Exploring the Synergistic Role of Zinc in NPK Fertilization on the Agronomic Performance of Safflower (Carthamus tinctorius)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location and Treatments
2.2. Soil Properties
2.3. Meteorological Data
2.4. Data Recorded
2.5. Statistical Analysis
3. Results and Discussion
3.1. Phenological Parameters
3.2. Growth Parameters
3.3. Yield Parameters
3.4. Quality Attributes
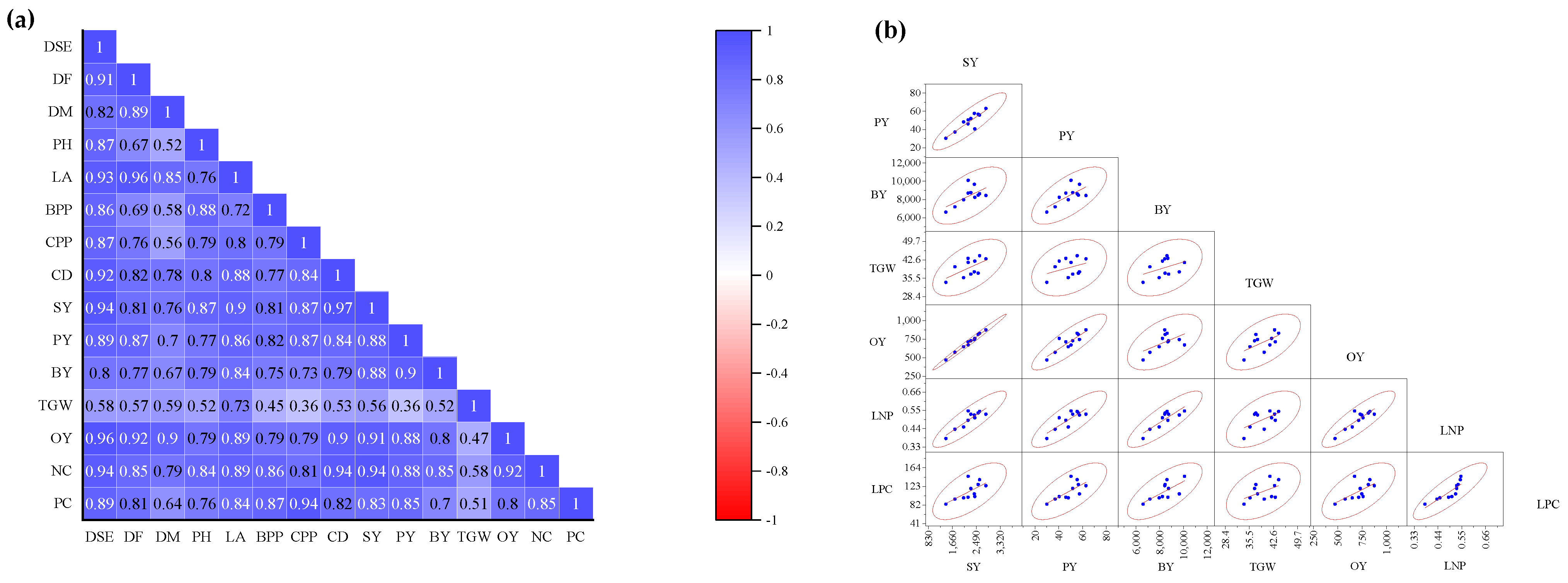
3.5. Correlation Analysis and Scatterplot Matrix
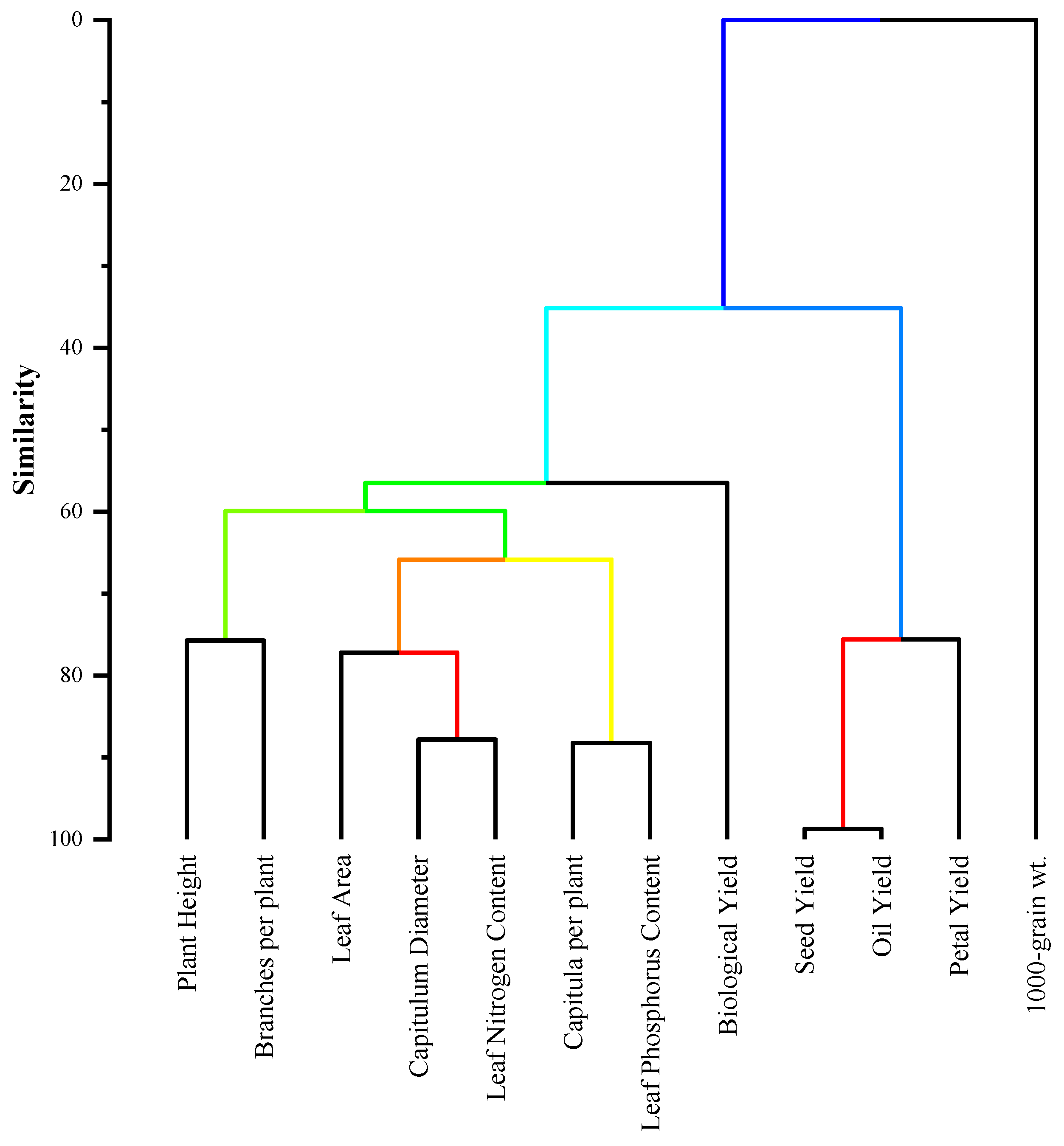
3.6. Hierarchical Cluster Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pakistan Bureau of Statistics. Agriculture Statistics. Available online: https://www.pbs.gov.pk/content/agriculture-statistics (accessed on 15 September 2024).
- Weiss, E.A. Oilseed Crops, 2nd ed.; Wiley: Hoboken, NJ, USA; Available online: https://www.wiley.com/en-us/Oilseed+Crops%2C+2nd+Edition-p-9780632052592 (accessed on 15 September 2024).
- FAO Statistical Yearbook 2013. Available online: https://www.fao.org/4/i3107e/i3107e00.htm (accessed on 15 September 2024).
- DE Queiroz, A.F.; Salviano, A.M.; DA Cunha, T.J.; Olszevski, N.; Júnior, V.S.D.S.; Neto, M.B.D.O. Potentialities and limitations of agricultural use in soils of semi-arid region of the state of Bahia. An. Acad. Bras. Ciências 2018, 90, 3373–3387. [Google Scholar] [CrossRef] [PubMed]
- Nouraein, M.; Skataric, G.; Spalevic, V.; Dudic, B.; Gregus, M. Short-term effects of tillage intensity and fertilization on sunflower yield, achene quality, and soil physicochemical properties under semi-arid conditions. Appl. Sci. 2019, 9, 5482. [Google Scholar] [CrossRef]
- Nosheen, A.; Naz, R.; Tahir, A.T.; Yasmin, H.; Keyani, R.; Mitrevski, B.; Bano, A.; Tong Chin, S.; Marriott, P.J.; Hussain, I. Improvement of safflower oil quality for biodiesel production by integrated application of PGPR under reduced amount of NP fertilizers. PLoS ONE 2018, 13, e0201738. [Google Scholar] [CrossRef] [PubMed]
- Yeloojeh, K.A.; Saeidi, G.; Sabzalian, M.R. Drought stress improves the composition of secondary metabolites in safflower flower at the expense of reduction in seed yield and oil content. Ind. Crops Prod. 2020, 154, 112496. [Google Scholar] [CrossRef]
- Joshi, S.; Thoday-Kennedy, E.; Daetwyler, H.D.; Hayden, M.; Spangenberg, G.; Kant, S. High-throughput phenotyping to dissect genotypic differences in safflower for drought tolerance. PLoS ONE 2021, 16, e0254908. [Google Scholar] [CrossRef]
- Abbadi, J.; Gerendás, J.; Sattelmacher, B. Effects of nitrogen supply on growth, yield and yield components of safflower and sunflower. Plant Soil. 2008, 306, 167–180. [Google Scholar] [CrossRef]
- Khalil, N.A.A.; Dagash, Y.M.; Yagoub, S.O. Effect of Sowing Date, Irrigation Intervals and Fertilizers on Safflower (Carthamus tinctorius L.) Yield. Discourse J. Agric. Food Sci. 2013, 1, 97–102. [Google Scholar]
- Emongor, V.; Oagile, O.; Phuduhudu, D.; Oarabile, P. Safflower Production. Botswana University of Agriculture and Natural ResourcesI Gaborone, 2017. Available online: http://repository.ruforum.org/system/tdf/Safflower%20Manual_BUAN.compressed.pdf?file=1&type=node&id=36745&force= (accessed on 15 September 2024).
- Bonfim-Silva, E.M.; Paludo, J.T.S.; Sousa, J.V.R.; Sousa, H.H.d.F.; da Silva, T.J.A. Development of safflower subjected to nitrogen rates in cerrado soil. Am. J. Plant Sci. 2015, 06, 2136–2143. [Google Scholar] [CrossRef]
- Kolanyane, M.O. The Influence of Nitrogen and Phosphorus Nutrition on Growth and Yield Components of Safflower (Carthamus tinctorius L.). Ph.D. Thesis, Botswana University of Agriculture & Natural Resources, Gaborone, Botswana, 2022. Available online: http://researchhub.buan.ac.bw/handle/13049/534 (accessed on 20 August 2024).
- Golzarfar, M.; Rad, A.H.S.; Delkhosh, B.; Bitarafan, Z. Safflower (Carthamus tinctorius L.) Response to Different Nitrogen and Phosphorus Fertilizer Rates in Two Planting Seasons. 2012. Available online: https://zemdirbyste-agriculture.lt/99(2)tomas/99_2_tomas_str6.pdf (accessed on 20 August 2024).
- Sharifi, R.S.; Namvar, A.; Sharifi, R.S. Grain filling and fatty acid composition of safflower fertilized with integrated nitrogen fertilizer and biofertilizers. Pesqui. Agropecuária Bras. 2017, 52, 236–243. [Google Scholar] [CrossRef]
- Liu, D.; Song, C.; Fang, C.; Xin, Z.; Xi, J.; Lu, Y. A recommended nitrogen application strategy for high crop yield and low environmental pollution at a basin scale. Sci. Total Environ. 2021, 792, 148464. [Google Scholar] [CrossRef]
- Razaq, M.; Zhang, P.; Shen, H.-L. Salahuddin Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Akhtar, K.; Karanja, J.K.; Noor-ul-Ain, N.; Haider, F.U. Understanding the adaptive mechanisms of plant in low phosphorous soil. In Plant Stress Physiology; CABI Books: London, UK, 2020; Available online: https://books.google.com/books?hl=en&lr=&id=ImwtEAAAQBAJ&oi=fnd&pg=PA285&dq=Understanding+the+adaptive+mechanisms+of+plant+in+low+phosphorous+soil.+In+Plant+stress+physiology&ots=AO8htTLSPO&sig=0mZGpY1p9ps5n-Z2RPErpNlsfQo (accessed on 20 August 2024).
- Sreekanth, N.; Singh, V.; Tiwari, D.; George, S.G. Effect of nitrogen and phosphorus levels on growth and yield of safflower (Carthamus tinctorius L.). Pharma Innov. J. 2021, 10, 2271–2273. [Google Scholar]
- Singh, R.K.; Singh, A.K. Effect of nitrogen, phosphorus and sulphur fertilization on productivity, nutrient-use efficiency and economics of safflower (Carthamus tinctorius) under late-sown condition. Indian J. Agron. 2001, 58, 583–587. [Google Scholar] [CrossRef]
- Lazar, T.; Taiz, L.; Zeiger, E. Plant physiology. 3rd edn. Ann. Bot. 2003, 91, 750–751. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Kikhazaleh, M.; Ramroudi, M.; Galavi, M.; Ghanbari, S.A.; Fanaei, H.R. Effect of potassium on yield and some qualitative and physiological traits of safflower (Carthamus tinctorius L.) under drought stress conditions. J. Plant Nutr. 2023, 46, 2380–2392. [Google Scholar] [CrossRef]
- Sein-Echaluce, V.C.; Mulet, J.M.; Barja, M.V.; Peleato, M.L.; Fillat, M.F. Exploring the ability of cyanobacterial ferric uptake regulator (FUR) proteins to increase yeast tolerance to abiotic stresses. In Cyanobacterial Lifestyle and Its Applications in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 179–196. Available online: https://www.sciencedirect.com/science/article/pii/B9780323906340000123 (accessed on 20 August 2024).
- Azimi Gandomani, M.; Faraji, H.; Dehdari, A.; Movahhedi Dehnavi, M.; Alinaghizadeh, M. Evaluation of the effect of salinity stress on ion accumulation, quantitative and qualitative yield of spring rapeseed cultivars. Environ. Stress. Crop Sci. 2009, 1, 27–37. [Google Scholar]
- Ravi, V.; Khurana, J.P.; Tyagi, A.K.; Khurana, P. An update on chloroplast genomes. Plant Syst. Evol. 2008, 271, 101–122. [Google Scholar] [CrossRef]
- Aslam, M.T.; Khan, I.; Chattha, M.U.; Nawaz, M.; Mustafa, A.; Athar, F.; Hassan, M.U.; Kanwal, H.; Shah, A.N. The Biofortification of Zinc in Legumes to Alleviate Zinc Deficiency. In Legumes Biofortification; Springer: Berlin/Heidelberg, Germany, 2023; pp. 327–346. Available online: https://link.springer.com/chapter/10.1007/978-3-031-33957-8_14 (accessed on 20 August 2024).
- Aslam, M.T.; Chattha, M.U.; Khan, I.; Haq, M.Z.U.; Mustafa, A.; Athar, F.; Bisma; Nawaz, M.; Shah, A.N.; Mahmood, F.; et al. Scope of Seed Priming in Inducing Biofortification in Plants. In Mineral Biofortification in Crop Plants for Ensuring Food Security; Springer: Berlin/Heidelberg, Germany, 2023; pp. 233–259. Available online: https://link.springer.com/chapter/10.1007/978-981-99-4090-5_11 (accessed on 20 August 2024).
- Ghiyasi, M.; Rezaee Danesh, Y.; Amirnia, R.; Najafi, S.; Mulet, J.M.; Porcel, R. Foliar Applications of ZnO and its nanoparticles increase safflower (Carthamus tinctorius L.) growth and yield under water stress. Agronomy 2023, 13, 192. [Google Scholar] [CrossRef]
- Rahmani, F.; Sayfzadeh, S.; Jabbari, H.; Valadabadi, S.A.; Masouleh, E.H. Alleviation of Drought Stress Effects on Safflower Yield by Foliar Application of Zinc. Int. J. Plant Prod. 2019, 13, 297–308. [Google Scholar] [CrossRef]
- Manvelian, J.; Weisany, W.; Tahir, N.A.R.; Jabbari, H.; Diyanat, M. Physiological and biochemical response of safflower (Carthamus tinctorius L.) cultivars to zinc application under drought stress. Ind. Crops Prod. 2021, 172, 114069. [Google Scholar] [CrossRef]
- Shukla, A.K.; Behera, S.K. Zinc Management in Indian Agriculture: Past, Present and Future. 2011. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20113337631 (accessed on 15 September 2024).
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2018, 302, 1–17. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Khanda, C.M.; Dixit, L. Effect of zinc and nitrogen fertilization on yield and, nutrient uptake of summer rice (Oryza sativu). Indian J. Agron. 1996, 41, 41_3. [Google Scholar]
- Thomas, G.W. Soil pH and Soil Acidity. In SSSA Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 2018; pp. 475–490. [Google Scholar] [CrossRef]
- Rhoades, J.D. Salinity: Electrical Conductivity and Total Dissolved Solids. In SSSA Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 2018; pp. 417–435. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Agronomy Monographs, 1st ed.; Page, A.L., Ed.; Wiley: Hoboken, NJ, USA, 1982; Volume 9, pp. 539–579. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic Forms. In Agronomy Monographs, 1st ed.; Page, A.L., Ed.; Wiley: Hoboken, NJ, USA, 1982; Volume 9, pp. 643–698. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, E.L. Phosphorus soluble in sodium bicarbonate. Methods Soil Anal. Part 1982, 2, 404–430. [Google Scholar]
- Knudsen, D.; Peterson, G.A.; Pratt, P.F. Lithium, Sodium, and Potassium. In Agronomy Monographs, 1st ed.; Page, A.L., Ed.; Wiley: Hoboken, NJ, USA, 1982; Volume 9, pp. 225–246. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil. Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Nachtergaele, F. Soil taxonomy—A basic system of soil classification for making and interpreting soil surveys. Geoderma 2001, 99, 336–337. [Google Scholar] [CrossRef]
- Gee, G.W. Particle size analysis. In Methods of Soil Analysis; ASA and SSSA: Madison, WI, USA, 1986. [Google Scholar]
- Horwitz, W. Official Methods of Analysis of the Association of Official Analytical Chemists; The Association: Singapore, 1925; Volume 2, Available online: https://books.google.com/books?hl=en&lr=&id=kiRtAAAAMAAJ&oi=fnd&pg=PP26&dq=AOAC+(1996)+Official+Method+of+Analysis+of+the+Association+of+Official+Analytical+Chemists.+AOAC+International,+Arlington.&ots=G-5JxhW56S&sig=Jaooi33vMATsRRBaH_rrpehVvsM (accessed on 20 August 2024).
- Roche, J.; Mouloungui, Z.; Cerny, M.; Merah, O. Effect of sowing dates on fatty acids and phytosterols patterns of Carthamus tinctorius L. Appl. Sci. 2019, 9, 2839. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Liu, Y.; Wei, D. High-efficient CO2-to-protein bioconversion by oleaginous Coccomyxa subellipsoidea using light quality shift and nitrogen supplementation strategy. Chem. Eng. J. 2023, 473, 145166. [Google Scholar] [CrossRef]
- Haghighati, A. Study on the effects of nitrogen and phosphorus fertilizers on the yield and oil content of safflower lines in drylands. Res. J. Agron. 2010, 4, 57–62. [Google Scholar] [CrossRef]
- Rathke, G.-W.; Behrens, T.; Diepenbrock, W. Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): A review. Agric. Ecosyst. Environ. 2006, 117, 80–108. [Google Scholar] [CrossRef]
- Ashkani, J.; Pakniyat, H.; Emam, Y.; Assad, M.T.; Bahrani, M.J. The Evaluation and Relationships of some Physiological Traits in Spring Safflower (Carthamus tinctorius L.) under Stress and Non-stress Water Regimes. J. Agric. Sci. Technol. 2007, 9, 267–277. [Google Scholar]
- Yeilaghi, H.; Arzani, A.; Ghaderian, M.; Fotovat, R.; Feizi, M.; Pourdad, S.S. Effect of salinity on seed oil content and fatty acid composition of safflower (Carthamus tinctorius L.) genotypes. Food Chem. 2012, 130, 618–625. [Google Scholar] [CrossRef]
- Tuncturk, M.; Yildirim, B. Effects of different forms and doses of nitrogen fertilizers on safflower (Carthamus tinctorius L.). Pak. J. Biol. Sci. 2004, 7, 1385–1389. [Google Scholar] [CrossRef][Green Version]
- Strašil, Z.; Vorlíček, Z. The effect of nitrogen fertilization, sowing rates and site on yields and yield components of selected varieties of safflower (Carthamus tinctorius L.). Plant Soil. Environ. 2002, 48, 307–311. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Oad, F.C. Nitrogen Requirement of Safflower (Carthamus tinctorius L.) for Growth and Yield Traits. 2006. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20063093528 (accessed on 16 September 2024).
- Ma, J.; Chen, T.; Lin, J.; Fu, W.; Feng, B.; Li, G.; Li, H.; Li, J.; Wu, Z.; Tao, L.; et al. Functions of nitrogen, phosphorus and potassium in energy status and their influences on rice growth and development. Rice Sci. 2022, 29, 166–178. [Google Scholar] [CrossRef]
- Elfadl, E.; Reinbrecht, C.; Frick, C.; Claupein, W. Optimization of nitrogen rate and seed density for safflower (Carthamus tinctorius L.) production under low-input farming conditions in temperate climate. Field Crops Res. 2009, 114, 2–13. [Google Scholar] [CrossRef]
- Emami, T.; Naseri, R.; Falahi, H.; Kazemi, E. Response of Yield, Yield Component and Oil Content of Safflower (cv. Sina) to Planting Date and Plant Spacing on Row in Rainfed Conditions of Western Iran. 2011. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20113327040 (accessed on 16 September 2024).
- Azari, A.; Khajehpour, M.R. Effects of planting pattern on growth, development, yield components and seed yield of safflower, local variety of Isfahan, Koseh, in spring planting. JWSS-Isfahan Univ. Technol. 2003, 7, 155–167. [Google Scholar]
- Santos, R.F.; de Almeida Silva, M. Carthamus tinctorius L.: Uma alternativa de cultivo para o Brasil. Acta Iguazu 2015, 4, 26–35. [Google Scholar]
- Malek, H.A.; Ferri, F. Effects of nitrogen and phosphorus fertilizers on safflower yield in dry land conditions. Int. J. Res. Agric. Sci. 2014, 1, 28–33. [Google Scholar]
- Kirkby, E.A.; Nikolic, M.; White, P.J.; Xu, G. Mineral nutrition, yield, and source–sink relationships. In Marschner’s Mineral Nutrition of Plants; Elsevier: Amsterdam, The Netherlands, 2023; pp. 131–200. Available online: https://www.sciencedirect.com/science/article/pii/B9780128197738000150 (accessed on 20 August 2024).
- Ahmed, M.A.; Rasheed, S.M.; Zeebaree, P.J. Responses of Two Red Cabbage Hybrids to Nano NPK and Zinc Fertilizers (Brassica oleracea L. var. capitata rubra). J. Environ. Sci. Stud. 2023, 6, 1. [Google Scholar] [CrossRef]
- Ravi, S.; Jadhav, R.L.; Bhat, S.N.; Shankar, K.A. Response of Iron and Boron on Uptake of Nutrients and Soil Nutrient Status after Harvest of Safflower Crop (Carthamus tinctorius L.) in Kalyan Karnataka, India. Int. J. Plant Soil. Sci. 2023, 35, 24–31. [Google Scholar] [CrossRef]
- Mishra, A.; Gupta, H.; Mishra, U.S.; Sirothia, P.; Darwai, P. Interaction Effect of Sulphur and NPK on Growth Parameters and Yield of Mustard. Int. J. Plant Soil. Sci. 2023, 35, 138–144. [Google Scholar] [CrossRef]
- Hiwale, S.D.; Chorey, A.B.; Deshmukh, M.R. Balanced crop nutrition to optimized productivity and quality of safflower under rainfed condition. In Advances in Agricultural and Horticultural Sciences; Kosmos Publishers: Harrison, NJ, USA, 2022; p. 125. Available online: https://www.researchgate.net/profile/Tejpal-Dahiya/publication/358899022_Biofloc_Technology_An_emerging_and_innovative_approach_for_sustainable_aquaculture_productivity/links/6253ec66ef0134206669a186/Biofloc-Technology-An-emerging-and-innovative-approach-for-sustainable-aquaculture-productivity.pdf#page=134 (accessed on 20 August 2024).
- Sahu, A.; Swaroop, N.; David, A.A.; Thomas, T. Effect of different levels of NPK and Zinc on soil health growth and yield of chickpea (Cicer arietinum L.) var. PUSA 362. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 591–597. [Google Scholar] [CrossRef]
- Omidi, A.H.; Sharifmogadas, M.R. Evaluation of Iranian safflower cultivars reaction to different sowing dates and plant densities. World Appl. Sci. J. 2010, 8, 953–958. [Google Scholar]
- Dordas, C.A.; Sioulas, C. Safflower yield, chlorophyll content, photosynthesis, and water use efficiency response to nitrogen fertilization under rainfed conditions. Ind. Crops Prod. 2008, 27, 75–85. [Google Scholar] [CrossRef]
- Rastgou, B.; Ebadi, A.; Vafaie, A.; Moghadam, S.H. The Effects of Nitrogen Fertilizer on Nutrient Uptake, Physiological Traits and Yield Components of Safflower (Carthamus tinctorius L.). 2013. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20133186691 (accessed on 16 September 2024).
- Mahasi, M.J.; Pathak, R.S.; Wachira, F.N.; Riungu, T.C.; Kinyua, M.G.; Kamundia, J.W. Correlations and Path Coefficient Analysis in Exotic Safflower (Carthamus tinctorious L.) Genotypes Tested in the Arid and Semi Arid Lands (Asals) of Kenya. 2006. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20063210358 (accessed on 16 September 2024).
- Arslan, B. The Path Analysis of Yield and its Components in Safflower (Carthamus tinctorius L.). J. Biol. Sci. 2007, 7, 668–672. [Google Scholar] [CrossRef][Green Version]
- Koutroubas, S.D.; Papakosta, D.K.; Doitsinis, A. Cultivar and seasonal effects on the contribution of pre-anthesis assimilates to safflower yield. Field Crops Res. 2004, 90, 263–274. [Google Scholar] [CrossRef]
- Eryiğit, T.; Yıldırım, B.; Kumlay, A.M.; Sancaktaroğlu, S. The effects of different row distances and nitrogen fertilizer rates on yield and yield components of safflower (Carthamus tinctorious) under micro-climate conditions of Iğdır Plain—Turkey. In Proceedings of the 3rd International Conference on Biological, Chemical & Environmental Sciences (BCES-2015), Kumpur, Malaysia, 21–25 September 2015; pp. 21–22. Available online: https://www.researchgate.net/profile/Tamer-Eryigit/publication/282246178_The_Effects_of_Different_Row_Distances_and_Nitrogen_Fertilizer_Rates_on_Yield_and_Yield_Components_of_Safflower_Carthamus_tinctorious_Under_Micro-Climate_Conditions_of_Igdir_Plain_-Turkey/links/56090dda08aea25fce3b9037/The-Effects-of-Different-Row-Distances-and-Nitrogen-Fertilizer-Rates-on-Yield-and-Yield-Components-of-Safflower-Carthamus-tinctorious-Under-Micro-Climate-Conditions-of-Igdir-Plain-Turkey.pdf (accessed on 16 September 2024).
- Ismail, A.M.; Azooz, M. Effect of Zinc Supply on Growth and Some Metabolic Characteristics of Safflower and Sunflower Plants. 2005. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20063011881 (accessed on 16 September 2024).
- Taha, M.H.; Shalaby, E.A.; Shanan, N.T. IMPROVING SAFFLOWER (Carthamus tinctorius L.) growth and biological activities under saline water irrigation by using iron and zinc foliar applications. J. Plant Prod. 2013, 4, 1219–1234. [Google Scholar] [CrossRef]
- Lakzayi, M. Influence of foliar application on safflower yield. Int. J. Multidiscip. Res. Dev. 2015, 2, 336–339. [Google Scholar]
- Gülmezoğlu, N.; Aytac, Z. The influences of various zinc applications on seed yield and zinc uptake of Safflower. J. Soil Water 2016, 5, 11–17. Available online: https://agris.fao.org/search/en/providers/122624/records/647461c12d3f560f80a6b6e4 (accessed on 16 September 2024).
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crops Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D. Response of tomato (Lycopersicon esculentum L.) cultivars to foliar application of zinc when grown in sand culture at low zinc. Sci. Hortic. 2002, 93, 53–64. [Google Scholar] [CrossRef]
- Dordas, C.A.; Sioulas, C. Dry matter and nitrogen accumulation, partitioning, and retranslocation in safflower (Carthamus tinctorius L.) as affected by nitrogen fertilization. Field Crops Res. 2008, 110, 35–43. [Google Scholar] [CrossRef]
- Gadallah, M.A.A. Effects of indole-3-acetic acid and zinc on the growth, osmotic potential and soluble carbon and nitrogen components of soybean plants growing under water deficit. J. Arid. Environ. 2000, 44, 451–467. [Google Scholar] [CrossRef]
- Hebbern, C.A.; Pedas, P.; Schjoerring, J.K.; Knudsen, L.; Husted, S. Genotypic differences in manganese efficiency: Field experiments with␣winter barley (Hordeum vulgare L.). Plant Soil 2005, 272, 233–244. [Google Scholar] [CrossRef]
- Mirzapour, M.H.; Khoshgoftar, A.H. Zinc Application Effects on Yield and Seed Oil Content of Sunflower Grown on a Saline Calcareous Soil. J. Plant Nutr. 2006, 29, 1719–1727. [Google Scholar] [CrossRef]
- Sarkar, D.; Mandal, B.; Kundu, M.C. Increasing use efficiency of boron fertilisers by rescheduling the time and methods of application for crops in India. Plant Soil 2007, 301, 77–85. [Google Scholar] [CrossRef]
- Safdar, M.E.; Qamar, R.; Javed, A.; Nadeem, M.A.; Javeed, H.M.R.; Farooq, S.; Głowacka, A.; Michałek, S.; Alwahibi, M.S.; Elshikh, M.S.; et al. Combined application of boron and zinc improves seed and oil yields and oil quality of oilseed rape (Brassica napus L.). Agronomy 2023, 13, 2020. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M.; Nadeem, F.; Rehman, A.; Hussain, M.; Nawaz, A.; Naveed, M. Zinc Application in Combination with Zinc Solubilizing Enterobacter sp. MN17 Improved Productivity, Profitability, Zinc Efficiency, and Quality of Desi Chickpea. J. Soil. Sci. Plant Nutr. 2020, 20, 2133–2144. [Google Scholar] [CrossRef]
- Mohammed, B.A.; Khandaker, M.M.; Arshad, A.M.; Nudin, N.F.H.; Majrashi, A.; Mohd, K.S. Effects of foliar NPK application on growth, yield and nutrient content of sweet corn grown on Rengam Series soil. Basrah J. Agric. Sci. 2023, 36, 254–270. [Google Scholar] [CrossRef]
- Mahmood, H.N. Foliar and Fertigation Application of NPK Fertilizers Impact on Growth, Seed Yield and Yield Components of Safflower (Carthamus tinctorius L.). Tikrit J. Agric. Sci. 2021, 21, 84–98. [Google Scholar] [CrossRef]
- Jaffar, R.A.-A.; Al-Refai, S.I. Response of safflower to (NPK) fertilizer combinations and plants distribution. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; p. 012056. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/923/1/012056/meta (accessed on 20 August 2024).
- Bellaloui, N.; Hanks, J.E.; Fisher, D.K.; Mengistu, A. Soybean Seed Composition Is Influenced by Within-Field Variability in Soil Nutrients; Crop Management—Wiley Online Library: Hoboken, NJ, USA, 2009; Available online: https://acsess.onlinelibrary.wiley.com/doi/full/10.1094/CM-2009-1203-01-RS (accessed on 20 August 2024).

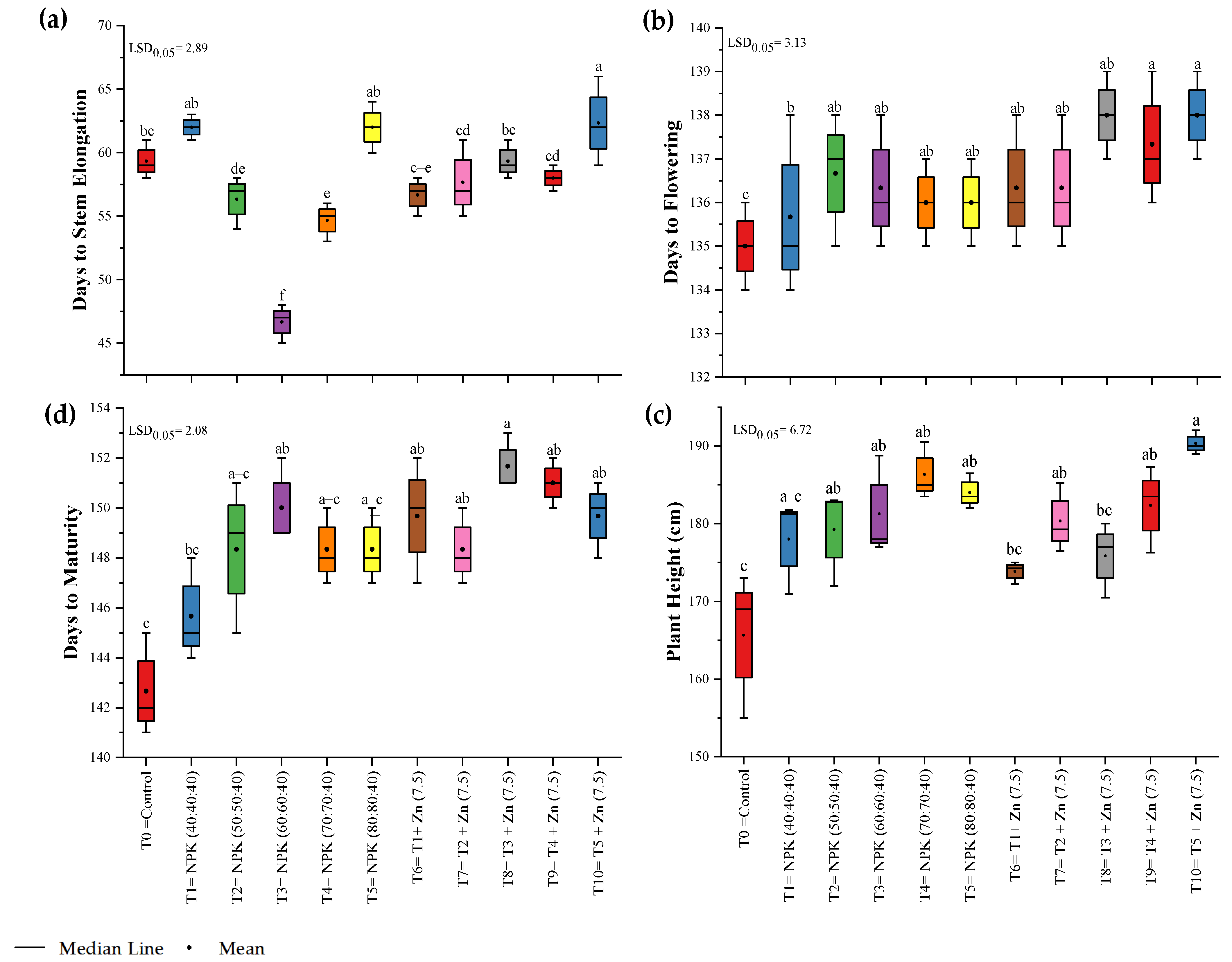
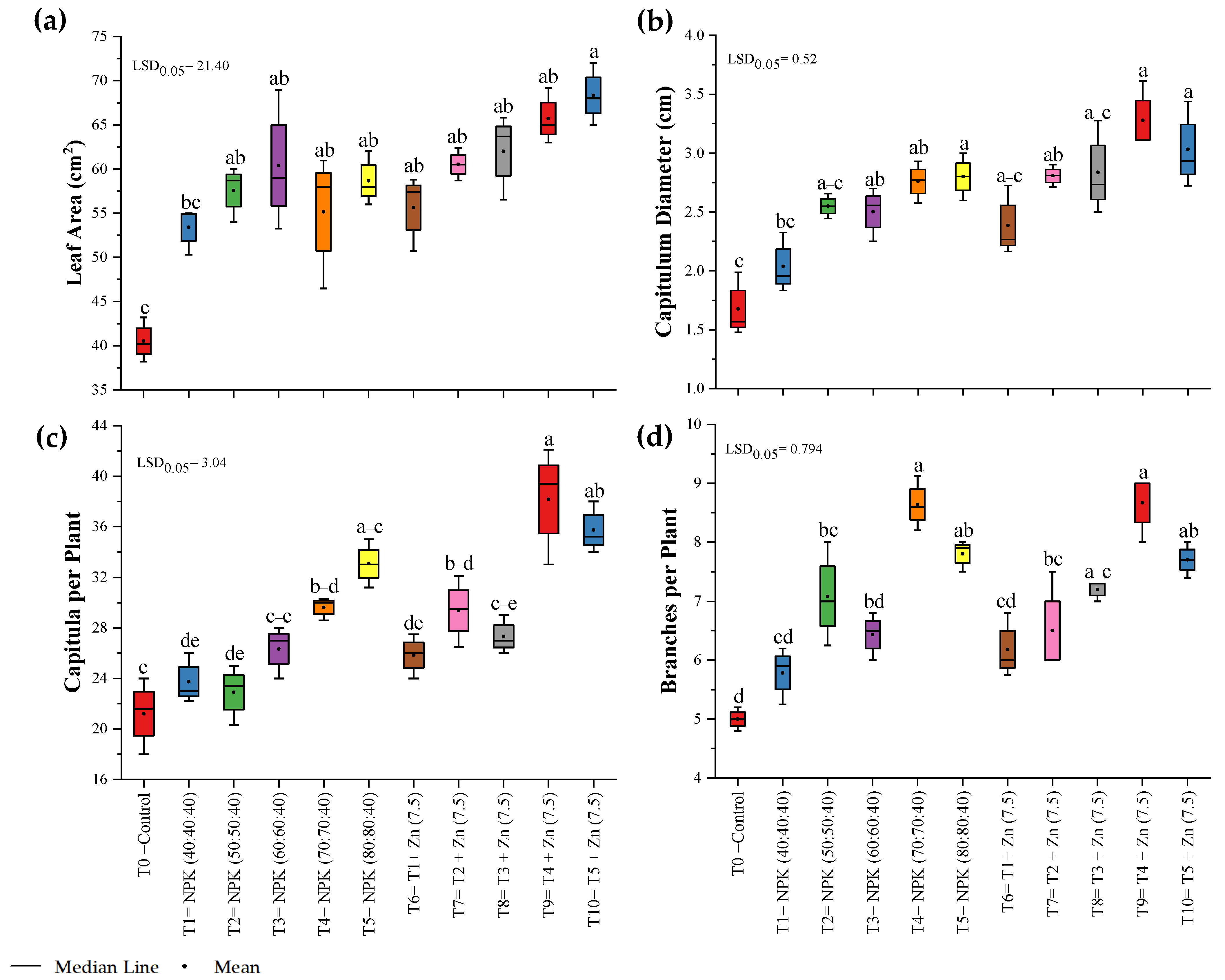
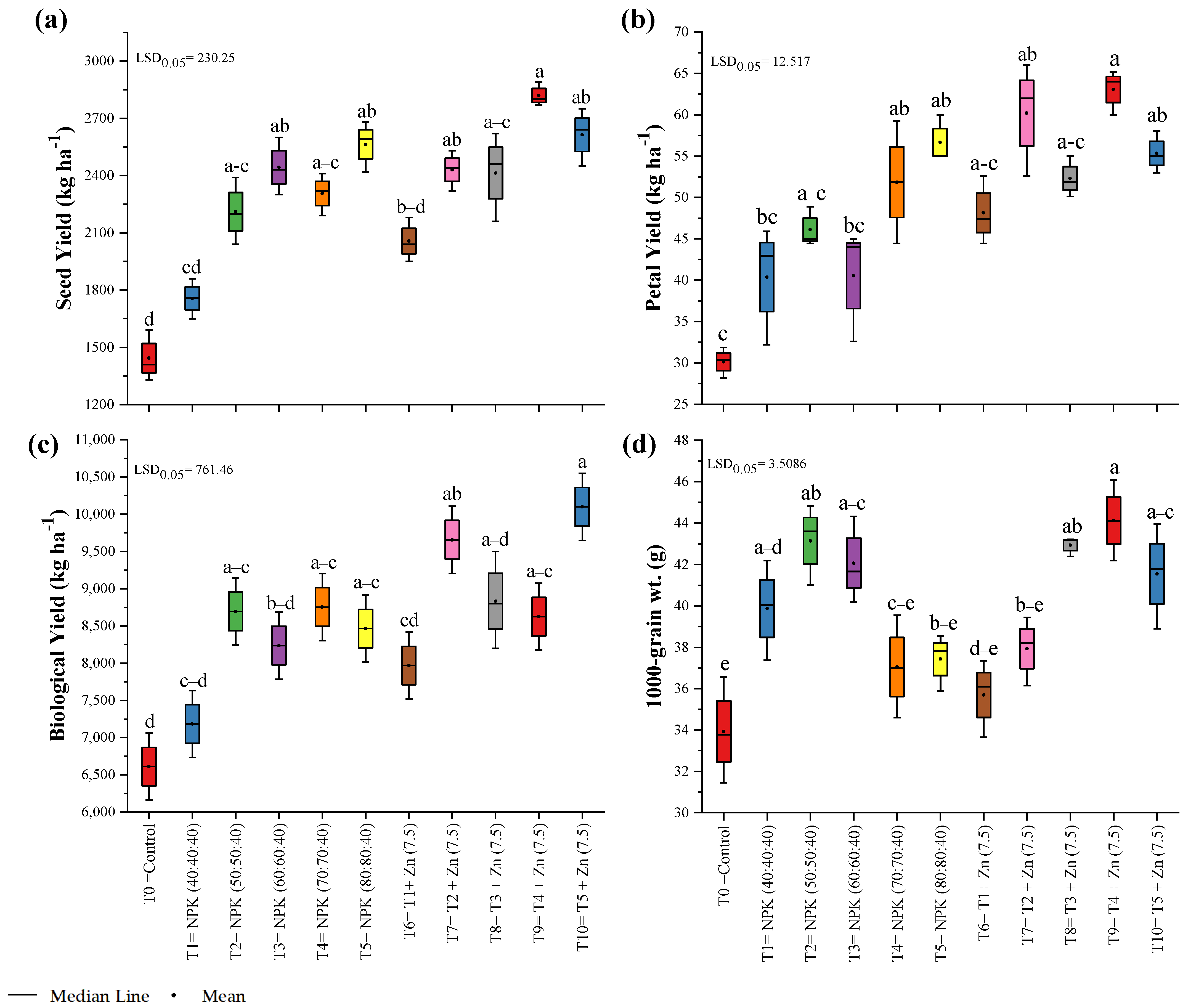
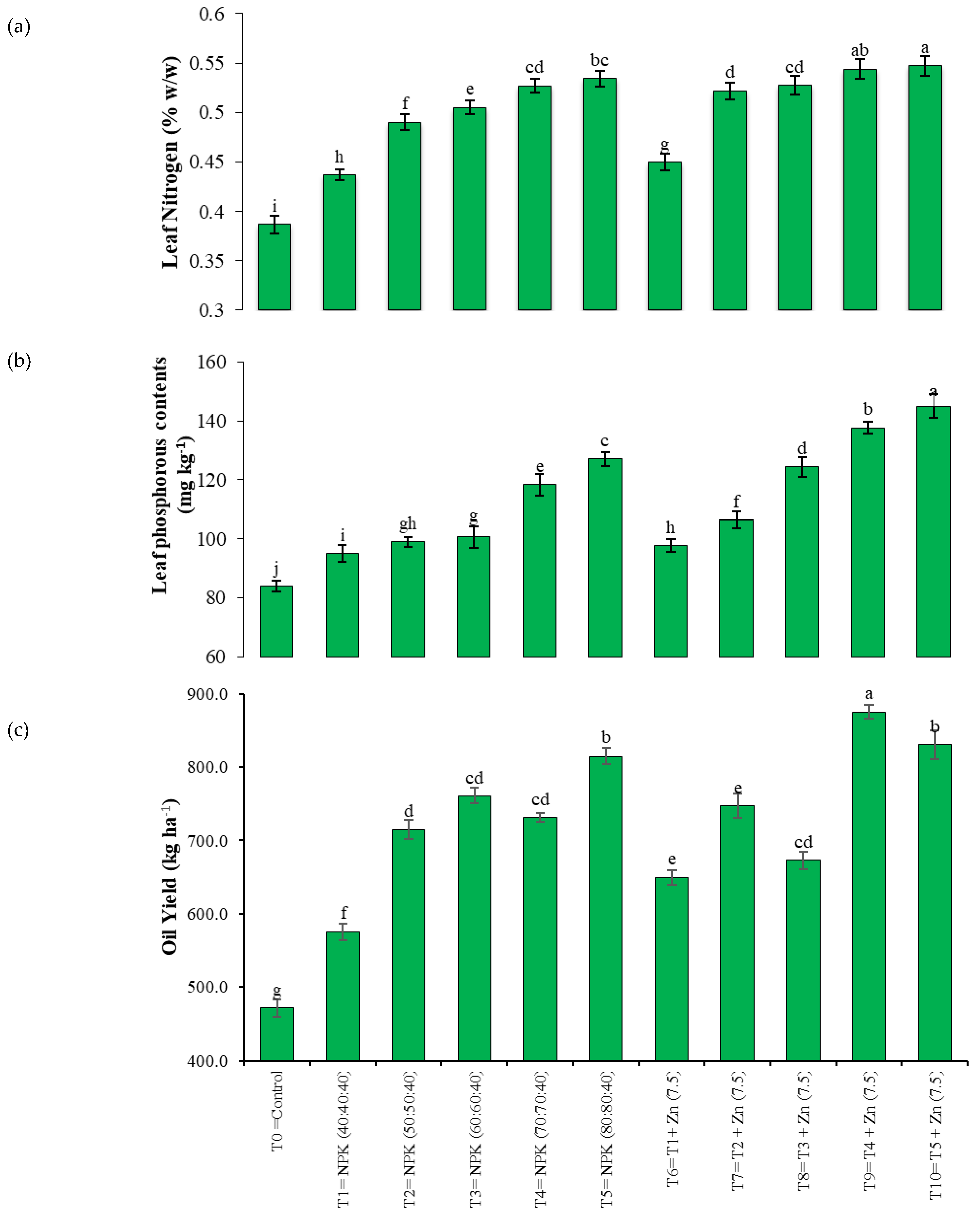
| Treatment Number | Fertilizer Dose |
|---|---|
| T0 | Control (no fertilizer application) |
| T1 | NPK at 40:40:40 kg ha−1 |
| T2 | NPK at 50:50:40 kg ha−1 |
| T3 | NPK at 60:60:40 kg ha−1 |
| T4 | NPK at 70:70:40 kg ha−1 |
| T5 | NPK at 80:80:40 kg ha−1 |
| T6 | T1 + Zinc at 7.5 kg ha−1 |
| T7 | T2 + Zinc at 7.5 kg ha−1 |
| T8 | T3 + Zinc at 7.5 kg ha−1 |
| T9 | T4 + Zinc at 7.5 kg ha−1 |
| T10 | T5 + Zinc at 7.5 kg ha−1 |
| Soil Properties | Results [Literature Followed for Analysis] |
|---|---|
| pH | 7.1 [37] |
| Electrical Conductivity (dS m−1) | 1.32 [38] |
| Organic Matter (%) | 1.10 [39] |
| Available Nitrogen (NO3−, mg kg−1) | 9.84 [40] |
| Available Phosphorus (mg kg−1) | 13.4 [41] |
| Available Potassium (mg kg−1) | 287 [42] |
| Zinc (mg kg−1) | 0.8 [43] |
| Saturation (%) | 34 [44] |
| Sand (% w/w) | 58 [45] |
| Silt (% w/w) | 28 [45] |
| Clay (% w/w) | 14 [45] |
| Texture | Sandy Loam |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamgeer, M.; Munir, H.; Hussain, S.; Adhikari, S.; Soufan, W.; Ahmed, J.; Aslam, M.; Rauf, S. Exploring the Synergistic Role of Zinc in NPK Fertilization on the Agronomic Performance of Safflower (Carthamus tinctorius). Horticulturae 2024, 10, 1243. https://doi.org/10.3390/horticulturae10121243
Alamgeer M, Munir H, Hussain S, Adhikari S, Soufan W, Ahmed J, Aslam M, Rauf S. Exploring the Synergistic Role of Zinc in NPK Fertilization on the Agronomic Performance of Safflower (Carthamus tinctorius). Horticulturae. 2024; 10(12):1243. https://doi.org/10.3390/horticulturae10121243
Chicago/Turabian StyleAlamgeer, Muhammad, Hassan Munir, Saddam Hussain, Sudeep Adhikari, Walid Soufan, Jahangir Ahmed, Maryam Aslam, and Saeed Rauf. 2024. "Exploring the Synergistic Role of Zinc in NPK Fertilization on the Agronomic Performance of Safflower (Carthamus tinctorius)" Horticulturae 10, no. 12: 1243. https://doi.org/10.3390/horticulturae10121243
APA StyleAlamgeer, M., Munir, H., Hussain, S., Adhikari, S., Soufan, W., Ahmed, J., Aslam, M., & Rauf, S. (2024). Exploring the Synergistic Role of Zinc in NPK Fertilization on the Agronomic Performance of Safflower (Carthamus tinctorius). Horticulturae, 10(12), 1243. https://doi.org/10.3390/horticulturae10121243









