Impact of Nitrogen Limitation, Irrigation Levels, and Nitrogen-Rich Biostimulant Application on Agronomical and Chemical Traits of Hydroponically Grown Cichorium spinosum L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Design
2.2. Measurements and Statistical Analysis
3. Results
3.1. Effects of Reduced Nitrogen Rates, Drought Stress, and Biostimulant Application on Agronomical Characteristics of Stamnagathi
3.2. Effects on Leaf Chemical Characteristics
4. Discussion
4.1. Dry Matter Content as a Stress Indicator
4.2. Nitrogen Rate, Water Availability, and Biostimulant Application: The Effects on Agronomical and Chemical Characteristics of C. spinosum L.
4.3. Nitrogen Limitations Overshadow the Effects of Water Stress in the Cultivation of C. spinosum L.
4.4. Promising Results and Limitations of Nitrogen Stress Alleviation by Foliar Nitrogen-Rich Biostimulant Application
4.5. Combined Nitrogen and Water Stress Affects Leaf Calcium Content of C. spinosum
4.6. Optimizing the Fertigation Deficit Cultivation of C. spinosum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-735-14%252FINFO-DOC%252FCF14_INF01x.pdf (accessed on 10 September 2023).
- Gouel, C.; Guimbard, H. Nutrition transition and the structure of global food demand. Am. J. Agric. Econ. 2019, 101, 383–403. [Google Scholar] [CrossRef]
- Schmitt, J.; Offermann, F.; Söder, M.; Frühauf, C.; Finger, R. Extreme weather events cause significant crop yield losses at the farm level in German agriculture. Food Policy 2022, 112, 102359. [Google Scholar] [CrossRef]
- Borrelli, P.; Robinson, D.A.; Panagos, P.; Lugato, E.; Yang, J.E.; Alewell, C.; Wuepper, D.; Montanarella, L.; Ballabio, C. Land use and climate change impacts on global soil erosion by water (2015–2070). Proc. Natl. Acad. Sci. USA 2020, 117, 21994–22001. [Google Scholar] [CrossRef] [PubMed]
- Wuepper, D.; Le Clech, S.; Zilberman, D.; Mueller, N.; Finger, R. Countries influence the trade-off between crop yields and nitrogen pollution. Nat. Food 2020, 1, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Cai, G.; Sang, J.; Chen, Y.; Wang, X. Agricultural environmental footprint index based on planetary boundary: Framework and case on Chinese agriculture. J. Clean. Prod. 2023, 385, 135699. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean Wild Edible Plants: Weeds or “New Functional Crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef]
- Pinela, J.; Carocho, M.; Dias, M.I.; Caleja, C.; Barros, L.; Ferreira, I.C.F.R. Wild Plant-Based Functional Foods, Drugs, and Nutraceuticals. In Wild Plants, Mushrooms and Nuts; Wiley: Hoboken, NJ, USA, 2016; pp. 315–351. [Google Scholar]
- Guarrera, P.M.; Savo, V. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: A review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef]
- Reyes-García, V.; Menendez-Baceta, G.; Aceituno-Mata, L.; Acosta-Naranjo, R.; Calvet-Mir, L.; Domínguez, P.; Garnatje, T.; Gómez-Baggethun, E.; Molina-Bustamante, M.; Molina, M.; et al. From famine foods to delicatessen: Interpreting trends in the use of wild edible plants through cultural ecosystem services. Ecol. Econ. 2015, 120, 303–311. [Google Scholar] [CrossRef]
- Martirosyan, D.; Stratton, S. Advancing functional food regulation. Bioact. Compd. Health Dis. 2023, 6, 166–171. [Google Scholar] [CrossRef]
- Jalali, M.; Fakhri, R. Evaluation of macro and trace elements content of wild edible Iranian plants and their contribution to dietary reference intakes. J. Food Compos. Anal. 2021, 102, 104049. [Google Scholar] [CrossRef]
- Sanchez-Bel, P.; Romojaro, A.; Egea, I.; Pretel, M.T. Wild edible plants as potential antioxidant or nutritional supplements for beverages minimally processed. LWT-Food Sci. Technol. 2015, 62, 830–837. [Google Scholar] [CrossRef]
- Pinela, J.; Carvalho, A.M.; Ferreira, I.C.F.R. Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today’s society. Food Chem. Toxicol. 2017, 110, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Psaroudaki, A.; Dimitropoulakis, P.; Constantinidis, T.; Katsiotis, A.; Skaracis, G.N. Ten Indigenous Edible Plants: Contemporary Use in Eastern Crete, Greece. Cult. Agric. Food Environ. 2012, 34, 172–177. [Google Scholar] [CrossRef]
- Cos, J.; Doblas-Reyes, F.; Jury, M.; Marcos, R.; Bretonnière, P.A.; Samsó, M. The Mediterranean climate change hotspot in the CMIP5 and CMIP6 projections. Earth Syst. Dyn. 2022, 13, 321–340. [Google Scholar] [CrossRef]
- Bonofiglio, D. Mediterranean Diet and Physical Activity as Healthy Lifestyles for Human Health. Nutrients 2022, 14, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Borelli, T.; Hunter, D.; Padulosi, S.; Amaya, N.; Meldrum, G.; de Oliveira Beltrame, D.M.; Samarasinghe, G.; Wasike, V.W.; Güner, B.; Tan, A.; et al. Local solutions for sustainable food systems: The contribution of orphan crops and wild edible species. Agronomy 2020, 10, 231. [Google Scholar] [CrossRef]
- Kiers, A.M. Endive, Chicory, and their wild relatives. A systematic and phylogenetic study of Cichorium (Asteraceae). Gorteria Dutch Bot. Arch. 2000, 5, 1–77. [Google Scholar]
- Melliou, E.; Magiatis, P.; Skaltsounis, A.L. Alkylresorcinol derivatives and sesquiterpene lactones from Cichorium spinosum. J. Agric. Food Chem. 2003, 51, 1289–1292. [Google Scholar] [CrossRef]
- Zeghichi, S.; Kallithraka, S.; Simopoulos, A.P. Nutritional Composition of Molokhia (Corchorus olitorius) and Stamnagathi (Cichorium spinosum). In Plants in Human Health and Nutrition Policy; KARGER: Basel, Switzerland, 2003; pp. 1–21. [Google Scholar]
- Chatzigianni, M.; Ntatsi, G.; Theodorou, M.; Stamatakis, A.; Livieratos, I.; Rouphael, Y.; Savvas, D. Functional Quality, Mineral Composition and Biomass Production in Hydroponic Spiny Chicory (Cichorium spinosum L.) Are Modulated Interactively by Ecotype, Salinity and Nitrogen Supply. Front. Plant Sci. 2019, 10, 1040. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Vasileios, A.; Ntatsi, G.; Barros, L.; Ferreira, I.C.F.R. Chemical composition and antioxidant activity of Cichorium spinosum L. leaves in relation to developmental stage. Food Chem. 2018, 239, 946–952. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Karkanis, A.; Ntatsi, G.; Barros, L.; Ferreira, I.C.F.R. Successive harvesting affects yield, chemical composition and antioxidant activity of Cichorium spinosum L. Food Chem. 2017, 237, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ntatsi, G.; Aliferis, K.A.; Rouphael, Y.; Napolitano, F.; Makris, K.; Kalala, G.; Katopodis, G.; Savvas, D. Salinity source alters mineral composition and metabolism of Cichorium spinosum. Environ. Exp. Bot. 2017, 141, 113–123. [Google Scholar] [CrossRef]
- Voutsinos-Frantzis, O.; Ntatsi, G.; Karavidas, I.; Neofytou, I.; Deriziotis, K.; Ropokis, A.; Consentino, B.B.; Sabatino, L.; Savvas, D. Exploring the Simultaneous Effect of Total Ion Concentration and K:Ca:Mg Ratio of the Nutrient Solution on the Growth and Nutritional Value of Hydroponically Grown Cichorium spinosum L. Agronomy 2022, 12, 2214. [Google Scholar] [CrossRef]
- Klados, E.; Tzortzakis, N. Effects of substrate and salinity in hydroponically grown Cichorium spinosum. J. Soil Sci. Plant Nutr. 2014, 14, 211–222. [Google Scholar] [CrossRef]
- Thompson, R.B.; Incrocci, L.; van Ruijven, J.; Massa, D. Reducing contamination of water bodies from European vegetable production systems. Agric. Water Manag. 2020, 240, 106258. [Google Scholar] [CrossRef]
- von Wirén, N.; Gazzarrini, S.; Gojon, A.; Frommer, W.B. The molecular physiology of ammonium uptake and retrieval. Curr. Opin. Plant Biol. 2000, 3, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Valadier, M.H.; Migge, A.; Becker, T.W. Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiol. 1998, 117, 283–292. [Google Scholar] [CrossRef]
- Bowsher, C.G.; Emes, M.J.; Cammack, R.; Hucklesby, D.P. Purification and properties of nitrite reductase from roots of pea (Pisum sativum cv. Meteor). Planta 1988, 175, 334–340. [Google Scholar] [CrossRef]
- Hoff, T.; Truong, H.N.; Caboche, M. The use of mutants and transgenic plants to study nitrate assimilation. Plant. Cell Environ. 1994, 17, 489–506. [Google Scholar] [CrossRef]
- Ferrario-Mery, S.; Hodges, M.; Hirel, B.; Foyer, C.H. Photorespiration-dependent increases in phosphoenolpyruvate carboxylase, isocitrate dehydrogenase and glutamate dehydrogenase in transformed tobacco plants deficient in ferredoxin-dependent glutamine-α-ketoglutarate aminotransferase. Planta 2002, 214, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Lancien, M.; Martin, M.; Hsieh, M.H.; Leustek, T.; Goodman, H.; Coruzzi, G.M. Arabidopsis glt1-T mutant defines a role for NADH-GOGAT in the non-photorespiratory ammonium assimilatory pathway. Plant J. 2002, 29, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Groenveld, T.; Argaman, A.; Šimůnek, J.; Lazarovitch, N. Numerical modeling to optimize nitrogen fertigation with consideration of transient drought and nitrogen stress. Agric. Water Manag. 2021, 254, 106971. [Google Scholar] [CrossRef]
- Van Diest, A. Means of preventing nitrate accumulation in vegetable and pasture plants. In Fundamental, Ecological and Agricultural Aspects of Nitrogen Metabolism in Higher Plants; Springer: Dordrecht, The Netherlands, 1986; pp. 455–471. ISBN 9024732581. [Google Scholar]
- Cantliffe, D.J. Nitrate Accumulation in Vegetable Crops as Affected by Photoperiod and Light Duration1. J. Am. Soc. Hortic. Sci. 1972, 97, 414–418. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Parente, A.; Serio, F. Fertilization strategies for lowering nitrate content in leafy vegetables: Chicory and rocket salad cases. J. Plant Nutr. 1998, 21, 1791–1803. [Google Scholar] [CrossRef]
- Reinink, K.; van Nes, M.; Groenwold, R. Genetic variation for nitrate content between cultivars of endive (Cichorium endiviae L.). Euphytica 1994, 75, 41–48. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Albornoz, F. Crop responses to nitrogen overfertilization: A review. Sci. Hortic. 2016, 205, 79–83. [Google Scholar] [CrossRef]
- Agusta, H.; Kartika, J.G.; Sari, K.R. Nitrate concentration and accumulation on vegetables related to altitude and sunlight intensity. IOP Conf. Ser. Earth Environ. Sci. 2021, 896, 012052. [Google Scholar] [CrossRef]
- Broadley, M.R.; Escobar-Gutierrez, A.J.; Burns, A.; Burns, I.G. What are the effects of nitrogen deficiency on growth components of lettuce? New Phytol. 2000, 147, 519–526. [Google Scholar] [CrossRef]
- Maynard, D.N.; Barker, A.V.; Minotti, P.L.; Peck, N.H. Nitrate Accumulation in Vegetables. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 1976; pp. 71–118. [Google Scholar]
- Blom-Zandrstra, M. Nitrate accumulation in vegetables and its relationship to quality. Ann. Appl. Biol. 1989, 115, 553–561. [Google Scholar] [CrossRef]
- Zhong, L.; Blekkenhorst, L.C.; Bondonno, N.P.; Sim, M.; Woodman, R.J.; Croft, K.D.; Lewis, J.R.; Hodgson, J.M.; Bondonno, C.P. A food composition database for assessing nitrate intake from plant-based foods. Food Chem. 2022, 394, 133411. [Google Scholar] [CrossRef] [PubMed]
- Takruri, H.R.; Humeid, M.A. Nitrate Levels in Edible Wild Herbs and Vegetables Common in Jordan. Nutr. Health 1988, 6, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.X.; Miao, Y.; Ruan, J.G.; Meng, S.P.; Da Dong, J.; Yin, H.; Huang, Y.; Chen, F.R.; Wang, Z.C.; Lai, Y.F. Association between nitrite and nitrate intake and risk of gastric cancer: A systematic review and meta-analysis. Med. Sci. Monit. 2019, 25, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- R Martin, K. Dietary Nitrates, Nitrites, and Food Safety: Risks Versus Benefits. Acta Sci. Nutr. Health 2021, 5, 65–76. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Dalgaard, F.; Blekkenhorst, L.C.; Murray, K.; Lewis, J.R.; Croft, K.D.; Kyrø, C.; Torp-Pedersen, C.; Gislason, G.; Tjønneland, A.; et al. Vegetable nitrate intake, blood pressure and incident cardiovascular disease: Danish Diet, Cancer, and Health Study. Eur. J. Epidemiol. 2021, 36, 813–825. [Google Scholar] [CrossRef]
- Guffanti, D.; Cocetta, G.; Franchetti, B.M.; Ferrante, A. The Effect of Flushing on the Nitrate Content and Postharvest Quality of Lettuce (Lactuca sativa L. Var. Acephala) and Rocket (Eruca sativa Mill.) Grown in a Vertical Farm. Horticulturae 2022, 8, 604. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Papa, G.; Serio, F. Nitrate and ammonium nutrition in chicory and rocket salad plants. J. Plant Nutr. 1998, 21, 1779–1789. [Google Scholar] [CrossRef]
- Chatzigianni, M.; Alkhaled, B.; Livieratos, I.; Stamatakis, A.; Ntatsi, G.; Savvas, D. Impact of nitrogen source and supply level on growth, yield and nutritional value of two contrasting ecotypes of Cichorium spinosum L. grown hydroponically. J. Sci. Food Agric. 2018, 98, 1615–1624. [Google Scholar] [CrossRef]
- Snyder, R. Climate Change Impacts on Water Use in Horticulture. Horticulturae 2017, 3, 27. [Google Scholar] [CrossRef]
- IPCC. IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. IPCC Summ. Policymalers 2019, 1–472. [Google Scholar]
- Vila-Traver, J.; Aguilera, E.; Infante-Amate, J.; González de Molina, M. Climate change and industrialization as the main drivers of Spanish agriculture water stress. Sci. Total Environ. 2021, 760, 143399. [Google Scholar] [CrossRef] [PubMed]
- Parkash, V.; Singh, S. A review on potential plant-basedwater stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Fathidarehnijeh, E.; Nadeem, M.; Cheema, M.; Thomas, R.; Krishnapillai, M.; Galagedara, L. Current perspective on nutrient solution management strategies to improve the nutrient and water use efficiency in hydroponic systems. Can. J. Plant Sci. 2023, 104, 88–102. [Google Scholar] [CrossRef]
- Costa França, M.G.; Pham Thi, A.T.; Pimentel, C.; Pereyra Rossiello, R.O.; Zuily-Fodil, Y.; Laffray, D. Differences in growth and water relations among Phaseolus vulgaris cultivars in response to induced drought stress. Environ. Exp. Bot. 2000, 43, 227–237. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Wu, F.; Bao, W.; Li, F.; Wu, N. Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ. Exp. Bot. 2008, 63, 248–255. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar Applications of Protein Hydrolysate, Plant and Seaweed Extracts Increase Yield but Differentially Modulate Fruit Quality of Greenhouse Tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Consentino, B.B.; Virga, G.; la Placa, G.G.; Sabatino, L.; Rouphael, Y.; Ntatsi, G.; Iapichino, G.; la Bella, S.; Mauro, R.P.; D’Anna, F.; et al. Celery (Apium graveolens L.) Performances as Subjected to Different Sources of Protein Hydrolysates. Plants 2020, 9, 1633. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, L.; Consentino, B.B.; Rouphael, Y.; De Pasquale, C.; Iapichino, G.; D’Anna, F.; La Bella, S. Protein Hydrolysates and Mo-Biofortification Interactively Modulate Plant Performance and Quality of ‘Canasta’ Lettuce Grown in a Protected Environment. Agronomy 2021, 11, 1023. [Google Scholar] [CrossRef]
- Sabatino, L.; Ntatsi, G.; La Bella, S.; Rouphael, Y.; De Pasquale, C.; Consentino, B.B. Impact of plant-based protein hydrolysate and different iodine doses on celery plant production and quality. Acta Hortic. 2023, 1377, 837–844. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [PubMed]
- Marketsandmarkets. Global Biostimulants Market (2021–2026) by Active Ingredient, Application Method, Crop Type, Form, Geography and the Impact of COVID-19 with Ansoff Analysis. 2021. Available online: https://www.marketresearch.com/Food-Beverage-c84/ (accessed on 19 April 2024).
- Fernandez, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. CRC Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef]
- Consentino, B.B.; Vultaggio, L.; Sabatino, L.; Ntatsi, G.; Rouphael, Y.; Bondì, C.; De Pasquale, C.; Guarino, V.; Iacuzzi, N.; Capodici, G.; et al. Combined effects of biostimulants, N level and drought stress on yield, quality and physiology of greenhouse-grown basil. Plant Stress 2023, 10, 100268. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Cristofano, F.; Colla, G.; Pii, Y.; Secomandi, E.; De Gregorio, M.; Buffagni, V.; Garcia-Perez, P.; Lucini, L.; Rouphael, Y. Vegetal-derived biostimulants distinctively command the physiological and metabolomic signatures of lettuce grown in depleted nitrogen conditions. Sci. Hortic. 2023, 317, 112057. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Voutsinos-Frantzis, O.; Savvas, D.; Antoniadou, N.; Karavidas, I.; Ntanasi, T.; Sabatino, L.; Ntatsi, G. Innovative Cultivation Practices for Reducing Nitrate Content in Baby Leaf Lettuce Grown in a Vertical Farm. Horticulturae 2024, 10, 375. [Google Scholar] [CrossRef]
- Voutsinos-Frantzis, O.; Karavidas, I.; Petropoulos, D.; Zioviris, G.; Fortis, D.; Ntanasi, T.; Ropokis, A.; Karkanis, A.; Sabatino, L.; Savvas, D.; et al. Effects of NaCl and CaCl2 as Eustress Factors on Growth, Yield, and Mineral Composition of Hydroponically Grown Valerianella locusta. Plants 2023, 12, 1454. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Haroon, M.H.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- The Perkin-Elmer Corporation. Analytical Methods for Atomic Absorption Spectroscopy, 4th ed.; The Perkin-Elmer Corporation: Waltham, MA, USA, 1996. [Google Scholar]
- Ramsay, J.; Brown, R.H.; Falloon, S.W.H. Simultaneous Determination of Sodium and Potassium in Small Volumes of Fluid by Flame Photometry. J. Exp. Biol. 1953, 30, 1–17. [Google Scholar] [CrossRef]
- Blumenthal, D.M.; Mueller, K.E.; Kray, J.A.; Ocheltree, T.W.; Augustine, D.J.; Wilcox, K.R. Traits link drought resistance with herbivore defence and plant economics in semi-arid grasslands: The central roles of phenology and leaf dry matter content. J. Ecol. 2020, 108, 2336–2351. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Schiattone, M.I.; Boari, F.; Cantore, V.; Castronuovo, D.; Denora, M.; Di Venere, D.; Perniola, M.; Sergio, L.; Todorovic, M.; Candido, V. Effect of water regime, nitrogen level and biostimulants application on yield and quality traits of wild rocket [Diplotaxis tenuifolia (L.) DC.]. Agric. Water Manag. 2023, 277, 108078. [Google Scholar] [CrossRef]
- Valenzuela, H. Optimizing the Nitrogen Use Efficiency in Vegetable Crops. Nitrogen 2024, 5, 106–143. [Google Scholar] [CrossRef]
- Whetton, R.L.; Harty, M.A.; Holden, N.M. Communicating Nitrogen Loss Mechanisms for Improving Nitrogen Use Efficiency Management, Focused on Global Wheat. Nitrogen 2022, 3, 213–246. [Google Scholar] [CrossRef]
- Lawlor, D.W. Carbon and nitrogen assimilation in relation to yield: Mechanisms are the key to understanding production systems. J. Exp. Bot. 2002, 53, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and Defence Mechanisms of Vegetable Crops against Drought, Heat and Salinity Stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Consentino, B.B.; Ciriello, M.; Sabatino, L.; Vultaggio, L.; Baldassano, S.; Vasto, S.; Rouphael, Y.; La Bella, S.; De Pascale, S. Current Acquaintance on Agronomic Biofortification to Modulate the Yield and Functional Value of Vegetable Crops: A Review. Horticulturae 2023, 9, 219. [Google Scholar] [CrossRef]
- Chatzigianni, M.; Aliferis, K.A.; Ntatsi, G.; Savvas, D. Effect of N Supply Level and N Source Ratio on Cichorium spinosum L. Metabolism. Agronomy 2020, 10, 952. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C.F.R. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Morad-Talab, N.; Abd-Allah, E.F.; Ahmad, P.; Hajiboland, R. Plant growth under drought stress. In Water Stress and Crop Plants; Wiley: Hoboken, NJ, USA, 2016; Volume 2, pp. 649–668. ISBN 9781119054450. [Google Scholar]
- The European Commission Commission Regulation (EU) No 1258/2011 of 2 December 2011 amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs Text with EEA Relevance. Available online: https://faolex.fao.org/docs/pdf/eur108181.pdf (accessed on 4 April 2023).
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Y.; Zhang, X.; Li, T.; Grundy, S.; Yang, Q.; Cheng, R. A Review of Environment Effects on Nitrate Accumulation in Leafy Vegetables Grown in Controlled Environments. Foods 2020, 9, 732. [Google Scholar] [CrossRef]
- Chen, B.M.; Wang, Z.H.; Li, S.X.; Wang, G.X.; Song, H.X.; Wang, X.N. Effects of nitrate supply on plant growth, nitrate accumulation, metabolic nitrate concentration and nitrate reductase activity in three leafy vegetables. Plant Sci. 2004, 167, 635–643. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A. Producing Nitrate-free Endive Heads: Effect of Nitrogen Form on Growth, Yield, and Ion Composition of Endive. J. Am. Soc. Hortic. Sci. 1997, 122, 140–145. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Nemali, K.S.; van Iersel, M.W. An automated system for controlling drought stress and irrigation in potted plants. Sci. Hortic. 2006, 110, 292–297. [Google Scholar] [CrossRef]
- Giuffrida, F.; Lipari, V. Leaching irigation to prevent salt accumulation in the substrate. Acta Hortic. 2003, 609, 435–440. [Google Scholar] [CrossRef]
- Giuffrida, F.; Argento, S.; Lipari, V.; Leonardi, C. Methods for controling salt accumulation in substrate cultivation. Acta Hortic. 2003, 614, 799–803. [Google Scholar] [CrossRef]
- Polyzos, N.; Paschoalinotto, B.H.; Compocholi, M.; Pinela, J.; Heleno, S.A.; Calhelha, R.C.; Dias, M.I.; Barros, L.; Petropoulos, S.A. Fertilization of Pot-Grown Cichorium spinosum L.: How It Can Affect Plant Growth, Chemical Profile, and Bioactivities of Edible Parts? Horticulturae 2022, 8, 890. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef]
- Ullah, I.; Mao, H.; Rasool, G.; Gao, H.; Javed, Q.; Sarwar, A.; Khan, M.I. Effect of deficit irrigation and reduced n fertilization on plant growth, root morphology and water use efficiency of tomato grown in soilless culture. Agronomy 2021, 11, 228. [Google Scholar] [CrossRef]
- Ru, C.; Wang, K.; Hu, X.; Chen, D.; Wang, W.; Yang, H. Nitrogen Modulates the Effects of Heat, Drought, and Combined Stresses on Photosynthesis, Antioxidant Capacity, Cell Osmoregulation, and Grain Yield in Winter Wheat. J. Plant Growth Regul. 2023, 42, 1681–1703. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y. Independent and combined influence of drought stress and nitrogen deficiency on physiological and proteomic changes of barley leaves. Environ. Exp. Bot. 2023, 210, 105346. [Google Scholar] [CrossRef]
- Hu, J.; Ma, W.; Wang, Z. Effects of nitrogen addition and drought on the relationship between nitrogen- and water-use efficiency in a temperate grassland. Ecol. Process. 2023, 12, 36. [Google Scholar] [CrossRef]
- Palta, J.P. Stress Interactions at the Cellular and Membrane Levels. HortScience 1990, 25, 1377–1381. [Google Scholar] [CrossRef]


| Culturing Practices | Date | Days after Sowing |
|---|---|---|
| Sowing | 30 December 2022 | 0 |
| Transplanting | 3 February 2023 | 35 |
| Harvest | 13 March 2023 | 73 |
| Element | Units | Control (NR100) | Limited Nitrogen (NR30) |
|---|---|---|---|
| NO3− | mmol L−1 | 12.00 | 4.00 |
| NH4+ | mmol L−1 | 1.64 | 0.55 |
| NH4+/Total-N | - | 0.12 | 0.12 |
| K+ | mmol L−1 | 6.71 | 6.98 |
| Ca2+ | mmol L−1 | 3.70 | 3.85 |
| Mg2− | mmol L−1 | 2.07 | 2.16 |
| SO42− | mmol L−1 | 3.11 | 4.41 |
| H2PO4− | mmol L−1 | 1.46 | 1.46 |
| Fe | μmol L−1 | 17.89 | 17.89 |
| Mn2+ | μmol L−1 | 9.36 | 9.36 |
| Zn2+ | μmol L−1 | 4.47 | 4.47 |
| Cu2+ | μmol L−1 | 0.73 | 0.73 |
| B | μmol L−1 | 27.56 | 27.56 |
| Mo | μmol L−1 | 0.52 | 0.52 |
| Si | mmol L−1 | 0.00 | 0.00 |
| Cl− | mmol L−1 | 0.40 | 6.00 |
| Na+ | mmol L−1 | 0.60 | 0.60 |
| HCO3− | mmol L−1 | 0.40 | 0.40 |
| Other properties | |||
| EC * | dS m−1 | 2.5 | 2.5 |
| pH | - | 5.6 | 5.6 |
| Ψs ** | MPa | −0.20 | −0.20 |
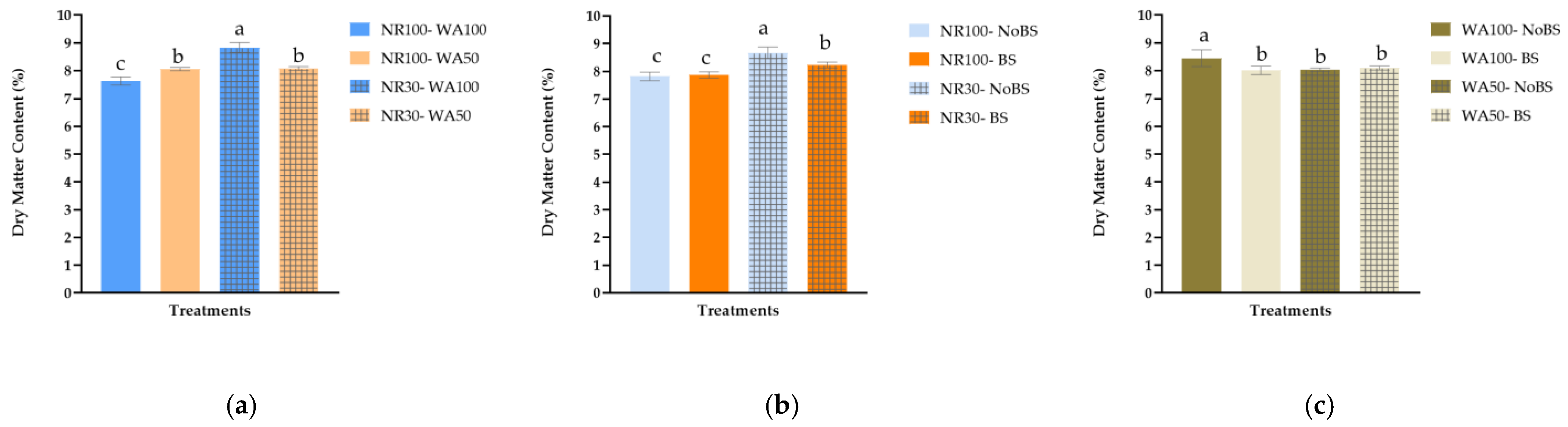
| Factor | Sources of Variation | LN (No. Plant−1) | LA (cm2 Plant−1) | LFW (g Plant−1) | LDW (g Plant−1) | DMC (%) |
|---|---|---|---|---|---|---|
| NR | NR100 | 16.78 ± 0.39 a | 252.88 ± 4.96 a | 17.87 ± 0.39 a | 1.40 ± 0.03 a | 7.85 ± 0.09 b |
| NR30 | 14.51 ± 0.28 b | 189.84 ± 5.65 b | 14.16 ± 0.31 b | 1.19 ± 0.02 b | 8.45 ± 0.12 a | |
| WA | WA100 | 16.35 ± 0.47 a | 229.61 ± 9.37 a | 16.47 ± 0.65 a | 1.33 ± 0.04 a | 8.23 ± 0.17 a |
| WA50 | 14.93 ± 0.28 b | 213.11 ± 7.01 b | 15.56 ± 0.32 b | 1.26 ± 0.03 b | 8.07 ± 0.05 a | |
| B | NoBS | 15.08 ± 0.46 b | 212.84 ± 9.59 b | 15.46 ± 0.59 b | 1.26 ± 0.04 b | 8.25 ± 0.15 a |
| BS | 16.21 ± 0.32 a | 229.88 ± 6.68 a | 16.57 ± 0.40 a | 1.33 ± 0.03 a | 8.05 ± 0.08 a |
| Factor Interaction | Sources of Variation | LN (No. Plant−1) | LA (cm2 Plant−1) | LDW (g Plant−1) | |
|---|---|---|---|---|---|
| NR × WA | NR100 | WA100 | 18.06 ± 0.48 a | 267.99 ± 6.27 a | 1.46 ± 0.04 a |
| WA50 | 15.50 ± 0.31 b | 237.77 ± 4.68 b | 1.34 ± 0.02 a | ||
| NR30 | WA100 | 14.65 ± 0.4 bc | 191.23 ± 7.74 c | 1.21 ± 0.03 a | |
| WA50 | 14.36 ± 0.4 c | 188.45 ± 8.55 c | 1.18 ± 0.04 a | ||
| NR × B | NR100 | NoBS | 16.71 ± 0.58 a | 252.55 ± 6.14 a | 1.38 ± 0.04 a |
| BS | 16.85 ± 0.53 a | 253.21 ± 8.07 a | 1.41 ± 0.04 a | ||
| NR30 | NoBS | 13.44 ± 0.21 c | 173.13 ± 7.75 c | 1.14 ± 0.03 a | |
| BS | 15.57 ± 0.26 b | 206.55 ± 4.74 b | 1.25 ± 0.02 a | ||
| WA × B | WA100 | NoBS | 15.82 ± 0.77 a | 221.53 ± 14.58 a | 1.32 ± 0.05 a |
| BS | 16.89 ± 0.52 a | 237.69 ± 11.95 a | 1.35 ± 0.05 a | ||
| WA50 | NoBS | 14.33 ± 0.41 a | 204.15 ± 12.58 a | 1.20 ± 0.04 a | |
| BS | 15.53 ± 0.28 a | 222.07 ± 5.72 a | 1.31 ± 0.03 a | ||
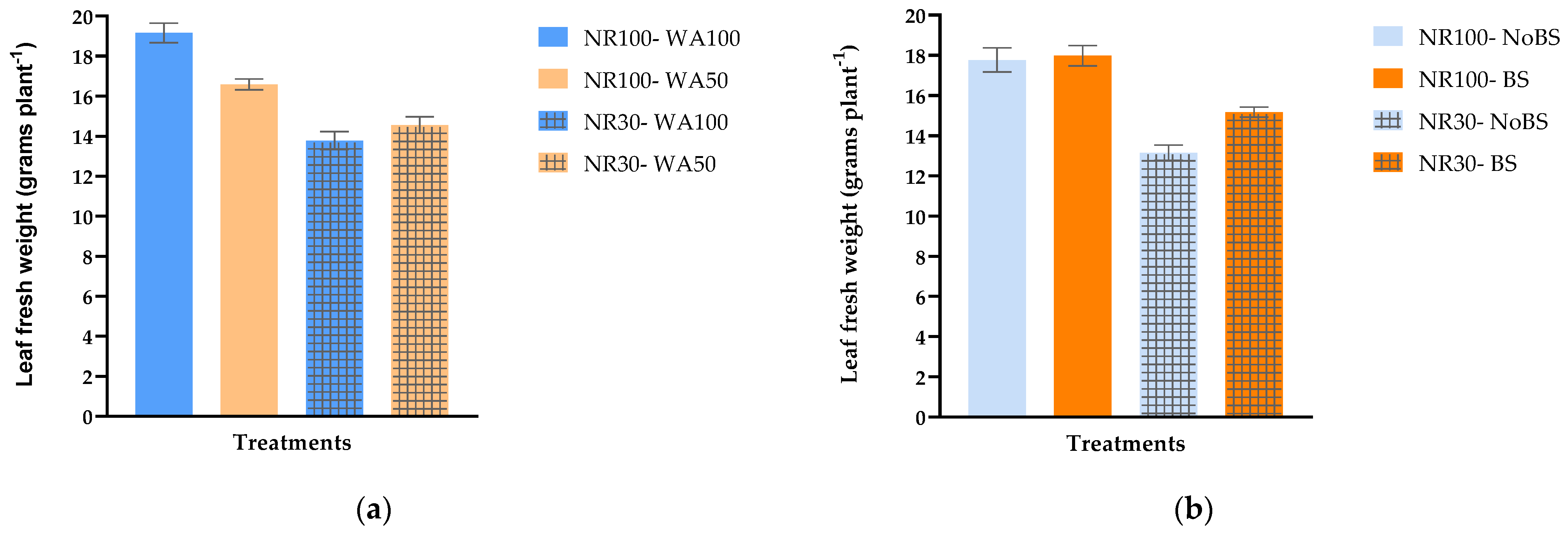
| NR | WA | B | LN (No. Plant−1) | LA (cm2 Plant−1) | LFW (g Plant−1) | LDW (g Plant−1) |
|---|---|---|---|---|---|---|
| NR100 | WA100 | NoBS | 18.08 ± 0.71 | 264.08 ± 8.22 | 19.22 ± 0.74 | 1.46 ± 0.05 |
| BS | 18.03 ± 0.72 | 271.91 ± 9.98 | 19.10 ± 0.73 | 1.46 ± 0.07 | ||
| WA50 | NoBS | 15.33 ± 0.49 | 241.02 ± 6.71 | 16.31 ± 0.43 | 1.31 ± 0.04 | |
| BS | 15.67 ± 0.42 | 234.51 ± 6.87 | 16.85 ± 0.34 | 1.36 ± 0.02 | ||
| NR30 | WA100 | NoBS | 13.56 ± 0.28 | 178.99 ± 12.00 | 12.70 ± 0.61 | 1.18 ± 0.05 |
| BS | 15.75 ± 0.36 | 203.46 ± 7.74 | 14.84 ± 0.20 | 1.24 ± 0.02 | ||
| WA50 | NoBS | 13.33 ± 0.34 | 167.27 ± 10.33 | 13.61 ± 0.42 | 1.10 ± 0.04 | |
| BS | 15.39 ± 0.41 | 209.63 ± 5.93 | 15.49 ± 0.44 | 1.25 ± 0.04 |
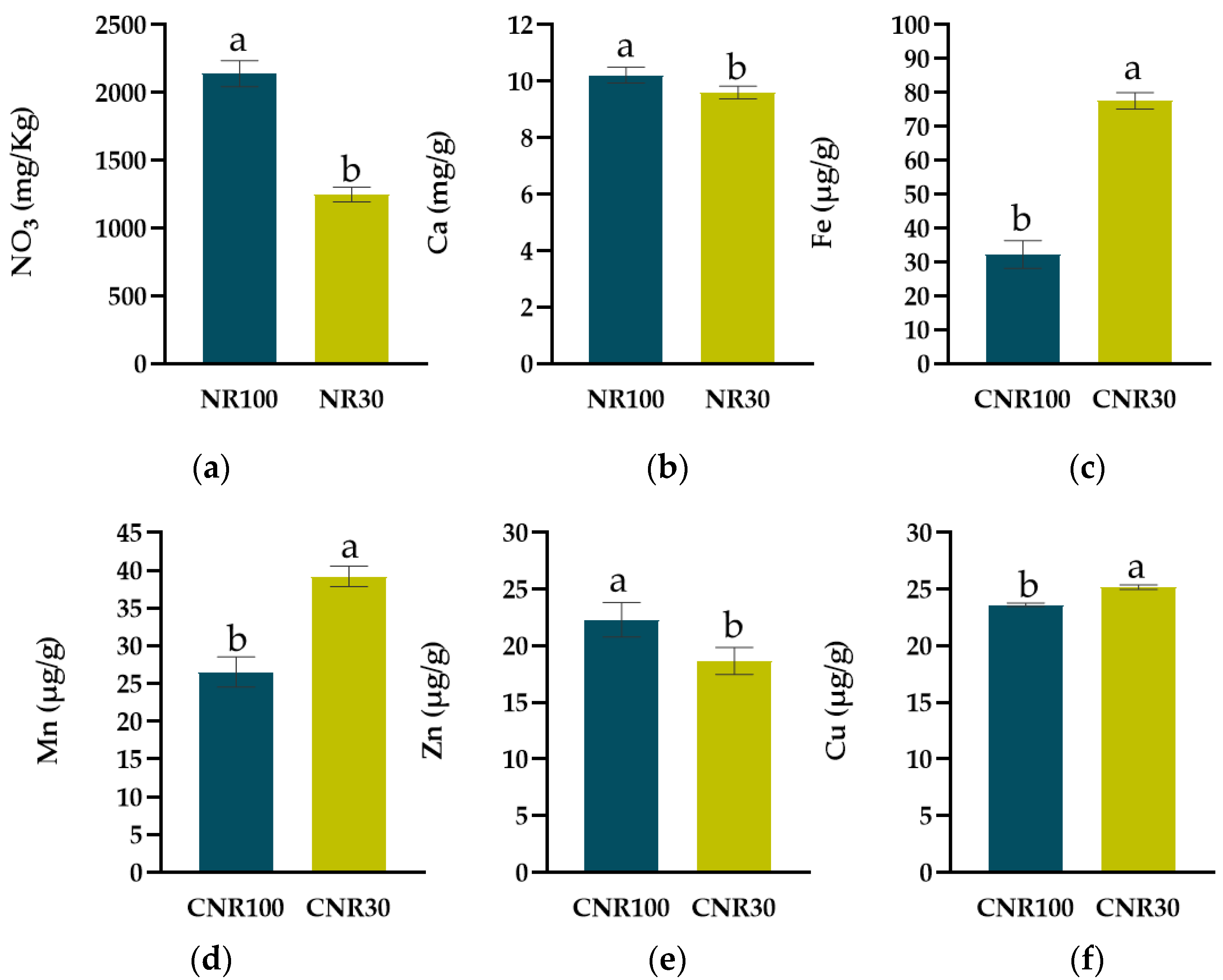
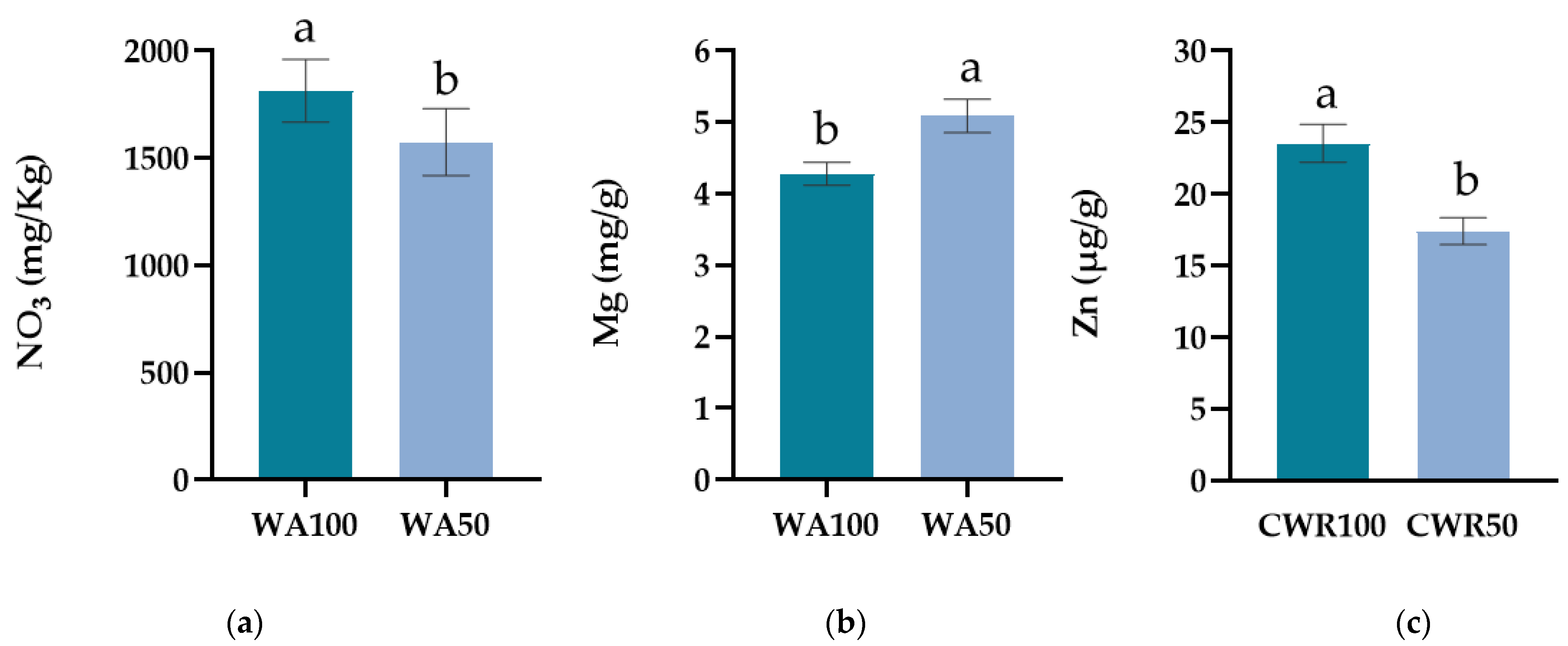
| NR | WA | B | NO3 (mg Kg−1) | K (mg g−1) | Mg (mg g−1) | Ca (mg g−1) |
|---|---|---|---|---|---|---|
| NR100 | WA100 | NoBS | 2186.71 ± 181.75 | 70.00 ± 1.15 | 4.20 ± 0.10 | 10.00 ± 0.24 |
| BS | 2301.36 ± 247.95 | 58.00 ± 4.16 | 3.96 ± 0.38 | 8.95 ± 0.35 | ||
| WA50 | NoBS | 1990.53 ± 158.94 | 69.33 ± 4.67 | 5.04 ± 0.26 | 11.06 ± 0.18 | |
| BS | 2084.01 ± 240.42 | 67.33 ± 3.53 | 4.83 ± 0.61 | 10.88 ± 0.39 | ||
| NR30 | WA100 | NoBS | 1464.52 ± 33.14 | 66.67 ± 1.33 | 4.41 ± 0.48 | 9.39 ± 0.03 |
| BS | 1305.19 ± 43.17 | 61.33 ± 0.67 | 4.56 ± 0.23 | 10.37 ± 0.23 | ||
| WA50 | NoBS | 1132.46 ± 79.41 | 63.33 ± 5.21 | 5.01 ± 0.22 | 9.38 ± 0.50 | |
| BS | 1092.81 ± 108.86 | 64.00 ± 1.15 | 5.48 ± 0.73 | 9.30 ± 0.64 |
| NR | WA | B | Fe (μg g−1) | Na (mg g−1) | Mn (μg g−1) | Zn (μg g−1) | Cu (μg g−1) |
|---|---|---|---|---|---|---|---|
| NR100 | WA100 | NoBS | 31.89 ± 8.18 | 2.64 ± 0.22 | 26.83 ± 6.42 | 23.61 ± 2.64 | 23.95 ± 0.31 |
| BS | 31.97 ± 3.36 | 2.56 ± 0.36 | 26.73 ± 1.47 | 27.74 ± 3.14 | 23.77 ± 0.25 | ||
| WA50 | NoBS | 40.61 ± 10.69 | 2.93 ± 0.37 | 28.46 ± 4.27 | 20.05 ± 0.54 | 23.05 ± 0.08 | |
| BS | 24.65 ± 10.65 | 3.12 ± 0.60 | 24.16 ± 4.70 | 17.86 ± 2.49 | 23.73 ± 0.27 | ||
| NR30 | WA100 | NoBS | 72.58 ± 4.02 | 2.48 ± 0.52 | 39.31 ± 2.31 | 21.64 ± 2.13 | 25.16 ± 0.48 |
| BS | 84.66 ± 0.36 | 2.08 ± 0.08 | 44.95 ± 2.11 | 21.25 ± 2.04 | 25.46 ± 0.44 | ||
| WA50 | NoBS | 76.77 ± 3.70 | 2.40 ± 0.24 | 37.77 ± 0.53 | 18.14 ± 0.34 | 25.11 ± 0.05 | |
| BS | 76.52 ± 7.94 | 2.45 ± 0.14 | 34.83 ± 1.92 | 13.67 ± 1.48 | 24.98 ± 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voutsinos-Frantzis, O.; Karavidas, I.; Savvas, D.; Ntanasi, T.; Kaimpalis, V.; Consentino, B.B.; Aliferis, K.A.; Karkanis, A.; Sabatino, L.; Ntatsi, G. Impact of Nitrogen Limitation, Irrigation Levels, and Nitrogen-Rich Biostimulant Application on Agronomical and Chemical Traits of Hydroponically Grown Cichorium spinosum L. Horticulturae 2024, 10, 1063. https://doi.org/10.3390/horticulturae10101063
Voutsinos-Frantzis O, Karavidas I, Savvas D, Ntanasi T, Kaimpalis V, Consentino BB, Aliferis KA, Karkanis A, Sabatino L, Ntatsi G. Impact of Nitrogen Limitation, Irrigation Levels, and Nitrogen-Rich Biostimulant Application on Agronomical and Chemical Traits of Hydroponically Grown Cichorium spinosum L. Horticulturae. 2024; 10(10):1063. https://doi.org/10.3390/horticulturae10101063
Chicago/Turabian StyleVoutsinos-Frantzis, Orfeas, Ioannis Karavidas, Dimitrios Savvas, Theodora Ntanasi, Vasileios Kaimpalis, Beppe Benedetto Consentino, Konstantinos A. Aliferis, Anestis Karkanis, Leo Sabatino, and Georgia Ntatsi. 2024. "Impact of Nitrogen Limitation, Irrigation Levels, and Nitrogen-Rich Biostimulant Application on Agronomical and Chemical Traits of Hydroponically Grown Cichorium spinosum L." Horticulturae 10, no. 10: 1063. https://doi.org/10.3390/horticulturae10101063
APA StyleVoutsinos-Frantzis, O., Karavidas, I., Savvas, D., Ntanasi, T., Kaimpalis, V., Consentino, B. B., Aliferis, K. A., Karkanis, A., Sabatino, L., & Ntatsi, G. (2024). Impact of Nitrogen Limitation, Irrigation Levels, and Nitrogen-Rich Biostimulant Application on Agronomical and Chemical Traits of Hydroponically Grown Cichorium spinosum L. Horticulturae, 10(10), 1063. https://doi.org/10.3390/horticulturae10101063












