Optimizing Lemon Balm (Melissa Officinalis L.) Cultivation: Effects of Different Manures on Plant Growth and Essential Oil Production During Consecutive Harvests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Site
2.2. Field Experiment and Cultivation Management
2.3. Measurements
2.3.1. Chlorophyll
2.3.2. Macro- and Micronutrient Concentration
2.3.3. Air-Dried Biomass and Essential Oil (LEO) Isolation
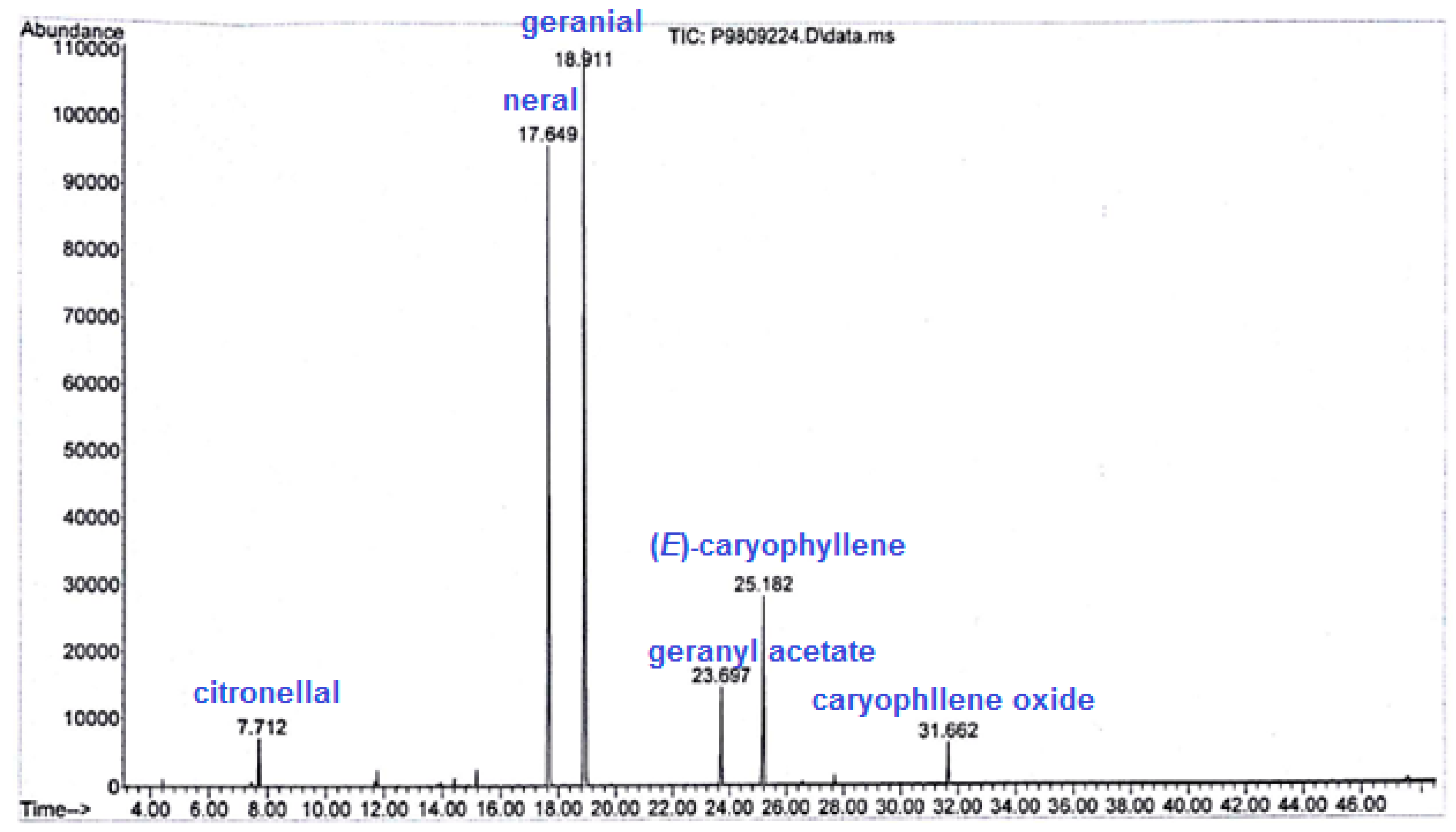
2.3.4. Qualitative and Semiquantitative Analysis of LEO Samples
2.3.5. Antioxidant Capacity
2.4. Statistical Analysis
3. Results
3.1. Effect on Photosynthesis Pigments
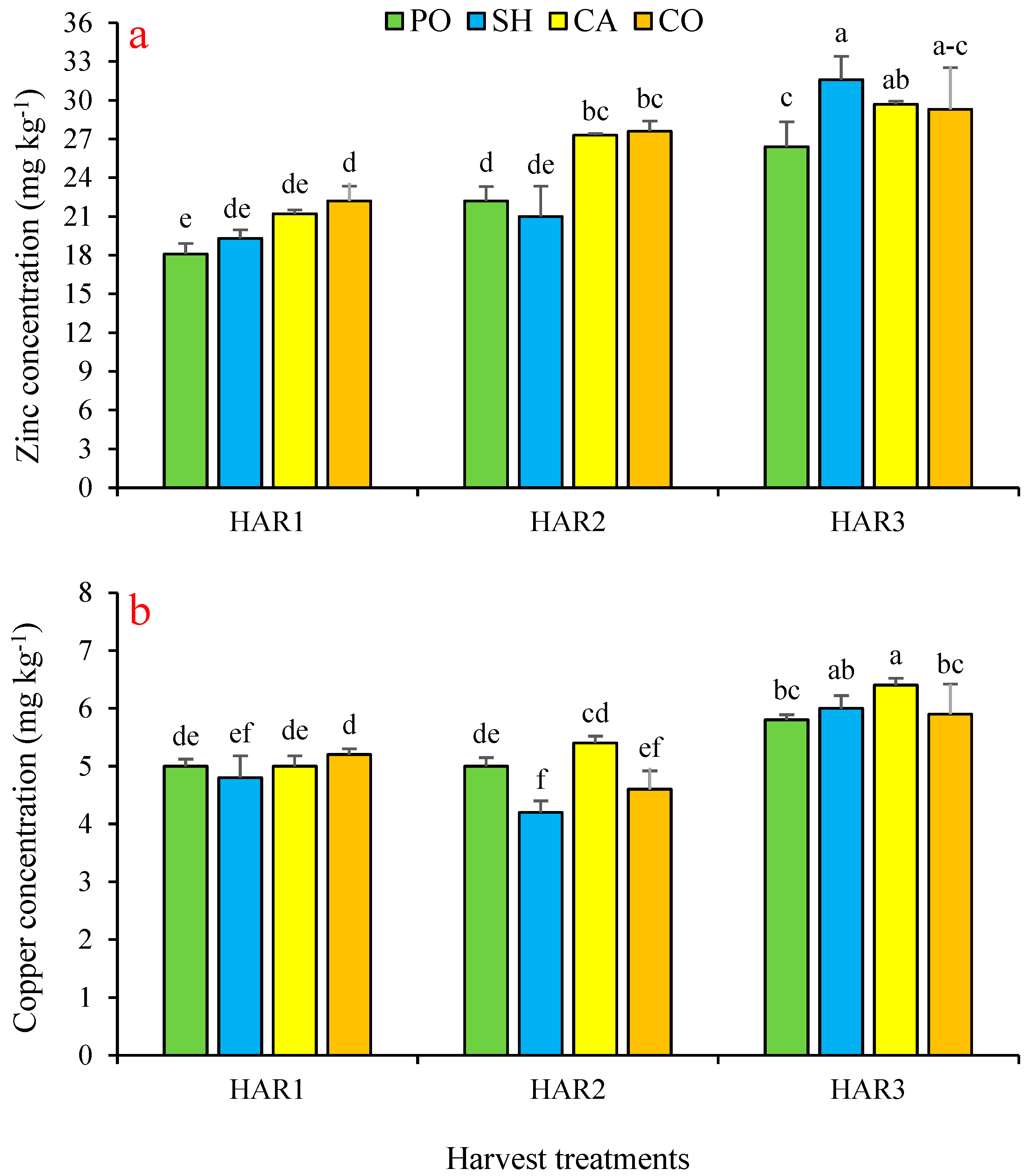
3.2. Effect on Nutrient Concentration
3.3. Effect on Biomass
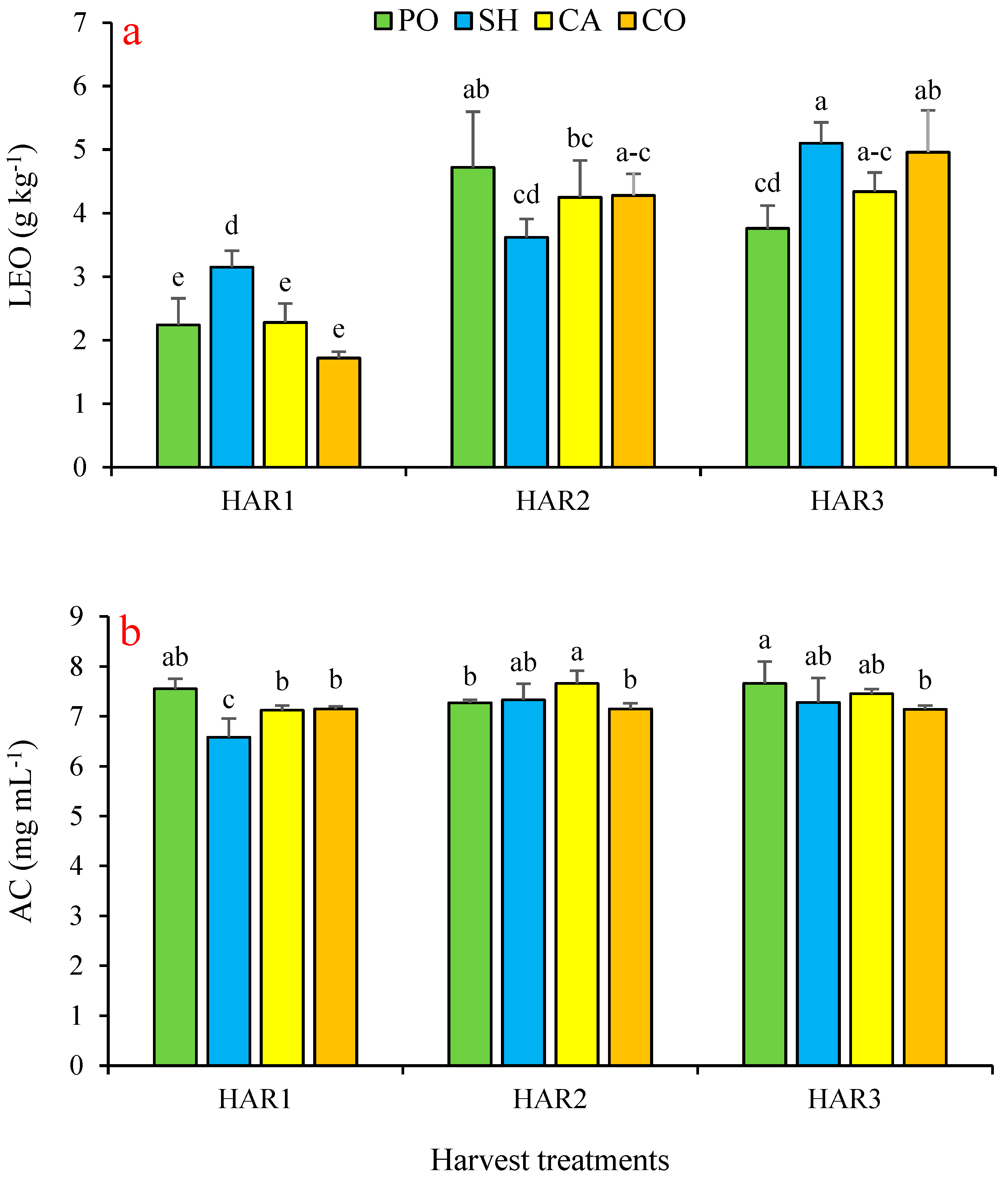
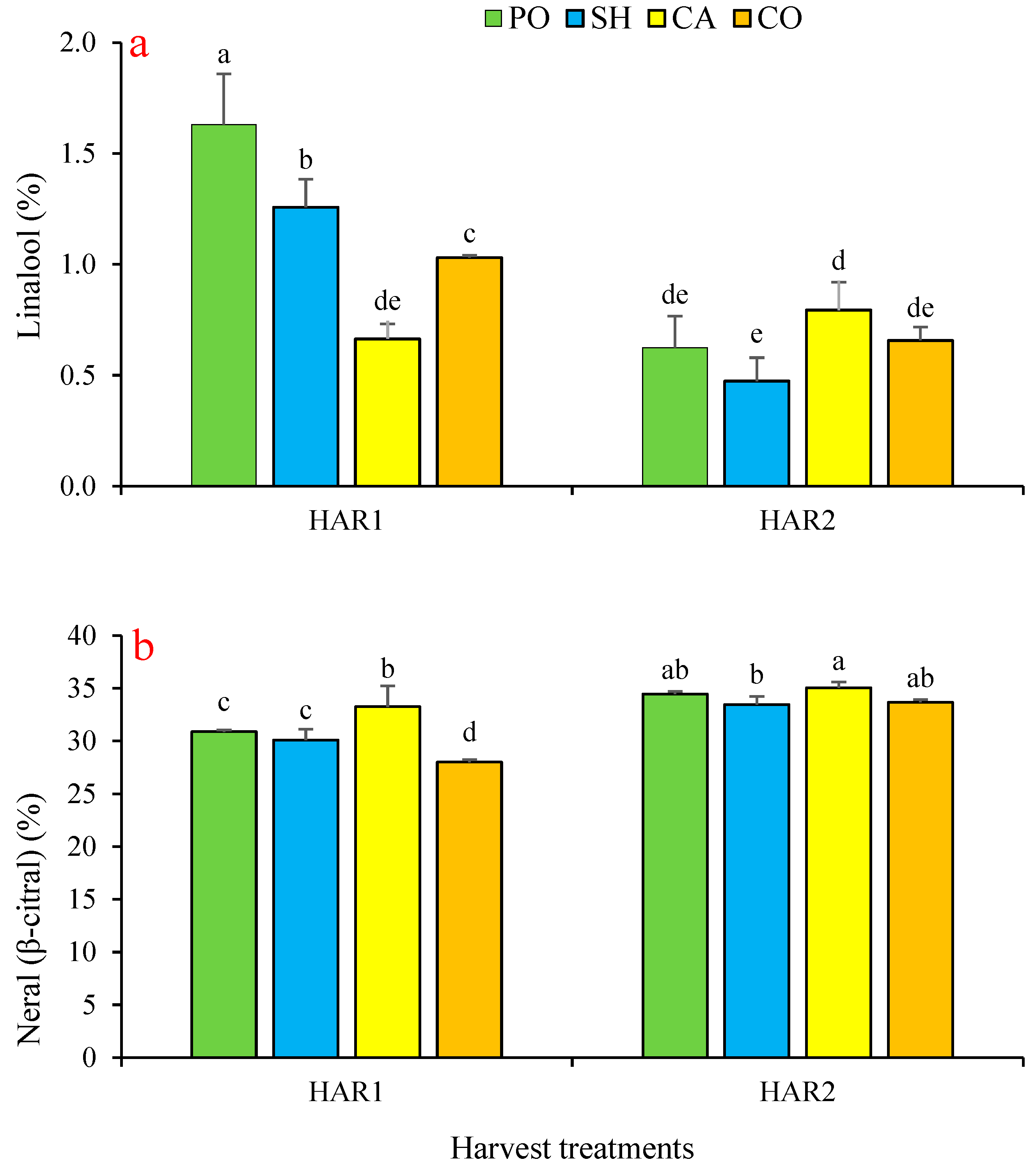
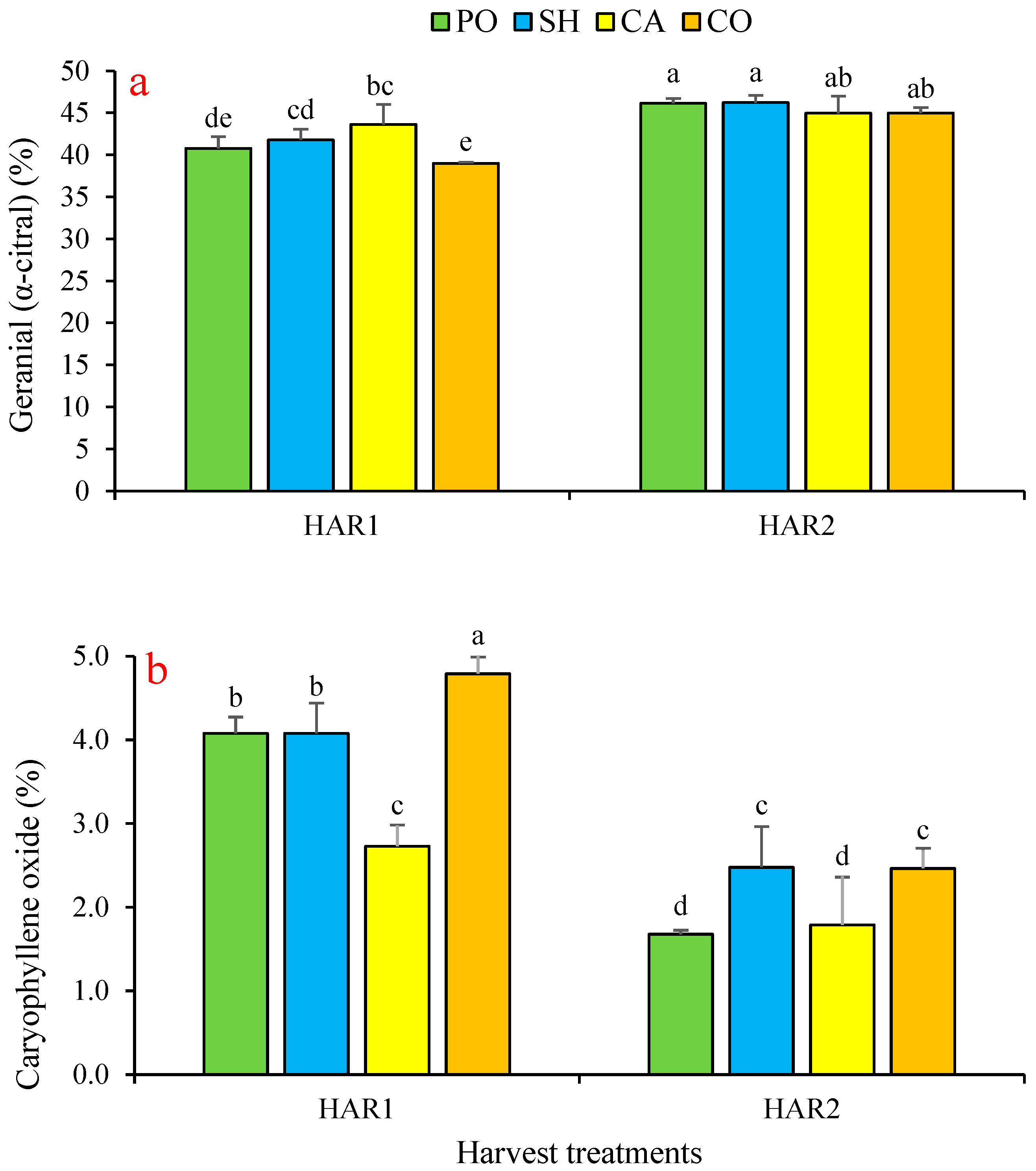
3.4. Effect on Lemon Balm’s Essential Oils
3.5. Effect on Antioxidant Capacity
4. Discussion
4.1. Photosynthesis Pigments
4.2. Nutrient Concentration
4.3. Lemon Balm Biomass
4.4. Lemon Balm’s Essential Oils
4.5. Antioxidant Capacity (AC)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Average Temperature (°C) | Average Minimum Temperature (°C) | Average Maximum Temperature (°C) | Precipitation (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| Month | 2016–2019 | 2019 | 2016–2019 | 2019 | 2016–2019 | 2019 | 2016–2019 | 2019 |
| January | −0.06 | 0.1 | −6.6 | −5.5 | 5.5 | 5.7 | 262.6 | 615.2 |
| February | 0.2 | −1.1 | −6.3 | −7.4 | 6.8 | 5.2 | 143.2 | 204.7 |
| March | 4.4 | 0.3 | −1.7 | −6.5 | 10.5 | 7.1 | 281.1 | 561.4 |
| April | 8.7 | 6.2 | 2.9 | 1.7 | 14.4 | 10.8 | 181.1 | 278.87 |
| May | 14.3 | 14.1 | 7.2 | 7.1 | 21.4 | 21.1 | 76.3 | 17.1 |
| June | 19.2 | 19.8 | 10.2 | 11.0 | 28.1 | 28.6 | 1.5 | 0.2 |
| July | 22.7 | 22.8 | 14.0 | 14.4 | 31.4 | 31.3 | 0.0 | 0.0 |
| August | 22.1 | 21.9 | 13.1 | 13.1 | 31.0 | 30.8 | 0.0 | 0.0 |
| September | 18.6 | 18.4 | 9.6 | 9.1 | 27.5 | 27.8 | 0.3 | 0.0 |
| October | 12.8 | 13.0 | 5.3 | 6.2 | 20.2 | 19.9 | 26.5 | 58.5 |
| November | 5.2 | 2.5 | −1.6 | −5.8 | 12.1 | 10.7 | 123.5 | 53.5 |
| December | 1.7 | −2.3 | −4.7 | −9.2 | 8.0 | 4.6 | 235.8 | 229.8 |
References
- Rostaei, M.; Fallah, S.; Lorigooini, Z.; Surki, A.A. Crop productivity and chemical compositions of black cumin essential oil in sole crop and intercropped with soybean under contrasting fertilization. Ind. Crop. Prod. 2018, 125, 622–629. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Stewart, B.A. Animal Waste Utilization: Effective Use of Manure as a Soil Resource; CRC Press: Boca Raton, FL, USA, 2019; p. 328. [Google Scholar]
- Aziz, T.; Ullah, S.; Sattar, A.; Nasim, M.; Farooq, M.; Khan, M.M. Nutrient availability and maize (Zea mays) growth in soil amended with organic manures. Int. J. Agric. Biol. 2010, 12, 621–624. [Google Scholar]
- Pandey, V.; Patel, A.; Patra, D.D. Integrated nutrient regimes ameliorate crop productivity, nutritive value, antioxidant activity and volatiles in basil (Ocimum basilicum L.). Ind. Crop. Prod. 2017, 87, 124–131. [Google Scholar] [CrossRef]
- Almasi, F. Organic fertilizer effects on morphological and biochemical traits and yield in coriander (Coriandrum sativum L.) as an industrial and medicinal plant. Agrotech. Ind. Crop. 2021, 1, 19–23. [Google Scholar] [CrossRef]
- Fallah, S.; Ghanbari-Odivi, A.; Rostaei, M.; Maggi, F.; Shahbazi, E. Improvement of production and quality of essential oils in multi-cut peppermint (Mentha × piperita L.) through eco-friendly fertilizers in the semi-arid highlands. Ind. Crop. Prod. 2024, 216, 118801. [Google Scholar] [CrossRef]
- Ghanbari-Odivi, A.; Fallah, S.; Carrubba, A. Optimizing hyssop (Hyssopus officinalis L.) cultivation: Effects of eifferent manures on plant growth and essential oil yield. Horticulturae 2024, 10, 894. [Google Scholar] [CrossRef]
- Nasiri, Y. Crop productivity and chemical compositions of dragonhead (Dracocephalum moldavica L.) essential oil under different cropping patterns and fertilization. Ind. Crop. Prod. 2021, 171, 113920. [Google Scholar] [CrossRef]
- Habibi Sharafabad, Z.; Abdipour, M.; Hosseinifarahi, M.; Kelidari, A.; Rashidi, L. Integrated humic acid and vermicomposting changes essential oil quantity, and quality in field-grown Lavandula angustifolia L. intercropped with Brassica nigra L. Ind. Crop. Prod. 2022, 178, 114635. [Google Scholar] [CrossRef]
- Sadat Darakeh, S.A.S.; Weisany, W.; Diyanat, M.; Ebrahimi, R. Bio-organic fertilizers induce biochemical changes and affect seed oil fatty acids composition in black cumin (Nigella sativa Linn). Ind. Crop. Prod. 2021, 164, 113383. [Google Scholar] [CrossRef]
- Serri, F.; Souri, M.K.; Rezapanah, M. Growth, biochemical quality and antioxidant capacity of coriander leaves under organic and inorganic fertilization programs. Chem. Biol. Technol. Agric. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Sajwan, M.Y.; Mishra, D.; Negi, M.S.; Bisht, P.S. Effect of organic manures on antioxidant activity and essential oil composition of artemisia annua cv. CIM arogya. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 3922–3930. [Google Scholar] [CrossRef]
- Khan, S. Lemon Balm. Available online: https://www.medicinenet.com/lemon_balm/article.htm (accessed on 15 September 2024).
- Miraj, S.; Rafieian-Kopaei, M.; Kiani, S. Melissa officinalis L: A review study with an antioxidant prospective. J. Evid. Based Complement. Altern. Med. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Sodre, A.; Luz, J.; Haber, L.; Marques, M.; Rodrigues, C.; Blank, A.F. Organic and mineral fertilization and chemical composition of lemon balm (Melissa officinalis L.) essential oil. Rev. Bras. Farmacogn. 2012, 22, 40–44. [Google Scholar] [CrossRef]
- Yaldiz, G.; Kasko Arici, Y.; Yilmaz, G. Phytochemical analysis, antioxidant and antibacterial activities of four Lamiaceae species cultivated in barnyard manure. J. Agric. Sci. 2017, 23, 95–108. [Google Scholar]
- de Assis, R.M.A.; Carneiro, J.J.; Medeiros, A.P.R.; de Carvalho, A.A.; da Cunha Honorato, A.; Carneiro, M.A.C.; Bertolucci, S.K.V.; Pinto, J.E.B.P. Arbuscular mycorrhizal fungi and organic manure enhance growth and accumulation of citral, total phenols, and flavonoids in Melissa officinalis L. Ind. Crop. Prod. 2020, 158, 112981. [Google Scholar] [CrossRef]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.A.; Elfeki, A.; Talarmin, H. Biological properties of citral and its potential protective effects against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. 2017, 87, 653–660. [Google Scholar] [CrossRef]
- Ling, Q.; Zhang, B.; Wang, Y.; Xiao, Z.; Hou, J.; Xiao, C.; Liu, Y.; Jin, Z. Chemical composition and antioxidant activity of the essential oils of citral-rich chemotype Cinnamomum camphora and Cinnamomum bodinieri. Molecules 2022, 27, 7356. [Google Scholar] [CrossRef]
- Alizadeh, P.; Fallah, S.; Raiesi, F. Potential N mineralization and availability to irrigated maize in a calcareous soil amended with organic manures and urea under field conditions. Int. J. Plant Prod. 2012, 6, 493–512. [Google Scholar] [CrossRef]
- Rajput, R.D.; Patil, R.P. The comparative study on spectrophotometric analysis of chlorophyll and carotenoids pigments from non-leguminous fodder crops. Int. J. Innov. Res. Sci. Eng. Technol. 2017, 7, 140–148. [Google Scholar]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis; Sparks, D.L., Ed.; part 3. SSSA book ser. 5; SSSA and ASA: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Ostadi, A.; Javanmard, A.; Machiani, M.A.; Morshedloo, M.R.; Nouraein, M.; Rasouli, F.; Maggi, F. Effect of different fertilizer sources and harvesting time on the growth characteristics, nutrient uptakes, essential oil productivity and composition of Mentha × piperita L. Ind. Crop. Prod. 2020, 148, 112290. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1958; pp. 178–182. [Google Scholar]
- Asadi, M.; Nasiri, Y.; Maggi, F.; Rasouli, F.; Morshedloo, M.R. Biomass yield and essential oil chemical composition of Mentha x piperita as affected by amino acids and different fertilizer resources. J. Soil Sci. Plant Nut. 2023, 23, 668–682. [Google Scholar] [CrossRef]
- British pharmacopoeia. British Pharmacopoeia; HMSO: London, UK, 1988; Volume 2, pp. 137–138. [Google Scholar]
- Javanmard, A.; Amani Machiani, M.; Haghaninia, M.; Pistelli, L.; Najar, B. Effects of green manures (in the form of monoculture and intercropping), biofertilizer and organic manure on the productivity and phytochemical properties of peppermint (Mentha piperita L.). Plants 2022, 11, 2941. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz-Mirzamohammadi, H.; Tohidi-Moghadam, H.R.; Hosseini, S.J. Is there any relationship between agronomic traits, soil properties and essential oil profile of peppermint (Mentha piperita L.) treated by fertiliser treatments and irrigation regimes? Annal. Appl. Bio. 2021, 179, 331–344. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Ranđelović, M.; Dimitrijević, M.; Otašević, S.; Stanojević, L.; Išljamović, M.; Ignjatović, A.; Arsić-Arsenijević, V.; Stojanović-Radić, Z. Antifungal activity and type of interaction of Melissa officinalis essential oil with antimycotics against biofilms of multidrug-resistant candida isolates from vulvovaginal mucosa. J. Fungi. 2023, 9, 1080. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Rostaei, M.; Fallah, S.; Lorigooini, Z.; Surki, A.A. The effect of organic manure and chemical fertilizer on essential oil, chemical compositions and antioxidant activity of dill (Anethum graveolens) in sole and intercropped with soybean (Glycine max). J. Clean. Prod. 2018, 199, 18–26. [Google Scholar] [CrossRef]
- Bojović, B.; Marković, A. Correlation between nitrogen and chlorophyll content in wheat (Triticum aestivum L.). Kragujevac J. Sci. 2009, 31, 69–74. [Google Scholar]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Gallardo, M.; Giménez, C.; Thompson, R.B. Different responses of various chlorophyll meters to increasing nitrogen supply in sweet pepper. Front. Plant Sci. 2018, 9, 1752. [Google Scholar] [CrossRef]
- Zandvakili, O.R.; Barker, A.V.; Hashemi, M.; Etemadi, F. Biomass and nutrient concentration of lettuce grown with organic fertilizers. J. Plant Nutr. 2019, 42, 444–457. [Google Scholar] [CrossRef]
- Fallah, S.; Mouguee, S.; Rostaei, M.; Adavi, Z.; Lorigooini, Z.; Shahbazi, E. Productivity and essential oil quality of Dracocephalum kotschyi under organic and chemical fertilization conditions. J. Clean. Prod. 2020, 255, 120189. [Google Scholar] [CrossRef]
- Baghdadi, A.; Halim, R.A.; Ghasemzadeh, A.; Fauzi Ramlan, M.; Sakimin, S.Z. Impact of organic and inorganic fertilizers on the yield and quality of silage corn intercropped with soybean. Peer J. 2018, 6, 5280. [Google Scholar] [CrossRef] [PubMed]
- Bajeli, J.; Tripathi, S.; Kumar, A.; Tripathi, A.; Upadhyay, R.K. Organic manures a convincing source for quality production of Japanese mint (Mentha arvensis L.). Ind. Crop. Prod. 2016, 83, 603–606. [Google Scholar] [CrossRef]
- Anwar, M.; Patra, D.D.; Chand, S.; Alpesh, K.; Naqvi, A.A.; Khanuja, S.P.S. Effect of organic manures and inorganic fertilizer on growth, herb and oil yield, nutrient accumulation, and oil quality of French basil. Commun. Soil Sci. Plant Anal. 2005, 36, 1737–1746. [Google Scholar] [CrossRef]
- Zhu, Y.; Merbold, L.; Leitner, S.; David, E.; Pelster, D.E.; Abwanda Okoma, S.; Ngetich, F.; Onyango, A.A.; Pellikka, P.; Butterbach-Bahl, K. The effects of climate on decomposition of cattle, sheep and goat manure in Kenyan tropical pastures. Plant Soil. 2020, 451, 325–343. [Google Scholar] [CrossRef]
- Markewich, H.A.; Pell, A.N.; Mbugua, D.M.; Cherney, D.J.R.; van Es, H.M.; Lehmann, J.; Robertson, J.B. Effects of storage methods on chemical composition of manure and manure decomposition in soil in small-scale Kenyan systems. Agric. Ecosyst. Environ. 2010, 139, 134–141. [Google Scholar] [CrossRef]
- Zhu, Y.; Merbold, L.; Leitner, S.; Xia, L.; Pelster, D.E.; Diaz-Pines, E.; Abwanda, S.; Mutuo, P.M.; Butterbach-Bahl, K. Influence of soil properties on N2O and CO2 emissions from excreta deposited on tropical pastures in Kenya. Soil Biol. Biochem. 2020, 140, 107636. [Google Scholar] [CrossRef]
- Singh, G.S. Advances in synthesis and chemistry of azetidines. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, A.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 130. [Google Scholar]
- Ehlers, B.K.; Berg, M.P.; Staudt, M.; Holmstrup, M.; Glasius, M.; Ellers, J.; Tomiolo, S.; Madsen, R.B.; Slotsbo, S.; Penuelas, J. Plant secondary compounds in soil and their role in belowground species interactions. Trends Ecol. Evol. 2020, 35, 716–730. [Google Scholar] [CrossRef]
- Al-Amier, H.; Toaima, V.; Mansour, B.M.M.; El Hela, A.A.; Sastry, K.S.; Craker, L. Use of mutations to improve essential oil yield and constituency in Pennyroyal. J. Herbs Spices Med. Plants. 2006, 11, 91–101. [Google Scholar] [CrossRef]
- Hasibeder, R.; Fuchslueger, L.; Richter, A.; Bahn, M. Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Physiologist. 2015, 205, 1117–1127. [Google Scholar] [CrossRef]
- Barthel, M.; Cieraad1, E.; Zakharova, A.; Hunt, J.E. Sudden cold temperature delays plant carbon transport and shifts allocation from growth to respiratory demand. Biogeosciences 2014, 11, 1425–1433. [Google Scholar] [CrossRef]
- Du, Y.; Cui, B.; Zhang, Q.; Wang, Z.; Sun, J.; Niu, W. Effects of manure fertilizer on crop yield and soil properties in China: A meta-analysis. Catena 2020, 193, 104617. [Google Scholar] [CrossRef]
- Abd-Elkader, E.H.; Ali, A.F.; Tawfik, O.H. Growth and essential oil of peppermint (Mentha piperita L.) plants as influenced by compost and some biostimulants. Arch. Agri. Sci. J. 2022, 5, 53–76. [Google Scholar] [CrossRef]
- Honorato, A.D.C.; de Assis, R.M.A.; Maciel, J.F.A.; Nohara, G.A.; de Carvalho, A.A.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Fertilization with different manure sources and doses provides quantitative-qualitative gains in the production of Thymus vulgaris L. S. Afr. J. Bot. 2024, 164, 345–355. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Maggi, F. Evaluation of yield, essential oil content and compositions of peppermint (Mentha piperita L.) intercropped with faba bean (Vicia faba L.). J. Clean. Prod. 2018, 171, 529–537. [Google Scholar] [CrossRef]
- Tripathi, Y.C.; Hazarika, P. Impact of harvesting cycle, maturity stage, drying and storage on essential oil content of patchouli leaves grown in Northeast region of India. J. Essent. Oil-Bear Plants 2014, 17, 1389–1396. [Google Scholar] [CrossRef]
- Eiasu, B.K.; Dyafta, V.; Araya, H.T. Effect of Leaf Age on Essential Oil Yield and Composition in Rose-scented Geranium. HortScience 2022, 57, 1524–1528. [Google Scholar] [CrossRef]
- Manjunatha, R.; Farooqi, A.A.; Vasundhara, M.; Srinivasappa, K.N. Effect of biofertilizers on growth, yield and essential oil content in patchouli (Pogostemon cablin Pellet.). Indian Perfum. 2002, 46, 97–104. [Google Scholar]
- Petropoulos, S.; Fernandes, Â.; Karkanis, A.; Ntatsi, G.; Barros, L.; Ferreira, I.C.F.R. Successive harvesting affects yield, chemical composition and antioxidant activity of Cichorium spinosum L. Food Chem. 2017, 237, 83–90. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, X.; Liu, Z.; Meng, R.; Chen, X.; Guo, N. Antimicrobial, antioxidant, and antitumor activity of epsilon-poly-L-lysine and citral, alone or in combination. Food Nutr. Res. 2016, 60, 31891. [Google Scholar] [CrossRef]
- Clara, M.B.G.; María, C.P.P.; Anders, L.; Antonio, M.; Dolores, R. Impact assessment of carvacrol and citral effect on Escherichia coli K12 and Listeria innocula growth. Food Control 2013, 33, 536–544. [Google Scholar] [CrossRef]
- Hirai, M.; Ota, Y.; Ito, M. Diversity in principal constituents of plants with a lemony scent and the predominance of citral. J. Nat. Med. 2022, 76, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Daci Ajvazi, M.; Folifack Tonfack, J.L.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen supply affects cannabinoid and terpenoid profile in medical cannabis (Cannabis sativa L.). Ind. Crop. Prod. 2021, 167, 113516. [Google Scholar] [CrossRef]
- Bustamante, M.Á.; Michelozzi, M.; Barra Caracciolo, A.; Grenni, P.; Verbokkem, J.; Geerdink, P.; Safi, C.; Nogues, I. Effects of soil fertilization on terpenoids and other carbon-based secondary metabolites in Rosmarinus officinalis plants: A comparative study. Plants 2020, 9, 830. [Google Scholar] [CrossRef]
- Cockson, P.; Schroeder-Moreno, M.; Veazie, P.; Barajas, G.; Logan, D.; Davis, M.; Whipker, B.E. Impact of phosphorus on Cannabis sativa reproduction, cannabinoids, and terpenes. Appl. Sci. 2020, 10, 7875. [Google Scholar] [CrossRef]
- Bhat, B.A.; Islam, S.T.; Ali, A.; Sheikh, B.A.; Tariq, L.; Ul Isalam, S.; Ul Hassan Dar, T. Role of micronutrients in secondary metabolism of plants. In Plant Micronutrients; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Ind. Crop. Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Blanch, J.S.; Peñuelas, J.; Sardans, J.; Llusià, J. Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol. Plant. 2009, 31, 207–218. [Google Scholar] [CrossRef]
- Derwich, E.; Benziane, Z.; Boukir, A. Antibacterial activity and chemical composition of the leaf essential oil of Mentha rotundifolia from Morocco. Electronic J. Environ. Agric. Food Chem. 2011, 9, 19–28. [Google Scholar]
- Benabdallah, A.; Boumendjel, M.; Aissi, O.; Rahmoune, C.; Boussaid, M.; Messaoud, C. Chemical composition, antioxidant activity and acetylcholinesterase inhibitory of wild Mentha species from northeastern Algeria. S. Afr. J. Bot. 2018, 116, 131–139. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit quality, antioxidant capacity and flavonoid content of organically and conventionally grown blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef]
- Deba, F.; Xuan, T.D.; Yasuda, M.; Tawata, S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa linn. Var. Radiata. Food Control 2008, 19, 346–352. [Google Scholar] [CrossRef]
- Fallah, S.; Rostaei, M.; Lorigooini, Z.; Surki, A.A. Chemical compositions of essential oil and antioxidant activity of dragonhead (Dracocephalum moldavica) in sole crop and dragonhead-soybean (Glycine max) intercropping system under organic manure and chemical fertilizers. Ind. Crop. Prod. 2018, 115, 158–165. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ozliman, S.; Yaldiz, G.; Camlica, M.; Ozsoy, N. Chemical components of essential oils and biological activities of the aqueous extract of Anethum graveolens L. grown under inorganic and organic conditions. Chem. Biol. Technol. Agric. 2021, 8, 20. [Google Scholar] [CrossRef]

| Manure Treatment | Harvest Treatment | ||
|---|---|---|---|
| HAR1 | HAR2 | HAR3 | |
| Poultry (PO) | June 21 | August 14 | October 6 |
| Sheep (SH) | June 21 | August 14 | October 6 |
| Cattle (CA) | June 21 | August 14 | October 6 |
| Control (CO) | June 21 | August 14 | October 6 |
| Parameter | Unit | Soil | PO | SH | CA |
|---|---|---|---|---|---|
| pH | 7.8 | 6.7 | 7.9 | 7.9 | |
| EC | µS cm−1 | 760 | 4750 | 4380 | 1980 |
| OC | g kg−1 | 7.6 | 312 | 175 | 195 |
| Nitrogen | % | 0.08 | 4.5 | 2.6 | 2.3 |
| Phosphorus | % | 8 × 10−4 | 1.7 | 0.59 | 0.56 |
| Potassium | % | 328 × 10−4 | 9.8 | 12.5 | 6.2 |
| Iron | mg kg−1 | 3.6 | 1475 | 3812 | 1718 |
| Zinc | mg kg−1 | 0.70 | 425 | 120 | 206 |
| Copper | mg kg−1 | 0.90 | 117 | 28.1 | 51.7 |
| Manganese | mg kg−1 | 8.1 | 493 | 331 | 220 |
| Source of Variance | Cha | Chb | N | P | K | Fe | Zn | Cu | Mn | Biomass | LEO Content | LEO Yield | AC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Block | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Manure type (Mt) | ** | * | NS | * | ** | NS | *** | ** | * | *** | NS | *** | ** |
| Harvest (H) | *** | ** | NS | NS | *** | ** | *** | *** | NS | *** | *** | *** | * |
| Mt × H | NS | NS | ** | ** | * | NS | * | ** | NS | NS | ** | NS | * |
| Treatments | Cha (µg mL−1) | Chb (µg mL−1) | Iron (mg kg−1) | Manganese (mg kg−1) | Biomass (kg ha−1) | LEO Yield (kg ha−1) |
|---|---|---|---|---|---|---|
| Harvest 1 | ||||||
| PO | 5.72 ab | 3.95 a | 417 c | 27.0 ab | 1448 a | 3.22 cd |
| SH | 4.68 b–d | 2.96 a–c | 345 c | 24.6 ab | 1228 ab | 3.84 b–d |
| CA | 5.41 a–c | 2.99 a–c | 278 c | 22.6 b | 1282 ab | 2.88 c–e |
| CO | 4.29 b–f | 2.40 b–d | 327 c | 21.6 b | 895 cd | 1.54 f |
| Harvest 2 | ||||||
| PO | 6.61 a | 3.15 ab | 399 c | 28.0 ab | 1152 bc | 5.43 a |
| SH | 4.87 b–d | 2.56 b–d | 1129 a | 30.9 a | 1088 bc | 3.89 bc |
| CA | 5.35 a–c | 2.71 bc | 945 ab | 31.6 a | 1164 b | 4.97 ab |
| CO | 4.48 b–e | 2.04 b–d | 492 bc | 24.8 ab | 771 d | 3.32 cd |
| Harvest 3 | ||||||
| PO | 4.07 c–f | 2.34 b–d | 440 c | 26.8 ab | 702 d | 2.64 d–f |
| SH | 3.15 ef | 1.82 cd | 443 ab | 26.5 ab | 729 d | 3.73 cd |
| CA | 3.55 d–f | 1.88 cd | 480 bc | 23.5 b | 678 d | 2.95 c–f |
| CO | 2.90 f | 1.47 d | 388 c | 27.0 ab | 386 e | 1.89 ef |
| Source of Variation | Retention Index (RI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 975 | 987 | 1100 | 1155 | 1230 | 1240 | 1265 | 1383 | 1415 | 1580 | |
| Block | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Manure type (Mt) | NS | NS | *** | ** | NS | *** | * | ** | ** | *** |
| Harvest (H) | NS | NS | *** | NS | NS | *** | *** | *** | *** | *** |
| Mt × H | NS | NS | *** | NS | NS | ** | * | NS | NS | ** |
| Treatments | Citronellal (%) | Geranyl Acetate (%) | (E)-Caryophyllene (%) |
|---|---|---|---|
| Harvest 1 | |||
| PO | 1.22 a–d | 3.82 cd | 7.45 b |
| SH | 0.92 d | 5.90 a | 7.56 b |
| CA | 1.23 a–d | 4.34 bc | 7.22 bc |
| CO | 1.38 ab | 5.29 ab | 10.7 a |
| Harvest 2 | |||
| PO | 1.09 b–d | 2.74 e | 4.72 d |
| SH | 1.04 cd | 3.59 c–e | 6.15 b–d |
| CA | 1.50 a | 3.12 de | 5.72 cd |
| CO | 1.30 a–c | 3.29 c–e | 6.41 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallah, S.; Maggi, F.; Ghanbari-Odivi, A.; Rostaei, M. Optimizing Lemon Balm (Melissa Officinalis L.) Cultivation: Effects of Different Manures on Plant Growth and Essential Oil Production During Consecutive Harvests. Horticulturae 2024, 10, 1105. https://doi.org/10.3390/horticulturae10101105
Fallah S, Maggi F, Ghanbari-Odivi A, Rostaei M. Optimizing Lemon Balm (Melissa Officinalis L.) Cultivation: Effects of Different Manures on Plant Growth and Essential Oil Production During Consecutive Harvests. Horticulturae. 2024; 10(10):1105. https://doi.org/10.3390/horticulturae10101105
Chicago/Turabian StyleFallah, Sina, Filippo Maggi, Askar Ghanbari-Odivi, and Maryam Rostaei. 2024. "Optimizing Lemon Balm (Melissa Officinalis L.) Cultivation: Effects of Different Manures on Plant Growth and Essential Oil Production During Consecutive Harvests" Horticulturae 10, no. 10: 1105. https://doi.org/10.3390/horticulturae10101105
APA StyleFallah, S., Maggi, F., Ghanbari-Odivi, A., & Rostaei, M. (2024). Optimizing Lemon Balm (Melissa Officinalis L.) Cultivation: Effects of Different Manures on Plant Growth and Essential Oil Production During Consecutive Harvests. Horticulturae, 10(10), 1105. https://doi.org/10.3390/horticulturae10101105







