Daily Light Integral and Far-Red Radiation Influence Morphology and Quality of Liners and Subsequent Flowering and Development of Petunia in Controlled Greenhouses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Propagation Stage
2.2. Radiation Treatments and Liner Stage
2.3. Adult Plant/Finish Stage
2.4. Experimental Design and Statistical Analysis
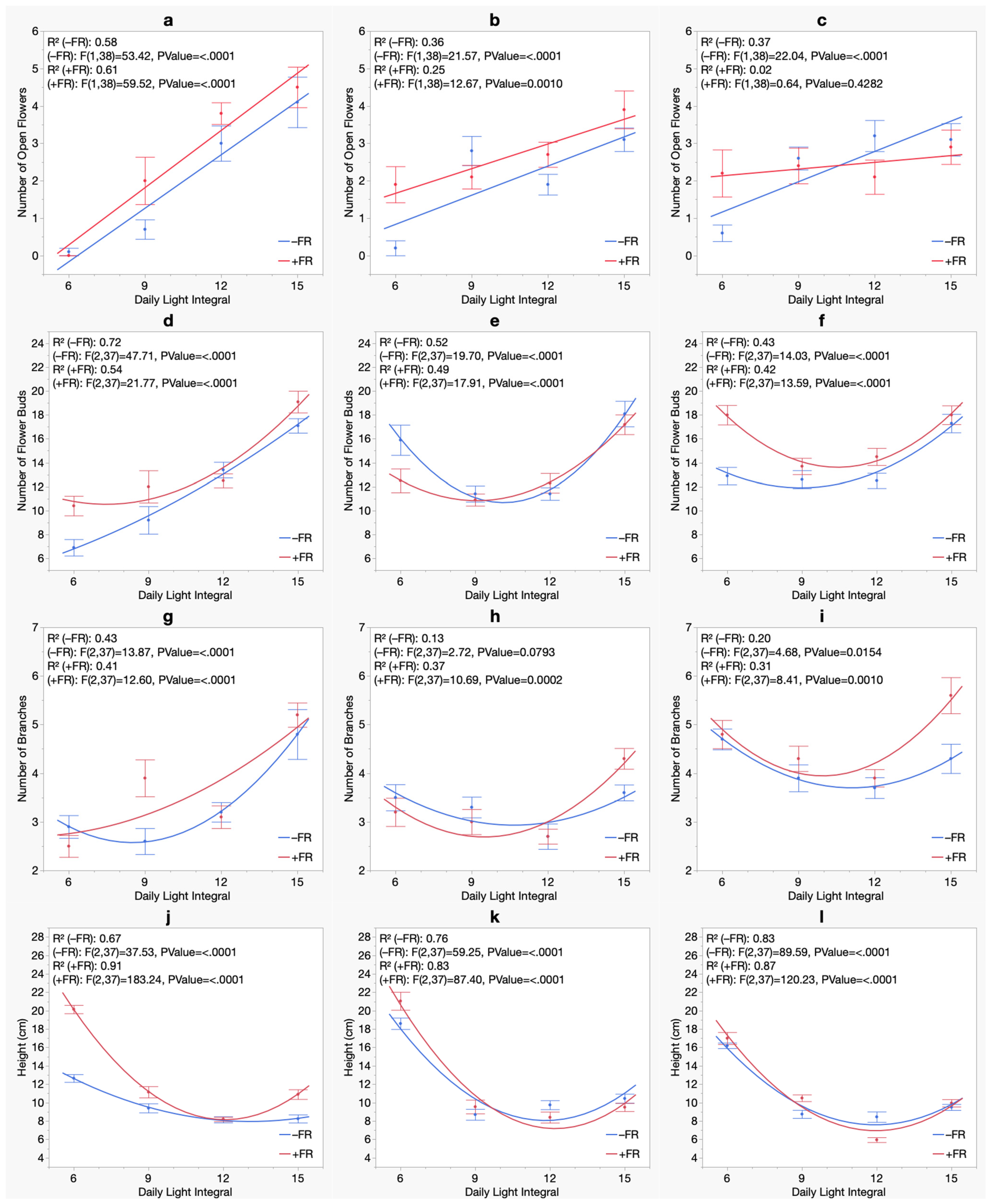
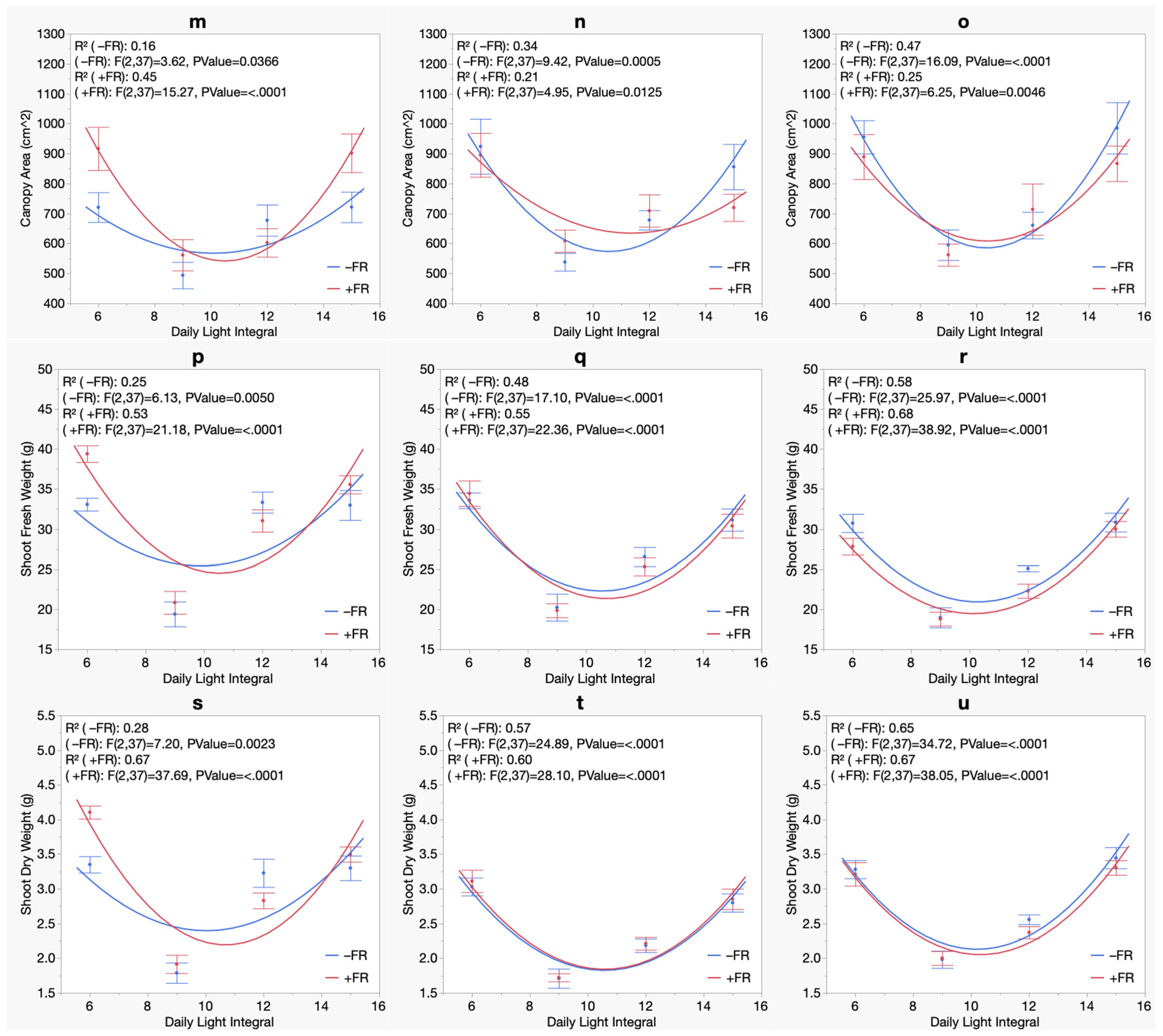
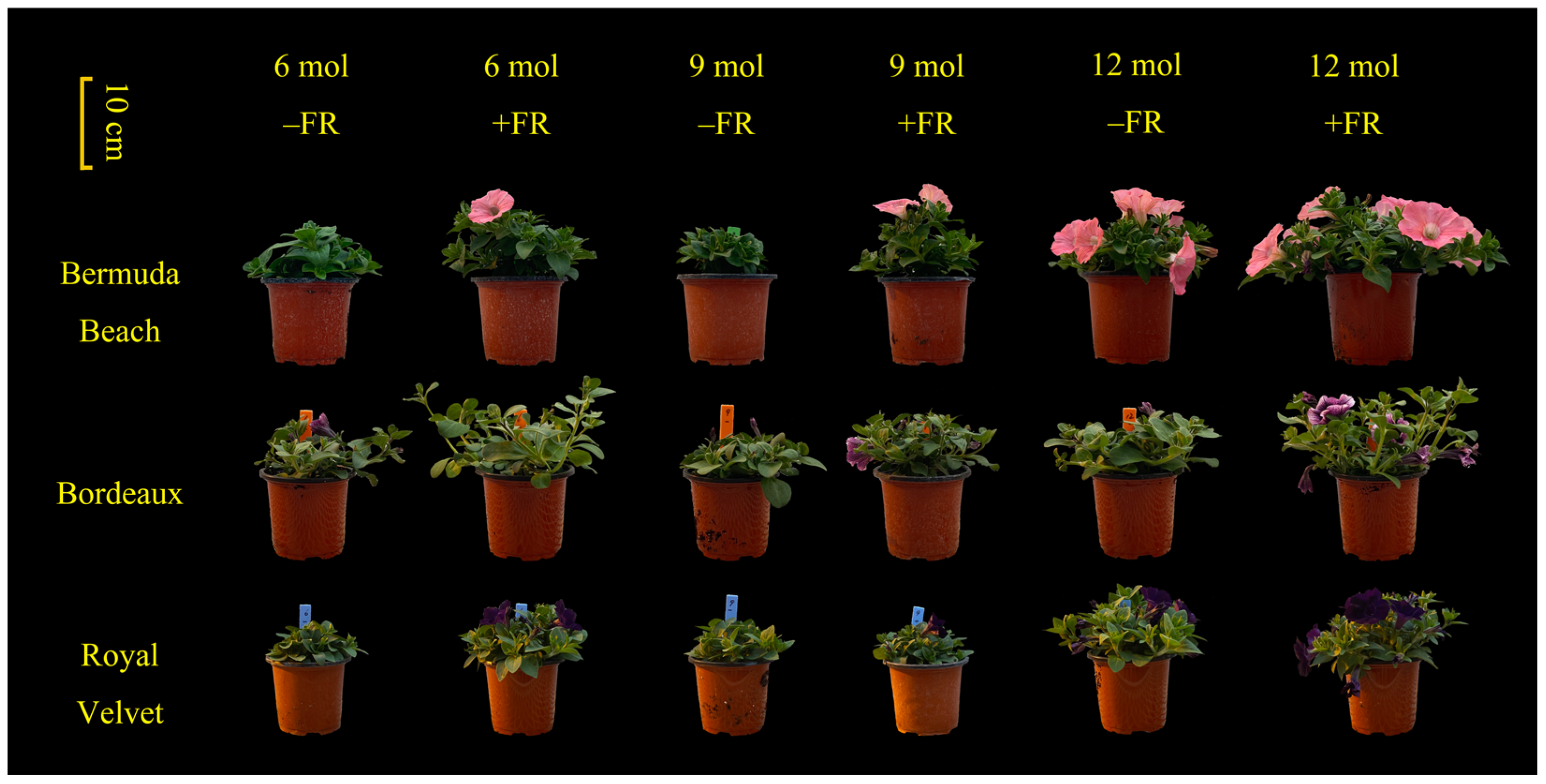
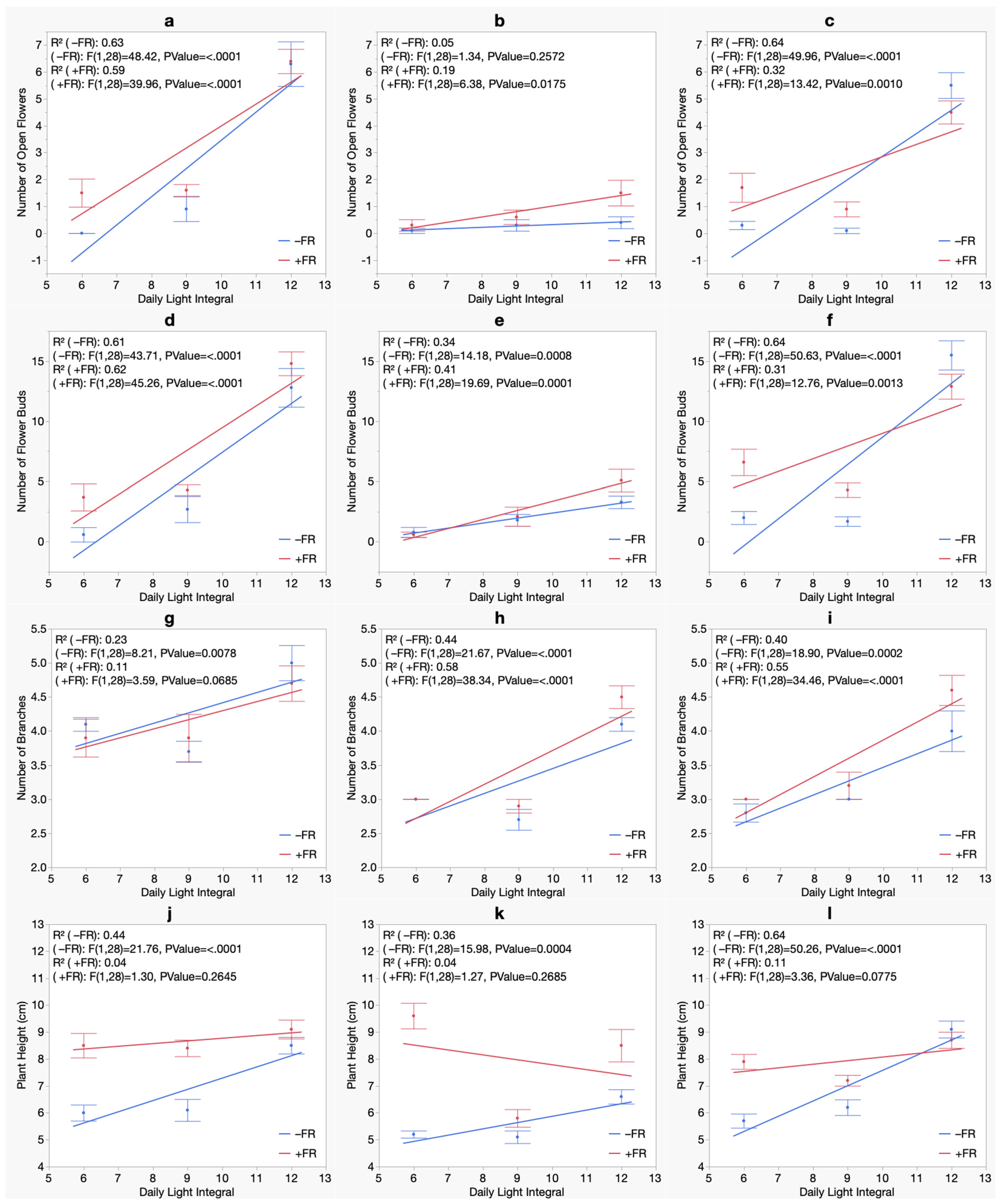
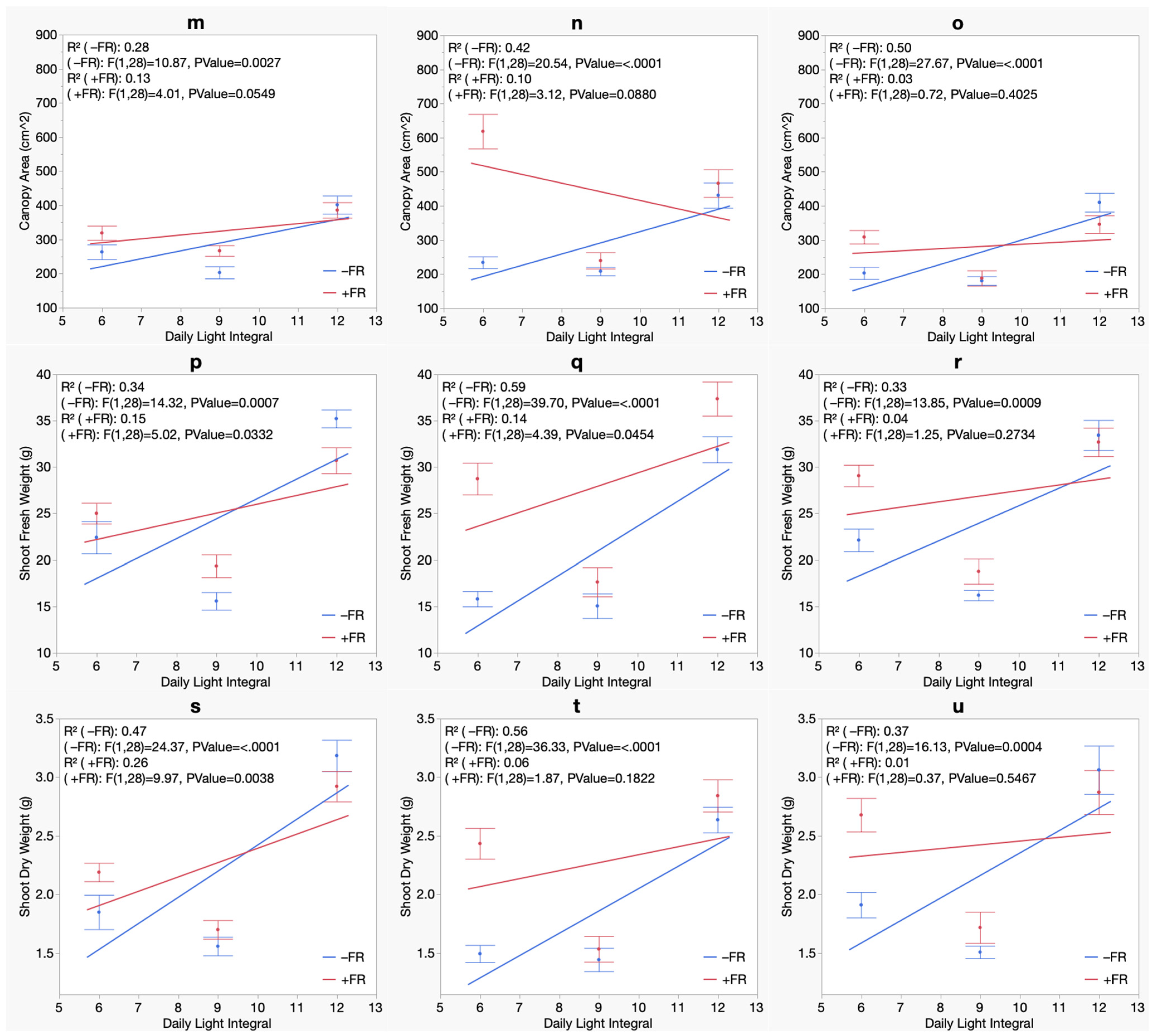
3. Results
3.1. Flowering
3.2. Branching
3.3. Morphology
3.4. Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Weather Scenario | Treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| 6− | 6+ | 9− | 9+ | 12− | 12+ | 15− | 15+ | |
| Experiment 1 | ||||||||
| Sunny/−Shade/−HPS | 1.0/0.9/1.0 | 1.1/0.8/0.8 | 1.3/1.3/1.3 | 1.2/1.1/1.1 | 1.3/1.3/1.2 | 1.2/1.0/1.0 | 1.3/1.3/1.3 | 1.2/1.1/1.0 |
| Sunny/+Shade/−HPS | 1.2/1.2/1.2 | 0.7/0.4/0.3 | 1.2/1.2/1.2 | 0.9/0.6/0.6 | 1.2/1.2/1.2 | 0.9/0.6/0.5 | 1.2/1.2/1.2 | 0.9/0.6/0.6 |
| Cloudy/−HPS | 1.1/1.3/1.3 | 0.4/0.1/0.1 | 1.1/1.2/1.2 | 0.5/0.2/0.2 | 1.1/1.2/1.2 | 0.5/0.3/0.2 | 1.1/1.1/1.1 | 0.5/0.3/0.3 |
| Cloudy/+HPS | 3.2/2.9/2.4 | 1.0/0.3/0.3 | 2.9/2.6/2.2 | 1.5/0.7/0.5 | 2.4/2.2/1.9 | 1.6/0.9/0.7 | 2.6/2.3/2.0 | 1.5/0.9/0.7 |
| Night/−HPS | N/A | 0.1/0.0/0.0 | N/A | 0.1/0.0/0.0 | N/A | 0.1/0.0/0.0 | N/A | 0.1/0.0/0.0 |
| Night/+HPS | 4.6/3.7/2.9 | 0.9/0.3/0.2 | 5.1/4.1/3.2 | 1.6/0.6/0.4 | 5.0/3.9/3.1 | 1.9/0.7/0.5 | 4.5/3.3/2.6 | 1.8/0.8/0.6 |
| Experiment 2 | ||||||||
| Sunny/−Shade/−HPS | 1.5/1.3/1.3 | 0.6/0.2/0.2 | 1.6/1.4/1.5 | 0.8/0.4/0.4 | 1.4/1.2/1.2 | 0.9/0.5/0.5 | 1.5/1.4/1.4 | 0.9/0.5/0.5 |
| Sunny/+Shade/−HPS | 2.4/2.0/1.7 | 1.3/0.7/0.6 | 2.5/2.1/1.9 | 1.5/0.7/0.6 | 1.8/1.6/1.5 | 1.3/0.8/0.8 | 3.5/2.9/2.4 | 1.5/0.7/0.6 |
| Cloudy/−HPS | 1.8/1.3/1.1 | 0.2/0.0/0.0 | 2.6/2.1/2.7 | 0.3/0.1/0.1 | 1.7/1.4/1.3 | 0.4/0.1/0.1 | 2.6/2.3/2.0 | 0.4/0.1/0.1 |
| Cloudy/+HPS | 4.2/3.4/2.7 | 1.3/0.5/0.4 | 4.5/3.6/3.1 | 1.7/0.7/0.5 | 3.9/3.3/2.6 | 1.8/0.8/0.6 | 4.4/3.6/2.8 | 1.5/0.6/0.4 |
| Night/−HPS | N/A | 0.1/0.0/0.0 | N/A | 0.1/0.0/0.0 | N/A | 0.1/0.0/0.0 | N/A | 0.1/0.0/0.0 |
| Night/+HPS | 4.6/3.7/2.9 | 0.9/0.3/0.2 | 5.1/4.1/3.2 | 1.6/0.6/0.4 | 5.0/3.9/3.1 | 1.9/0.7/0.5 | 4.5/3.3/2.6 | 1.8/0.8/0.6 |
References
- U.S. Department of Agriculture, National Agricultural Statistics Service. 2019 Census of Horticultural Specialties; United States Department of Agriculture: Washington, DC, USA, 2019. [Google Scholar]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T., Jr.; Geneve, R.L. Plant Propagation: Principles and Practices, 8th ed.; Pearson: London, UK, 2017. [Google Scholar]
- Kelly, R.O.; Deng, Z.; Harbaugh, B.K. Evaluation of 125 petunia cultivars as bedding plants and establishment of class standards. Horttechnology 2007, 17, 386–396. [Google Scholar] [CrossRef]
- Adams, S.; Hadley, P.; Pearson, S. The effects of temperature, photoperiod, and photosynthetic photon flux on the time to flowering of petunia ‘Express Blush Pink’. J. Am. Soc. Hortic. Sci. 1998, 123, 577–580. [Google Scholar] [CrossRef]
- Blanchard, M.G.; Runkle, E.S.; Fisher, P.R. Modeling plant morphology and development of petunia in response to temperature and photosynthetic daily light integral. Sci. Hortic. 2011, 129, 313–320. [Google Scholar] [CrossRef]
- Kaczperski, M.; Carlson, W.; Karlsson, M. Growth and development of Petunia × hybrida as a function of temperature and irradiance. J. Am. Soc. Hortic. Sci. 1991, 116, 232–237. [Google Scholar] [CrossRef]
- Kang, J.-G.; van Iersel, M.W. Interactions between temperature and fertilizer concentration affect growth of subirrigated petunias. J. Plant Nutr. 2001, 24, 753–765. [Google Scholar] [CrossRef]
- Shvarts, M.; Weiss, D.; Borochov, A. Temperature effects on growth, pigmentation and post-harvest longevity of petunia flowers. Sci. Hortic. 1997, 69, 217–227. [Google Scholar] [CrossRef]
- Warner, R.M. Temperature and photoperiod influence flowering and morphology of four Petunia spp. HortScience 2010, 45, 365–368. [Google Scholar] [CrossRef]
- Erwin, J.; Switras-Meyer, S.; Bennette, K.; Hadley, C.; Harris, D.; Peterson, A.; Schuelke, H. Photoperiodic responses of annual bedding plants (cont.). Minn. Commer. Flower Grow. Bull. 2000, 49, 4–9. [Google Scholar]
- Jackson, S.D. Plant responses to photoperiod. New Phytol. 2009, 181, 517–531. [Google Scholar] [CrossRef]
- Jalal-Ud-Din, B.; Khan, M.Q.; Zubair, M.; Munir, M. Effects of different photoperiods on flowering time of facultative long day ornamental annuals. Int. J. Agric. Biol. 2009, 11, 251–256. [Google Scholar]
- Plringer, A.; Cathey, H. Effect of photoperiod, kind of supplemental light and temperature on the growth and flowering of petunia plants. J. Am. Soc. Hortic. Sci. 1960, 76, 649–660. [Google Scholar]
- Reekie, J.; Hicklenton, P.; Reekie, E. The interactive effects of carbon dioxide enrichment and daylength on growth and development in Petunia hybrida. Ann. Bot. 1997, 80, 57–64. [Google Scholar] [CrossRef]
- Albright, L.; Both, A.-J.; Chiu, A. Controlling greenhouse light to a consistent daily integral. Trans. ASAE 2000, 43, 421. [Google Scholar] [CrossRef]
- Erwin, J.; Warner, R. Determination of photoperiodic response group and effect of supplemental irradiance on flowering of several bedding plant species. Acta Hortic. 2000, 580, 95–99. [Google Scholar] [CrossRef]
- Mattson, N.S.; Erwin, J.E. The impact of photoperiod and irradiance on flowering of several herbaceous ornamentals. Sci. Hortic. 2005, 104, 275–292. [Google Scholar] [CrossRef]
- Lopez, R.G.; Runkle, E.S. Photosynthetic daily light integral during propagation influences rooting and growth of cuttings and subsequent development of New Guinea impatiens and petunia. HortScience 2008, 43, 2052–2059. [Google Scholar] [CrossRef]
- Currey, C.J.; Lopez, R.G. Biomass accumulation and allocation, photosynthesis, and carbohydrate status of New Guinea impatiens, geranium, and petunia cuttings are affected by photosynthetic daily light integral during root development. J. Am. Soc. Hortic. Sci. 2015, 140, 542–549. [Google Scholar] [CrossRef]
- Faust, J.E.; Holcombe, V.; Rajapakse, N.C.; Layne, D.R. The effect of daily light integral on bedding plant growth and flowering. HortScience 2005, 40, 645–649. [Google Scholar] [CrossRef]
- Emerson, R.; Lewis, C.M. The dependence of the quantum yield of Chlorella photosynthesis on wavelength of light. Am. J. Bot. 1943, 30, 165–178. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; Van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic quantum yield dynamics: From photosystems to leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef]
- Inada, K. Action spectra for photosynthesis in higher plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agro Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Haidekker, M.; van Iersel, M.W. Far-red light enhances photochemical efficiency in a wavelength-dependent manner. Physiol. Plant. 2019, 167, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R.; Chalmers, R.; Cederstrand, C. Some factors influencing the long-wave limit of photosynthesis. Proc. Natl. Acad. Sci. USA 1957, 43, 133–143. [Google Scholar] [CrossRef]
- Gobets, B.; van Grondelle, R. Energy transfer and trapping in photosystem I. Biochim. Biophys. Acta Bioenerg. 2001, 1507, 80–99. [Google Scholar] [CrossRef]
- Pettai, H.; Oja, V.; Freiberg, A.; Laisk, A. Photosynthetic activity of far-red light in green plants. Biochim. Biophys. Acta Bioenerg. 2005, 1708, 311–321. [Google Scholar] [CrossRef]
- Lee, M.; Xu, J.; Wang, W.; Rajashekar, C. The effect of supplemental blue, red and far-red light on the growth and the nutritional quality of red and green leaf lettuce. Am. J. Plant Sci. 2019, 10, 2219–2235. [Google Scholar] [CrossRef]
- Lee, M.-J.; Son, K.-H.; Oh, M.-M. Increase in biomass and bioactive compounds in lettuce under various ratios of red to far-red LED light supplemented with blue LED light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Jiang, H.; He, R.; Song, S.; Su, W.; Liu, H. Supplementary far-red and blue lights influence the biomass and phytochemical profiles of two lettuce cultivars in plant factory. Molecules 2021, 26, 7405. [Google Scholar] [CrossRef]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F. Adding far-red to red-blue light-emitting diode light promotes yield of lettuce at different planting densities. Front. Plant Sci. 2021, 11, 609977. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Bachman, G.R.; McMahon, M.J. Day and night temperature differential (DIF) or the absence of far-red light alters cell elongation in ‘Celebrity White’ petunia. J. Am. Soc. Hortic. Sci. 2006, 131, 309–312. [Google Scholar] [CrossRef]
- Chen, X.-L.; Xue, X.-Z.; Guo, W.-Z.; Wang, L.-C.; Qiao, X.-J. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Kong, Y.; Nemali, K. Blue and far-red light affect area and number of individual leaves to influence vegetative growth and pigment synthesis in lettuce. Front. Plant Sci. 2021, 12, 667407. [Google Scholar] [CrossRef]
- Zhang, M.; Park, Y.; Runkle, E.S. Regulation of extension growth and flowering of seedlings by blue radiation and the red to far-red ratio of sole-source lighting. Sci. Hortic. 2020, 272, 109478. [Google Scholar] [CrossRef]
- Kubota, C.; Chia, P.; Yang, Z.; Li, Q. Applications of far-red light emitting diodes in plant production under controlled environments. Acta Hortic. 2011, 952, 59–66. [Google Scholar] [CrossRef]
- Legendre, R.; van Iersel, M.W. Supplemental far-red light stimulates lettuce growth: Disentangling morphological and physiological effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Gommers, C.M.; Visser, E.J.; St Onge, K.R.; Voesenek, L.A.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef]
- Henry, H.A.; Aarssen, L.W. On the relationship between shade tolerance and shade avoidance strategies in woodland plants. Oikos 1997, 80, 575–582. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Xie, X.; Cheng, H.; Hou, C.; Ren, M. Integration of light and auxin signaling in shade plants: From mechanisms to opportunities in urban agriculture. Int. J. Mol. Sci. 2022, 23, 3422. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Mah, J.J.; Llewellyn, D.; Zheng, Y. Morphology and flowering responses of four bedding plant species to a range of red to far red ratios. HortScience 2018, 53, 472–478. [Google Scholar] [CrossRef]
- Kohler, A.E.; Lopez, R.G. Duration of light-emitting diode (LED) supplemental lighting providing far-red radiation during seedling production influences subsequent time to flower of long-day annuals. Sci. Hortic. 2021, 281, 109956. [Google Scholar] [CrossRef]
- Kim, H.; Heins, R.; Carlson, W. Development and flowering of petunia grown in a far-red deficient light environment. Acta Hortic. 2000, 580, 127–135. [Google Scholar] [CrossRef]
- Fletcher, J.; Tatsiopoulou, A.; Mpezamihigo, M.; Carew, J.; Henbest, R.; Hadley, P. Far-red light filtering by plastic film, greenhouse-cladding materials: Effects on growth and flowering in Petunia and Impatiens. J. Hortic. Sci. Biotechnol. 2005, 80, 303–306. [Google Scholar] [CrossRef]
- Meng, Q. White LEDs: A Cost-Effective Alternative to Red + Far-Red LEDs. American Floral Endowment. Available online: https://endowment.org/white-leds-a-cost-effective-alternative-to-red-far-red-leds/ (accessed on 1 February 2024).
- Park, Y.; Runkle, E.S. Far-red radiation and photosynthetic photon flux density independently regulate seedling growth but interactively regulate flowering. Environ. Exp. Bot. 2018, 155, 206–216. [Google Scholar] [CrossRef]
- Gautam, P.; Terfa, M.T.; Olsen, J.E.; Torre, S. Red and blue light effects on morphology and flowering of Petunia × hybrida. Sci. Hortic. 2015, 184, 171–178. [Google Scholar] [CrossRef]
- Kong, Y.; Stasiak, M.; Dixon, M.A.; Zheng, Y. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: A comparison with red light in four bedding plant species. Environ. Exp. Bot. 2018, 155, 345–359. [Google Scholar] [CrossRef]
- Owen, W.G.; Meng, Q.; Lopez, R.G. Promotion of flowering from far-red radiation depends on the photosynthetic daily light integral. HortScience 2018, 53, 465–471. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W.; Bugbee, B. Photosynthesis in sun and shade: The surprising importance of far-red photons. New Phytol. 2022, 236, 538–546. [Google Scholar] [CrossRef]
- Ciolkosz, D.E.; Both, A.J.; Albright, L.D. Selection and placement of greenhouse luminaires for uniformity. Appl. Eng. Agric. 2001, 17, 875. [Google Scholar] [CrossRef]
- Kusuma, P.; Bugbee, B. Far-red fraction: An improved metric for characterizing phytochrome effects on morphology. J. Am. Soc. Hortic. Sci. 2021, 146, 3–13. [Google Scholar] [CrossRef]
- Smith, H. Light quality, photoperception, and plant strategy. Annu. Rev. Plant Physiol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Haliapas, S.; Yupsanis, T.A.; Syros, T.D.; Kofidis, G.; Economou, A.S. Petunia × hybrida during transition to flowering as affected by light intensity and quality treatments. Acta Physiol. Plant. 2008, 30, 807–815. [Google Scholar] [CrossRef]
- Armitage, A.; Wetzstein, H.Y. Influence of light intensity on flower initiation and differentiation in hybrid geranium. HortScience 1984, 19, 114–116. [Google Scholar] [CrossRef]
- Cockshull, K.; Hughes, A. The effects of light intensity at different stages in flower initiation and development of Chrysanthemum morifolium. Ann. Bot. 1971, 35, 915–926. [Google Scholar] [CrossRef]
- Friend, D.; Fisher, J.; Helson, V. The effect of light intensity and temperature on floral initiation and inflorescence development of Marquis wheat. Can. J. Bot. 1963, 41, 1663–1674. [Google Scholar] [CrossRef]
- Seddigh, M.; Jolliff, G.D. Light intensity effects on meadowfoam growth and flowering. Crop Sci. 1994, 34, 497–503. [Google Scholar] [CrossRef]
- Casal, J.J. Shade avoidance. Arab. Book 2012, 10, e0157. [Google Scholar] [CrossRef] [PubMed]
- De Wit, M.; Galvao, V.C.; Fankhauser, C. Light-mediated hormonal regulation of plant growth and development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Sessa, G.; Carabelli, M.; Possenti, M.; Morelli, G.; Ruberti, I. Multiple pathways in the control of the shade avoidance response. Plants 2018, 7, 102. [Google Scholar] [CrossRef]
- Yang, C.; Li, L. Hormonal regulation in shade avoidance. Front. Plant Sci. 2017, 8, 1527. [Google Scholar] [CrossRef]
- Soster, A.J.; Lopez, R.G. A Single Substrate Drench of Paclobutrazol Can Prevent Undesired Extension Growth of Potted Succulents under Low Indoor Radiation Intensities. Influence of Light Quantity and Duration, and Temperature on Growth and Development of Succulents. Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2021; pp. 64–95. [Google Scholar]
- Collado, C.E.; Whipker, B.E.; Hernández, R. Morphology and growth of ornamental seedlings grown under supplemental light-emitting diode lighting and chemical plant-growth regulators. Acta Hortic. 2000, 1227, 517–524. [Google Scholar] [CrossRef]
- Oh, W.; Kim, J.; Kim, Y.H.; Lee, I.J.; Kim, K.S. Shoot elongation and gibberellin contents in Cyclamen persicum are influenced by temperature and light intensity. Hortic. Environ. Biotechnol. 2015, 56, 762–768. [Google Scholar] [CrossRef]
- Niu, G.; Heins, R.D.; Cameron, A.C.; Carlson, W.H. Day and night temperatures, daily light integral, and CO2 enrichment affect growth and flower development of Campanula carpatica ‘Blue Clips’. Sci. Hortic. 2001, 87, 93–105. [Google Scholar] [CrossRef]
- Moccaldi, L.A.; Runkle, E.S. Modeling the effects of temperature and photosynthetic daily light integral on growth and flowering of Salvia splendens and Tagetes patula. J. Am. Soc. Hortic. Sci. 2007, 132, 283–288. [Google Scholar] [CrossRef]
- Hoang, Q.T.; Han, Y.-J.; Kim, J.-I. Plant phytochromes and their phosphorylation. Int. J. Mol. Sci. 2019, 20, 3450. [Google Scholar] [CrossRef] [PubMed]
- Eylands, N.J. Anatomical, Physiological, and Photomorphogenic Responses of Lettuce and Basil to Far-Red Radiation under Sole-Source Lighting. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, May 2023. [Google Scholar]







| Weather Scenario | Treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| 6− | 6+ | 9− | 9+ | 12− | 12+ | 15− | 15+ | |
| Experiment 1 | ||||||||
| Sunny/−Shade/−HPS | 16.6% | 17.4% | 12.8% | 14.0% | 13.4% | 15.1% | 13.2% | 15.0% |
| Sunny/+Shade/−HPS | 14.2% | 30.9% | 13.8% | 20.7% | 13.8% | 21.9% | 14.0% | 20.7% |
| Cloudy/−HPS | 13.0% | 45.3% | 13.6% | 38.5% | 12.6% | 33.5% | 13.7% | 34.0% |
| Cloudy/+HPS | 7.2% | 31.7% | 7.6% | 19.5% | 8.3% | 15.7% | 8.4% | 16.1% |
| Night/−HPS | 0.0% | 87.8% | 0.0% | 89.7% | 0.0% | 88.0% | 0.0% | 92.4% |
| Night/+HPS | 5.8% | 25.8% | 5.1% | 21.2% | 5.1% | 18.6% | 6.1% | 18.2% |
| Experiment 2 | ||||||||
| Sunny/−HPS | 10.7% | 32.7% | 12.0% | 28.3% | 12.1% | 23.0% | 12.1% | 22.6% |
| Sunny/+HPS | 9.0% | 19.1% | 9.0% | 18.5% | 10.9% | 15.8% | 6.5% | 17.9% |
| Cloudy/−HPS | 11.7% | 67.8% | 10.3% | 58.4% | 10.9% | 49.4% | 8.2% | 55.0% |
| Cloudy/+HPS | 6.0% | 25.3% | 5.7% | 19.7% | 6.4% | 18.0% | 5.5% | 21.8% |
| Night/−HPS | 0.0% | 87.8% | 0.0% | 89.7% | 0.0% | 88.0% | 0.0% | 92.4% |
| Night/+HPS | 5.8% | 25.8% | 5.1% | 21.2% | 5.1% | 18.6% | 6.1% | 18.2% |
| Summer Crop Cycle (Experiment 1) | Winter Crop Cycle (Experiment 2) | |||||
|---|---|---|---|---|---|---|
| Bermuda Beach | Bordeaux | Royal Velvet | Bermuda Beach | Bordeaux | Royal Velvet | |
| Number of Open Flowers | ||||||
| Model | Linear | Linear | Linear | Linear | Linear | Linear |
| DLI | *** | *** | *** | N/A | N/A | N/A |
| DLI2 | N/A | N/A | N/A | N/A | N/A | N/A |
| FR | NS | * | NS | N/A | N/A | N/A |
| DLI × FR | NS | NS | * | N/A | N/A | N/A |
| Number of Flower Buds | ||||||
| Model | Quadratic | Quadratic | Quadratic | Linear | Linear | Linear |
| DLI | *** | *** | ** | N/A | N/A | N/A |
| DLI2 | * | *** | *** | N/A | N/A | N/A |
| FR | ** | NS | *** | N/A | N/A | N/A |
| DLI × FR | NS | NS | * | N/A | N/A | N/A |
| Number of Branches | ||||||
| Model | Quadratic | Quadratic | Quadratic | Linear | Linear | Linear |
| DLI | *** | NS | NS | *** | *** | *** |
| DLI2 | ** | *** | *** | N/A | N/A | N/A |
| FR | NS | NS | * | NS | NS | NS |
| DLI × FR | NS | * | NS | *** | *** | NS |
| Plant Height | ||||||
| Model | Quadratic | Quadratic | Quadratic | Linear | Linear | Linear |
| DLI | *** | *** | *** | *** | NS | *** |
| DLI2 | *** | *** | *** | N/A | N/A | N/A |
| FR | *** | NS | NS | *** | NS | NS |
| DLI × FR | *** | ** | NS | ** | *** | NS |
| Shoot Fresh Weight | ||||||
| Model | Quadratic | Quadratic | Quadratic | Linear | Linear | Linear |
| DLI | *** | *** | *** | *** | *** | *** |
| DLI2 | *** | *** | *** | N/A | N/A | N/A |
| FR | NS | NS | NS | NS | NS | NS |
| DLI × FR | NS | NS | NS | NS | *** | NS |
| Shoot Dry Weight | ||||||
| Model | Quadratic | Quadratic | Quadratic | Linear | Linear | Linear |
| DLI | *** | *** | *** | *** | *** | *** |
| DLI2 | *** | *** | *** | N/A | N/A | N/A |
| FR | NS | *** | *** | NS | NS | NS |
| DLI × FR | NS | NS | NS | NS | *** | NS |
| Root Fresh Weight | ||||||
| Model | Linear | Linear | Quadratic | Linear | Linear | Linear |
| DLI | *** | * | * | *** | *** | *** |
| DLI2 | N/A | N/A | *** | N/A | N/A | N/A |
| FR | * | NS | NS | NS | ** | NS |
| DLI × FR | NS | NS | NS | NS | NS | NS |
| Root Dry Weight | ||||||
| Model | Linear | Linear | Quadratic | Linear | Linear | Linear |
| DLI | *** | *** | *** | *** | ** | ** |
| DLI2 | N/A | N/A | *** | N/A | N/A | N/A |
| FR | NS | *** | NS | NS | *** | NS |
| DLI × FR | NS | NS | NS | NS | NS | NS |
| Summer Crop Cycle (Experiment 1) | Winter Crop Cycle (Experiment 2) | |||||
|---|---|---|---|---|---|---|
| Bermuda Beach | Bordeaux | Royal Velvet | Bermuda Beach | Bordeaux | Royal Velvet | |
| Number of Open Flowers | ||||||
| Model | Quadratic | Quadratic | Quadratic | Linear | Linear | Linear |
| DLI | NS | *** | ** | *** | ** | *** |
| DLI2 | *** | *** | *** | N/A | N/A | N/A |
| FR | NS | NS | NS | NS | * | NS |
| DLI × FR | NS | NS | NS | NS | NS | * |
| Number of Flower Buds | ||||||
| Model | Quadratic | Quadratic | Quadratic | Linear | Linear | Linear |
| DLI | NS | ** | *** | *** | *** | *** |
| DLI2 | *** | *** | *** | N/A | N/A | N/A |
| FR | NS | NS | NS | * | NS | NS |
| DLI × FR | NS | NS | NS | NS | NS | ** |
| Number of Branches | ||||||
| Model | Linear | Linear | Quadratic | Linear | Linear | Linear |
| DLI | ** | *** | *** | ** | *** | *** |
| DLI2 | N/A | N/A | *** | N/A | N/A | N/A |
| FR | NS | NS | NS | NS | NS | * |
| DLI × FR | NS | NS | NS | NS | NS | NS |
| Plant Height | ||||||
| Model | Quadratic | Quadratic | Quadratic | Linear | Linear | Linear |
| DLI | *** | *** | *** | *** | NS | *** |
| DLI2 | *** | *** | *** | N/A | N/A | N/A |
| FR | NS | NS | NS | *** | *** | *** |
| DLI × FR | NS | NS | NS | NS | * | *** |
| Canopy Area | ||||||
| Model | Quadratic | Quadratic | Quadratic | Linear | Linear | Linear |
| DLI | NS | NS | NS | *** | NS | *** |
| DLI2 | *** | *** | *** | N/A | N/A | N/A |
| FR | * | NS | NS | NS | *** | NS |
| DLI × FR | NS | NS | NS | NS | *** | ** |
| Shoot Fresh Weight | ||||||
| Model | Quadratic | Quadratic | Quadratic | Linear | Linear | Linear |
| DLI | NS | NS | * | *** | *** | ** |
| DLI2 | *** | *** | *** | N/A | N/A | N/A |
| FR | NS | NS | * | NS | *** | NS |
| DLI × FR | NS | NS | NS | NS | NS | NS |
| Shoot Dry Weight | ||||||
| Model | Quadratic | Quadratic | Quadratic | Linear | Linear | Linear |
| DLI | NS | NS | * | *** | *** | ** |
| DLI2 | *** | *** | *** | N/A | N/A | N/A |
| FR | NS | NS | NS | NS | ** | NS |
| DLI × FR | NS | NS | NS | NS | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Mattson, N. Daily Light Integral and Far-Red Radiation Influence Morphology and Quality of Liners and Subsequent Flowering and Development of Petunia in Controlled Greenhouses. Horticulturae 2024, 10, 1106. https://doi.org/10.3390/horticulturae10101106
Xia J, Mattson N. Daily Light Integral and Far-Red Radiation Influence Morphology and Quality of Liners and Subsequent Flowering and Development of Petunia in Controlled Greenhouses. Horticulturae. 2024; 10(10):1106. https://doi.org/10.3390/horticulturae10101106
Chicago/Turabian StyleXia, Jiaqi, and Neil Mattson. 2024. "Daily Light Integral and Far-Red Radiation Influence Morphology and Quality of Liners and Subsequent Flowering and Development of Petunia in Controlled Greenhouses" Horticulturae 10, no. 10: 1106. https://doi.org/10.3390/horticulturae10101106
APA StyleXia, J., & Mattson, N. (2024). Daily Light Integral and Far-Red Radiation Influence Morphology and Quality of Liners and Subsequent Flowering and Development of Petunia in Controlled Greenhouses. Horticulturae, 10(10), 1106. https://doi.org/10.3390/horticulturae10101106









