Optimization of Cannabinoid Production in Hemp Through Methyl Jasmonate Application in a Vertical Farming System
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. Methyl Jasmonate Treatment
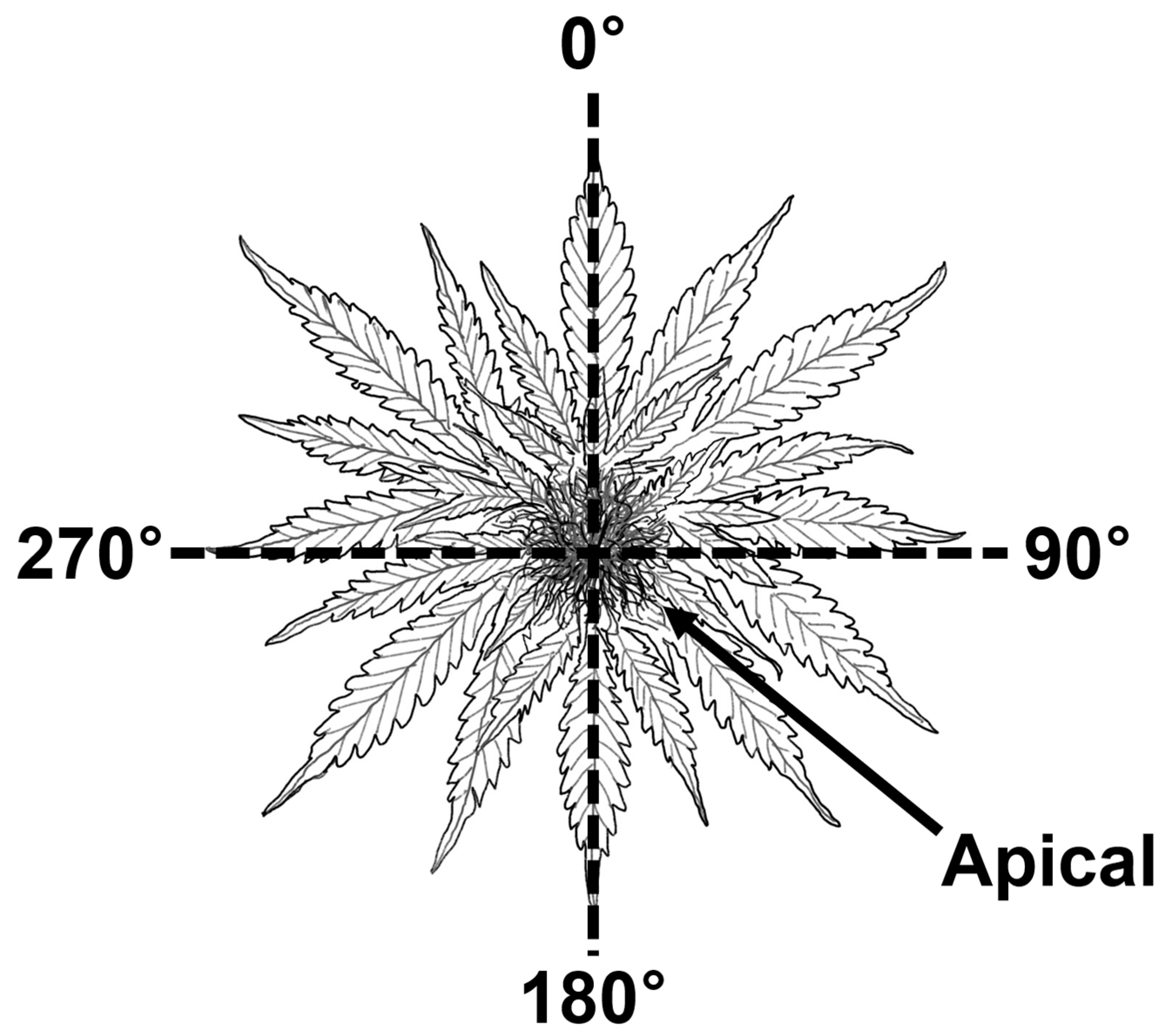
2.3. Analysis of Plant Growth Parameters
2.4. Glandular Trichome Density Measurement
2.5. Analysis of Phytocannabinoids Using HPLC
2.6. Yield of Major Cannabinoids
2.7. Statistical Analysis
3. Results
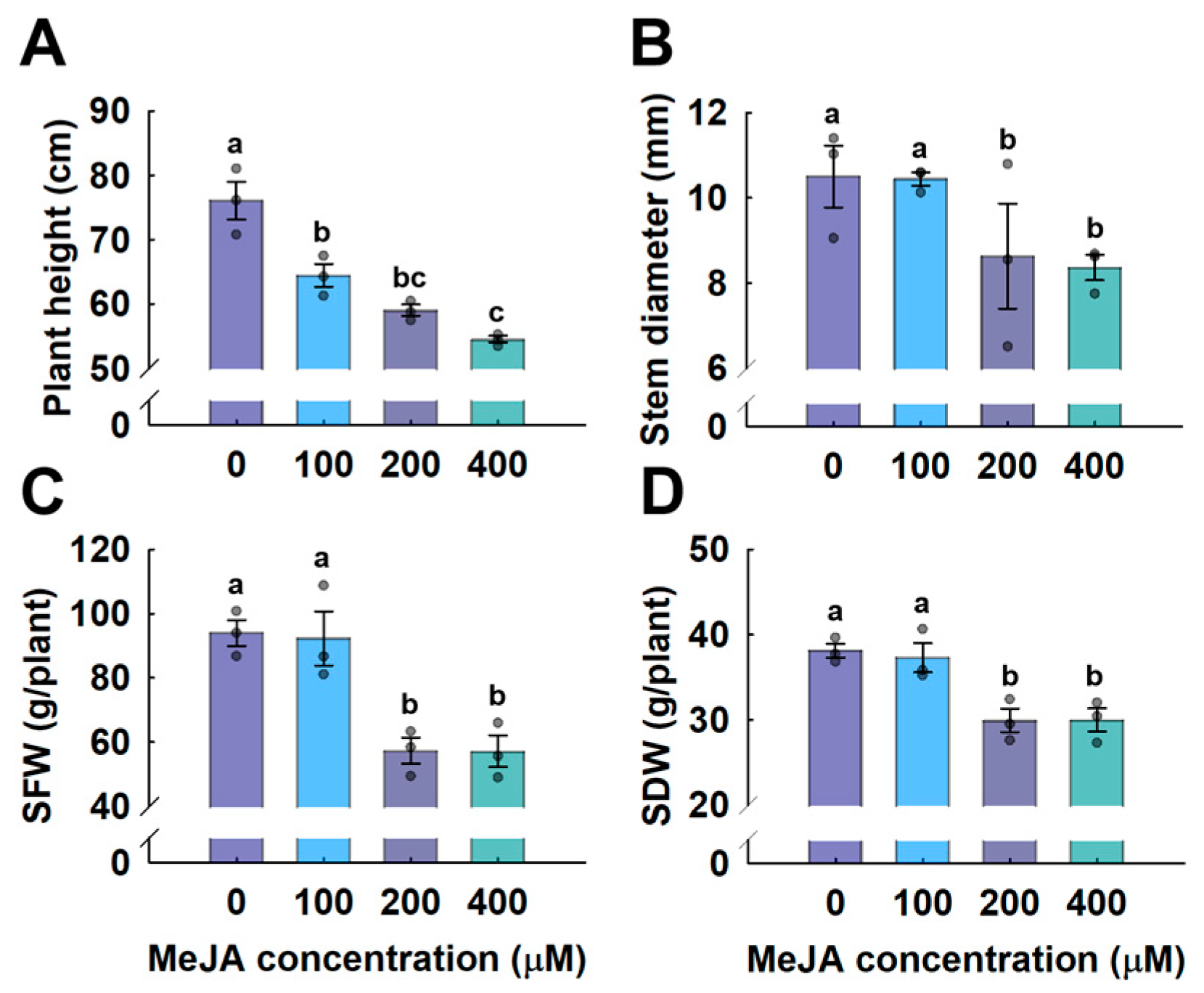
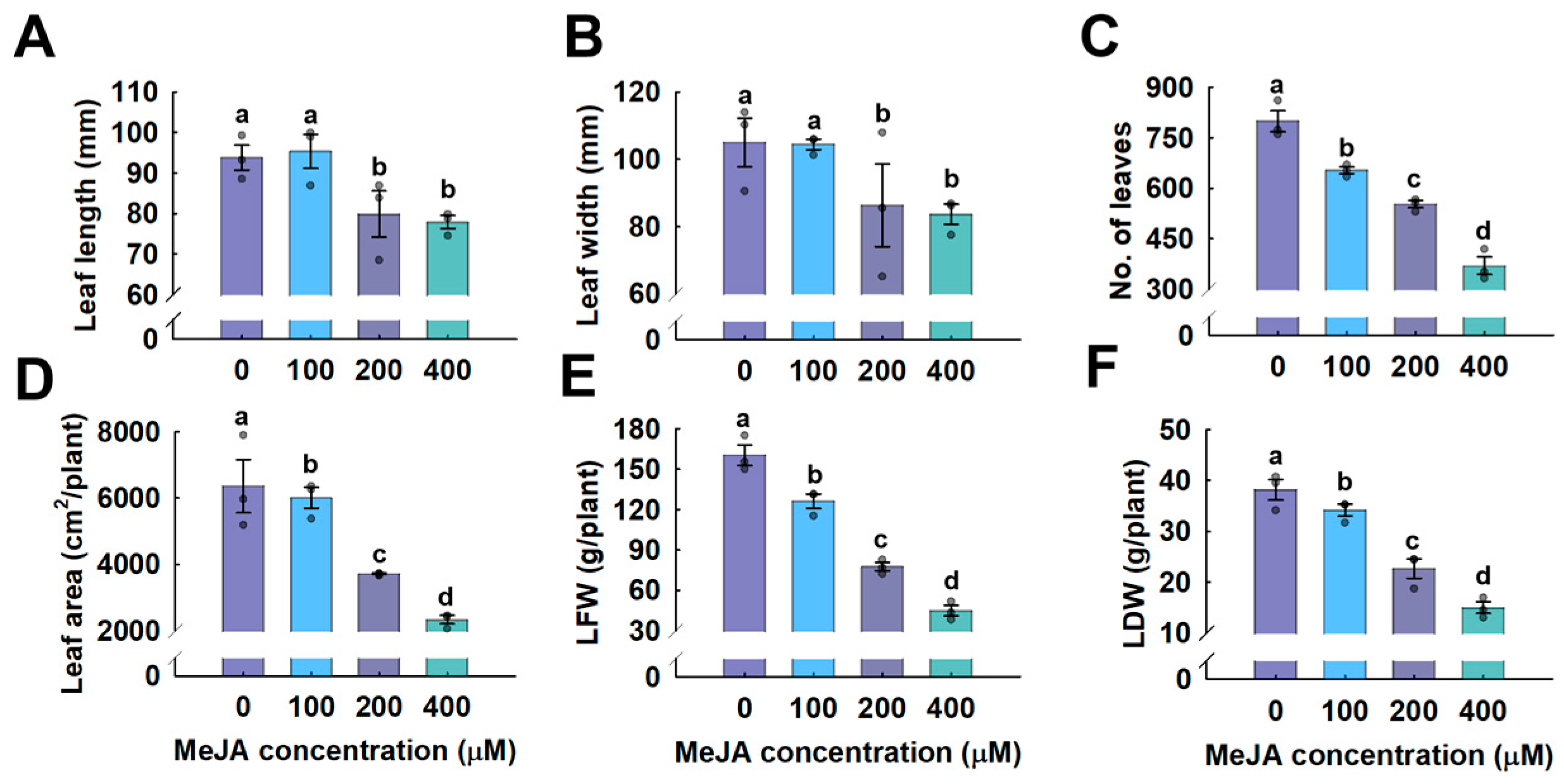
3.1. Growth Parameter Analysis
3.2. Glandular Trichome Density Analysis
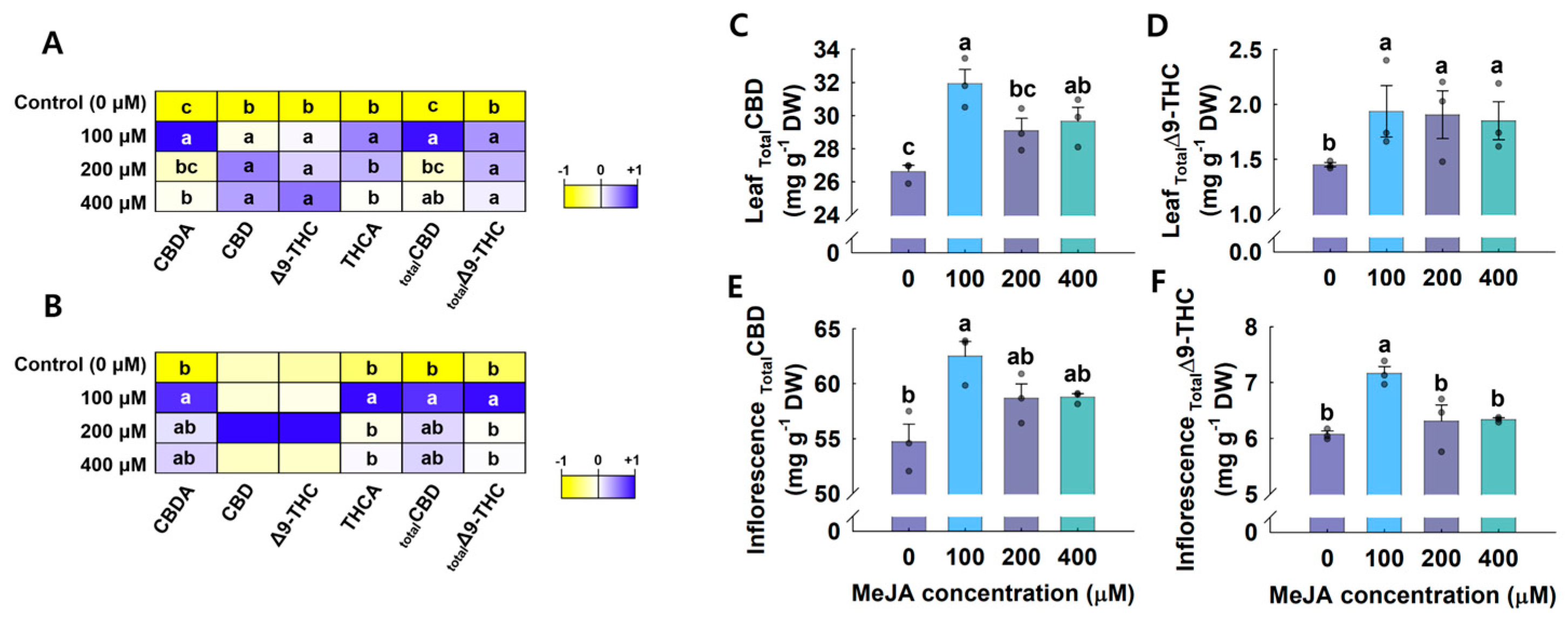
3.3. Cannabinoid Content Analysis Using HPLC
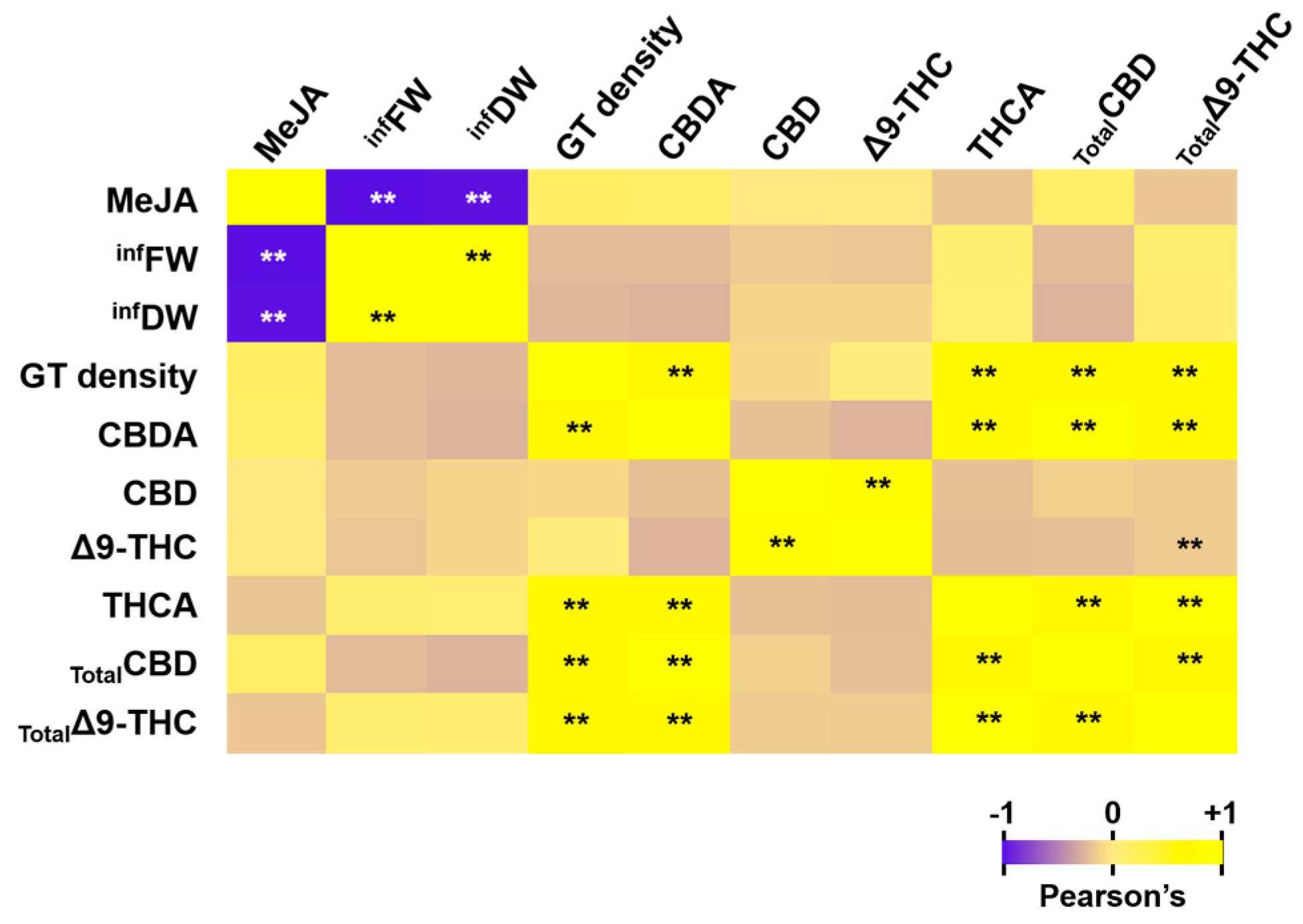
3.4. Correlation Coefficient Analysis
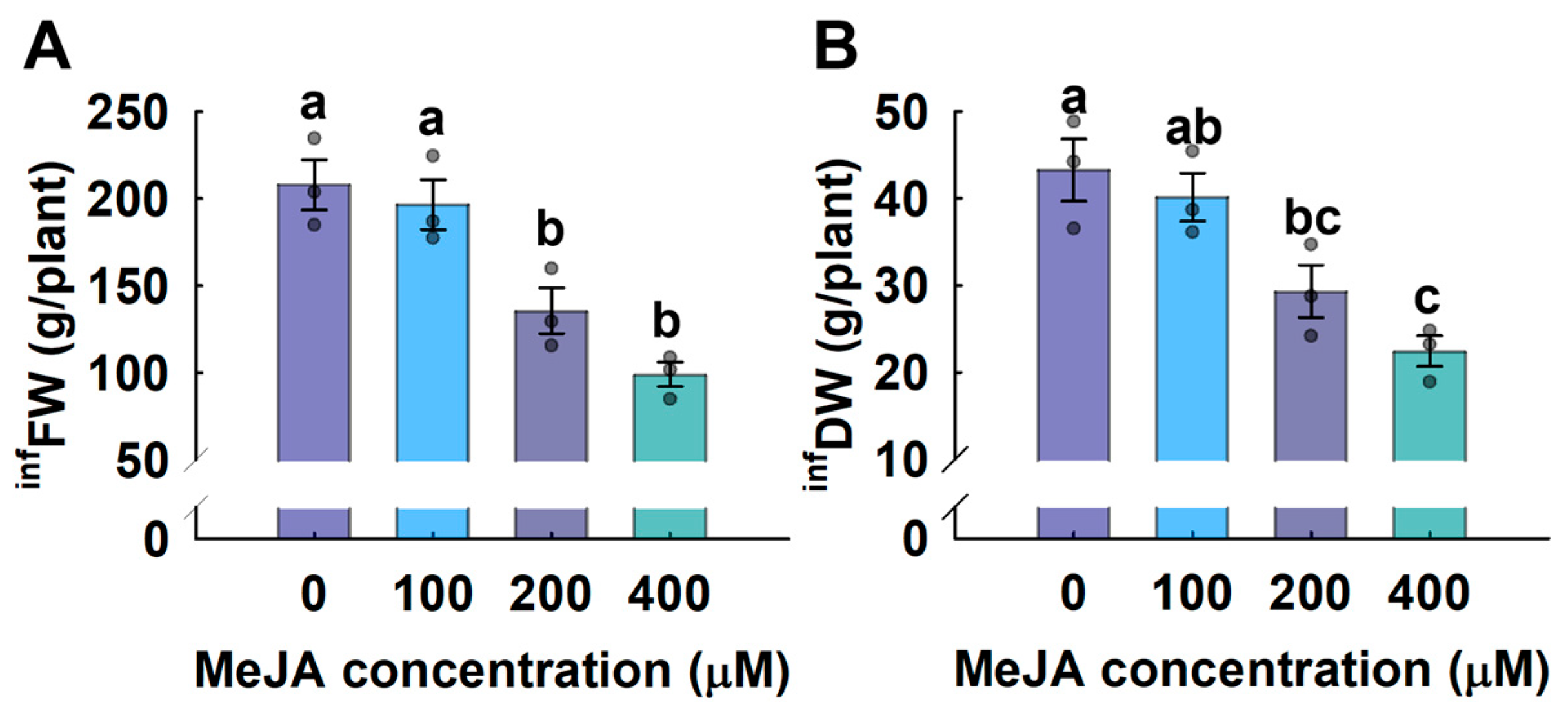
3.5. Cannabinoid Yield Analysis
4. Discussion
4.1. Methyl Jasmonate Reduces the Growth of Hemp
4.2. Methyl Jasmonate Influences Cannabinoid Synthesis and Trichome Development in Hemp
4.3. Methyl Jasmonate Increases the Efficiency of Cannabinoid Yield in Vertical Farms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Khazaleh, A.K.; Zhou, X.; Bhuyan, D.J.; Münch, G.W.; Al-Dalabeeh, E.A.; Jaye, K.; Chang, D. The Neurotherapeutic Arsenal in Cannabis sativa: Insights into Anti-Neuroinflammatory and Neuroprotective Activity and Potential Entourage Effects. Molecules 2024, 29, 410. [Google Scholar] [CrossRef]
- Pisanti, S.; Bifulco, M. Medical Cannabis: A plurimillennial history of an evergreen. J. Cell. Physiol. 2019, 234, 8342–8351. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef] [PubMed]
- Happyana, N.; Kayser, O. Monitoring metabolite profiles of Cannabis sativa L. trichomes during flowering period using 1H NMR-based metabolomics and real-time PCR. Planta Med. 2016, 82, 1217–1223. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Yang, R.; Berthold, E.C.; McCurdy, C.R.; da Silva Benevenute, S.; Brym, Z.T.; Freeman, J.H. Development of cannabinoids in flowers of industrial hemp (Cannabis sativa L.): A pilot study. J. Agric. Food Chem. 2020, 68, 6058–6064. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Karschner, E.L.; Goodwin, R.S.; Scheidweiler, K.B.; Hirvonen, J.; Queiroz, R.H.; Huestis, M.A. Impact of prolonged cannabinoid excretion in chronic daily cannabis smokers’ blood on per se drugged driving laws. Clin. Chem. 2013, 59, 519–526. [Google Scholar] [CrossRef]
- Capano, A.; Weaver, R.; Burkman, E. Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: A prospective cohort study. Postgrad. Med. 2020, 132, 56–61. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2017, 2, e93760. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed]
- Shannon, S.; Opila-Lehman, J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: A case report. Perm. J. 2016, 20, 16-005. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Chu, R.-M.; Wang, C.-C.; Lee, C.-Y.; Lin, S.-H.; Jan, T.-R. Cannabidiol-induced apoptosis in primary lymphocytes is associated with oxidative stress-dependent activation of caspase-8. Toxicol. Appl. Pharmacol. 2008, 226, 260–270. [Google Scholar]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl jasmonate and salicylic acid as powerful elicitors for enhancing the production of secondary metabolites in medicinal plants: An updated review. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 153, 447–458. [Google Scholar] [CrossRef]
- Kumari, R.; Kotecha, M. A review on the standardization of herbal medicines. Int. J. Pharma Sci. Res. 2016, 7, 97–106. [Google Scholar]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef]
- Kandoudi, W.; Radácsi, P.; Gosztola, B.; Zámboriné Németh, É. Elicitation of medicinal plants in vivo—Is it a realistic tool? The effect of methyl jasmonate and salicylic acid on lamiaceae species. Horticulturae 2021, 8, 5. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Zhou, L.-G.; Wu, J.-Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol. 2010, 87, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Vasconsuelo, A.; Boland, R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Biotic elicitors effectively increase the glucosinolates content in Brassicaceae sprouts. J. Agric. Food Chem. 2014, 62, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, H.; Qi, Y.; Liu, H.; Zhang, X.; Ma, P.; Liang, Z.; Dong, J. Salicylic acid-induced cytosolic acidification increases the accumulation of phenolic acids in Salvia miltiorrhiza cells. Plant Cell Tissue Organ Culture (PCTOC) 2016, 126, 333–341. [Google Scholar] [CrossRef]
- Popova, L.; Ananieva, E.; Hristova, V.; Christov, K.; Georgieva, K.; Alexieva, V.; Stoinova, Z. Salicylic acid-and methyl jasmonate-induced protection on photosynthesis to paraquat oxidative stress. Bulg. J. Plant Physiol. 2003, 29, 133–152. [Google Scholar]
- Rao, M.V.; Lee, H.-i.; Creelman, R.A.; Mullet, J.E.; Davis, K.R. Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 2000, 12, 1633–1646. [Google Scholar] [CrossRef]
- Szabo, E.; Thelen, A.; Petersen, M. Fungal elicitor preparations and methyl jasmonate enhance rosmarinic acid accumulation in suspension cultures of Coleus blumei. Plant Cell Rep. 1999, 18, 485–489. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Vivanco, J.M. Jasmonic acid-induced hypericin production in cell suspension cultures of Hypericum perforatum L. (St. John’s wort). Phytochemistry 2002, 60, 289–293. [Google Scholar] [CrossRef]
- Yu, K.-W.; Gao, W.; Hahn, E.-J.; Paek, K.-Y. Jasmonic acid improves ginsenoside accumulation in adventitious root culture of Panax ginseng CA Meyer. Biochem. Eng. J. 2002, 11, 211–215. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic press: Cambridge, MA, USA, 2019. [Google Scholar]
- García-Tejero, I.F.; Hernández, A.; Ferreiro-Vera, C.; Zuazo, V.H.D.; García, J.H.; Sánchez-Carnerero, C.; Casano, S. Yield of new hemp varieties for medical purposes under semi-arid Mediterranean environment conditions. Comun. Sci. 2020, 11, e3264. [Google Scholar] [CrossRef]
- Stutte, G.W. Commercial transition to LEDs: A pathway to high-value products. HortScience 2015, 50, 1297–1300. [Google Scholar] [CrossRef]
- Hahm, S.; Lee, B.; Bok, G.; Kim, S.; Park, J. Diniconazole Promotes the Yield of Female Hemp (Cannabis sativa) Inflorescence and Cannabinoids in a Vertical Farming System. Agronomy 2023, 13, 1497. [Google Scholar] [CrossRef]
- Caplan, D.; Stemeroff, J.; Dixon, M.; Zheng, Y. Vegetative propagation of cannabis by stem cuttings: Effects of leaf number, cutting position, rooting hormone, and leaf tip removal. Can. J. Plant Sci. 2018, 98, 1126–1132. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Rokhzadi, A.; Amini, S.; Karami, E. Effect of salicylic acid and methyl jasmonate on growth and secondary metabolites in Cuminum cyminum L. J. Biodivers. Environ. Sci. 2013, 3, 140–149. [Google Scholar]
- Hesami, M.; Pepe, M.; Jones, A.M.P. Morphological characterization of Cannabis sativa L. throughout its complete life cycle. Plants 2023, 12, 3646. [Google Scholar] [CrossRef]
- Punja, Z.K.; Sutton, D.B.; Kim, T. Glandular trichome development, morphology, and maturation are influenced by plant age and genotype in high THC-containing cannabis (Cannabis sativa L.) inflorescences. J. Cannabis Res. 2023, 5, 12. [Google Scholar] [CrossRef]
- Stack, G.; Toth, J.; Carlson, C.; Cala, A.; Marrero-González, M.; Wilk, R.; Gentner, D.; Crawford, J.; Philippe, G.; Rose, J. Season-long characterization of high-cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance. GCB Bioenergy 2021, 13, 546–561. [Google Scholar] [CrossRef]
- AL Ubeed, H.M.S.; Wills, R.B.H.; Chandrapala, J. Post-Harvest Operations to Generate High-Quality Medicinal Cannabis Products: A Systemic Review. Molecules 2022, 27, 1719. [Google Scholar] [CrossRef]
- Hädener, M.; König, S.; Weinmann, W. Quantitative determination of CBD and THC and their acid precursors in confiscated cannabis samples by HPLC-DAD. Forensic Sci. Int. 2019, 299, 142–150. [Google Scholar] [CrossRef]
- Tura, M.; Mandrioli, M.; Valli, E.; Toschi, T.G. Quality indexes and composition of 13 commercial hemp seed oils. J. Food Compos. Anal. 2023, 117, 105112. [Google Scholar] [CrossRef]
- CAS 13956-29-1; Cannabidiol Solution. Accu Standard: New Haven, CN, USA, 2024.
- CAS 23978-85-0; Delta 9-Tetrahydrocannabinolic Acid A (THCA-A) 1000 µg/mL in Acetonitrile. LGC Standards Ltd.: Teddington, UK, 2024.
- CAS 1972-08-3; Delta-9-Tetrahydrocannabinol (THC). Accu Standard: New Haven, CN, USA, 2024.
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Traw, M.B.; Bergelson, J. Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol. 2003, 133, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Chen, W.; Xu, Z.; Chen, M.; Wang, H.; Yu, D. The emerging role of jasmonate in the control of flowering time. J. Exp. Bot. 2022, 73, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, P.; Menzies, N.W.; Lombi, E.; Kopittke, P.M. Effects of methyl jasmonate on plant growth and leaf properties. J. Plant Nutr. Soil Sci. 2018, 181, 409–418. [Google Scholar] [CrossRef]
- Heijari, J.; Nerg, A.M.; Kainulainen, P.; Viiri, H.; Vuorinen, M.; Holopainen, J.K. Application of methyl jasmonate reduces growth but increases chemical defence and resistance against Hylobius abietis in Scots pine seedlings. Entomol. Exp. Appl. 2005, 115, 117–124. [Google Scholar] [CrossRef]
- Gould, N.; Reglinski, T.; Northcott, G.L.; Spiers, M.; Taylor, J.T. Physiological and biochemical responses in Pinus radiata seedlings associated with methyl jasmonate-induced resistance to Diplodia pinea. Physiol. Mol. Plant Pathol. 2009, 74, 121–128. [Google Scholar] [CrossRef]
- Sampedro, L.; Moreira, X.; Zas, R. Resistance and response of Pinus pinaster seedlings to Hylobius abietis after induction with methyl jasmonate. Plant Ecol. 2011, 212, 397–401. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Li, Y.; Pan, J.; Lou, D.; Hu, Y.; Yu, D. The bHLH transcription factors MYC2, MYC3, and MYC4 are required for jasmonate-mediated inhibition of flowering in Arabidopsis. Mol. Plant 2017, 10, 1461–1464. [Google Scholar] [CrossRef]
- Diallo, A.O.; Agharbaoui, Z.; Badawi, M.A.; Ali-Benali, M.A.; Moheb, A.; Houde, M.; Sarhan, F. Transcriptome analysis of an mvp mutant reveals important changes in global gene expression and a role for methyl jasmonate in vernalization and flowering in wheat. J. Exp. Bot. 2014, 65, 2271–2286. [Google Scholar] [CrossRef]
- Albrechtova, J.; Ullmann, J. Methyl jasmonate inhibits growth and flowering in Chenopodium rubrum. Biol. Plant. 1994, 36, 317–319. [Google Scholar] [CrossRef]
- Beydler, B.; Osadchuk, K.; Cheng, C.-L.; Manak, J.R.; Irish, E.E. The juvenile phase of maize sees upregulation of stress-response genes and is extended by exogenous jasmonic acid. Plant Physiol. 2016, 171, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, M.; Cai, X.; Han, Z.; Si, J.; Chen, D. Jasmonate signaling pathway modulates plant defense, growth, and their trade-offs. Int. J. Mol. Sci. 2022, 23, 3945. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Zhang, X.; Wu, F.; Feng, H.; Deng, L.; Xu, L.; Zhang, M.; Wang, Q.; Li, C. Transcriptional mechanism of jasmonate receptor COI1-mediated delay of flowering time in Arabidopsis. Plant Cell 2015, 27, 2814–2828. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, G.; Tang, B.; Zhang, J.; Zhou, S.; Hu, Z. The Jasmonate ZIM-domain protein gene SlJAZ2 regulates plant morphology and accelerates flower initiation in Solanum lycopersicum plants. Plant Sci. 2018, 267, 65–73. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, N.; Wang, W.; Ahmed, S.; Cheng, Y.; Chen, S.; Wang, X.; Wang, Y.; Hu, X.; Wang, T. Involvement of ABA responsive SVB genes in the regulation of trichome formation in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 6790. [Google Scholar] [CrossRef]
- An, L.; Zhou, Z.; Yan, A.; Gan, Y. Progress on trichome development regulated by phytohormone signaling. Plant Signal. Behav. 2011, 6, 1959–1962. [Google Scholar] [CrossRef]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef]
- Rodziewicz, P.; Loroch, S.; Marczak, Ł.; Sickmann, A.; Kayser, O. Cannabinoid synthases and osmoprotective metabolites accumulate in the exudates of Cannabis sativa L. glandular trichomes. Plant Sci. 2019, 284, 108–116. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J. Chem. Ecol. 2005, 31, 2211–2216. [Google Scholar]
- Tian, D.; Tooker, J.; Peiffer, M.; Chung, S.H.; Felton, G.W. Role of trichomes in defense against herbivores: Comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 2012, 236, 1053–1066. [Google Scholar]
- Hua, B.; Chang, J.; Xu, Z.; Han, X.; Xu, M.; Yang, M.; Yang, C.; Ye, Z.; Wu, S. HOMEODOMAIN PROTEIN8 mediates jasmonate-triggered trichome elongation in tomato. New Phytol. 2021, 230, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-C.; Hu, Q.-Q.; Li, W.; Chen, Y.; Han, L.-H.; Tao, M.; Wu, W.-Y.; Li, X.-B.; Huang, G.-Q. Cotton (Gossypium hirsutum) JAZ3 and SLR1 function in jasmonate and gibberellin mediated epidermal cell differentiation and elongation. Plant Cell Tissue Organ Culture (PCTOC) 2018, 133, 249–262. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, P.; Cai, S.; Haughn, G.; Page, J.E. Three novel transcription factors involved in cannabinoid biosynthesis in Cannabis sativa L. Plant Mol. Biol. 2021, 106, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Kwon, C.-T.; Heo, J.; Lemmon, Z.H.; Capua, Y.; Hutton, S.F.; Van Eck, J.; Park, S.J.; Lippman, Z.B. Rapid customization of Solanaceae fruit crops for urban agriculture. Nat. Biotechnol. 2020, 38, 182–188. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Touliatos, D.; Dodd, I.C.; McAinsh, M. Vertical farming increases lettuce yield per unit area compared to conventional horizontal hydroponics. Food Energy Secur. 2016, 5, 184–191. [Google Scholar] [CrossRef]
- Beacham, A.M.; Vickers, L.H.; Monaghan, J.M. Vertical farming: A summary of approaches to growing skywards. J. Hortic. Sci. Biotechnol. 2019, 94, 277–283. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahm, S.; Lee, Y.; Lee, K.; Park, J. Optimization of Cannabinoid Production in Hemp Through Methyl Jasmonate Application in a Vertical Farming System. Horticulturae 2024, 10, 1165. https://doi.org/10.3390/horticulturae10111165
Hahm S, Lee Y, Lee K, Park J. Optimization of Cannabinoid Production in Hemp Through Methyl Jasmonate Application in a Vertical Farming System. Horticulturae. 2024; 10(11):1165. https://doi.org/10.3390/horticulturae10111165
Chicago/Turabian StyleHahm, Seungyong, Yongjae Lee, Kwangya Lee, and Jongseok Park. 2024. "Optimization of Cannabinoid Production in Hemp Through Methyl Jasmonate Application in a Vertical Farming System" Horticulturae 10, no. 11: 1165. https://doi.org/10.3390/horticulturae10111165
APA StyleHahm, S., Lee, Y., Lee, K., & Park, J. (2024). Optimization of Cannabinoid Production in Hemp Through Methyl Jasmonate Application in a Vertical Farming System. Horticulturae, 10(11), 1165. https://doi.org/10.3390/horticulturae10111165





