Studies on Distant Hybridization Compatibility between the Azalea (Rhododendron × hybridum hort.) and the Rhododendron decorum Franch. Native to China
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Pollination
2.3. Evaluation of Cross Fertility
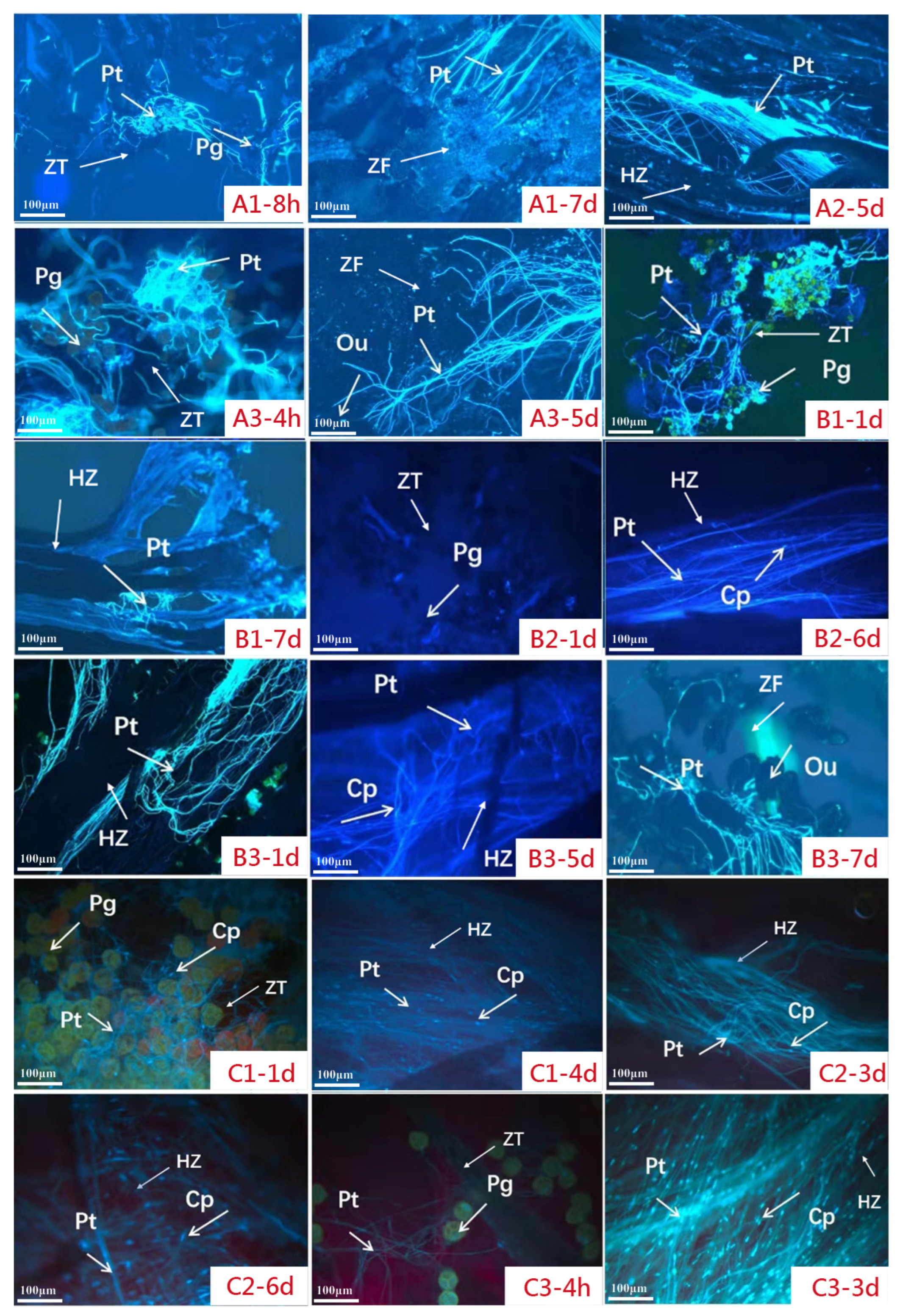
2.4. Fluorescence Observation of the Pollen Tube
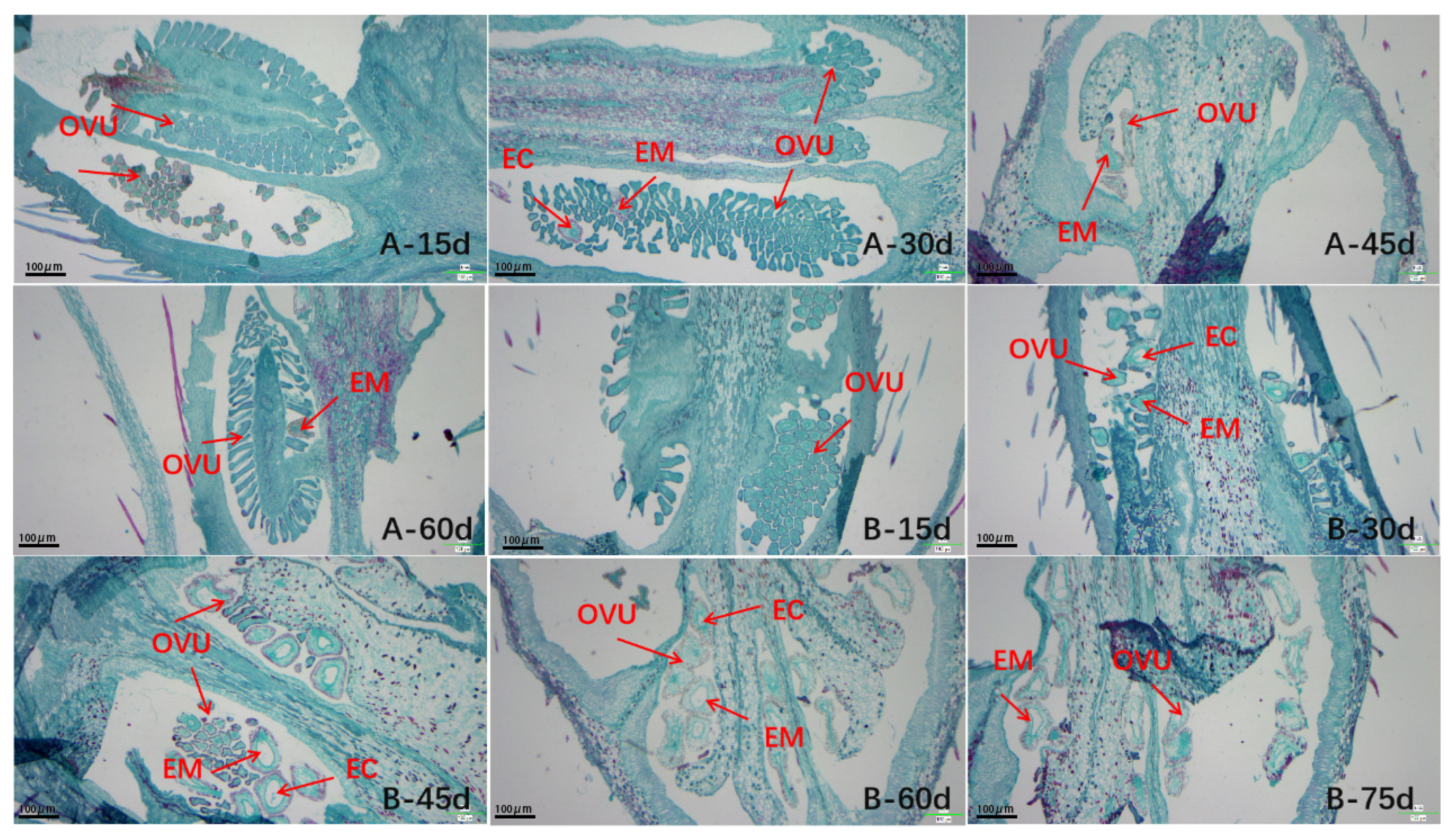
2.5. Anatomical Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of Pollination Methods on Fruit Setting
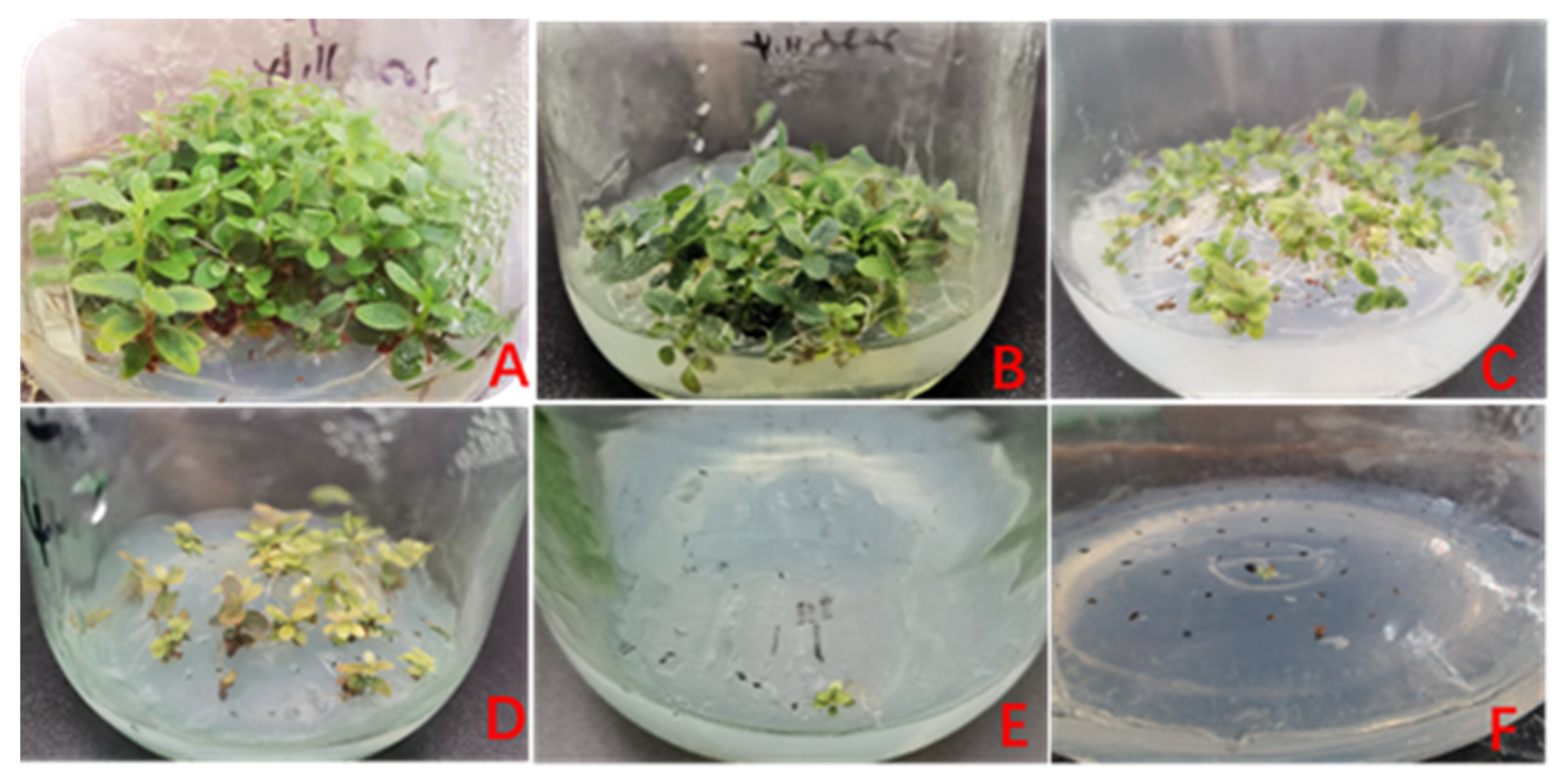
3.2. Fertility Evaluation
3.3. Pollen Tube Analysis
3.4. Observation of the Process of Embryonic Development by Paraffin Section
4. Discussion
4.1. Hybridization Compatibility Analysis
4.2. Comprehensive Evaluation of Intersubgeneric Hybridization
4.3. Overcoming the Barrier of Distant Hybridization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, X.; Yang, M.; Li, C.; Huang, S.; Wang, F.; Li, X.; Yoichi, W.; Zhang, L.; Zheng, Y.; Wang, X.; et al. Spatiotemporal evolution of the global species diversity of Rhododendron. Mol. Biol. Evol. 2022, 39, msab314. [Google Scholar] [CrossRef]
- Ma, Y.; Mao, X.; Wang, J.; Zhang, L.; Jiang, Y.; Geng, Y.; Ma, T.; Cai, L.; Huang, S.; Hollingsworth, P.; et al. Pervasive hybridization during evolutionary radiation of Rhododendron subgenus Hymenanthes in mountains of southwest China. Natl. Sci. Rev. 2022, 9, nwac276. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Fan, H.; Shen, Z.; Xie, X.; Lv, S.; Jiang, B.; Yang, G.; Yan, Y.; Wu, Z.; Wu, Y. Molecular characterization of a chalcone synthase gene RhCHS from Rhododendron × hybridum Hort. Gene 2023, 857, 147176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, Y.; Long, C. Isolation and characterization of Twenty-four Microsatellite Loci for Rhododendron decorum Franch. (Ericaceae). Hortscience 2009, 44, 2028–2030. [Google Scholar] [CrossRef]
- Min, T.L. A revision of subgenus Hymenanthes (Rhododendron, L.) in Yunnan and Xizang. Acta Bot. Yunnanica 1984, 6, 141–171. [Google Scholar]
- Zheng, S.; Yi, C.; Liu, Q.; Zhang, J. Primary study on hybridization breeding among several Rhododendron species in Yunnan province. J. Yunnan Agric. Univ. (Nat. Sci.) 2016, 31, 1052–1057. [Google Scholar]
- Sorokhaibam, S.S.; Chandra, A.; Baishya, R.; Barik, S.K.; Goel, S.; Tandon, R. Contradistinctive floral attributes, pollination guilds and their consequence on the outcrossing rate in two elevational morphs of Rhododendron arboreum Sm. Front. Plant Sci. 2004, 15, 1355680. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, S.; Oba, E.G.; Peng, L.; Wang, J.; Zhang, L.; Song, J.; Huang, H. Microscopic observation and transcriptome analysis provide insights into mechanisms of hybrid incompatibility in Rhododendron. Sci. Hortic. 2024, 336, 113417. [Google Scholar] [CrossRef]
- Huang, J.; Yang, L.; Yang, L.; Wu, X.; Cui, X.; Zhang, L.; Hui, J.; Zhao, Y.; Yang, H.; Liu, S.; et al. Stigma receptors control intraspecies and interspecies barriers in Brassicaceae. Nature 2023, 614, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Tomiczak, K.; Adamus, A.; Cegielska-Taras, T.; Kiełkowska, A.; Smyda-Dajmund, P.; Sosnowska, K.; Szała, L. Tissue Culture Techniques for the Production of Interspecific Hybrids in Poland: History and Achievements. Acta Soc. Bot. Pol. 2022, 91, 9119. [Google Scholar] [CrossRef]
- Yin, Y.; Guan, W.; Song, J.; Li, Y.; Du, J. Studies on the cross affinity of Rhododendron hybridum and Rhododendron spinuliferum (Rhododendron). J. Southwest For. Univ. 2023, 43, 173–178. [Google Scholar]
- Zhuang, P. Cross fertility of inter-subgen. Rhododendron of 32 Rhododendron species. Guihaia 2018, 38, 1566–1580. [Google Scholar]
- Williams, E.G.; Rouse, J.L.; Palser, B.F.; Knox, R.B. Reproductive Biology of Rhododendron. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1990; Volume 12, pp. 1–68. [Google Scholar]
- Eeckhaut, T.; Keyser, E.D.; Huylenbroeck, J.V.; Riek, J.D.; Bockstaele, E.J. Application of embryo rescue after interspecific crosses in the genus Rhododendron. Plant Cell Tissue Organ Cult. 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Xie, W.; Wang, J.; Peng, L.; Song, J.; Lu, L.; Xu, H.; Li, S. The compatibility and fruit growth dynamics of the cross between Rhododendron decorum and R. irroratum. Acta Agric. Univ. Jiangxiensis 2016, 38, 90–96. [Google Scholar]
- Xiong, W.; Wu, Y.; Bai, T.; Xie, W.; Dong, Y.; Zhang, J. Breeding system and hybridization affinity of Rhododendron irroratum. Bull. Bot. Res. 2024, 44, 370–379. [Google Scholar]
- Geng, X.; Zhao, H.; Wu, Y.; Zhang, Y. Cross-compatibility of wild Rhododendron and the effective evaluation indicators. Guihaia 2017, 37, 979–988. [Google Scholar]
- Ureshino, K.; Kawai, M.; Miyajima, I. Factors of intersectional unilateral cross incompatibility between several evergreen Azalea cpecies and Rhododendron japonicum f. flavum. J. Jpn. Soc. Hortic. Sci. 2000, 69, 261–265. [Google Scholar] [CrossRef]
- Lu, Y.; Kermicle, J.L.; Evans, M.M. Genetic and cellular analysis of cross-incompatibility in Zea mays. Plant Reprod. 2014, 27, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Mizuta, D.; Nakatsuka, A.; Akabane, M. Attaining inter-subgeneric hybrids in fragrant azalea breeding and the inheritance of organelle DNA. Euphytica 2008, 159, 67–72. [Google Scholar] [CrossRef]
- Xie, W.; Song, J.; Tang, L.; Li, S.; Wang, J. Cross compatibility of the cross between Rhododendron ‘XXL’ and R. cyanocarpum. Mol. Plant Breed. 2023, 21, 582–588. [Google Scholar]
- Sandstedt, G.D.; Wu, C.A.; Sweigart, A.L. Evolution of multiple postzygotic barriers between species of the Mimulus tilingii complex. Evolution 2021, 75, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, J.M.; Matute, D.R. The importance of intrinsic postzygotic barriers throughout the speciation process. Philos. Trans. B 2020, 375, 20190533. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gui, M.; Zhao, D.; Chen, M.; Ju, S.; Li, S.; Lu, Z.; Mo, X.; Wang, J. Study on reproductive barriers in 4x–2x crosses in Dianthus caryophyllus L. Euphytica 2013, 189, 471–483. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Y.; Abbas, F.; Yan, F.; Zou, X.; Yu, Y.; Gao, T.; He, J.; Wang, Q.; Yu, R.; et al. Distant heteroploid hybridization improved Hedychium floral scent, floral color and morphologcal traits. Ind. Crops Prod. 2023, 194, 116357. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, Q.; Dong, Z.; Sun, H.; Meng, Z.; Chen, X.; Tao, J.; Yu, X. Cross affinity and progeny ploidy of different ploidy herbaceous peony cultivars. J. Northeast. For. Univ. 2021, 49, 29–33+39. [Google Scholar]
- Wang, S.; Zhou, L.; Qiu, J.; Dong, J.; Yu, H.; Chai, W.; Lai, W. Comparative study on traits of progeny and parents of strawberry interspecific cross. Acta Agric. Zhejiangensis 2020, 32, 1780–1787. [Google Scholar]

| Index | Sterile | Weakly Fertile | Fertile | |||
|---|---|---|---|---|---|---|
| Threshold | Value | Threshold | Value | Threshold | Value | |
| Green seedling rate (%) | 0 < Gs < 10 | 1.0 | 10 ≤ Gs < 50 | 3.0 | Gs ≥ 50 | 5.0 |
| Green seedling coefficient | 0 < Gc < 0.6 | 0.5 | 0.6 ≤ Gc < 0.9 | 1.5 | Gc ≥ 0.9 | 2.0 |
| Fruit setting rate (%) | 0 < St < 20 | 0.5 | 20 ≤ St < 40 | 1.0 | St ≥ 40 | 1.5 |
| Number of fertile seeds per unit | 0 < Sf < 20 | 0.5 | 20 ≤ Sf < 200 | 1.0 | Sf ≥ 200 | 1.5 |
| Cross Combination (Female × Male) | Pollination Methods | Ovary Swelling Rate | Fruit Bearing Rate | Number of Seeds per Fruit | Weight of 1000 Seeds |
|---|---|---|---|---|---|
| ‘Sima’ × R. decorum | Conventional | 5.88 | 1.96 | 113.67 ± 21.13 c | 0.0683 ± 0.0081 bc |
| Early | 30.61 | 18.37 | |||
| Delayed | 13.73 | 5.88 | |||
| ‘Sima’ × ‘Fuchsia Parasol’ | Conventional | 63.46 | 55.69 | 376.33 ± 43.47 a | 0.082 ± 0.0056 a |
| Early | 71.43 | 65.71 | |||
| Delayed | 83.33 | 77.78 | |||
| ‘Yin Taohong’ × R. decorum | Conventional | 13.16 | 5.26 | 75 ± 23 c | 0.0587 ± 0.0055 c |
| Early | 11.11 | 7.41 | |||
| Delayed | 52.83 | 22.64 | |||
| ‘Yin Taohong’ × ‘Red Tiara’ | Conventional | 68.42 | 55.26 | 272.67 ± 52.70 b | 0.081 ± 0.0035 a |
| Early | 74.29 | 65.71 | |||
| Delayed | 83.78 | 72.97 | |||
| ‘Little Taohong’ × R. decorum | Conventional | 70.59 | 0.00 | 60 ± 2.64 c | 0.0423 ± 0.0093 d |
| Early | 76.92 | 3.85 | |||
| Delayed | 75.00 | 2.08 | |||
| ‘Little Taohong’ × ‘Red Tiara’ | Conventional | 63.64 | 52.27 | 304.67 ± 50.14 b | 0.0793 ± 0.0035 ab |
| Early | 74.51 | 68.63 | |||
| Delayed | 69.57 | 65.22 |
| Cross Combination (Female × Male) | Germination Rate (%) | Green Seedling Rate (%) | Green Seedling Coefficient | Number of Fertile Seeds per Unit | Comprehensive Score |
|---|---|---|---|---|---|
| ‘Sima’ × R. decorum | 29 ± 7.94 b | 23 ± 8.89 b | 0.79 | 26.14 | 5.5 |
| ‘Sima’ × ‘Fuchsia Parasol’ | 80.33 ± 1.57 a | 80.33 ± 1.57 a | 1.00 | 302.31 | 10.0 |
| ‘Yin Taohong’ × R. decorum | 2.67 ± 1.15 c | 2.00 ± 2 c | 0.88 | 1.50 | 3.0 |
| ‘Yin Taohong’ × ‘Red Tiara’ | 76.33 ± 6.81 a | 76.33 ± 6.81 a | 1.00 | 208.13 | 10.0 |
| ‘Little Taohong’ × R. decorum | 3.33 ± 2.31 c | 0.00 | 0.00 | 0.00 | 0.0 |
| ‘Little Taohong’ × ‘Red Tiara’ | 84 ± 10.58 a | 84 ± 10.58 a | 1.00 | 255.92 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Li, Y.; Yang, Y.; Song, J.; Meng, J.; Guan, W. Studies on Distant Hybridization Compatibility between the Azalea (Rhododendron × hybridum hort.) and the Rhododendron decorum Franch. Native to China. Horticulturae 2024, 10, 1089. https://doi.org/10.3390/horticulturae10101089
Hao Z, Li Y, Yang Y, Song J, Meng J, Guan W. Studies on Distant Hybridization Compatibility between the Azalea (Rhododendron × hybridum hort.) and the Rhododendron decorum Franch. Native to China. Horticulturae. 2024; 10(10):1089. https://doi.org/10.3390/horticulturae10101089
Chicago/Turabian StyleHao, Ziyao, Yefang Li, Yingying Yang, Jie Song, Jing Meng, and Wenling Guan. 2024. "Studies on Distant Hybridization Compatibility between the Azalea (Rhododendron × hybridum hort.) and the Rhododendron decorum Franch. Native to China" Horticulturae 10, no. 10: 1089. https://doi.org/10.3390/horticulturae10101089
APA StyleHao, Z., Li, Y., Yang, Y., Song, J., Meng, J., & Guan, W. (2024). Studies on Distant Hybridization Compatibility between the Azalea (Rhododendron × hybridum hort.) and the Rhododendron decorum Franch. Native to China. Horticulturae, 10(10), 1089. https://doi.org/10.3390/horticulturae10101089





