Applicability Analysis of Peanut Addition to Button Mushroom Substrate Supplement Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Substrate and Supplements
2.3. Cultivation
2.4. Analyses of Aflatoxins
2.5. Variables Analyzed
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dedousi, M.; Melanouri, E.-M.; Karayannis, D.; Kaminarides, E.-I.; Diamantopoulou, P. Utilização de Substratos Usados e Resíduos Do Cultivo de Cogumelos Para Produzir Novas Culturas de Pleurotus ostreatus, Pleurotus eryngii e Agaricus bisporus. Carbon Resour. Convers. 2024, 7, 100196. [Google Scholar] [CrossRef]
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, Medicinal, and Cosmetic Value of Bioactive Compounds in Button Mushroom (Agaricus bisporus): A Review. Appl. Sci. 2021, 11, 5943. [Google Scholar] [CrossRef]
- Wasser, S.P.; Akavia, E. Regulatory issues of mushrooms as functional foods and dietary supplements: Safety and efficacy. In Mushrooms as Functional Foods; Cheung, P.C.K., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 199–226. [Google Scholar]
- Pardo-Gimenez, A.; Pardo-Gonzalez, J.E.; Cunha-Zied, D. Supplementation of High Nitrogen Agaricus Compost: Yield and Mushroom Quality. J. Agric. Sci. Technol. 2017, 19, 1589–1601. [Google Scholar]
- Buth, J. Compost as a Food Base for Agaricus bisporus. In Edible and Medicinal Mushrooms; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 129–147. [Google Scholar]
- Burton, K.; Noble, R.; Rogers, S.; Wilson, J. Understanding Mushroom Nutri-Tion: Project Aimed at Improving Yield, Substrate Efficiency and Utilisation and Flavor; M056 Final Report; Agriculture and Horticulture Development Board: Warwickshire, UK, 2015; 54p. [Google Scholar]
- Carrasco, J.; Zied, D.C.; Pardo, J.E.; Preston, G.M.; Pardo-Giménez, A. Supplementation in Mushroom Crops and Its Impact on Yield and Quality. AMB Express 2018, 8, 146. [Google Scholar] [CrossRef]
- Zied, D.C.; Caitano, C.E.C.; Pardo-Gimenez, A.; Dias, E.S.; Zeraik, M.L.; Pardo, J.E. Using of Appropriated Strains in the Practice of Compost Supplementation for Agaricus subrufescens Production. Front. Sustain. Food Syst. 2018, 2, 26. [Google Scholar] [CrossRef]
- Santos, R.C.; Freire, R.M.M.; de Lima, L.M. O Agronegócio Do Amendoim No Brasil, 2nd ed.; Bases de Dados da Pesquisa Agropecuária: Brasilia, Brazil, 2013. [Google Scholar]
- Pardo-Giménez, A.; Catalán, L.; Carrasco, J.; Álvarez-Ortí, M.; Zied, D.; Pardo, J. Effect of Supplementing Crop Substrate with Defatted Pistachio Meal on Agaricus bisporus and Pleurotus ostreatus Production. J. Sci. Food Agric. 2016, 96, 3838–3845. [Google Scholar] [CrossRef]
- Ferrari, A.B.S.; Azevedo de Oliveira, G.; Mannochio Russo, H.; de Carvalho Bertozo, L.; da Silva Bolzani, V.; Zied, D.C.; Farias Ximenes, V.; Zeraik, M.L. Pleurotus ostreatus and Agaricus subrufescens: Investigation of Chemical Composition and Antioxidant Properties of These Mushrooms Cultivated with Different Handmade and Commercial Supplements. Int. J. Food Sci. Technol. 2021, 56, 452–460. [Google Scholar] [CrossRef]
- Otieno, O.D.; Mulaa, F.J.; Obiero, G.; Midiwo, J. Utilization of Fruit Waste Substrates in Mushroom Production and Manipulation of Chemical Composition. Biocatal. Agric. Biotechnol. 2022, 39, 102250. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Che Yunus, M.A.; Abdul Aziz, A.H.; Md Yasir, A.S.H.; Irianto, I.; Jumakir, J.; Waluyo, W.; Suparwoto, S.; Qomariyah, L. Valorization of Peanut Skin as Agricultural Waste Using Various Extraction Methods: A Review. Molecules 2023, 28, 4325. [Google Scholar] [CrossRef]
- Mohammadi Torkashvand, A.; Alidoust, M.; Mahboub Khomami, A. The Reuse of Peanut Organic Wastes as a Growth Medium for Ornamental Plants. Int. J. Recycl. Org. Waste Agric. 2015, 4, 85–94. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Du, F. Potential Use of Peanut By-Products in Food Processing: A Review. J. Food Sci. Technol. 2012, 49, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Verma, P.; Shahi, S.K. Degradation of Polycyclic Aromatic Hydrocarbons (Phenanthrene and Pyrene) by the Ligninolytic Fungi Ganoderma lucidum Isolated from the Hardwood Stump. Bioresour. Bioprocess. 2018, 5, 11. [Google Scholar] [CrossRef]
- Bankole, P.O.; Adekunle, A.A.; Jeon, B.-H.; Govindwar, S.P. Novel Cobiomass Degradation of NSAIDs by Two Wood Rot Fungi, Ganoderma applanatum and Laetiporus sulphureus: Ligninolytic Enzymes Induction, Isotherm and Kinetic Studies. Ecotoxicol. Environ. Saf. 2020, 203, 110997. [Google Scholar] [CrossRef] [PubMed]
- Hanafiah, Z.M.; Wan Mohtar, W.H.M.; Hasan, H.A.; Jensen, H.S.; Klaus, A.; Sharil, S.; Wan-Mohtar, W.A.A.Q.I. Ability of Ganoderma lucidum Mycelial Pellets to Remove Ammonia and Organic Matter from Domestic Wastewater. Int. J. Environ. Sci. Technol. 2022, 19, 7307–7320. [Google Scholar] [CrossRef]
- Yadav, A.; Rene, E.R.; Kanti Mandal, M.; Kumar Dubey, K. Biodegradation of Cyclophosphamide and Etoposide by White Rot Fungi and Their Degradation Kinetics. Bioresour. Technol. 2022, 346, 126355. [Google Scholar] [CrossRef]
- Noble, R.; Thai, M.; Kertesz, M.A. Nitrogen Balance and Supply in Australasian Mushroom Composts. Appl. Microbiol. Biotechnol. 2024, 108, 151. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, M.K.; Yang, N. Mushroom Consumption and Cardiometabolic Health Outcomes in the General Population: A Systematic Review. Nutr. Res. Pract. 2024, 18, 165–179. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Zied, D.C.; Álvarez-Ortí, M.; Rubio, M.; Pardo, J.E. Effect of Supplementing Compost with Grapeseed Meal on Production. J. Sci. Food Agric. 2012, 92, 1665–1671. [Google Scholar] [CrossRef]
- Kamal, A.; Khair, A.; Dawlatana, M.; Hassan, M.; Begum, F.; Rahim, M. Evaluation of Aflatoxins and Pesticide Residues in Fresh and Different Processed Mushrooms. Bangladesh J. Sci. Ind. Res. 2009, 44, 193–198. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Pardo-González, J.E.; Zied, D.C. Evaluation of Harvested Mushrooms and Viability of Agaricus bisporus Growth Using Casing Materials Made from Spent Mushroom Substrate. Int. J. Food Sci. Technol. 2011, 46, 787–792. [Google Scholar] [CrossRef]
- Ferreira, D.F. SISVAR: Um Programa Para Análises e Ensino de Estatística. Rev. Cient. Symp. 2008, 6, 36–41. [Google Scholar]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 12 August 2024).
- El-Shemy, H. Soybean and Nutrition, 1st ed.IntechOpen: London, UK, 2011. [Google Scholar]
- FAO—Food and Agriculture Organizations of the United Nations. FAO Data Base. Available online: https://www.fas.usda.gov/data/world-agricultural-production (accessed on 12 August 2024).
- Zied, D.C.; Prado, E.P.; Dias, E.S.; Pardo, J.E.; Pardo-Gimenez, A. Use of Peanut Waste for Oyster Mushroom Substrate Supplementation—Oyster Mushroom and Peanut Waste. Braz. J. Microbiol. 2019, 50, 1021–1029. [Google Scholar] [CrossRef]
- Davis, J.P.; Dean, L.L. Peanut composition, flavor and nutrition. In Peanuts; Stalker, H.T., Wilson, R.F., Eds.; AOCS Press: Washington, DC, USA, 2016; pp. 289–345. [Google Scholar]
- Caitano, C.E.C.; Iossi, M.R.; Pardo-Giménez, A.; Vieira Júnior, W.G.; Dias, E.S.; Zied, D.C. Design of a Useful Diagrammatic Scale for the Quantification of Lecanicillium fungicola Disease in Agaricus bisporus Cultivation. Curr. Microbiol. 2020, 77, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Zied, D.C.; Nunes, J.S.; Nicolini, V.F.; Gimenez, A.P.; Rinker, D.L.; Dias, E.S. Tolerance to Lecanicillium fungicola and Yield of Agaricus bisporus Strains Used in Brazil. Sci. Hortic. 2015, 190, 117–122. [Google Scholar] [CrossRef]
- Harfi, T.; Alireza, M.-A.; Farzad, R.; Fariborz, Z.-N. Induced Mutation in Agaricus bisporus by Gamma Ray to Improve Genetic Variability, Degradation Enzyme Activity, and Yield. Int. J. Radiat. Biol. 2021, 97, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Foulongne-Oriol, M.; Rodier, A.; Rousseau, T.; Savoie, J.-M. Quantitative Trait Locus Mapping of Yield-Related Components and Oligogenic Control of the Cap Color of the Button Mushroom, Agaricus bisporus. Appl. Environ. Microbiol. 2012, 78, 2422–2434. [Google Scholar] [CrossRef]
- Zied, D.C.; Pardo, J.E.; Noble, R.; Pardo-Giménez, A. Efficiency of Mushrooms for Food Production—Fundamental Strategic Decision-Making. J. Food Compos. Anal. 2024, 125, 105734. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, T.; Juan, J.; Qian, W.; Zhang, J.; Chen, H.; Shen, X.; Huang, J. Lignocellulose Degradation Efficiency of Agaricus bisporus Strains Grown on Wheat Straw-Based Compost. J. Agric. Food Chem. 2023, 71, 10607–10615. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, S.; Devi, R.; Kapoor, S. Wheat Straw and Maize Stalks Based Compost for Cultivation of Agaricus bisporus. Pharma Innov. J. 2019, 8, 393–397. [Google Scholar]
- Li, C.; Xu, S. Edible Mushroom Industry in China: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- Dawadi, E.; Magar, P.B.; Bhandari, S.; Subedi, S.; Shrestha, S.; Shrestha, J. Nutritional and Post-Harvest Quality Preservation of Mushrooms: A Review. Heliyon 2022, 8, e12093. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Shivani, P.C.S.; Kumar, M.; Rani, N. Processing of Mushrooms: A Viable Option to Sustain the Growing Population of the Developing Countries. Int. J. Chem. Stud. 2020, 8, 1416–1423. [Google Scholar] [CrossRef]
- Kwiatkowski, A.; Alves, A.P.F. Importância da detecção e do controle de aflatoxinas em alimentos. SaBios 2007, 2, 45–54. [Google Scholar]
- Branà, M.T.; Cimmarusti, M.T.; Haidukowski, M.; Logrieco, A.F.; Altomare, C. Bioremediation of Aflatoxin B1-Contaminated Maize by King Oyster Mushroom (Pleurotus eryngii). PLoS ONE 2017, 12, e0182574. [Google Scholar] [CrossRef]
- Branà, M.T.; Sergio, L.; Haidukowski, M.; Logrieco, A.F.; Altomare, C. Degradation of Aflatoxin B1 by a Sustainable Enzymatic Extract from Spent Mushroom Substrate of Pleurotus eryngii. Toxins 2020, 12, 49. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Zhao, Y.; Kakumyan, P. Impact of Cultivation Substrate and Microbial Community on Improving Mushroom Productivity: A Review. Biology 2022, 11, 569. [Google Scholar] [CrossRef]
- Carrasco, J.; Tello, M.L.; de Toro, M.; Tkacz, A.; Poole, P.; Pérez-Clavijo, M.; Preston, G. Casing Microbiome Dynamics during Button Mushroom Cultivation: Implications for Dry and Wet Bubble Diseases. Microbiology 2019, 165, 611–624. [Google Scholar] [CrossRef]
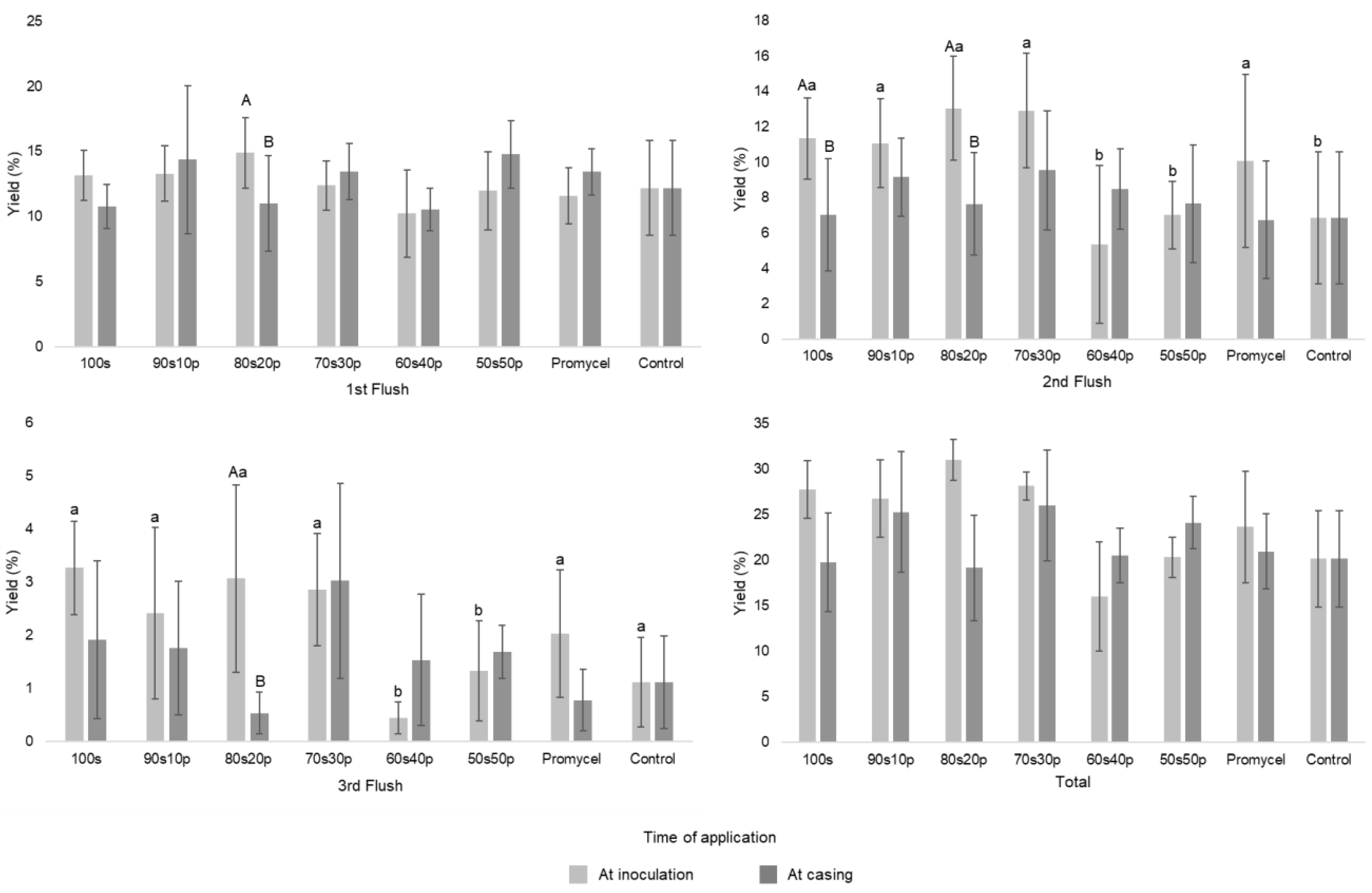
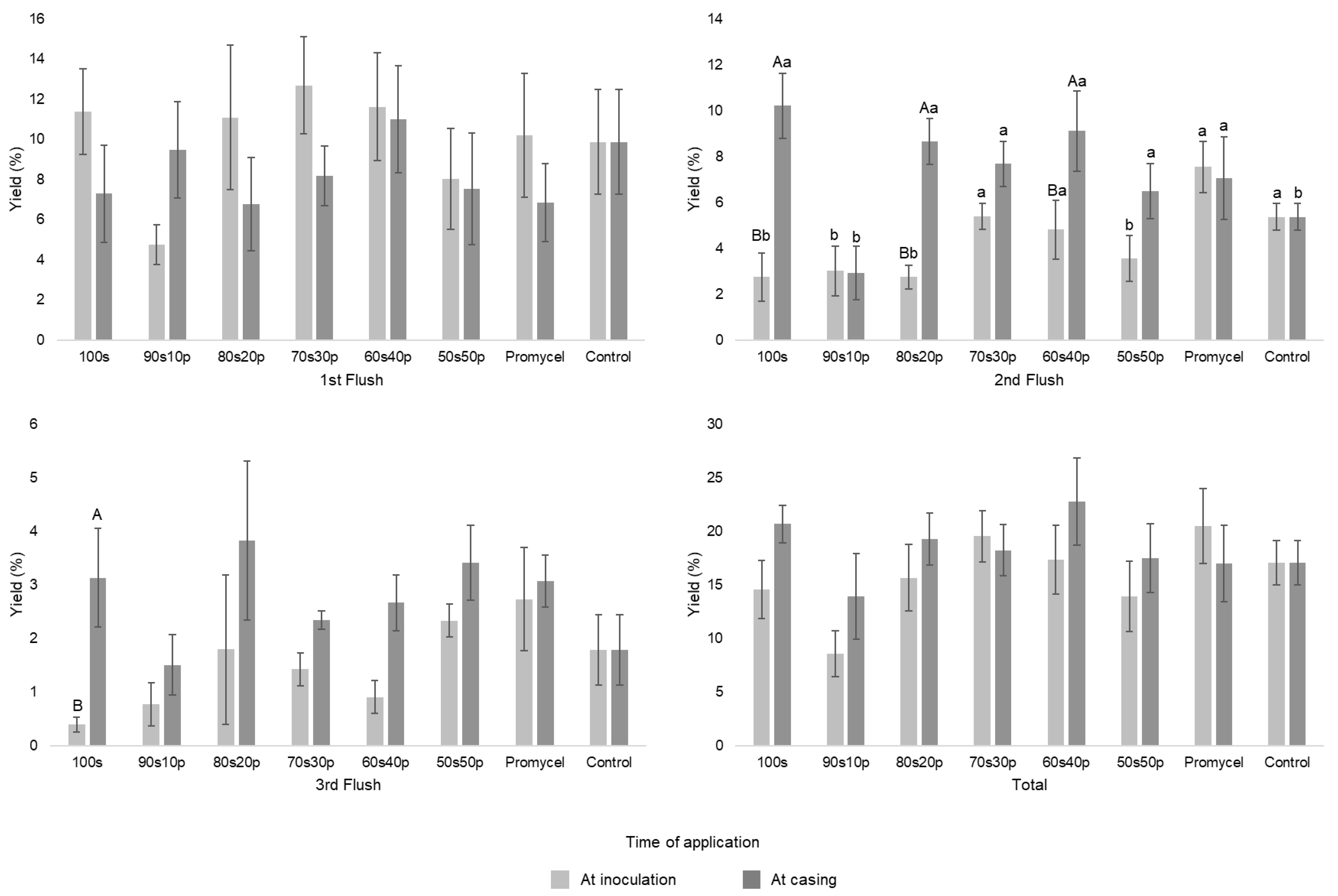

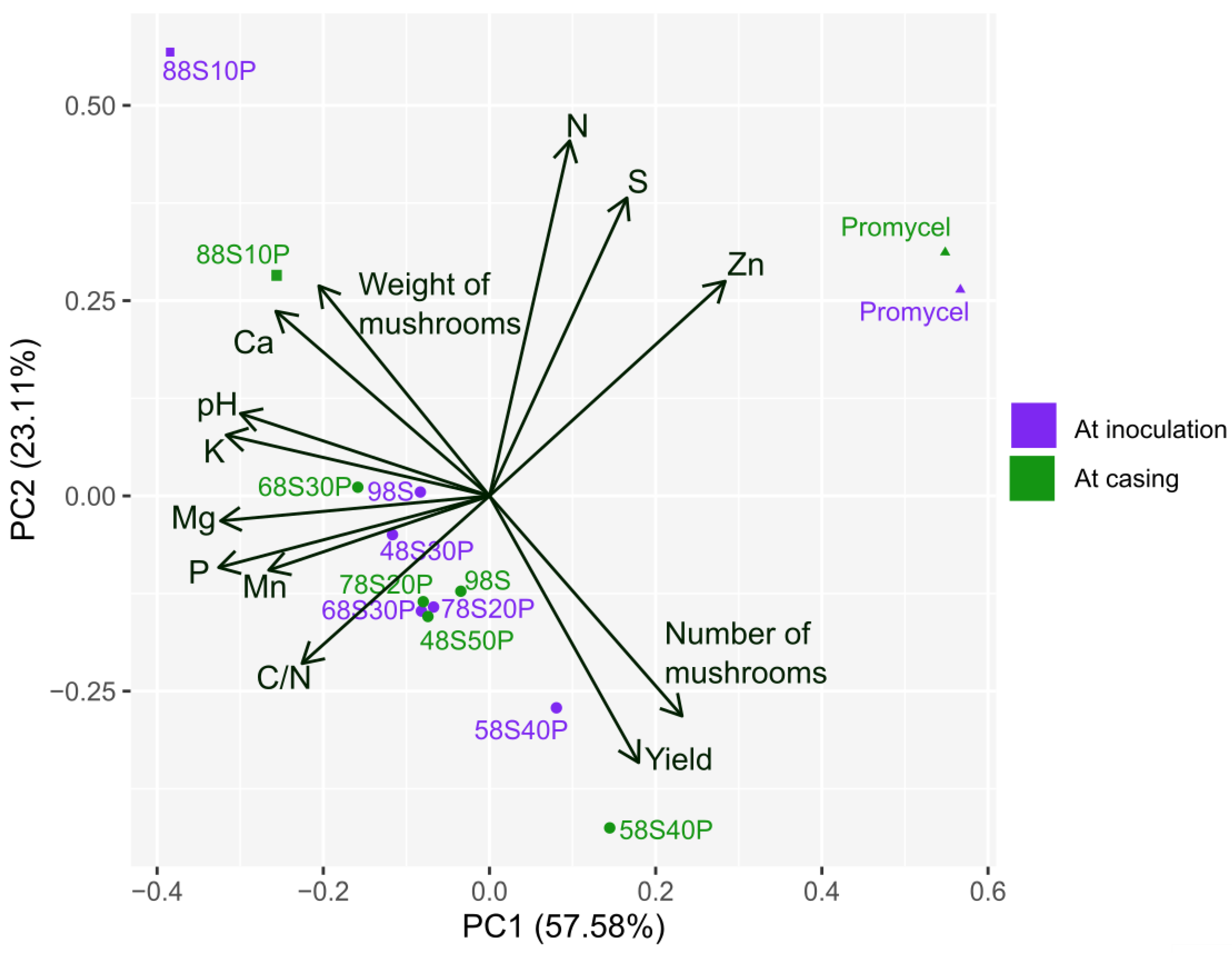
| Materials | N | P2O5 | K2O | Ca | Mg | S | M.O. | Rel. C/N | Na | Cu | Fe | Mn | Zn | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (Natural) | mg/kg (Natural) | Natural | ||||||||||||
| Substrate | 2.34 | 0.91 | 2.88 | 3.61 | 0.44 | 0.86 | 89 | 21/1 | 1899 | 120 | 1908 | 137 | 118 | 7.3 |
| Supplements | ||||||||||||||
| 100s | 5.6 | 0.98 | 2 | 0.7 | 0.4 | 0.2 | 66 | 7 | 100 | 11 | 163 | 27 | 40 | 6 |
| 90s10p | 5.7 | 1.14 | 2.4 | 0.8 | 0.5 | 0.2 | 80 | 7 | 124 | 9 | 184 | 25 | 41 | 6.6 |
| 80s20p | 5.5 | 0.96 | 1.9 | 0.7 | 0.4 | 0.1 | 76 | 7 | 105 | 8 | 432 | 25 | 37 | 6.2 |
| 70s30p | 6.1 | 1.05 | 2.1 | 0.7 | 0.4 | 0.1 | 83 | 7 | 109 | 9 | 475 | 25 | 37 | 6 |
| 60s40p | 5.6 | 0.86 | 1.8 | 0.6 | 0.3 | 0 | 86 | 7 | 110 | 7 | 200 | 22 | 38 | 6 |
| 50s50p | 5.7 | 0.96 | 1.9 | 0.7 | 0.4 | 0.1 | 86 | 8 | 110 | 7 | 200 | 22 | 39 | 6.1 |
| Promycel | 6.8 | 0.34 | 1.4 | 0.6 | 0.2 | 0.3 | 87 | 6 | na | 11 | 197 | 18 | 67 | 5.7 |
| Time of Application | Supplement | |||||||
|---|---|---|---|---|---|---|---|---|
| 100S | 90S10P * | 80S20P | 70S30P | 60S40P | 50S50P | Promycel | Control | |
| Biological Efficiency, % | ||||||||
| At inoculation | 85.15 ± 9.76 Aa | 82.19 ± 13.02 a | 95.09 ± 6.84 Aa | 86.36 ± 4.80 a | 49.20 ± 18.36 b | 62.34 ± 6.68 b | 72.60 ± 18.84 a | 61.92 ± 16.22 b |
| At casing | 60.56 ± 16.61 Bb | 77.58 ± 20.24 a | 58.82 ± 17.73 Bb | 79.85 ± 18.57 a | 63.03 ± 9.22 b | 74.02 ± 8.88 a | 64.34 ± 12.67 b | 61.92 ± 16.22b |
| Weight of mushrooms, g | ||||||||
| At inoculation | 8.59 ± 1.32 b | 9.15 ± 1.08 b | 7.44 ± 1.17 Ab | 7.73 ± 0.99 b | 10.28 ± 1.86 a | 11.75 ± 2.90 a | 10.02 ± 1.07 a | 7.82 ± 1.46 b |
| At casing | 10.95 ± 2.49 b | 9.40 ± 1.35 b | 14.36 ± 3.92 Ba | 9.68 ± 1.68 b | 10.90 ± 1.54 b | 10.23 ± 1.39 b | 10.92 ± 1.64 b | 7.82 ± 1.46 b |
| Number of mushrooms, un | ||||||||
| At inoculation | 166.8 ± 44.64 Aa | 147.8 ± 29.26 a | 213 ± 42.63 Aa | 184 ± 23.20 a | 83 ± 44.05 b | 90.4 ± 32.28 b | 120.4 ± 40.43 b | 129.6 ± 24.18 b |
| At casing | 99.6 ± 52.79 Bb | 138.4 ± 47.20 a | 70 ± 22.04 Bb | 141.2 ± 54.81 a | 97.6 ± 30.98 b | 120.8 ± 29.28 a | 98.8 ± 27.95 b | 129.6 ± 24.18 a |
| Precocity, % | ||||||||
| At inoculation | 69.51 ± 9.68 | 72.93 ± 17.01 | 70.05 ± 14.57 | 77.8 ± 8.16 | 89 ± 8.99 | 78.51 ± 11.16 | 86.53 ± 6.20 | 80.18 ± 15.03 |
| At casing | 75.96 ± 15.72 | 84.52 ± 10.41 | 79.65 ± 11.96 | 87.27 ± 7.97 | 86.49 ± 3.89 | 78.08 ± 6.00 | 91.49 ± 8.88 | 80.18 ± 15.03 |
| Time of Application | Supplement | |||||||
|---|---|---|---|---|---|---|---|---|
| 100S | 90S10P * | 80S20P | 70S30P | 60S40P | 50S50P | Promycel | Control | |
| Biological Efficiency, % | ||||||||
| At inoculation | 44.63 ± 18.61 | 26.24 ± 14.59 | 48 ± 21.13 | 59.9 ± 16.27 | 53.23 ± 22.03 | 42.74 ± 20.63 | 62.88 ± 24.03 | 52.3 ± 14.40 |
| At casing | 63.39 ± 11.87 | 42.66 ± 27.62 | 59.1 ± 16.49 | 55.88 ± 16.58 | 69.89 ± 27.98 | 53.63 ± 21.95 | 52.05 ± 24.65 | 52.3 ± 14.40 |
| Weight of mushrooms, g | ||||||||
| At inoculation | 13.25 ± 1.67 b | 23.58 ± 14.47 Aa | 12.77 ± 2.36 b | 12.12 ± 2.51 b | 12.87 ± 0.68 b | 16.79 ± 5.30b | 13.67 ± 3.98b | 15.63 ± 3.25 b |
| At casing | 14.7 ± 2.92 | 15.37 ± 3.97 B | 16.16 ± 5.18 | 15.96 ± 2.29 | 13.48 ± 2.17 | 14.46 ± 2.37 | 11.35 ± 1.07 | 15.63 ± 3.25 |
| Number of mushrooms, un | ||||||||
| At inoculation | 57.4 ± 26.28 | 34.2 ± 13.14 | 68.6 ± 40.82 | 85.8 ± 33.70 | 68.4 ± 30.24 | 60 ± 33.49 | 84.4 ± 44.93 | 54.4 ± 29.58 |
| At casing | 73.2 ± 23.30 | 57.6 ± 45.09 | 64.6 ± 27.96 | 61.4 ± 26.96 | 91.8 ± 54.09 | 64 ± 35.64 | 77.2 ± 35.64 | 54.4 ± 29.58 |
| Precocity, % | ||||||||
| At inoculation | 61.51 ± 22.06Aa | 39.54 ± 31.08 Ba | 54.72 ± 33.51 Aa | 47.02 ± 16.51 a | 53.94 ± 11.54 Aa | 20.12 ± 36.68 b | 23.39 ± 11.96 b | 43.99 ± 18.49 a |
| At casing | 27.44 ± 22.38 Bc | 68.33 ± 12.23 Aa | 20.26 ± 9.66 Bc | 28.84 ± 5.35 c | 28.76 ± 4.04 Bc | 26.51 ± 19.04 c | 16.87 ± 12.28 c | 43.99 ± 18.49 b |
| Formulation | Supplement | Mushroom (Supplementation during Inoculation) | Mushroom (Supplementation in the Casing Layer) |
|---|---|---|---|
| 90S10P | 1.50 | ND * | ND |
| 80S20P | 2.00 | ND | ND |
| 70S30P | 2.00 | ND | ND |
| 60S40P | 1.50 | ND | ND |
| 50S50P | 1.20 | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caitano, C.E.C.; Vieira Júnior, W.G.; Alves, L.d.S.; Teixeira, P.A.G.; de Paula, L.C.; Zied, D.C. Applicability Analysis of Peanut Addition to Button Mushroom Substrate Supplement Formulation. Horticulturae 2024, 10, 1088. https://doi.org/10.3390/horticulturae10101088
Caitano CEC, Vieira Júnior WG, Alves LdS, Teixeira PAG, de Paula LC, Zied DC. Applicability Analysis of Peanut Addition to Button Mushroom Substrate Supplement Formulation. Horticulturae. 2024; 10(10):1088. https://doi.org/10.3390/horticulturae10101088
Chicago/Turabian StyleCaitano, Cinthia Elen Cardoso, Wagner Gonçalves Vieira Júnior, Lucas da Silva Alves, Pedro Afonso Gomes Teixeira, Laura Cristina de Paula, and Diego Cunha Zied. 2024. "Applicability Analysis of Peanut Addition to Button Mushroom Substrate Supplement Formulation" Horticulturae 10, no. 10: 1088. https://doi.org/10.3390/horticulturae10101088
APA StyleCaitano, C. E. C., Vieira Júnior, W. G., Alves, L. d. S., Teixeira, P. A. G., de Paula, L. C., & Zied, D. C. (2024). Applicability Analysis of Peanut Addition to Button Mushroom Substrate Supplement Formulation. Horticulturae, 10(10), 1088. https://doi.org/10.3390/horticulturae10101088







