Molecular Research Progress on Gametophytic Self-Incompatibility in Rosaceae Species
Abstract
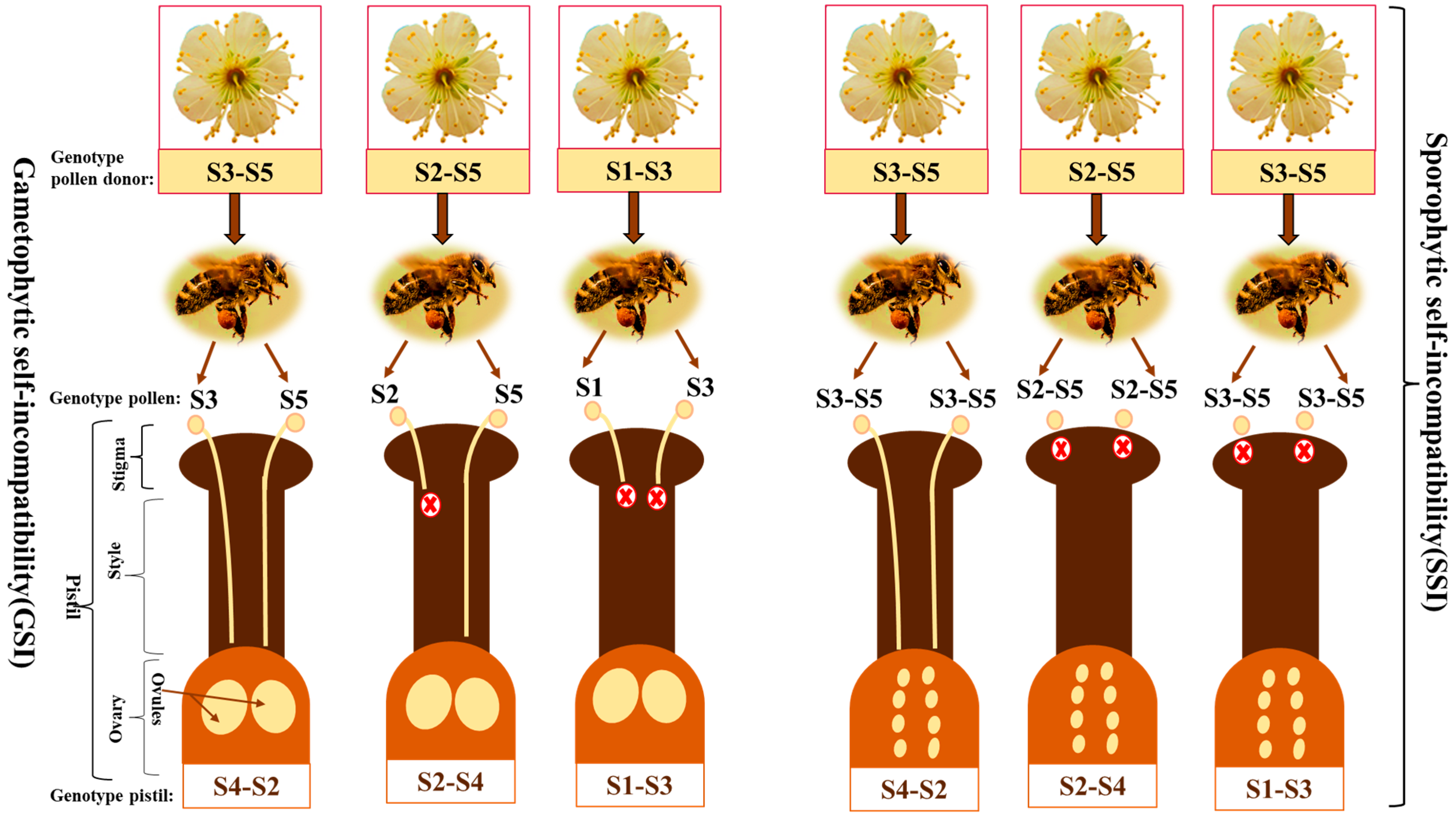
1. Introduction
2. Chronological Exploration of S-Locus Components Related to the Male and Female Components
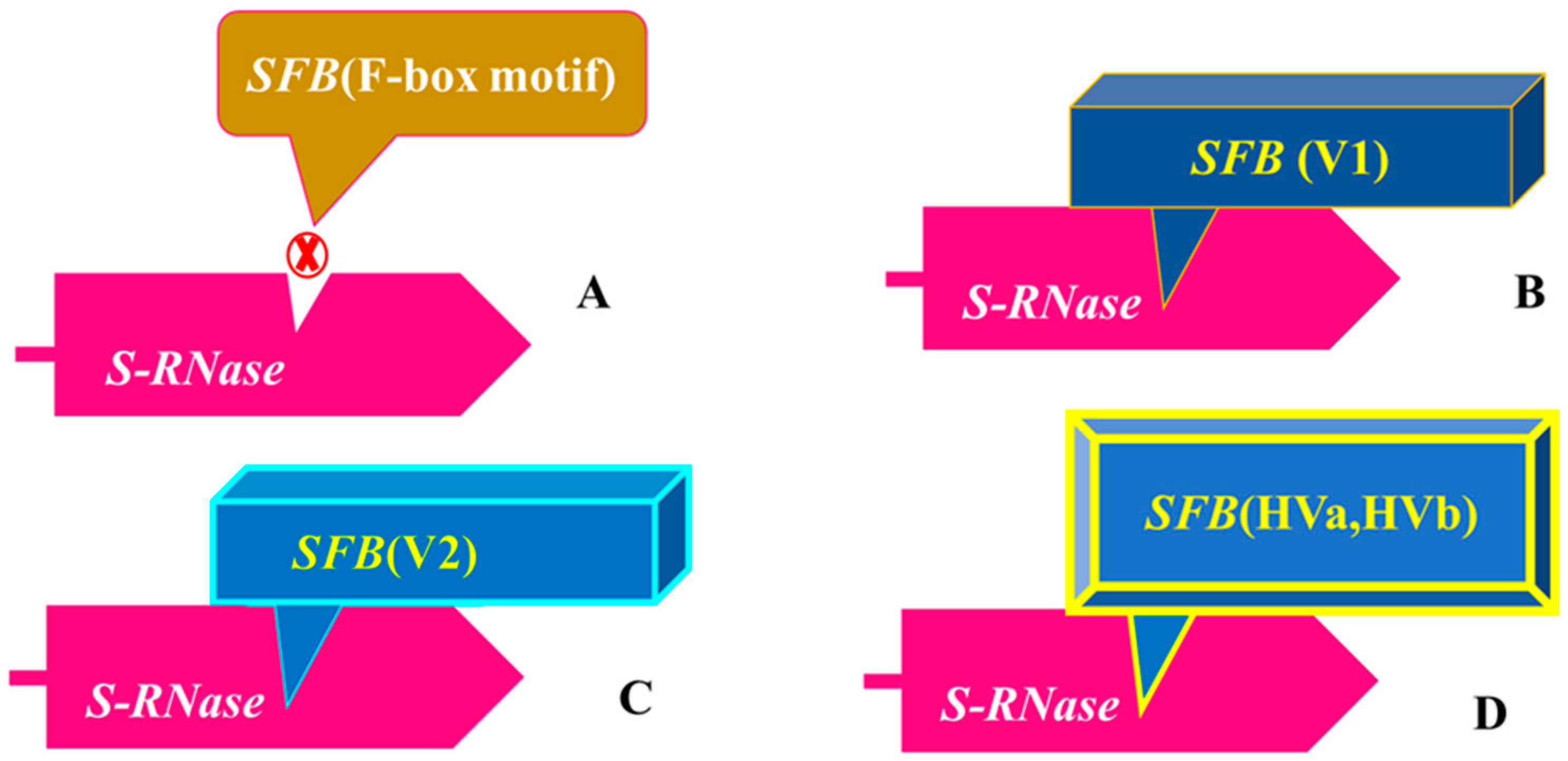
2.1. The Female or Pistil Determinant—S-RNase
| Cultivar/Accession | Latin Name | Origin | S-Genotype | SC/SI | References |
|---|---|---|---|---|---|
| Huang Yan | Prunus persica (L.) Batsch | Yunnan | S2S2 | SC | [25] |
| Bai Nian He | Prunus persica (L.) Batsch | Yunnan | S2S2 | SC | [25] |
| Qing Si | Prunus persica (L.) Batsch | Yunnan | S2S2 | SC | [25] |
| Long 1-2-4 | Prunus kansuensis Rehder | Gansu | S1S2 | SC | [25] |
| Zhang Bai 5 | Prunus kansuensis Rehder | Gansu | S2S2 | SC | [25] |
| Xinjiang Huang Rou | Prunus ferganensis (Kost. and Rjab.) Y.Y.Yao | Xinjiang | S1S2 | SC | [25] |
| Hong Gan Lu | Prunus persica (L.) Batsch | Liaoning | S2S2 | SC | [25] |
| Hun Chun Tao | Prunus persica (L.) Batsch | Jilin | S1S2 | SC | [25] |
| Da Hong Pao | Prunus persica (L.) Batsch | Hubei | S2S2 | SC | [25] |
| Kharfi | Prunus persica (L.) Batsch | Tunisia | S1S2 | SC | [60] |
| Meski | Prunus persica (L.) Batsch | Tunisia | S1S2 | SC | [60] |
| Blanco Mollar | Prunus persica (L.) Batsch | Spain | S1S1 | SC | [60] |
| Rojo Mollar | Prunus persica (L.) Batsch | Spain | S2S2 | SC | [60] |
| Rubby Rich | Prunus persica (L.) Batsch | USA | S1S1 | SC | [60] |
| Spring Lady | Prunus persica (L.) Batsch | USA | S1S1 | SC | [60] |
| Sun Late | Prunus persica (L.) Batsch | USA | S1S2 | SC | [60] |
| Rich May | Prunus persica (L.) Batsch | USA | S1S2 | SC | [60] |
| Tasstour Hamra Tardive | Prunus salicina Lindl. | NA | SfSk | SI | [53] |
| Ain Bagra1 | Prunus salicina Lindl. | NA | SfSc | SI | [53] |
| Sauvage | Prunus salicina Lindl. | NA | SfSc | SI | [53] |
| Tasstour Hamra précoce | Prunus salicina Lindl. | NA | SfSg | SI | [53] |
| Bedri1 | Prunus salicina Lindl. | NA | SeSh | SC | [53] |
| Santa Rosa (C2) | Prunus salicina Lindl. | NA | ScSe | SC | [53] |
| Mulata | Prunus domestica L. | NA | S2S5S6S7 S12 | NA | [53] |
| President | Prunus domestica L. | NA | S1S5S7S9 | NA | [53] |
| Meski Kbira Kahla | Prunus insititia L. | NA | S6S7S8S10 | NA | [53] |
| Zenou | Prunus insititia L. | NA | S1S2S10 S11 | NA | [53] |
| Bebecou | Prunus armeniaca L. | NA | S6SC | SC | [61] |
| Castlebrite | Prunus armeniaca L. | NA | S2S2 | SC | [61] |
| Castleton | Prunus armeniaca L. | NA | S1S2 | SC | [61] |
| Orange Red | Prunus armeniaca L. | NA | S6S17 | SI | [61] |
| SEO | Prunus armeniaca L. | NA | S6S17 | SI | [61] |
| Velázquez | Prunus armeniaca L. | NA | S5S20 | SI | [61] |
| Cheyenne | Prunus armeniaca L. | NA | S6S9 | SI | [62] |
| Primaya | Prunus armeniaca L. | NA | S1S6 | SI | [62] |
| Fiamma | Prunus armeniaca L. | NA | ScSc | SC | [21] |
| Summit | Prunus avium (L.) | NA | S1S2 | NA | [63] |
| Sunburst | Prunus avium (L.) | NA | S3S4 | NA | [63] |
| Emperor Francis | Prunus avium (L.) | NA | S3S4 | NA | [63] |
| Vanda | Prunus avium (L.) | NA | S1S6 | NA | [63] |
| Fragaria viridis 42 | Fragaria viridis Weston | NA | SaSb | SI | [15] |
| Xiyeqing | Prunus mume Siebold and Zucc. | China | S2S15 | SI | [64] |
| Zaohong | Prunus mume Siebold and Zucc. | China | S2S15 | SC | [64] |
| Ruantiaohongmei | Prunus mume Siebold and Zucc. | NA | S14S13 | NA | [65] |
| Xiaoyezhugan | Prunus mume Siebold and Zucc. | NA | S3S33 | NA | [65] |
| Zhonghong | Prunus mume Siebold and Zucc. | NA | S3S24 | NA | [65] |
| Meilinhong | Prunus mume Siebold and Zucc. | NA | S37S1 | NA | [65] |
| Dalongmei | Prunus mume Siebold and Zucc. | NA | S20S30 | NA | [65] |
| Huasu | Pyrus communis L. | NA | S5S108 | NA | [66] |
| Huanghua | Pyrus communis L. | NA | S1S2 | NA | [66] |
| Manfeng | Pyrus communis L. | NA | S3S4 | NA | [66] |
| Yunnan Mali | Pyrus communis L. | NA | S12S51 | NA | [66] |
| Ambrosia | Malus domestica (Suckow) Borkh. | NA | S3S28 | NA | [67] |
| Goodland | Malus domestica Borkh. | NA | S11S55 | NA | [67] |
| Newtown Pippin | Malus domestica (Suckow) Borkh. | NA | S2S7 | NA | [67] |
| Minneiska | Malus domestica (Suckow) Borkh. | NA | S24S55 | NA | [67] |
| Marubakaidou | Malus prunifolia (Willd.) Borkh. | NA | S1S2 | NA | [68] |
| A18 | Prunus tenella Batsch | Serbia | S2S3 | NA | [69] |
| A10 | Prunus tenella Batsch | Serbia | S1S5 | NA | [69] |
| A18 | Prunus tenella Batsch | Serbia | S2S3 | NA | [69] |
| A3 | Prunus tenella Batsch | Serbia | S8S9 | NA | [69] |
| A14 | Prunus tenella Batsch | Serbia | S2S5 | NA | [69] |
| A17 | Prunus tenella Batsch | Serbia | S2S3 | NA | [69] |
| Cigany | Prunus cerasus L. | Azerbaijan | S6m2S9S26S36b2 | [70] | |
| Erdi Botermo | Prunus cerasus L. | Azerbaijan | S4S6mS35S36a | [70] | |
| A8 | Prunus tenella Batsch | Serbia | S4S9 | NA | [69] |
| A6 | Prunus tenella Batsch | Serbia | S7S8 | NA | [69] |
| A9 | Prunus tenella Batsch | Serbia | S7S8 | NA | [69] |
| A11 | Prunus tenella Batsch | Serbia | S1S5 | NA | [69] |
| A15 | Prunus tenella Batsch | Serbia | S6S8 | NA | [69] |
| Muir Beauty | Prunus domestica L. | NA | S10S17 | SC | [71] |
| G45N-35 | Prunus domestica L. | NA | S8S14S16 | SI | [71] |
| G40N-34 | Prunus domestica L. | NA | S4S7 | SI | [71] |
| Improved French | Prunus domestica L. | NA | S10S12S17 | SC | [71] |
| 3-8E-46RR | Prunus domestica L. | NA | S1S3S6S17 | SC | [71] |
| Sutter | Prunus domestica L. | NA | S3S10S12S17 | SC | [71] |
| D3-39 | Prunus domestica L. | NA | S6S17 | SC | [71] |
| G16N-19 | Prunus domestica L. | NA | S11S14S17 | SC | [71] |
| Zempléni | Prunus spinosa L. | Nagykapos | S12SCSJSQ | SC | [72] |
| Dabai | Prunus pseudocerasus Lindl. | China | S1S2S5S8 | SC | [73] |
| Taishanganying | Prunus pseudocerasus Lindl. | China | S1S2S4S6 | NA | [73] |
| Satsuma | Prunus salicina Lindl. | USA | SfSh | NA | [74] |
| Huahongli | Prunus salicina Lindl. | China | SbS8 | NA | [74] |
| Sordum | Prunus salicina Lindl. | Japan | SaSb | NA | [75] |
| Fenghuali | SdSg | NA | [74] | ||
| Pingguoli | Prunus salicina Lindl. | China | S15S116 | NA | [76] |
| Honeyrosa | Prunus salicina Lindl. | Japan | SbSg | SC | [77] |
| Bullbank | Prunus salicina Lindl. | USA | SKS8 | NA | [74] |
| Daqingke | Prunus salicina Lindl. | China | SeS20 | NA | [74] |
| Huangguli | Prunus salicina Lindl. | China | SeS12 | NA | [74] |
| Wanshuhuanai | Prunus salicina Lindl. | China | S8S9 | NA | [74] |
| Zhengzhouzaoli | Prunus salicina Lindl. | China | S10/32S15 | NA | [74] |
| Sanyueli | Prunus salicina Lindl. | China | S10/32Ssany | NA | [74] |
| Saozouli | Prunus salicina Lindl. | China | S11S16 | NA | [74] |
2.2. Male or Pollen Determinant—SFB Genes
| Cultivar /Accession | Latin Name | Origin | SFB Alleles-or Genotype | SC/SI | References |
|---|---|---|---|---|---|
| Meiguili | Prunus salicina Lindl. | NA | PsSFB-c/PsSFB-e | NA | [82] |
| Nvgelei | Prunus salicina Lindl. | NA | PsSFB-c/PsSFB-h | NA | [82] |
| Daqiandi | Prunus mume Siebold and Zucc. | Nanjing | PmSFB2/ PmSFB22 | NA | [83] |
| Huangjiazuanshi | Prunus salicina Lindl. | NA | PsSFB-e/PsSFB-h | NA | [82] |
| Heibaoshi | Prunus salicina Lindl. | NA | PsSFB-b/PsSFB-h | NA | [82] |
| Nanko | Prunus mume Siebold and Zucc. | NA | PmSFB1/PmSFB7 | SI | [10] |
| Younai | Prunus salicina Lindl. | NA | PsSFB-f/PsSFB-h | NA | [82] |
| Huangpili | Prunus salicina Lindl. | NA | PsSFB-f/PsSFB-7 | NA | [82] |
| Dalizhong | Prunus mume Siebold and Zucc. | Nanjing | PmSFB7/PmSFB14 | NA | [83] |
| Ozarkpremier | Prunus salicina Lindl. | NA | PsSFB-a/PsSFB-f | NA | [82] |
| Qiuji | Prunus salicina Lindl. | NA | PsSFB-b/PsSFB-h | NA | [82] |
| Xiangjiaoli | Prunus salicina Lindl. | NA | PsSFB-e/PsSFB-10 | NA | [82] |
| Orleans-171 | Prunus avium (L.) | NA | PaSFB7/ PaSFB10 | SI | [84] |
| Zaohong | Prunus mume Siebold and Zucc. | Nanjing | PmSFB2/PmSFB15 | SC | [28] |
| Katy | Prunus armeniaca L. | NA | Par-SFB8/ Par-SFB1 | SC | [85] |
| Jiangjishantao (Wild peach) | Prunus davidiana (Carrière) Franch. | NA | PdSFB1/ PdSFB2 | NA | [86] |
| Hongding | Prunus mume Siebold and Zucc. | Zhejiang | SFB18/SFB42 | NA | [80] |
| Fupinggansutao | Prunus kansuensis Rehder | NA | PkSFB1/ PkSFB2 | NA | [86] |
| Dabai | Prunus pseudocerasus Lindl. | China | PpsSFB1/PpsSFB5 | SC | [73] |
| Taishanganying | Prunus pseudocerasus Lindl. | China | PpsSFB1/PpsSFB4/PpsSFB6 | SC | [73] |
| Wickson | Prunus salicina Lindl. | NA | PsSFBk/SFBf | NA | [87] |
| Black Diamond | Prunus salicina Lindl. | NA | PsSFBe/SFBh | NA | [87] |
| Royal-Zee | Prunus salicina Lindl. | NA | PsSFBc/SFBe | NA | [87] |
| Songold | Prunus salicina Lindl. | NA | PsSFBh/SFBk | NA | [87] |
| Methely | Prunus salicina Lindl. | NA | PsSFBb/SFBg | NA | [87] |
| Shiro | Prunus salicina Lindl. | NA | PsSFBg/SFBf | NA | [87] |
| Golfrose | Prunus salicina Lindl. | NA | PsSFBb/SFBc | NA | [87] |
| Newyorker | Prunus salicina Lindl. | NA | PsSFBe/SFBk | NA | [87] |
3. Research Achievements on Self-(in)Compatibility Mechanism Understanding and Perspectives in Fruit Tree Species
3.1. Self-Compatibility Mutation, S-RNase Recognition System
| Accessions/ Cultivars | Latin Name | Affected Part at the S-Locus | Gene/ Allele | Types of the Mutation and Genetic Variations | References |
|---|---|---|---|---|---|
| Currot | Prunus armeniaca L. | Pollen | SFB(c) | An insertion of 358 bp | [104] |
| Canino | Prunus armeniaca L. | Pollen | SFB(c) | An insertion of 358 bp | [104] |
| Fiamma | Prunus armeniaca L. | Pollen | SFB | Insertion of a transposable element at a position +904 to 1261 bp in SFB | [21] |
| Orihime | Prunus mume Siebold and Zucc. | Pollen | SFBf | An insertion of 6.8 Kbp in the middle of the SFBf coding region | [81,94] |
| Cristobalina | Prunus avium (L.) | Outside S-locus | MGST | Insertion of a transposon-like sequence in MGST promoter region | [27] |
| Feicheng Hong Li 6 | Prunus persica L. Batsch | Pollen | SFB4m | An insertion of 4949 bp fragment | [25] |
| Guang He Tao | Prunus mira Koehne | Pollen | SFB2m | An insertion of 5 bp in SFB2m sequence | [25] |
| Xinjiang Huang Rou | Prunus kansuensis Rehder | Pollen | SFB1m | An insertion of 155 bp Insertion in SFB1m sequence | [25] |
| ErdiB-termo | Prunus cerasus L. | Style | S6-Nasem | An insertion of 2600 bp upstream in S6-RNase | [106] |
| Osa-N-jisseiki | Pyrus pyrifolia (Burm.f.) Nakai | Style | S4-RNase | Deletion of a 236 kb region | [107] |
| Yanzhuang | Pyrus × bretschneideri Rehder | Style | S21-RNase | The residue of glycine-to-valine mutation in the conserved region C2 inhibits RNase activity of mutated S-RNase. This substitution breakdowns self-incompatibility in some plants | [108] |
| Unknown | Fragaria nilgerrensis Schlecht. ex J.Gay | Style | S-RNase | Loss of S-RNase | [109] |
| Wild Fragaria vesca V4 | Fragaria vesca L. | Style | S-RNase | Loss of S-RNase | [109] |
| Unknown | Fragaria iinumae Makino | Style | S-RNase | Loss of S-RNase | [109] |
| Fragaria vesca 41 | Fragaria vesca L. | Style | S-RNase | Loss of S-RNase | [15] |
| Fragaria nilgerrensis 45 | Fragaria nilgerrensis Schlecht. ex J.Gay | Style | S-RNase | Loss of S-RNase | [15] |
| Fragaria mandshurica 43 | Fragaria mandshurica Staudt | Style | S-RNase | Loss of S-RNase | [15] |
| Benihoppe | Fragaria × ananassa (Weston) Duchesne ex Rozier | Style | S-RNase | Loss of S-RNase | [15] |
| Blanquerna | Prunus dulcis D. A. Webb | Style | Sf-RNase | DNA methylation in Sf -RNase (epigenetic changes in several cytosine residues were detected in a fragment of 4700 bp of the 5′ upstream region) | [97] |
| Soleta | Prunus dulcis D. A. Webb | Style | Sf-RNase | DNA methylation in Sf -RNase (epigenetic changes in several cytosine residues were detected in a fragment of 4700 bp of the 5′ upstream region) | [97] |
3.2. Modifiers and Genetic Factors Involving in Self-(in)-Compatibility Regulation
3.3. Overview of Molecular Recognition Mechanism between Pollen and Pistil Determinants Genes
4. Approaches Used for Self-Compatible Cultivar Breeding and Improvement
4.1. Classical Breeding Techniques
4.2. Conventional Breeding Approaches
4.2.1. Marker-Assisted Selection
4.2.2. Gene Transformation Technologies
4.2.3. Genome Editing Technologies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Full Names |
| MGST | M-locus-encoded glutathione S-transferase |
| PmSFB | SFB allele of Prunus mume Siebold and Zucc. |
| PpsSFB | SFB allele of Prunus pseudocerasus Lindl. |
| PkSFB | SFB allele of Prunus kansuensis Rehder |
| PaSFB | SFB allele of Prunus avium (L.) |
| PsSFB | SFB allele of Prunus salicina Lindl. |
| PdSFB | SFB allele of Prunus davidiana (Carrière) Franch. |
| SC | Self-compatibility |
| SI | Self-incompatibility |
| SFB/SLF | S haplotype-specific F-box/S-Locus F-box |
| S-RNase | S-ribonucleases |
| GSI | Gametophytic Self-incompatibility |
| PPMs | Pollen Part Mutations |
| SPMs | Style Part Mutations |
| MAS | Marker-Assisted Selection |
| NA | Not Applicable |
| SNP | Single-Nucleotide Polymorphism |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
References
- De Nettancourt, D. Incompatibility and Incongruity in Wild and Cultivated Plants; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; Volume 3. [Google Scholar]
- McCubbin, A.G.; Kao, T.-h. Molecular recognition and response in pollen and pistil interactions. Annu. Rev. Cell Dev. Biol. 2000, 16, 333–364. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Hiscock, S. Evolution and phylogeny of self-incompatibility systems in angiosperms. In Self-Incompatibility in Flowering Plants; Springer: Berlin/Heidelberg, Germany, 2008; pp. 73–101. [Google Scholar]
- Tao, R.; Iezzoni, A.F. The S-RNase-based gametophytic self-incompatibility system in Prunus exhibits distinct genetic and molecular features. Sci. Hortic. 2010, 124, 423–433. [Google Scholar] [CrossRef]
- McClure, B.A.; Haring, V.; Ebert, P.R.; Anderson, M.A.; Simpson, R.J.; Sakiyama, F.; Clarke, A.E. Style self-incompatibility gene products of Nicotlana alata are ribonucleases. Nature 1989, 342, 955–957. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, K.; Sassa, H.; Tamura, M.; Kusaba, M.; Tao, R.; Gradziel, T.M.; Dandekar, A.M.; Hirano, H. Characterization of the S-locus region of almond (Prunus dulcis): Analysis of a somaclonal mutant and a cosmid contig for an S haplotype. Genetics 2001, 158, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Entani, T.; Iwano, M.; Shiba, H.; Che, F.S.; Isogai, A.; Takayama, S. Comparative analysis of the self-incompatibility (S-) locus region of Prunus mume: Identification of a pollen-expressed F-box gene with allelic diversity. Genes Cells 2003, 8, 203–213. [Google Scholar] [CrossRef]
- Ushijima, K.; Sassa, H.; Dandekar, A.M.; Gradziel, T.M.; Tao, R.; Hirano, H. Structural and transcriptional analysis of the self-incompatibility locus of almond: Identification of a pollen-expressed F-box gene with haplotype-specific polymorphism. Plant Cell 2003, 15, 771–781. [Google Scholar] [CrossRef]
- Yamane, H.; Tao, R.; Mori, H.; Sugiura, A. Identification of a non-S RNase, a possible ancestral form of S-RNases, in Prunus. Mol. Genet. Genom. 2003, 269, 90–100. [Google Scholar] [CrossRef]
- Yamane, H.; Ushijima, K.; Sassa, H.; Tao, R. The use of the S haplotype-specific F-box protein gene, SFB, as a molecular marker for S-haplotypes and self-compatibility in Japanese apricot (Prunus mume). Theor. Appl. Genet. 2003, 107, 1357–1361. [Google Scholar] [CrossRef]
- Ishimizu, T.; Shinkawa, T.; Sakiyama, F.; Norioka, S. Primary structural features of rosaceous S-RNases associated with gametophytic self-incompatibility. Plant Mol. Biol. 1998, 37, 931–941. [Google Scholar] [CrossRef]
- Ushijima, K.; Sassa, H.; Tao, R.; Yamane, H.; Dandekar, A.; Gradziel, T.; Hirano, H. Cloning and characterization of cDNAs encoding S-RNases from almond (Prunus dulcis): Primary structural features and sequence diversity of the S-RNases in Rosaceae. Mol. Gen. Genet. MGG 1998, 260, 261–268. [Google Scholar] [CrossRef]
- Ikeda, K.; Igic, B.; Ushijima, K.; Yamane, H.; Hauck, N.R.; Nakano, R.; Sassa, H.; Iezzoni, A.F.; Kohn, J.R.; Tao, R. Primary structural features of the S haplotype-specific F-box protein, SFB, in Prunus. Sex. Plant Reprod. 2004, 16, 235–243. [Google Scholar] [CrossRef]
- Halász, J.; Molnár, A.B.; Ilhan, G.; Ercisli, S.; Hegedűs, A. Identification and Molecular Analysis of Putative Self-Incompatibility Ribonuclease Alleles in an Extreme Polyploid Species, Prunus laurocerasus L. Front. Plant Sci. 2021, 12, 715414. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Ge, C.; Li, T.; Wang, S.; Gao, Z.; Sassa, H.; Qiao, Y. Molecular characteristics of S-RNase alleles as the determinant of self-incompatibility in the style of Fragaria viridis. Hortic. Res. 2021, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Claessen, H.; Van de Poel, B.; Keulemans, W.; De Storme, N. A semi in vivo pollination technique to assess the level of gametophytic self-incompatibility and pollen tube growth in pear (Pyrus communis L.). Plant Reprod. 2022, 35, 127–140. [Google Scholar] [CrossRef]
- Wu, L.; Xu, Y.; He, M.; Jiang, X.-T.; Qi, K.-J.; Gu, C.; Zhang, S.-L. Involvement of three ABRE-binding factors in the gametophytic self-incompatibility reaction in pear. Sci. Hortic. 2022, 301, 111089. [Google Scholar] [CrossRef]
- Serra, S.; Roeder, S.; Sheick, R.; Musacchi, S. Pistil Biology of ‘WA 38’Apple and Effect of Pollen Source on Pollen Tube Growth and Fruit Set. Agronomy 2022, 12, 123. [Google Scholar] [CrossRef]
- Uzun, A.; Ozer, L.; Turgunbaev, K.; Pınar, H.; Yaman, M.; Yılmaz, K.U.; Abdullaev, A. Identification of Self-Incompatibility in Kyrgyzstan-Originated Apple Genotypes with Molecular Marker Technique. Erwerbs-Obstbau 2022, 64, 401–406. [Google Scholar] [CrossRef]
- Certal, A.C.; Almeida, R.B.; Bošković, R.; Oliveira, M.M.; Feijó, J.A. Structural and molecular analysis of self-incompatibility in almond (Prunus dulcis). Sex. Plant Reprod. 2002, 15, 13–20. [Google Scholar] [CrossRef]
- Orlando Marchesano, B.M.; Chiozzotto, R.; Baccichet, I.; Bassi, D.; Cirilli, M. Development of an HRMA-Based Marker Assisted Selection (MAS) Approach for Cost-Effective Genotyping of S and M Loci Controlling Self-Compatibility in Apricot (Prunus armeniaca L.). Genes 2022, 13, 548. [Google Scholar] [CrossRef]
- Younes, A.; María, N.-A.; Pedro, M.-G.; Fayçal, B. Reproductive biology of a diverse apricot (Prunus armeniaca L.) Germplasm from the regions of hodna and aurès in algeria. Rev. Agrobiol. 2021, 11, 2359–2365. [Google Scholar]
- Tsukamoto, T.; Hauck, N.R.; Tao, R.; Jiang, N.; Iezzoni, A.F. Molecular characterization of three non-functional S-haplotypes in sour cherry (Prunus cerasus). Plant Mol. Biol. 2006, 62, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Fukuta, K.; Matsumoto, D.; Hanada, T.; Mei, G.; Esumi, T.; Habu, T.; Fuyuhiro, Y.; Ogawa, S.; Yaegaki, H. Characterization of a novel self-compatible S3′ haplotype leads to the development of a universal PCR marker for two distinctly originated self-compatible S haplotypes in Japanese apricot (Prunus mume Sieb. et Zucc.). J. Jpn. Soc. Hortic. Sci. 2009, 78, 40–48. [Google Scholar] [CrossRef]
- Chen, Q.; Meng, D.; Gu, Z.; Li, W.; Yuan, H.; Duan, X.; Yang, Q.; Li, Y.; Li, T. SLFL genes participate in the ubiquitination and degradation reaction of S-RNase in self-compatible peach. Front. Plant Sci. 2018, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Tao, R. Molecular and Developmental Biology: Self-incompatibility. In The Prunus mume Genome; Springer: Berlin/Heidelberg, Germany, 2019; pp. 119–135. [Google Scholar]
- Ono, K.; Akagi, T.; Morimoto, T.; Wünsch, A.; Tao, R. Genome re-sequencing of diverse sweet cherry (Prunus avium) individuals reveals a modifier gene mutation conferring pollen-part self-compatibility. Plant Cell Physiol. 2018, 59, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-P.; Gao, Z.-H.; Ni, Z.-J.; Zhang, Z.; Cai, B.-H. Self-compatibility in ‘Zaohong’ Japanese apricot is associated with the loss of function of pollen S genes. Mol. Biol. Rep. 2013, 40, 6485–6493. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, C.; Zhang, B.; Tang, F.; Li, F.; Liao, Q.; Tang, D.; Peng, Z.; Jia, Y.; Gao, M. A nonS-locus F-box gene breaks self-incompatibility in diploid potatoes. Nat. Commun. 2021, 12, 4142. [Google Scholar] [CrossRef]
- Potter, D.; Still, S.M.; Grebenc, T.; Ballian, D.; Božič, G.; Franjiæ, J.; Kraigher, H. Phylogenetic relationships in tribe Spiraeeae (Rosaceae) inferred from nucleotide sequence data. Plant Syst. Evol. 2007, 266, 105–118. [Google Scholar] [CrossRef]
- Zhang, S.D.; Jin, J.J.; Chen, S.Y.; Chase, M.W.; Soltis, D.E.; Li, H.T.; Yang, J.B.; Li, D.Z.; Yi, T.S. Diversification of Rosaceae since the Late Cretaceous based on plastid phylogenomics. New Phytol. 2017, 214, 1355–1367. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Cheng, T.; Zhang, W.; Liu, Y.; Wu, P.; Yang, X.; Wang, L.; Zhou, S. Molecular systematics of Rosoideae (Rosaceae). Plant Syst. Evol. 2020, 306, 9. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, C.; Hu, Y.; Wen, J.; Li, S.; Yi, T.; Chen, H.; Xiang, J.; Ma, H. Evolution of Rosaceae fruit types based on nuclear phylogeny in the context of geological times and genome duplication. Mol. Biol. Evol. 2017, 34, 262–281. [Google Scholar] [CrossRef]
- Gordillo-Romero, M.; Correa-Baus, L.; Baquero-Méndez, V.; de Lourdes Torres, M.; Vintimilla, C.; Tobar, J.; Torres, A.F. Gametophytic self-incompatibility in Andean capuli (Prunus serotina subsp. capuli): Allelic diversity at the S-RNase locus influences normal pollen-tube formation during fertilization. PeerJ 2020, 8, e9597. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, D.; Tao, R. Distinct self-recognition in the Prunus S-RNase-based gametophytic self-incompatibility system. Hortic. J. 2016, 85, 289–305. [Google Scholar] [CrossRef]
- Rafel Socias i Company; Kodad, O.; Martí, A.F.I.; Alonso, J.M. Mutations conferring self-compatibility in Prunus species: From deletions and insertions to epigenetic alterations. Sci. Hortic. 2015, 192, 125–131. [Google Scholar]
- Anderson, M.A.; Cornish, E.; Mau, S.-L.; Williams, E.; Hoggart, R.; Atkinson, A.; Bonig, I.; Grego, B.; Simpson, R.; Roche, P. Cloning of cDNA for a stylar glycoprotein associated with expression of self-incompatibility in Nicotiana alata. Nature 1986, 321, 38–44. [Google Scholar] [CrossRef]
- Anderson, M.A.; McFadden, G.I.; Bernatzky, R.; Atkinson, A.; Orpin, T.; Dedman, H.; Tregear, G.; Fernley, R.; Clarke, A.E. Sequence variability of three alleles of the self-incompatibility gene of Nicotiana alata. Plant Cell 1989, 1, 483–491. [Google Scholar]
- Kawata, Y.; Sakiyama, F.; Tamaoki, H. Amino-acid sequence of ribonuclease T2 from Aspergillus oryzae. Eur. J. Biochem. 1988, 176, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Roalson, E.H.; McCubbin, A.G. S-RNases and sexual incompatibility: Structure, functions, and evolutionary perspectives. Mol. Phylogenetics Evol. 2003, 29, 490–506. [Google Scholar] [CrossRef]
- Steinbachs, J.; Holsinger, K. S-RNase–mediated gametophytic self-incompatibility is ancestral in Eudicots. Mol. Biol. Evol. 2002, 19, 825–829. [Google Scholar] [CrossRef]
- Igic, B.; Kohn, J.R. Evolutionary relationships among self-incompatibility RNases. Proc. Natl. Acad. Sci. USA 2001, 98, 13167–13171. [Google Scholar] [CrossRef]
- Honsho, C.; Ushijima, K.; Anraku, M.; Ishimura, S.; Yu, Q.; Gmitter, F.G., Jr.; Tetsumura, T. Association of T2/S-RNase with Self-Incompatibility of Japanese Citrus Accessions Examined by Transcriptomic, Phylogenetic, and Genetic Approaches. Front. Plant Sci. 2021, 121, 638321. [Google Scholar] [CrossRef]
- Xue, Y.; Carpenter, R.; Dickinson, H.G.; Coen, E.S. Origin of allelic diversity in antirrhinum S locus RNases. Plant Cell 1996, 8, 805–814. [Google Scholar] [PubMed]
- Ramanauskas, K.; Igić, B. The evolutionary history of plant T2/S-type ribonucleases. PeerJ 2017, 5, e3790. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Z.; Katoh, N.; Miyairi, K.; Okuno, T. S-RNase is secreted from transmitting tract cells into the intercellular spaces after pollen tubes enter the style in apple (Malus pumila Mill.). J. Hortic. Sci. Biotechnol. 2007, 82, 433–438. [Google Scholar] [CrossRef]
- Cruz-Garcia, F.; Nathan Hancock, C.; Kim, D.; McClure, B. Stylar glycoproteins bind to S-RNase in vitro. Plant J. 2005, 42, 295–304. [Google Scholar] [CrossRef]
- Sassa, H. Molecular mechanism of the S-RNase-based gametophytic self-incompatibility in fruit trees of Rosaceae. Breed. Sci. 2016, 66, 116–121. [Google Scholar] [CrossRef]
- Aguiar, B.; Vieira, J.; Cunha, A.E.; Fonseca, N.A.; Iezzoni, A.; van Nocker, S.; Vieira, C.P. Convergent evolution at the gametophytic self-incompatibility system in Malus and Prunus. PLoS ONE 2015, 10, e0126138. [Google Scholar] [CrossRef]
- Sassa, H.; Nishio, T.; Kowyama, Y.; Hirano, H.; Koba, T.; Ikehashi, H. Self-incompatibility (S) alleles of the Rosaceae encode members of a distinct class of the T2/S ribonuclease superfamily. Mol. Gen. Genet. MGG 1996, 250, 547–557. [Google Scholar]
- Boubakri, A.; Krichen, L.; Batnini, M.-A.; Trifi-Farah, N.; Roch, G.; Audergon, J.-M.; Bourguiba, H. Self-(in) compatibility analysis of apricot germplasm in Tunisia: S-RNase allele identification, S-genotype determination and crop history evolution. Sci. Hortic. 2021, 276, 109758. [Google Scholar] [CrossRef]
- Kubo, K.-i.; Entani, T.; Takara, A.; Wang, N.; Fields, A.M.; Hua, Z.; Toyoda, M.; Kawashima, S.-i.; Ando, T.; Isogai, A. Collaborative non-self recognition system in S-RNase–based self-incompatibility. Science 2010, 330, 796–799. [Google Scholar] [CrossRef]
- Abdallah, D.; Baraket, G.; Perez, V.; Ben Mustapha, S.; Salhi-Hannachi, A.; Hormaza, J.I. Analysis of self-incompatibility and genetic diversity in diploid and hexaploid plum genotypes. Front. Plant Sci. 2019, 10, 896. [Google Scholar] [CrossRef]
- Tao, R.; Yamane, H.; Sassa, H.; Mori, H.; Gradziel, T.M.; Dandekar, A.M.; Sugiura, A. Identification of stylar RNases associated with gametophytic self-incompatibility in almond (Prunus dulcis). Plant Cell Physiol. 1997, 38, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Yamane, H.; Sugiura, A.; Murayama, H.; Sassa, H.; Mori, H. Molecular typing of S-alleles through identification, characterization and cDNA cloning for S-RNases in sweet cherry. J. Am. Soc. Hortic. Sci. 1999, 124, 224–233. [Google Scholar] [CrossRef]
- Yaegaki, H.; Shimada, T.; Moriguchi, T.; Hayama, H.; Haji, T.; Yamaguchi, M. Molecular characterization of S-RNase genes and S-genotypes in the Japanese apricot (Prunus mume Sieb. et Zucc.). Sex. Plant Reprod. 2001, 13, 251–257. [Google Scholar] [CrossRef]
- Tao, R.; Habu, T.; Yamane, H.; Sugiura, A. Characterization and cDNA cloning for Sf-RNase, a molecular marker for self-compatibility, in Japanese apricot (Prunus mume). J. Jpn. Soc. Hortic. Sci. 2002, 71, 595–600. [Google Scholar] [CrossRef]
- Ioerger, T.; Gohlke, J.; Xu, B.; Kao, T.-h. Primary structural features of the self-incompatibility protein in Solanaceae. Sex. Plant Reprod. 1991, 4, 81–87. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Skirpan, A.L.; Kao, T.h. S-RNase-mediated self-incompatibility. J. Exp. Bot. 2003, 54, 115–122. [Google Scholar] [CrossRef]
- Abdallah, D.; Baraket, G.; Perez, V.; Salhi Hannachi, A.; Hormaza, J.I. Self-compatibility in peach [Prunus persica (L.) Batsch]: Patterns of diversity surrounding the S-locus and analysis of SFB alleles. Hortic. Res. 2020, 7, 170. [Google Scholar] [CrossRef]
- Muñoz-Sanz, J.V.; Zuriaga, E.; López, I.; Badenes, M.L.; Romero, C. Self-(in) compatibility in apricot germplasm is controlled by two major loci, S and M. BMC Plant Biol. 2017, 17, 82. [Google Scholar] [CrossRef]
- Herrera, S.; Lora, J.; Hormaza, J.I.; Herrero, M.; Rodrigo, J. Optimizing production in the new generation of apricot cultivars: Self-incompatibility, S-RNase allele identification, and incompatibility group assignment. Front. Plant Sci. 2018, 9, 527. [Google Scholar] [CrossRef]
- Patzak, J.; Henychová, A.; Paprštein, F.; Sedlák, J. Evaluation of S-incompatibility locus, genetic diversity and structure of sweet cherry (Prunus avium L.) genetic resources by molecular methods and phenotypic characteristics. J. Hortic. Sci. Biotechnol. 2020, 95, 84–92. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Z.; Zhang, Z. Identification of S-genotypes and novel S-RNase alleles in Japanese apricot cultivars native to China. Sci. Hortic. 2010, 123, 459–463. [Google Scholar] [CrossRef]
- Hu, G.; Daouda, C.; Gao, Z. Analysis of S Genotypes of 11 Plum Cultivars and Identification of New S Genes. J. Plant Genet. Resour. 2021, 22, 860–872. [Google Scholar]
- He, M.; Li, L.; Xu, Y.; Mu, J.; Xie, Z.; Gu, C.; Zhang, S. Identification of S-genotypes and a novel S-RNase in 84 native Chinese pear accessions. Hortic. Plant J. 2022, 8, 713–726. [Google Scholar] [CrossRef]
- Sheick, R.; Serra, S.; Tillman, J.; Luby, J.; Evans, K.; Musacchi, S. Characterization of a novel S-RNase allele and genotyping of new apple cultivars. Sci. Hortic. 2020, 273, 109630. [Google Scholar] [CrossRef]
- Kim, H.-T.; Moriya, S.; Okada, K.; Abe, K.; Park, J.-I.; Yamamoto, T.; Nou, I.-S. Identification and characterization of S-RNase genes in apple rootstock and the diversity of S-RNases in Malus species. J. Plant Biotechnol. 2016, 43, 49–57. [Google Scholar] [CrossRef]
- Šurbanovski, N.; Tobutt, K.R.; Konstantinović, M.; Maksimović, V.; Sargent, D.J.; Stevanović, V.; Ortega, E.; Bošković, R.I. Self-incompatibility of Prunus tenella and evidence that reproductively isolated species of Prunus have different SFB alleles coupled with an identical S-RNase allele. Plant J. 2007, 50, 723–734. [Google Scholar] [CrossRef]
- Nazari, S.A.; Hajilou, J.; Zeinalabedini, M.; Imani, A. Determination of s-alleles in Iranian sour cherry (Prunus cerasus) using consensus primers. J. Agric. Sci. Belgrade 2024, 69, 169–180. [Google Scholar] [CrossRef]
- Fernandez i Marti, A.; Castro, S.; DeJong, T.M.; Dodd, R.S. Evaluation of the S-locus in Prunus domestica, characterization, phylogeny and 3D modelling. PLoS ONE 2021, 16, e0251305. [Google Scholar] [CrossRef]
- Halász, J.; Makovics-Zsohár, N.; Szőke, F.; Ercisli, S.; Hegedűs, A. Simple sequence repeat and S-Locus genotyping to assist the genetic characterization and breeding of polyploid Prunus species, P. spinosa and P. domestica subsp. insititia. Biochem. Genet. 2021, 59, 1065–1087. [Google Scholar] [CrossRef]
- Goeckeritz, C.Z.; Rhoades, K.E.; Childs, K.L.; Iezzoni, A.F.; VanBuren, R.; Hollender, C.A. Genome of tetraploid sour cherry (Prunus cerasus L.) ‘Montmorency’ identifies three distinct ancestral Prunus genomes. Hortic. Res. 2023, 10, uhad097. [Google Scholar] [CrossRef]
- Hedhly, A.; Guerra, M.E.; Grimplet, J.; Rodrigo, J. S-Locus Genotyping in Japanese Plum by High Throughput Sequencing Using a Synthetic S-Loci Reference Sequence. Int. J. Mol. Sci. 2023, 24, 3932. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Tao, R.; Sugiura, A. Identification and cDNA cloning for S-RNases in self-incompatible Japanese plum (Prunus salicina Lindl. cv. Sordum). Plant Biotechnol. 1999, 16, 389–396. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, S.; Heng, W.; Wu, H.; Wu, J.; Zhang, S. Identification of S-genotypes in 17 Chinese cultivars of Japanese plum (Prunus salicina Lindl.) and molecular characterisation of 13 novel S-alleles. J. Hortic. Sci. Biotechnol. 2008, 83, 635–640. [Google Scholar] [CrossRef]
- Beppu, K.; Takemoto, Y.; Yamane, H.; Yaegaki, H.; Yamaguchi, M.; Kataoka, I.; Tao, R. Determination of S-haplotypes of Japanese plum (Prunus salicina Lindl.) cultivars by PCR and cross-pollination tests. J. Hortic. Sci. Biotechnol. 2003, 78, 315–318. [Google Scholar] [CrossRef]
- Ikeda, K.; Ushijima, K.; Yamane, H.; Tao, R.; Hauck, N.R.; Sebolt, A.M.; Iezzoni, A.F. Linkage and physical distances between the S-haplotype S-RNase and SFB genes in sweet cherry. Sex. Plant Reprod. 2005, 17, 289–296. [Google Scholar] [CrossRef]
- Gómez, E.M.; Buti, M.; Sargent, D.J.; Dicenta, F.; Ortega, E. Transcriptomic analysis of pollen-pistil interactions in almond (Prunus dulcis) identifies candidate genes for components of gametophytic self-incompatibility. Tree Genet. Genomes 2019, 15, 53. [Google Scholar] [CrossRef]
- Coulibaly, D.; Hu, G.; Ni, Z.; Ouma, K.O.; Huang, X.; Iqbal, S.; Ma, C.; Shi, T.; Hayat, F.; Karikari, B. A Key Study on Pollen-Specific SFB Genotype and Identification of Novel SFB Alleles from 48 Accessions in Japanese Apricot (Prunus mume Sieb. et Zucc.). Forests 2022, 13, 1388. [Google Scholar] [CrossRef]
- Halasz, J.; Kodad, O.; Hegedűs, A. Identification of a recently active Prunus-specific non-autonomous Mutator element with considerable genome shaping force. Plant J. 2014, 79, 220–231. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Huang, S.-X.; Kitashiba, H.; Nishio, T. Identification of S-haplotype-specific F-box gene in Japanese plum (Prunus salicina Lindl.). Sex. Plant Reprod. 2007, 20, 1–8. [Google Scholar] [CrossRef]
- Wang, P.; Gao, Z.; Ni, Z.; Zhuang, W.; Zhang, Z. Isolation and identification of new pollen-specific SFB genes in Japanese apricot (Prunus mume). Genet. Mol. Res. 2013, 12, 3286–3295. [Google Scholar] [CrossRef]
- Vaughan, S.; Russell, K.; Sargent, D.; Tobutt, K. Isolation of S-locus F-box alleles in Prunus avium and their application in a novel method to determine self-incompatibility genotype. Theor. Appl. Genet. 2006, 112, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gu, C.; Du, Y.-H.; Wu, H.-Q.; Liu, W.-S.; Liu, N.; Lu, J.; Zhang, S.-L. Self-compatibility of ‘Katy’apricot (Prunus armeniaca L.) is associated with pollen-part mutations. Sex. Plant Reprod. 2011, 24, 23–35. [Google Scholar] [CrossRef]
- Gu, C.; Wang, L.; Korban, S.S.; Han, Y. Identification and characterization of S-RNase genes and S-genotypes in Prunus and Malus species. Can. J. Plant Sci. 2015, 95, 213–225. [Google Scholar] [CrossRef]
- Sapir, G.; Stern, R.A.; Goldway, M.; Shafir, S. SFBs of Japanese plum (Prunus salicina): Cloning seven alleles and determining their linkage to the S-RNase gene. HortScience 2007, 42, 1509–1512. [Google Scholar] [CrossRef]
- Morimoto, T.; Akagi, T.; Tao, R. Evolutionary analysis of genes for S-RNase-based self-incompatibility reveals S locus duplications in the ancestral Rosaceae. Hortic. J. 2015, 84, 233–242. [Google Scholar] [CrossRef]
- Halász, J.; Pedryc, A.; Hegedűs, A. Origin and dissemination of the pollen-part mutated SC haplotype which confers self-compatibility in apricot (Prunus armeniaca). New Phytol. 2007, 176, 792–803. [Google Scholar] [CrossRef]
- Hegedűs, A.; Lénárt, J.; Halász, J. Sexual incompatibility in Rosaceae fruit tree species: Molecular interactions and evolutionary dynamics. Biol. Plant. 2012, 56, 201–209. [Google Scholar] [CrossRef]
- Zuriaga, E.; Munoz-Sanz, J.V.; Molina, L.; Gisbert, A.D.; Badenes, M.L.; Romero, C. An S-locus independent pollen factor confers self-compatibility in ‘Katy’apricot. PLoS ONE 2013, 8, e53947. [Google Scholar] [CrossRef]
- Golz, J.; Su, V.; Clarke, A.; Newbigin, E. A molecular description of mutations affecting the pollen component of the Nicotiana alata S locus. Genetics 1999, 152, 1123–1135. [Google Scholar] [CrossRef]
- Golz, J.F.; Oh, H.-Y.; Su, V.; Kusaba, M.; Newbigin, E. Genetic analysis of Nicotiana pollen-part mutants is consistent with the presence of an S-ribonuclease inhibitor at the S locus. Proc. Natl. Acad. Sci. USA 2001, 98, 15372–15376. [Google Scholar] [CrossRef]
- Ushijima, K.; Yamane, H.; Watari, A.; Kakehi, E.; Ikeda, K.; Hauck, N.R.; Iezzoni, A.F.; Tao, R. The S haplotype-specific F-box protein gene, SFB, is defective in self-compatible haplotypes of Prunus avium and P. mume. Plant J. 2004, 39, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, D.; Tao, R. Recognition of S-RNases by an S locus F-box like protein and an S haplotype-specific F-box like protein in the Prunus-specific self-incompatibility system. Plant Mol. Biol. 2019, 100, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Watari, A.; Hanada, T.; Habu, T.; Yaegaki, H.; Yamaguchi, M.; Yamane, H. Self-compatible peach (Prunus persica) has mutant versions of the S haplotypes found in self-incompatible Prunus species. Plant Mol. Biol. 2007, 63, 109–123. [Google Scholar] [CrossRef]
- Fernández i Martí, A.; Gradziel, T.M. Methylation of the S f locus in almond is associated with S-RNase loss of function. Plant Mol. Biol. 2014, 86, 681–689. [Google Scholar] [CrossRef]
- Jones, P.A.; Takai, D. The role of DNA methylation in mammalian epigenetics. Science 2001, 293, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mohapatra, T. Dynamics of DNA methylation and its functions in plant growth and development. Front. Plant Sci. 2021, 12, 858. [Google Scholar] [CrossRef]
- Tao, R.; Habu, T.; Yamane, H.; Sugiura, A.; Iwamoto, K. Molecular markers for self-compatibility in Japanese apricot (Prunus mume). HortScience 2000, 35, 1121–1123. [Google Scholar] [CrossRef]
- Tao, R.; Habu, T.; Namba, A.; Yamane, H.; Fuyuhiro, F.; Iwamoto, K.; Sugiura, A. Inheritance of S f-RNase in Japanese apricot (Prunus mume) and its relation to self-compatibility. Theor. Appl. Genet. 2002, 105, 222–228. [Google Scholar] [CrossRef]
- Tao, R.; Namba, A.; Yamane, H.; Fuyuhiro, Y.; Watanabe, T.; Habu, T.; Sugiura, A. Development of the Sf-RNase gene-specific PCR primer set for Japanese apricot (Prunus mume Sieb. et Zucc.). Hortic. Res. 2003, 2, 237–240. [Google Scholar] [CrossRef][Green Version]
- Habu, T.; Kishida, F.; Morikita, M.; Kitajima, A.; Yamada, T.; Tao, R. A simple and rapid procedure for the detection of self-compatible individuals in Japanese apricot (Prunus mume Sieb. et Zucc.) using the loop-mediated isothermal amplification (LAMP) method. HortScience 2006, 41, 1156–1158. [Google Scholar] [CrossRef]
- Vilanova, S.; Badenes, M.L.; Burgos, L.; Martínez-Calvo, J.; Llácer, G.; Romero, C. Self-compatibility of two apricot selections is associated with two pollen-part mutations of different nature. Plant Physiol. 2006, 142, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; Lora, J.; Hormaza, J.I.; Rodrigo, J. Self-Incompatibility in Apricot: Identifying Pollination Requirements to Optimize Fruit Production. Plants 2022, 11, 2019. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Ikeda, K.; Hauck, N.R.; Iezzoni, A.F.; Tao, R. Self-incompatibility (S) locus region of the mutated S 6-haplotype of sour cherry (Prunus cerasus) contains a functional pollen S allele and a non-functional pistil S allele. J. Exp. Bot. 2003, 54, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Tonaka, N.; Moriya, Y.; Norioka, N.; Sawamura, Y.; Matsumoto, T.; Nakanishi, T.; Takasaki-Yasuda, T. Deletion of a 236 kb region around S 4-RNase in a stylar-part mutant S 4 sm-haplotype of Japanese pear. Plant Mol. Biol. 2008, 66, 389–400. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Wu, C.; Yu, J.; Liu, C.; Fan, W.; Li, T.; Li, W. A mutation near the active site of S-RNase causes self-compatibility in S-RNase-based self-incompatible plants. Plant Mol. Biol. 2020, 103, 129–139. [Google Scholar] [CrossRef]
- Chen, W.; Wan, H.; Liu, F.; Du, H.; Zhang, C.; Fan, W.; Zhu, A. Rapid evolution of T2/S-RNase genes in Fragaria linked to multiple transitions from self-incompatibility to self-compatibility. Plant Divers. 2022, 49, 219–228. [Google Scholar] [CrossRef]
- Franklin-Tong, V.E.; Franklin, F. The different mechanisms of gametophytic self–incompatibility. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003, 358, 1025–1032. [Google Scholar] [CrossRef]
- Wu, J.; Gu, C.; Khan, M.A.; Wu, J.; Gao, Y.; Wang, C.; Korban, S.S.; Zhang, S. Molecular determinants and mechanisms of gametophytic self-incompatibility in fruit trees of Rosaceae. Crit. Rev. Plant Sci. 2013, 32, 53–68. [Google Scholar] [CrossRef]
- McClure, B.; Mou, B.; Canevascini, S.; Bernatzky, R. A small asparagine-rich protein required for S-allele-specific pollen rejection in Nicotiana. Proc. Natl. Acad. Sci. USA 1999, 96, 13548–13553. [Google Scholar] [CrossRef]
- Goldraij, A.; Kondo, K.; Lee, C.B.; Hancock, C.N.; Sivaguru, M.; Vazquez-Santana, S.; Kim, S.; Phillips, T.E.; Cruz-Garcia, F.; McClure, B. Compartmentalization of S-RNase and HT-B degradation in self-incompatible Nicotiana. Nature 2006, 439, 805–810. [Google Scholar] [CrossRef]
- Gu, Z.; Meng, D.; Yang, Q.; Yuan, H.; Wang, A.; Li, W.; Chen, Q.; Zhang, Y.; Wang, D.; Li, T. A CBL gene, MdCBL5, controls the calcium signal and influences pollen tube growth in apple. Tree Genet. Genomes 2015, 11, 27. [Google Scholar] [CrossRef]
- Qu, H.-y.; Zhang, Z.; Wu, F.; Wang, Y. The role of Ca2+ and Ca2+ channels in the gametophytic self-incompatibility of Pyrus pyrifolia. Cell Calcium 2016, 60, 299–308. [Google Scholar] [CrossRef]
- Wang, C.-L.; Wu, J.; Xu, G.-H.; Gao, Y.-b.; Chen, G.; Wu, J.-Y.; Wu, H.-q.; Zhang, S.-L. S-RNase disrupts tip-localized reactive oxygen species and induces nuclear DNA degradation in incompatible pollen tubes of Pyrus pyrifolia. J. Cell Sci. 2010, 123, 4301–4309. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Tang, C.; Wang, R.; Gu, C.; Wu, X.; Hu, S.; Jiao, J.; Zhang, S. Transcriptome and phytohormone analysis reveals a comprehensive phytohormone and pathogen defence response in pear self-/cross-pollination. Plant Cell Rep. 2017, 36, 1785–1799. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.; Kim, S.; McClure, B. A pollen protein, NaPCCP, that binds pistil arabinogalactan proteins also binds phosphatidylinositol 3-phosphate and associates with the pollen tube endomembrane system. Plant Physiol. 2009, 149, 791–802. [Google Scholar] [CrossRef]
- Qu, H.; Guan, Y.; Wang, Y.; Zhang, S. PLC-mediated signaling pathway in pollen tubes regulates the gametophytic self-incompatibility of Pyrus species. Front. Plant Sci. 2017, 8, 1164. [Google Scholar] [CrossRef]
- De Graaf, B.H.; Knuiman, B.; Derksen, J.; Mariani, C. Characterization and localization of the transmitting tissue-specific PELPIII proteins of Nicotiana tabacum. J. Exp. Bot. 2003, 54, 55–63. [Google Scholar] [CrossRef]
- Liu, Z.-q.; Xu, G.-h.; Zhang, S.-l. Pyrus pyrifolia stylar S-RNase induces alterations in the actin cytoskeleton in self-pollen and tubes in vitro. Protoplasma 2007, 232, 61–67. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, S.; Yang, Y.; Zhao, C.; Wolukau, J.N. Influence of endogenous and exogenous RNases on the variation of pollen cytosolic-free Ca2+ in Pyrus serotina Rehd. Acta Physiol. Plant. 2008, 30, 233–241. [Google Scholar] [CrossRef]
- Matsumoto, D.; Tao, R. Isolation of Pollen-expressed Actin as a Candidate Protein Interacting with S-RNase in Prunus avium L. J. Jpn. Soc. Hortic. Sci. 2012, 81, 41–47. [Google Scholar] [CrossRef]
- Meng, D.; Gu, Z.; Li, W.; Wang, A.; Yuan, H.; Yang, Q.; Li, T. Apple MdABCF assists in the transportation of S-RN ase into pollen tubes. Plant J. 2014, 78, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Meng, D.; Gu, Z.; Li, W.; Chen, Q.; Li, Y.; Yuan, H.; Yu, J.; Liu, C.; Li, T. Apple S-RN ase interacts with an actin-binding protein, Md MVG, to reduce pollen tube growth by inhibiting its actin-severing activity at the early stage of self-pollination induction. Plant J. 2018, 95, 41–56. [Google Scholar] [CrossRef]
- Gu, Z.; Li, W.; Doughty, J.; Meng, D.; Yang, Q.; Yuan, H.; Li, Y.; Chen, Q.; Yu, J.; Liu, C.s. A gamma-thionin protein from apple, MdD1, is required for defence against S-RNase-induced inhibition of pollen tube prior to self/non-self recognition. Plant Biotechnol. J. 2019, 17, 2184–2198. [Google Scholar] [CrossRef]
- Muñoz-Sanz, J.V.; Zuriaga, E.; Badenes, M.L.; Romero, C. A disulfide bond A-like oxidoreductase is a strong candidate gene for self-incompatibility in apricot (Prunus armeniaca) pollen. J. Exp. Bot. 2017, 68, 5069–5078. [Google Scholar] [CrossRef] [PubMed]
- Yeting, X.; Zhang, Q.; Zhang, X.; Jian, W.; Mubarek, A.; Bo, Y.; Chunmiao, G.; Peng, G.; Wenxuan, D. The proteome reveals the involvement of serine/threonine kinase in the recognition of self-incompatibility in almond. J. Proteom. 2022, 256, 104505. [Google Scholar]
- Gómez, E.M.; Prudencio, Á.S.; Ortega, E. Protein Profiling of Pollen–Pistil Interactions in Almond (Prunus dulcis) and Identification of a Transcription Regulator Presumably Involved in Self-Incompatibility. Agronomy 2022, 12, 345. [Google Scholar] [CrossRef]
- Matsumoto, D.; Tao, R. Yeast Two-Hybrid screening for the general inhibitor detoxifying S-RNase in Prunus. Acta Hortic. 2012, 967, 167–170. [Google Scholar] [CrossRef]
- Fujii, S.; Kubo, K.-i.; Takayama, S. Non-self-and self-recognition models in plant self-incompatibility. Nat. Plants 2016, 2, 16130. [Google Scholar] [CrossRef]
- Erez, K.; Jangid, A.; Feldheim, O.N.; Friedlander, T. The role of promiscuous molecular recognition in the evolution of RNase-based self-incompatibility in plants. Nat. Commun. 2024, 15, 4864. [Google Scholar] [CrossRef]
- Abd-Hamid, N.-A.; Ahmad-Fauzi, M.-I.; Zainal, Z.; Ismail, I. Diverse and dynamic roles of F-box proteins in plant biology. Planta 2020, 251, 68. [Google Scholar] [CrossRef]
- Claessen, H.; Keulemans, W.; Van de Poel, B.; De Storme, N. Finding a compatible partner: Self-incompatibility in European pear (Pyrus communis); molecular control, genetic determination, and impact on fertilization and fruit set. Front. Plant Sci. 2019, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Lora, J.; Hormaza, J.I.; Herrero, M. The diversity of the pollen tube pathway in plants: Toward an increasing control by the sporophyte. Front. Plant Sci. 2016, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Isogai, A. Self-incompatibility in plants. Annu. Rev. Plant Biol. 2005, 56, 467. [Google Scholar] [CrossRef] [PubMed]
- Weckwerth, W.; Ghatak, A.; Bellaire, A.; Chaturvedi, P.; Varshney, R.K. PANOMICS meets germplasm. Plant Biotechnol. J. 2020, 18, 1507–1525. [Google Scholar] [CrossRef]
- Yang, Y.; Saand, M.A.; Huang, L.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Sirohi, M.H.; Wang, F. Applications of multi-omics technologies for crop improvement. Front. Plant Sci. 2021, 12, 563953. [Google Scholar] [CrossRef]
- Singha, D.L.; Das, D.; Paswan, R.R.; Chikkaputtaiah, C.; Kumar, S. Novel Approaches and Advanced Molecular Techniques for Crop Improvement. In Plant-Microbe Interactions: Harnessing Next-Generation Molecular Technologies for Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–27. [Google Scholar]
- Begna, T. Conventional breeding methods widely used to improve self-pollinated crops. Int. J. Res. 2021, 7, 1–16. [Google Scholar]
- Zhang, D.; Li, Y.-Y.; Zhao, X.; Zhang, C.; Liu, D.-K.; Lan, S.; Yin, W.; Liu, Z.-J. Molecular insights into self-incompatibility systems: From evolution to breeding. Plant Commun. 2024, 5, 100719. [Google Scholar] [CrossRef]
- Rommens, C.M. Intragenic crop improvement: Combining the benefits of traditional breeding and genetic engineering. J. Agric. Food Chem. 2007, 55, 4281–4288. [Google Scholar] [CrossRef]
- Gradziel, T.M. Transfer of Self-Fruitfulness to Cultivated Almond from Peach and Wild Almond. Horticulturae 2022, 8, 965. [Google Scholar] [CrossRef]
- Kumawat, G.; Kumawat, C.K.; Chandra, K.; Pandey, S.; Chand, S.; Mishra, U.N.; Lenka, D.; Sharma, R. Insights into marker assisted selection and its applications in plant breeding. In Plant Breeding-Current and Future Views; Intechopen: Rijeka, Croatia, 2020. [Google Scholar]
- Muñoz-Espinoza, C.; Espinosa, E.; Bascuñán, R.; Tapia, S.; Meneses, C.; Almeida, A.M. Development of a molecular marker for self-compatible S4′ haplotype in sweet cherry (Prunus avium L.) using high-resolution melting. Plant Breed. 2017, 136, 987–993. [Google Scholar] [CrossRef]
- Lewis, D.; Crowe, L.K. The induction of self-fertility in tree fruits. J. Hortic. Sci. 1954, 29, 220–225. [Google Scholar] [CrossRef]
- Ono, K.; Masui, K.; Tao, R. Artificial control of the Prunus self-incompatibility system using antisense oligonucleotides against pollen genes. Hortic. J. 2022, 91, 437–447. [Google Scholar] [CrossRef]
- Tuncel, A.; Qi, Y. CRISPR/Cas mediated genome editing in potato: Past achievements and future directions. Plant Sci. 2022, 325, 111474. [Google Scholar] [CrossRef] [PubMed]
- Bánfalvi, Z.; Csákvári, E.; Villányi, V.; Kondrák, M. Generation of transgene-free PDS mutants in potato by Agrobacterium-mediated transformation. BMC Biotechnol. 2020, 20, 25. [Google Scholar] [CrossRef]
- Ye, M.; Peng, Z.; Tang, D.; Yang, Z.; Li, D.; Xu, Y.; Zhang, C.; Huang, S. Generation of self-compatible diploid potato by knockout of S-RNase. Nat. Plants 2018, 4, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Enciso-Rodriguez, F.; Manrique-Carpintero, N.C.; Nadakuduti, S.S.; Buell, C.R.; Zarka, D.; Douches, D. Overcoming self-incompatibility in diploid potato using CRISPR-Cas9. Front. Plant Sci. 2019, 10, 376. [Google Scholar] [CrossRef]
- Ma, C.; Zhu, C.; Zheng, M.; Liu, M.; Zhang, D.; Liu, B.; Li, Q.; Si, J.; Ren, X.; Song, H. CRISPR/Cas9-mediated multiple gene editing in Brassica oleracea var. capitata using the endogenous tRNA-processing system. Hortic. Res. 2019, 6, 20. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coulibaly, D.; Gao, F.; Bai, Y.; Ouma, K.O.; Antwi-Boasiako, A.; Zhou, P.; Iqbal, S.; Bah, A.A.; Huang, X.; Diarra, S.T.; et al. Molecular Research Progress on Gametophytic Self-Incompatibility in Rosaceae Species. Horticulturae 2024, 10, 1101. https://doi.org/10.3390/horticulturae10101101
Coulibaly D, Gao F, Bai Y, Ouma KO, Antwi-Boasiako A, Zhou P, Iqbal S, Bah AA, Huang X, Diarra ST, et al. Molecular Research Progress on Gametophytic Self-Incompatibility in Rosaceae Species. Horticulturae. 2024; 10(10):1101. https://doi.org/10.3390/horticulturae10101101
Chicago/Turabian StyleCoulibaly, Daouda, Feng Gao, Yang Bai, Kenneth Omondi Ouma, Augustine Antwi-Boasiako, Pengyu Zhou, Shahid Iqbal, Amadou Apho Bah, Xiao Huang, Sabaké Tianégué Diarra, and et al. 2024. "Molecular Research Progress on Gametophytic Self-Incompatibility in Rosaceae Species" Horticulturae 10, no. 10: 1101. https://doi.org/10.3390/horticulturae10101101
APA StyleCoulibaly, D., Gao, F., Bai, Y., Ouma, K. O., Antwi-Boasiako, A., Zhou, P., Iqbal, S., Bah, A. A., Huang, X., Diarra, S. T., Segbo, S., Hayat, F., & Gao, Z. (2024). Molecular Research Progress on Gametophytic Self-Incompatibility in Rosaceae Species. Horticulturae, 10(10), 1101. https://doi.org/10.3390/horticulturae10101101








