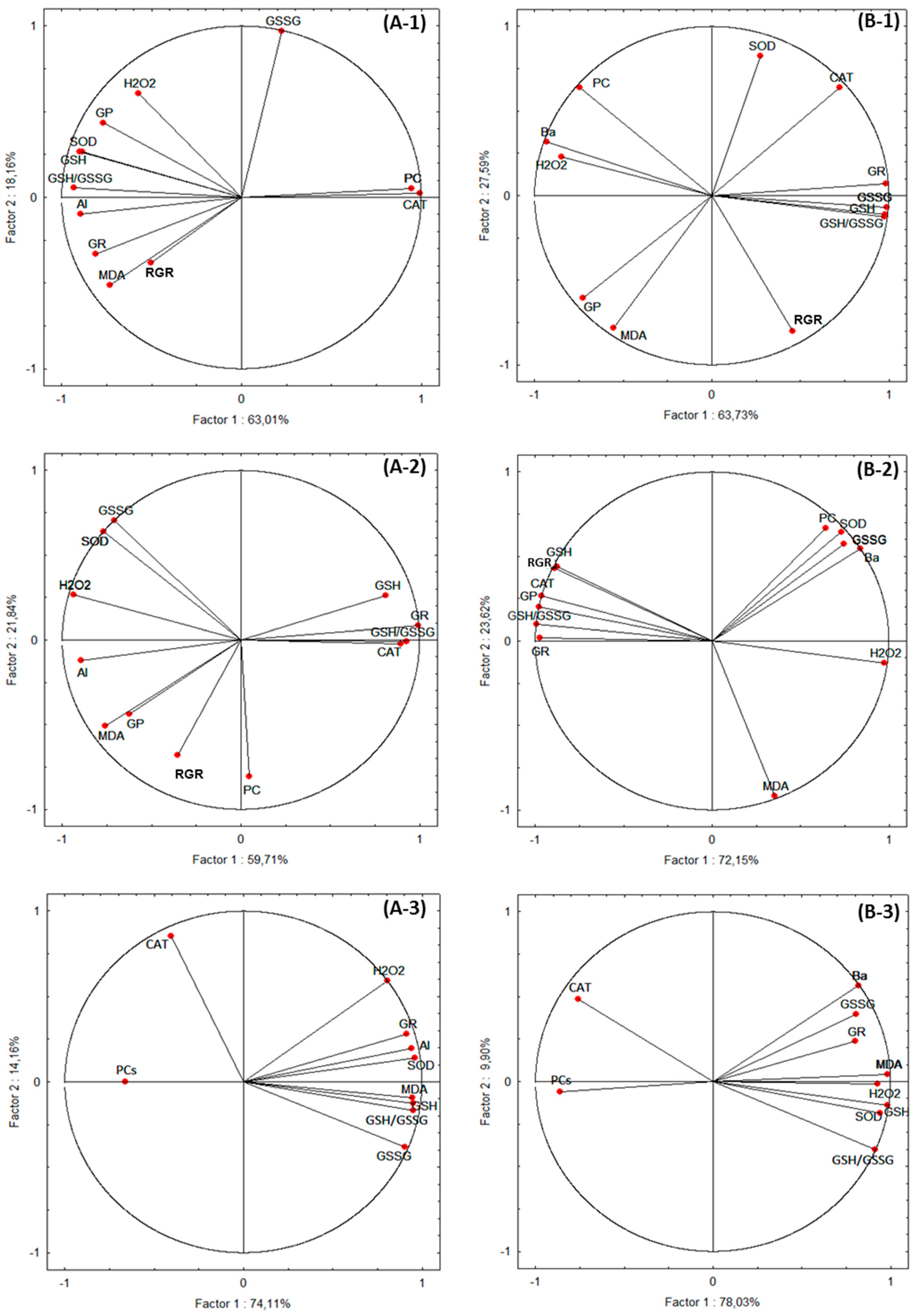
3.8. Statistical Analyses
4. Discussion
4.1. Growth
4.2. TME Contents
4.3. Malondialdehyde (MDA)
4.4. Hydrogen Peroxide (H2O2)
4.5. Antioxidant Enzymes
4.6. Reduced Glutathione (GSH) and Phytochelatins (PCs)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahimi, G.; Kolahchi, Z.; Charkhabi, A. Uptake and translocation of some heavy metals by rice crop (Oryza sativa) in paddy soils. Agriculture 2017, 63, 163–175. [Google Scholar] [CrossRef]
- Ratul, A.K.; Hassan, M.; Uddin, M.K.; Sultana, M.S.; Akbor, M.A.; Ahsan, M.A. Potential health risk of heavy metals accumulation in vegetables irrigated with polluted river water. Int. Food Res. J. 2018, 25, 329–338. [Google Scholar]
- Herrero, M.; Rovira, J.; Nadal, M.; Domingo, J.L. Risk assessment due to dermal exposure of trace elements and indigo dye in jeans: Migration to artificial sweat. Environ. Res. 2019, 172, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Choudhary, P.; Khan, S.A. GIS-based evaluation of groundwater geochemistry and statistical determination of the fate of contaminants in shallow aquifers from different functional areas of Agra city, India: Levels and spatial distributions. RSC Adv. 2018, 8, 15876. [Google Scholar] [CrossRef]
- Maurya, P.K.; Malik, D.S.; Yadav, K.K.; Kumar, A.; Kumar, S.; Kamyab, H. Bioaccumulation and potential sources of heavy metal contamination in fish species in River Ganga basin: Possible human health risks evaluation. Toxicol. Rep. 2019, 6, 472–481. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Rahman, R.; Upadhyaya, H. Aluminium Toxicity and Its Tolerance in Plant: A Review. J. Plant Biol. 2021, 64, 101–121. [Google Scholar] [CrossRef]
- Davidson, T.; Ke, Q.; Costa, M. Chapter 5: Selected Molecular Mechanism of Metal Toxicity and Carcinogenicity. In Handbook on the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L., Eds.; Academic Press: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Çanlı, M. A new perspective to aberrations caused by barium and vanadium ions on Lens culinaris Medik. Ecotoxicol. Environ. Saf. 2018, 160, 19–23. [Google Scholar] [CrossRef]
- Omole, J.G.; Alabi, Q.K.; Aturamu, A.; Adefisayo, M.A.; Oluwayomi, O.; Dada, M.B.; Ige, M.S. Barium chloride dose-dependently induced heart and lung injury in Wistar rats. Environ. Toxicol. 2019, 34, 1303–1312. [Google Scholar] [CrossRef]
- Lu, Q.; Xu, X.; Liang, L.; Xu, Z.; Shang, L.; Guo, J.; Xiao, D.; Qiu, G. Barium concentration, phytoavailability, and risk assessment in soil-rice systems from an active barium mining region. Appl. Geochem. 2019, 106, 142–148. [Google Scholar] [CrossRef]
- Devi, S.S.; Saha, B.; Panda, S.K. Differential Loss of ROS homeostasis and activation of anti oxidative defense response in tea cultivar due to aluminum toxicity in acidic soil. Curr. Trends Biotechnol. Pharm. 2014, 14, 33–43. [Google Scholar] [CrossRef]
- Shahnawaz, M.D.; Sanadhya, D. Aluminium induced oxidative stress and antioxidants system in two barley varieties and its alleviation through ascorbic acid and salicylic acid seed priming approach. Int. J. Life Sci. Pharm. Res. 2017, 7, 26–37. [Google Scholar]
- Kouki, R.; Ayachi, R.; Ferreira, R.; Sleimi, N. Behavior of Cucumis sativus L. in presence of aluminum stress: Germination, plant growth, and antioxidant enzymes. Food Sci. Nutr. 2021, 9, 3280–3288. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Broadley, M.R.; Robbrecht, E.; Smets, E. Aluminum hyperaccumulation in angiosperms: A review of if phylogenetic significance. Bot. Rev. 2002, 68, 235–269. [Google Scholar] [CrossRef]
- Peana, M.; Medici, S.; Dadar, M.; Zoroddu, M.A.; Pelucelli, A.; Chasapis, C.T.; Bjørklund, G. Environmental barium: Potential exposure and health-hazards. Arch. Toxicol. 2021, 95, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Sleimi, N.; Kouki, R.; Hadj-Ammar, M.; Ferreira, R.; Pérez-Clemente, R.M. Barium effect on germination, plant growth, and antioxidant enzymes in Cucumis sativus L. plants. Food Sci. Nutr. 2021, 9, 2086–2094. [Google Scholar] [CrossRef]
- Kouki, R.; Dridi, N.; Vives-Peris, V.; Gómez-Cadenas, A.; Caçador, I.; Pérez-Clemente, R.M.; Sleimi, N. Appraisal of Abelmoschus esculentus L. Response to Aluminum and Barium Stress. Plants 2023, 12, 179. [Google Scholar] [CrossRef]
- Dridi, N.; Bouslimi, H.; Duarte, B.; Caçador, I.; Sleimi, N. Evaluation of Physiological and Biochemical Parameters and Some Bioindicators of Barium Tolerance in Limbarda crithmoides and Helianthus annuus. Int. J. Plant Biol. 2022, 13, 115–131. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Al Mahmud, J.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Sleimi, N.; Guerfalli, S.; Bankaji, I. Biochemical indicators of salt stress in Plantago maritima: Implications for environmental stress assessment. Ecol. Indic. 2015, 48, 570–577. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Ahmad, I.; Mohmood, I.; Pacheco, M.; Duarte, A.C.; Pereira, E.; Umar, S.; Ahmad, A.; Khan, N.; Iqbal, M.; et al. Modulationf glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—A review. Environ. Exp. Bot. 2012, 75, 307–324. [Google Scholar]
- Benchasri, S. Okra (Abelmoschus esculentus (L.) Moench) as a Valuable Vegetable of the World. Ratar. Povrt. 2012, 49, 105–112. [Google Scholar]
- Yao, X.; Chu, J.; Wang, G. Effects of selenium on wheat seedlings under drought stress. Biol. Trace Elem. Res. 2009, 130, 283–290. [Google Scholar] [CrossRef]
- Hewitt, E.J. Sand and water culture methods used in the study of plant nutrition. J. Assoc. Off. Anal. Chem. 1966, 49, 888–889. [Google Scholar]
- Stolt, J.P.; Sneller, F.E.C.; Brynelsson, T.; Lundborg, T.; Schat, H. Phytochelatin and cadmium accumulation in wheat. Environ. Exp. Bot. 2003, 49, 21–28. [Google Scholar] [CrossRef]
- Hunt, R. Basic Growth Analysis: Plant Growth Analysis for Beginners; Unwin Hyman: London, UK, 1990; p. 112. [Google Scholar]
- Sleimi, N.; Abdely, C. Salt-tolerance strategy of two halophytes species: Spartina alterniflora and Suaeda fruticosa. In Tasks for Vegetation Science; Cash Crop Halophytes: Recent Studies; Lieth, H., Mochtchenko, M., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2003; Volume 38, pp. 79–85. [Google Scholar] [CrossRef]
- Wilkins, D.A. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978, 91, 255–261. [Google Scholar] [CrossRef]
- Sleimi, N.; Bankaji, I.; Kouki, R.; Dridi, N.; Duarte, B.; Cacador, I. Assessment of extraction methods of trace metallic elements in plants: Approval of a common method. Sustainability 2022, 14, 1428. [Google Scholar] [CrossRef]
- Bankaji, I.; Kouki, R.; Dridi, N.; Ferreira, R.; Hidouri, S.; Duarte, B.; Sleimi, N.; Caçador, I. Comparison of digestion methods using atomic absorption spectrometry for the determination of metal levels in plants. Separations 2023, 10, 40. [Google Scholar] [CrossRef]
- Hodges, D.; DeLong, J.; Forney, C.; Prange, R. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Flors, V.; Jacas, J.; GarcÍa-AgustÍn, P.; Gomez-Cadenas, A. Enzymatic and non-enzymatic antioxidant responses of Carrizo citrange, a salt-sensitive citrus rootstock, to different levels of salinity. Plant Cell Physiol. 2003, 44, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Labidi, O.; Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M.; Sleimi, N. Assessing of growth, antioxidant enzymes, and phytohormone regulation in Cucurbita pepo under cadmium stress. Food Sci. Nutr. 2021, 9, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- López-Climent, M.F.; Arbona, V.; Pérez-Clemente, R.M.; Zandalinas, S.I.; Gómez-Cadenas, A. Effect of cadmium and calcium treatments on phytochelatin and glutathione levels in citrus plants. Plant Biol. 2014, 16, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Meth. Enzym. 1985, 113, 548–555. [Google Scholar]
- De Vos, C.H.R.; Vonk, M.J.; Vooijs, R.; Schat, H. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol. 1992, 98, 853–858. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 8.0; StatSoft, Inc.: Tulsa, OK, USA, 2007; Available online: https://statistica.software.informer.com/8.0/ (accessed on 20 February 2024).
- Li, C.; Xu, H.; Xu, J.; Chun, X.; Ni, D. Effects of aluminium on ultrastructure and antioxidant activity in leaves of tea plant. Acta Physiol. Plant 2010, 33, 973–978. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, J.; Cao, J.; Zeng, Y.; Li, X.; Ma, J.; Huang, Z.; Jiang, M.; Sun, L. The Beneficial Effects of Aluminum on the Plant Growth in Camellia japonica. J. Soil Sci. Plant Nutr. 2020, 20, 1799–1809. [Google Scholar] [CrossRef]
- Muhammad, N.; Zvobgo, G.; Zhang, G.P. The beneficial effect and possible mechanisms of aluminum on plant growth in acid soil: A review. J. Integr. Agric. 2019, 18, 1518–1528. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.T.; Qi, Y.P.; Guo, P.; Lu, Y.B.; Chen, L.S. Aluminum toxicity-induced alterations of leaf proteome in two citrus species differing in aluminum tolerance. Int. J. Mol. Sci. 2016, 17, 1180. [Google Scholar] [CrossRef]
- Bouslimi, H.; Ferreira, R.; Dridi, N.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Effects of Barium stress in Brassica juncea and Cakile maritima: The indicator role of some antioxidant enzymes and secondary metabolites. Phyton 2021, 90, 145–158. [Google Scholar] [CrossRef]
- De Souza Cardoso, A.A.; Monteiro, F.A. Sulfur supply reduces barium toxicity in Tanzania guinea grass (Panicum maximum) by inducing antioxidant enzymes and proline metabolism. Ecotoxicol. Environ. Saf. 2021, 208, 111643. [Google Scholar] [CrossRef] [PubMed]
- Suwa, R.K.; Jayachandran, N.T.; Nguyen, A.; Boulenouar, K.; Fujita, K.; Saneoka, H. Barium toxicity effects in soybean plants. Arch. Environ. Contam. Toxicol. 2008, 55, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kumara, S.; Prasad, S.; Yadav, K.K.; Shrivastavaa, M.; Guptab, N.; Nagarc, S.; Bachd, Q.; Kamyabe, H.; Khana, S.A.; Yadava, S.; et al. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculík, M. Toxicity of aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Bragadin, M.; Manente, S.; Scutari, G.; Rigobello, M.P.; Bindoli, A. Possible transport mechanism for aluminium in biological membranes. J. Inorg. Biochem. 2004, 98, 1169–1173. [Google Scholar] [CrossRef]
- Negishi, T.; Oshima, K.; Hattori, M.; Kanai, M.; Mano, S.; Nishimura, M.; Yoshida, K. Tonoplast- and plasma membrane-localised aquaporin-family transporters in blue hydrangea sepals of aluminium hyperaccumulating plant. PLoS ONE 2012, 7, e43189. [Google Scholar] [CrossRef]
- González-Weller, D.; Gutiérrez, A.J.; Rubio, C.; Revert, C.; Hardisson, A. Dietary Intake of Aluminum in a Spanish Population (Canary Islands). J. Agric. Food Chem. 2010, 58, 10452–10457. [Google Scholar] [CrossRef]
- Bawwab, M.; Qutob, A.; Al Khatib, M.; Malassa, H.; Shawahna, A.; Qutob, M. Evaluation of Heavy Metal Concentrations in Soil and Edible Vegetables Grown in Compost from Unknown Sources in Al-Jiftlik, Palestine. J. Environ. Prot. 2022, 13, 112–125. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Amtmann, A.; Rubio, F. Potassium in Plants, ELS; Article a0023737; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar] [CrossRef]
- Kamachi, H.; Kitamura, N.; Sakatoku, A.; Tanaka, D.; Nakamura, S. Barium accumulation in the metalliferous fern Athyrium Yokoscense. Theor. Exp. Plant Physiol. 2015, 27, 99–107. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.J.; Li, G.; Jin, C.W.; Liu, W.J.; Zhang, S.S.; Zhang, Y.S.; Lin, X.Y. Aluminum-induced changes in reactive oxygen species accumulation, lipid peroxidation and antioxidant capacity in wheat root tips. Biol. Plant 2012, 56, 89–96. [Google Scholar] [CrossRef]
- Hosseini, M.; Boojar, M.M.; Delkhosh, B.; Moradi, P. Effects of foliar spraying acetyl-coA on dry weight of leaves, chlorophyll a, and antioxidant enzymes of rosemary (Rosmarinus officinalis L.). J. Plant Physiol. 2014, 4, 977–981. [Google Scholar]
- Hussain, I.; Siddique, A.; Ashraf, M.A.; Rasheed, R.; Ibrahim, M.; Iqbal, M.; Akbar, S.; Imran, M. Does exogenous application of ascorbic acid modulate growth, photosynthetic pigments and oxidative defense in okra (Abelmoschus esculentus (L.) Moench) under lead stress? Acta Physiol. Plant 2017, 39, 144. [Google Scholar] [CrossRef]
- Bankaji, I.; Sleimi, N.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. NaCl protects against Cd and Cu-induced toxicity in the halophyte Atriplex halimus. Span. J. Agric. Res. 2016, 14, e0810. [Google Scholar] [CrossRef]
- Tamás, L.; Mistrík, I.; Zelinová, V. Heavy metal-induced reactive oxygen species and cell death in barley root tip. Environ. Exp. Bot. 2017, 140, 34–40. [Google Scholar] [CrossRef]
- Dridi, N.; Brito, P.; Bouslimi, H.; Ferreira, R.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Physiological and biochemical behaviours and antioxidant response of Helianthus annuus under lanthanum and cerium stress. Sustainability 2022, 14, 4153. [Google Scholar] [CrossRef]
- Huan, C.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Ma, R.; Yu, Z. Potential role of reactive oxygen species and antioxidant genes in the regulation of peach fruit development and ripening. Plant Physiol. Biochem. 2016, 104, 294–303. [Google Scholar] [CrossRef]
- Resende, E.C.O.; Martins, P.F.; de Azevedo, R.A.; Jacomino, A.P.; Bron, I.U. Oxidative processes during “Golden” papaya fruit ripening. Braz. J. Plant Physiol. 2012, 24, 85–94. [Google Scholar] [CrossRef]
- Berni, R.; Luyckxc, M.; Xud, X.; Legayd, S.; Sergeantd, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Song, A.; Xue, G.; Cui, P.; Fan, F.; Liu, H.; Yin, C.; Sun, W.; Liang, Y. The role of silicon in enhancing resistance to bacterial blight of hydroponic-and soil-cultured rice. Sci. Rep. 2016, 6, 24640. [Google Scholar] [CrossRef] [PubMed]
- Pirzadah, T.B.; Malika, B.; Tahirc, I.; Rehman, U.; Hakeem, O.R.; Alharby, H.F. Aluminium stress modulates the osmolytes and enzyme defense system in Fagopyrum species. Plant Physiol. Biochem. 2019, 144, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Suresh Babu, B.; Muniswamy, D.; Radhaiah, A.; Reddy, P.L.N. Aluminium induced phytotoxic effect on antioxidant enzyme activities in pearl millet varieties Nandi 32 and Nirmal 9. Indian J. Fund Appl. Life Sci. 2013, 3, 252–258. [Google Scholar]
- Hameed, A.; Qadri, T.N.; Muazaffar, M.; Siddiqi, T.O.; Iqbal, M. Differential activation of the enzymatic antioxidant system of Abelmoschus esculentus (L.) Moench under CdCl2 and HgCl2 exposure. Braz. J. Plant Physiol. 2011, 23, 45–55. [Google Scholar] [CrossRef]
- Samad, R.; Rashid, P.; Karmoker, J.L. Aluminum toxicity on the activities of antioxidant enzymes in rice (Oryza Sativa L.) and chickpea (Cicer Arietinum M.) seedlings grown in hydroponic culture. Bangladesh J. Bot. 2020, 49, 619–624. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Shaw, A.K.; Ghosh, S.; Kalaji, H.M.; Bosa, K.; Brestic, M.; Zivcak, M.; Hossain, Z. Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley Hordeum vulgare L. Environ. Exp. Bot. 2014, 102, 37–47. [Google Scholar] [CrossRef]
- Li, F.T.; Qi, J.M.; Zhang, G.Y.; Lin, L.H.; Fang, P.P.; Tao, A.F.; Xu, J.T. Effect of cadmium stress on the growth antioxidative enzymes and lipid peroxidation in two kenaf (Hibiscus cannabinus L.) plant seedlings. J. Integ. Agric. 2013, 12, 610–620. [Google Scholar] [CrossRef]
- Awasthi, J.P.; Saha, B.; Panigrahi, J.; Yanase, E.; Koyama, H.; Panda, S.K. Redox balance, metabolic fingerprint and physiological characterization in contrasting North East Indian rice for aluminum stress tolerance. Sci. Rep. 2019, 9, 8681. [Google Scholar] [CrossRef]
- Kao, C.H. Role of glutathione in abiotic stress tolerance of rice plants. J. Taiwan Agric. Res. 2015, 164, 167–176. [Google Scholar]
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Fujita, M. EDTA reduces cadmium toxicity in mustard (Brassica juncea L.) by enhancing metal chelation, antioxidant defense and glyoxalase systems. Acta Agro. Bot. 2019, 72, 1722. [Google Scholar] [CrossRef]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.Y.; Ahammed, G.J.; Qi, Z.Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, e1492. [Google Scholar] [CrossRef] [PubMed]
- Sheetal, K.R.; Singh, S.D.; Anand, A.; Prasad, S. Heavy metal accumulation and effects on growth, biomass and physiological processes in mustard. Indian J. Plant Physiol. 2016, 21, 219–223. [Google Scholar] [CrossRef]

| RGR (mg·Day−1) | ||||
| Treatment | Al | Ba | ||
| Roots | Shoots | Roots | Shoots | |
| 0 µM | 0.0557 ± 0.004 a | 0.0485 ± 0.004 b | 0.0557 ± 0.0003 a | 0.0485 ± 0.003 a |
| 100 µM | 0.0550 ± 0.002 a | 0.0481 ± 0.003 b | 0.0555 ± 0.0003 a | 0.0419 ± 0.003 b |
| 200 µM | 0.0554 ± 0.003 a | 0.0523 ± 0.003 a | 0.0564 ± 0.0004 a | 0.0423 ± 0.004 b |
| 400 µM | 0.0562 ± 0.003 a | 0.0478 ± 0.003 b | 0.0560 ± 0.0003 a | 0.0426 ± 0.003 b |
| 600 µM | 0.0567 ± 0.003 a | 0.0476 ± 0.003 b | 0.0562 ± 0.0004 a | 0.043 ± 0.004 b |
| GP (%) | ||||
| Treatment | Al | Ba | ||
| Roots | Shoots | Roots | Shoots | |
| 100 µM | 101.59 ± 4.53 a | 100.75 ± 2.83 b | 101.11 ± 1.79 a | 88.12 ± 1.84 a |
| 200 µM | 103.36 ± 8.17 a | 110.45 ± 3.38 a | 102.22 ± 5.42 a | 87.42 ± 2.05 a |
| 400 µM | 103.44 ± 6.30 a | 103.40 ± 3.18 b | 102.09 ± 5.00 a | 87.14 ± 1.04 a |
| 600 µM | 101.75 ± 5.34 a | 103.49 ± 2.68 b | 101.20 ± 2.85 a | 87.15 ± 1.82 a |
| SOD (U/mg protein−1) | (µM) | Al | Ba | ||||
| Roots | Shoots | Fruits | Roots | Shoots | Fruits | ||
| 0 | 3758.2 ± 231.1 c | 1773.5 ± 59.6 c | 1036.8 ± 34.7 b | 3758.2 ± 231.1 a | 1773.5 ± 59.6 c | 1036.8 ± 34.7 c | |
| 100 | 4936.95 ± 251.5 b | 2169.4 ± 55.6 c | 1370.01 ± 20 b | 3595.4 ± 667.2 a | 2325.09 ± 172 c | 1266.3 ± 59.9 c | |
| 200 | 6229.4 ± 498.2 a | 2979.1 ± 191.9 c | 1973.5 ± 96.5 a | 3235.3 ± 228.9 a | 2279.2 ± 168.5 c | 1161.9 ± 62.7 c | |
| 400 | 5632.98 ± 453.2 b | 3834.2 ± 63.6 b | 2025.5 ± 192.6 a | 3597.7 ± 540.6 a | 3402.6 ± 337.5 b | 1325.7 ± 203 b | |
| 600 | 5512.90 ± 112 b | 5961.2 ± 636 a | 2330.5 ± 251.8 a | 3637.01 ± 379.8 a | 4506.7 ± 447.3 a | 1693.05 ± 103.4 a | |
| CAT (U/mg protein−1) | (µM) | Al | Ba | ||||
| Roots | Shoots | Fruits | Roots | Shoots | Fruits | ||
| 0 | 2.74 ± 0.2 a | 9.75 ± 1.04 a | 0.82 ± 0.03 a | 2.74 ± 0.20 a | 9.75 ± 1.04 a | 0.82 ± 0.03 a | |
| 100 | 1.95 ± 0.45 b | 9.16 ± 0.68 a | 1.00 ± 0.11 a | 0.80 ± 0.05 b | 0.79 ± 0.03 b | 0.79 ± 0.08 a | |
| 200 | 0.97 ± 0.09 c | 0.83 ± 0.09 b | 0.83 ± 0.04 a | 0.77 ± 0.11 b | 0.79 ± 0.05 b | 0.71 ± 0.09 a | |
| 400 | 1.09 ± 0.11 c | 0.83 ± 0.15 b | 0.85 ± 0.02 a | 0.94 ± 0.13 b | 0.65 ± 0.07 b | 0.84 ± 0.10 a | |
| 600 | 0.72 ± 0.16 c | 1.50 ± 0.02 b | 0.82 ± 0.06 a | 1.06 ± 0.05 b | 0.95 ± 0.18 b | 0.62 ± 0.09 a | |
| GR (U/mg protein−1) | (µM) | Al | Ba | ||||
| Roots | Shoots | Fruits | Roots | Shoots | Fruits | ||
| 0 | 0.089 ± 0.008 a | 0.07 ± 0.002 b | 0.018 ± 0.004 c | 0.089 ± 0.008 a | 0.07 ± 0.002 a | 0.018 ± 0.004 b | |
| 100 | 0.045 ± 0.01 b | 0.068 ± 0.003 b | 0.031 ± 0.003 b | 0.07 ± 0.009 b | 0.046 ± 0.003 b | 0.046 ± 0.001 a | |
| 200 | 0.015 ± 0.006 c | 0.072 ± 0.003 b | 0.045 ± 0.003 a | 0.05 ± 0.003 c | 0.036 ± 0.002 c | 0.05 ± 0.005 a | |
| 400 | 0.012 ± 0.002 c | 0.088 ± 0.003 a | 0.047 ± 0.002 a | 0.044 ± 0.004 c | 0.033 ± 0.001 c | 0.047 ± 0.005 a | |
| 600 | 0.013 ± 0.006 c | 0.091 ± 0.004 a | 0.05 ± 0.005 a | 0.036 ± 0.005 c | 0.037 ± 0.002 c | 0.052 ± 0.001 a | |
| GSH/GSSG | (µM) | Al | Ba | ||||
| Roots | Shoots | Fruits | Roots | Shoots | Fruits | ||
| 0 | 0.64 ± 0.02 c | 7.08 ± 0.35 a | 0.01 ± 0.001 c | 0.64 ± 0.02 | 7.08 ± 0.35 a | 0.01 ± 0.001 c | |
| 100 | 0.77 ± 0.05 b | 3.65 ± 0.15 b | 0.01 ± 0.004 c | 0.59 ± 0.06 | 3.05 ± 0.35 b | 0.10 ± 0.03 b | |
| 200 | 0.82 ± 0.02 a | 3.92 ± 0.34 b | 0.03 ± 0.01 c | 0.51 ± 0.05 | 2.88 ± 0.33 b | 0.08 ± 0.03 b | |
| 400 | 0.77 ± 0.04 b | 2.44 ± 0.37 b | 0.08 ± 0.03 b | 0.39 ± 0.04 | 2.00 ± 0.11 b | 0.07 ± 0.02 | |
| 600 | 0.87 ± 0.02 a | 2.63 ± 0.43 b | 0.13 ± 0.01 a | 0.41 ± 0.04 | 2.40 ± 0.39 b | 0.27 ± 0.02 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouki, R.; Bankaji, I.; Hidouri, S.; Bouzahouane, H.; Caçador, I.; Pérez-Clemente, R.M.; Sleimi, N. Physiological Behavior and Antioxidant Responses of Abelmoschus esculentus (L.) Exposed to Different Concentrations of Aluminum and Barium. Horticulturae 2024, 10, 1338. https://doi.org/10.3390/horticulturae10121338
Kouki R, Bankaji I, Hidouri S, Bouzahouane H, Caçador I, Pérez-Clemente RM, Sleimi N. Physiological Behavior and Antioxidant Responses of Abelmoschus esculentus (L.) Exposed to Different Concentrations of Aluminum and Barium. Horticulturae. 2024; 10(12):1338. https://doi.org/10.3390/horticulturae10121338
Chicago/Turabian StyleKouki, Rim, Insaf Bankaji, Saida Hidouri, Hana Bouzahouane, Isabel Caçador, Rosa María Pérez-Clemente, and Noomene Sleimi. 2024. "Physiological Behavior and Antioxidant Responses of Abelmoschus esculentus (L.) Exposed to Different Concentrations of Aluminum and Barium" Horticulturae 10, no. 12: 1338. https://doi.org/10.3390/horticulturae10121338
APA StyleKouki, R., Bankaji, I., Hidouri, S., Bouzahouane, H., Caçador, I., Pérez-Clemente, R. M., & Sleimi, N. (2024). Physiological Behavior and Antioxidant Responses of Abelmoschus esculentus (L.) Exposed to Different Concentrations of Aluminum and Barium. Horticulturae, 10(12), 1338. https://doi.org/10.3390/horticulturae10121338






