Effects of Water-Deficit Stress on the Growth and Physiological Characteristics of Chloranthus spicatus Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Materials
2.2. Experimental Set-Up
2.3. Measurements
2.3.1. Growth Parameters
2.3.2. Photosynthesis Parameters
2.3.3. Physiological Indicators
2.4. Statistics and Data Analysis
3. Results
3.1. Shoot Length and Diameter Differed among C. spicatus Seedlings Subjected to Different WD Stress Treatments
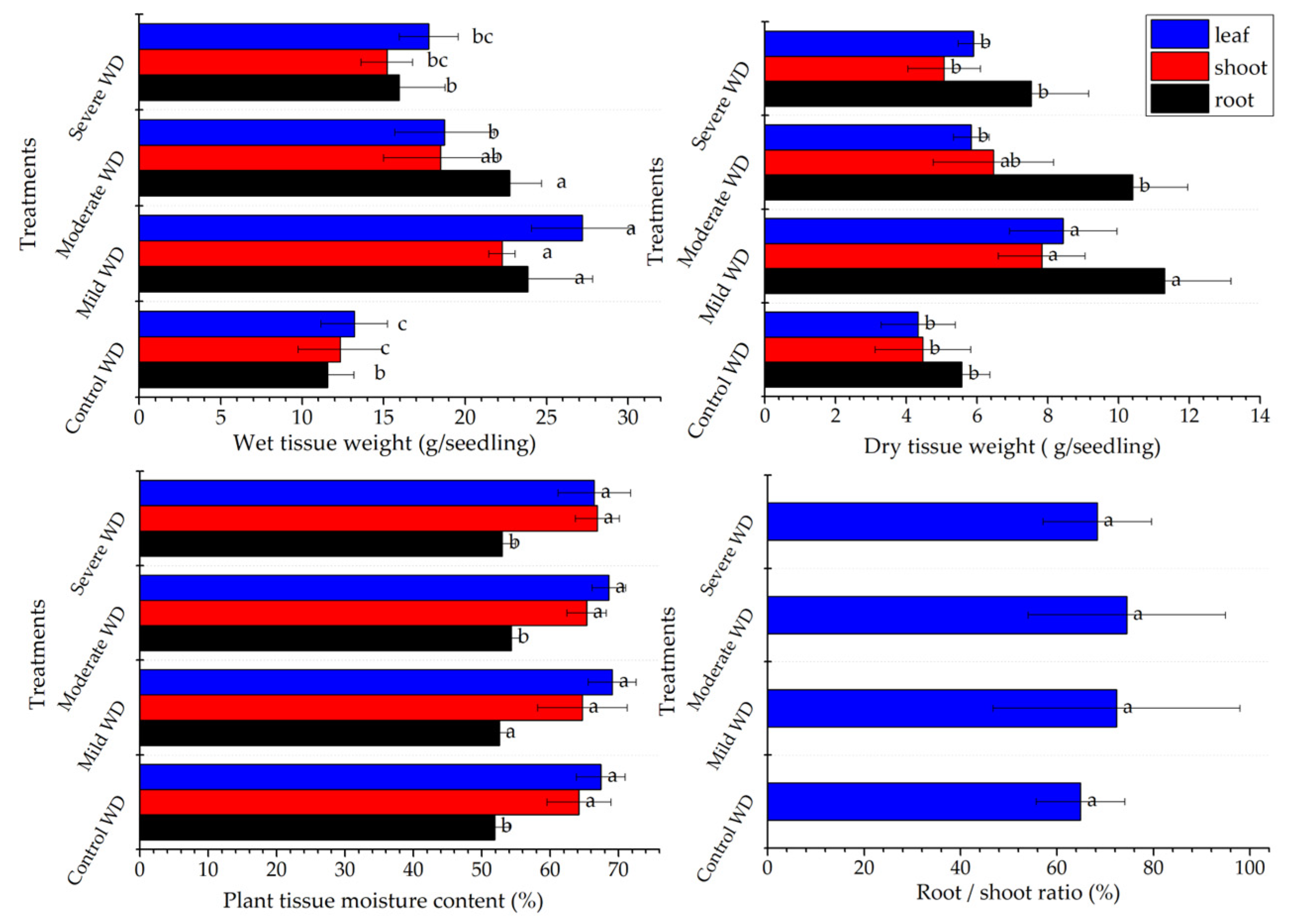
3.2. C. spicatus Seedling Biomass Varied in Response to WD Stress Treatments
3.3. Changes in Photosynthesis Parameters of C. spicatus in Response to WD Stress
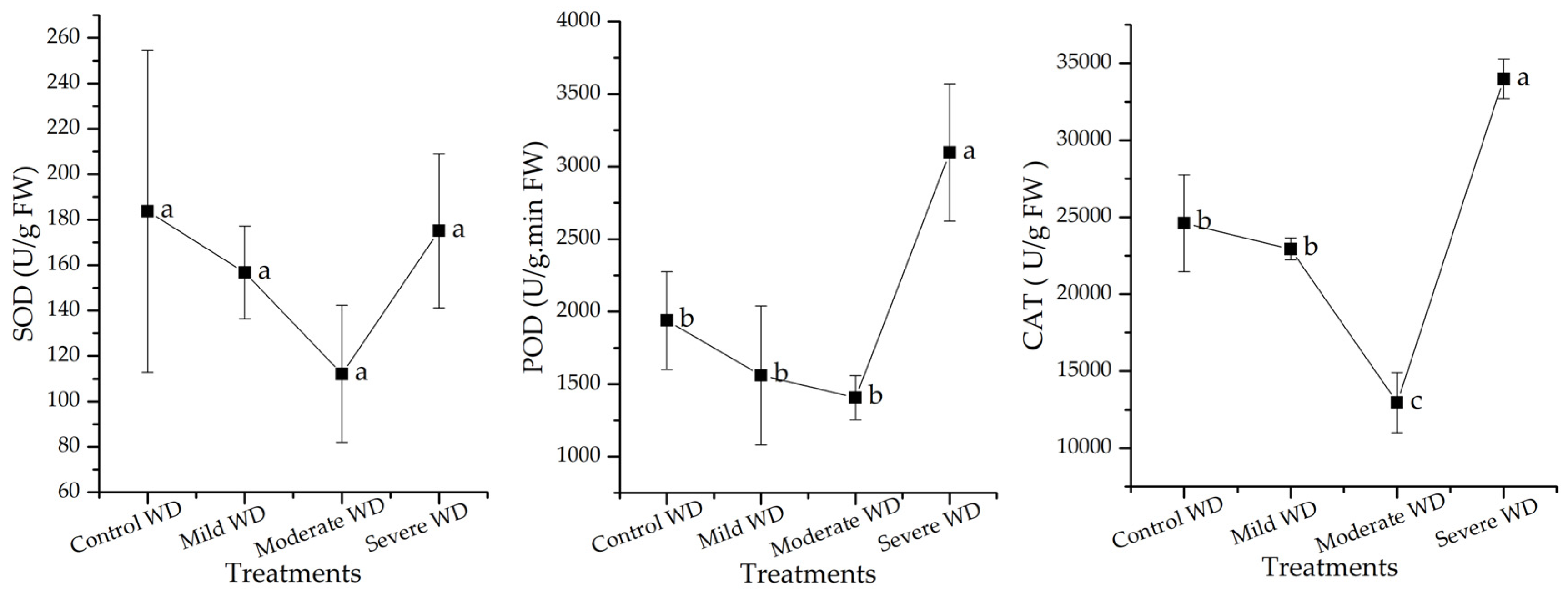
3.4. Activity of Antioxidant Enzymes of C. spicatus Seedlings Varied in Response to Different WD Stress Treatments
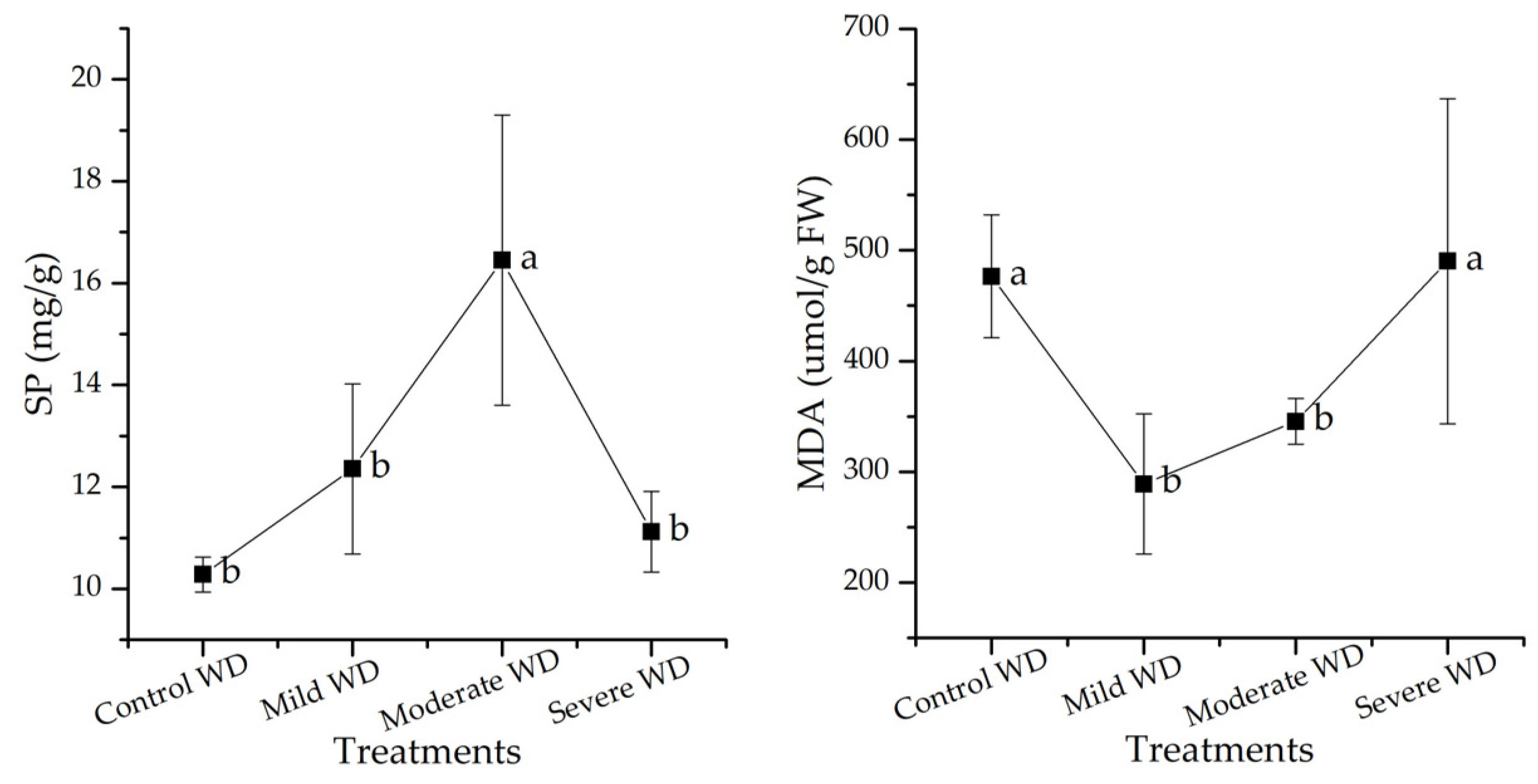
3.5. SP and MDA Contents of C. spicatus Seedlings Changed in Response to WD Stress
4. Discussion
4.1. Plant Growth and Biomass under WD Stress
4.2. Plant Photosynthesis Differences under WD Stress
4.3. Antioxidant Enzyme Activities Differ among Plants Treated with Varying WD Intensities
4.4. Osmoregulatory Substances of Plants under WD Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Huang, Y.; Sun, M.; Zhao, M.; Wu, Y.; Wan, Z.; Su, X.; Zhang, C. Chemical Constituents of the Volatile Oil from Chloranthus spicatus Leaves and Its Antioxidant and Antimicrobial Activity. J. Northeast Foreatry Univ. 2021, 49, 52–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H. The industry evolution and manufacturing skills of chloranthus tea. China Tea 2021, 43, 62–65. [Google Scholar]
- Zhang, K. Shexian County “Three Flowers”. Chin. Nativ. Prod. 1996, 3, 27–28. [Google Scholar]
- Yisheng, Y. Specialties of Huizhou—Chloranthus flowers tea in shexian county. J. Tea Bus. 1980, 1, 106–108. [Google Scholar] [CrossRef]
- Maogui, T.; Xing, S. Integrated cultivation and key technology application of Phalaenopsis greenhouse facilities. J. Tea Bus. 2020, 42, 068–072. [Google Scholar] [CrossRef]
- Wang, X. Climate suitability division of actinidia chinensis in the mountain areas of south anhui. Resour. Dev. Mark. 1996, 12, 73–74+84. [Google Scholar]
- Zhan, L.; Cai, Y.; Zhu, Z.; Ma, L.; Jia, Z.; Wu, N.; Luo, Q.; Zhu, G.; Deng, Z.; Wang, J. Physiological Response of Magnolia wufengensis ‘Jiaohong 1’ to Water Stress. J. Southwest For. Univ. 2023, 44, 18–26. [Google Scholar]
- Silva, J.d.S.; da Costa, R.S.; Tomaz, F.L.d.S.; Bezerra, A.E.; Mesquita, R.O. Mechanisms of tolerance to water deficit and physiological responses to rehydration in cowpea. Rev. CiÊncia AgronÔmica 2021, 52, e20207198. [Google Scholar] [CrossRef]
- Mira-García, A.B.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Effect of Water Stress and Shading on Lime Yield and Quality. Plants 2023, 12, 503. [Google Scholar] [CrossRef]
- Aroca, R.; Světlíková, P.; Hájek, T.; Těšitel, J. Water-stress physiology of Rhinanthus alectorolophus, a root-hemiparasitic plant. PLoS ONE 2018, 13, e0200927. [Google Scholar] [CrossRef]
- Watt, M.S.; de Silva, D.; Estarija, H.J.C.; Yorston, W.; Massam, P. Detecting the Short-Term Effects of Water Stress on Radiata Pine Physiology Using Thermal Imagery. Forests 2023, 15, 28. [Google Scholar] [CrossRef]
- Syaripah Najihah, T.; Hafiz Ibrahim, M.; Amalina Mohd Zain, N.; Nulit, R.; Edaroyati Megat Wahab, P. Activity of the oil palm seedlings exposed to a different rate of potassium fertilizer under water stress condition. AIMS Environ. Sci. 2020, 7, 46–68. [Google Scholar] [CrossRef]
- Hultine, K.; Ferrini, F.; Giordani, E.; Sebastiani, F.; Loreto, F.; Moura, B.B.; Gori, A.; Brunetti, C. Phenotypic plasticity of two M. oleifera ecotypes from different climatic zones under water stress and re-watering. Conserv. Physiol. 2020, 8, coaa028. [Google Scholar] [CrossRef]
- Gisbert-Mullor, R.; Martín-García, R.; Zidari, I.B.; Pascual-Seva, N.; Pascual, B.; Padilla, Y.G.; Calatayud; López-Galarza, S. A Water Stress–Tolerant Pepper Rootstock Improves the Behavior of Pepper Plants under Deficit Irrigation through Root Biomass Distribution and Physiological Adaptation. Horticulturae 2023, 9, 362. [Google Scholar] [CrossRef]
- Yanping, H.; Weidong, L.; Wensheng, P.; Min, Z.; Minjie, L.; Qijun, W.; Mingjun, H.; Diao, Z.; Dangren, L. Effects of water stress on the growth, physiological and biochemical characteristics of Sapium discolor seedlings. J. Cent. South Univ. For. Technol. 2023, 43, 62–72. [Google Scholar] [CrossRef]
- Song, Y.; Qiao, C.; Liu, D.; He, Y.; Wang, L. Nutrient Heterogeneity of Pinus massoniana Forests Soil in Tunxi District of Huangshan City. Guizhou Agric. Sci. 2015, 43, 92–94. [Google Scholar]
- Baoping, Z.; Peng, R.; Zhongshan, X.; Junying, W.; Ruifang, L.; Jinghui, L. Effects of water stress on photosynthetic characteristics and yield formation in Oats (Avena sativa L.) with different drought resistance. J. Triticeae Crops 2020, 40, 1399–1407. [Google Scholar] [CrossRef]
- Zoghi, Z.; Hosseini, S.M.; Kouchaksaraei, M.T.; Kooch, Y.; Guidi, L. The effect of biochar amendment on the growth, morphology and physiology of Quercus castaneifolia seedlings under water-deficit stress. Eur. J. For. Res. 2019, 138, 967–979. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, M.; Peng, F.; Yang, B.; Chen, T.; Li, Y. Characteristics of Photosynthesis and Fast Chlorophyll Fluorescence Induction Dynamics in Different Pecan Cultivars. J. Northeast Foreatry Univ. 2017, 45, 36–42. [Google Scholar] [CrossRef]
- Dong, J.; Liu, C.; Zhao, Y.; Yuan, Z. Effects of Salt Stress on Growth, Physiological and Biochemical Characteristics of Punica granatum. J. Southwest For. Univ. 2021, 41, 47–54. [Google Scholar]
- Major, J.E.; Johnsen, K.H. Family variation in photosynthesis of 22-year-old black spruce: A test of two models of physiological response to water stress. Can. J. For. Res. 1996, 26, 1922–1933. [Google Scholar] [CrossRef]
- Hu, H.; Fei, L.; Li, Z.; Liu, X.; Xv, X.; Wang, Y.; Han, Z. Physiological responses of two apple rootstocks to drought stress under different water retention treatments. J. China Agric. Univ. 2023, 28, 50–60. [Google Scholar] [CrossRef]
- Cui, Q.; Li, Y.; He, X.; Li, S.; Zhong, X.; Liu, B.; Zhang, D.; Li, Q. Physiological and iTRAQ based proteomics analyses reveal the mechanism of elevated CO2 concentration alleviating drought stress in cucumber (Cucumis sativus L.) seedlings. Plant Physiol. Biochem. 2019, 143, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Xv, X. A Review on Plant Stress Physiology. World For. Res. 2003, 16, 6–11. [Google Scholar] [CrossRef]
- Su, C.; Duan, Y.; Gu, Z.; Zheng, P.; Huang, X.; Fei, Y.; Zhou, M. Effects of water stress on the physiological and photosynthetic characteristics of Phoebe bournei seedlings. J. Cent. South Univ. For. Technol. 2022, 42, 85–93. [Google Scholar] [CrossRef]
- Jiyao, L. Mechanisms of Drought Tolerance in Plants. J. Beijing For. Univ. 1991, 13, 3–14. [Google Scholar]
- Villar-Salvador, P.; Planelles, R.; Oliet, J.; Peñuelas-Rubira, J.L.; Jacobs, D.F.; González, M. Drought tolerance and transplanting performance of holm oak (Quercus ilex) seedlings after drought hardening in the nursery. Tree Physiol. 2004, 24, 1147–1155. [Google Scholar] [CrossRef]
- Coopman, R.E.; Jara, J.C.; Bravo, L.A.; Sáez, K.L.; Escobar, R. Changes in morpho-physiological attributes of Eucalyptus globulus plants in response to different drought hardening treatments. Electron. J. Biotechnol. 2008, 11, 1535–1541. [Google Scholar] [CrossRef]
- Gheysari, M.; Sadeghi, S.H.; Loescher, H.W.; Amiri, S.; Zareian, M.J.; Majidi, M.M.; Asgarinia, P.; Payero, J.O. Comparison of deficit irrigation management strategies on root, plant growth and biomass productivity of silage maize. Agric. Water Manag. 2017, 182, 126–138. [Google Scholar] [CrossRef]
- Yu, W.; Ji, R.; Feng, R.; Zhao, X.; Zhang, Y. Response of water stress on photosynthetic characteristics and water use efficiency of maize leaves in different growth stage. Acta Ecol. Sin. 2015, 35, 2902–2909. [Google Scholar] [CrossRef][Green Version]
- O’Brien, M.J.; Burslem, D.F.R.P.; Caduff, A.; Tay, J.; Hector, A. Contrasting nonstructural carbohydrate dynamics of tropical tree seedlings under water deficit and variability. New Phytol. 2015, 205, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Daferera, D.; Polissiou, M.; Passam, H. The effect of water deficit stress on the growth, yield and composition of essential oils of parsley. Sci. Hortic. 2008, 115, 393–397. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Li, J.; Xiong, X.; Chen, Y.; Yin, X.; Feng, D. The response of mulberry trees after seedling hardening to summer drought in the hydro-fluctuation belt of Three Gorges Reservoir Areas. Environ. Sci. Pollut. Res. 2013, 20, 7103–7111. [Google Scholar] [CrossRef] [PubMed]
- Kolyva, F.; Nikolopoulos, D.; Bresta, P.; Liakopoulos, G.; Karabourniotis, G.; Rhizopoulou, S. Acclimation of the Grapevine Vitis vinifera L. cv. Assyrtiko to Water Deficit: Coordination of Structural and Functional Leaf Traits and the Dynamic of Calcium Oxalate Crystals. Plants 2023, 12, 3992. [Google Scholar] [CrossRef] [PubMed]
- Amuji, C.F.; Beaumont, L.J.; Esperón-Rodríguez, M. Simulating the impact of projected West African heatwaves and water stress on the physiology and yield of three tomato varieties. Adv. Hortic. Sci. 2020, 34, 147–156. [Google Scholar] [CrossRef]
- Chanbusarakum, L.; Bragg, J.; Cheng, P.; Aucar, S.; Sarath, G.; Palmer, N.; Edme, S.; Tobias, C.M. Gene Expression and Physiological Differences in Neo-Octoploid Switchgrass Subjected to Drought Stress. BioEnergy Res. 2020, 13, 63–78. [Google Scholar] [CrossRef]
- Chukalla, A.D.; Krol, M.S.; Hoekstra, A.Y. Grey water footprint reduction in irrigated crop production: Effect of nitrogen application rate, nitrogen form, tillage practice and irrigation strategy. Hydrol. Earth Syst. Sci. Discuss. 2018, 22, 3245–3259. [Google Scholar] [CrossRef]
- Kato, Y.; Kamoshita, A.; Yamagishi, J.; Imoto, H.; Abe, J. Growth of Rice (Oryza sativa L.) Cultivars under Upland Conditions with Different Levels of Water Supply. Plant Prod. Ence 2015, 9, 3–13. [Google Scholar] [CrossRef][Green Version]
- Mamnouie, E.; Fotouhi Ghazvini, R.; Esfahani, M.; Nakhoda, B. The Effects of Water Deficit on Crop Yield and the Physiological Characteristics of Barley (Hordeum vulgare L.) Varieties. J. Agric. Sci. Technol. 2018, 8, 211–219. [Google Scholar]


| Treatments | Relative Water Content of Soil | Stress Intension |
|---|---|---|
| control | 95–100% | Control WD |
| mild | 75–80% | Mild WD |
| moderate | 55–60% | Moderate WD |
| severe | 35–40% | Severe WD |
| Treatments | Shoot Length (cm) | Shoot Diameter (mm) | ||||
|---|---|---|---|---|---|---|
| H1 | H2 | H | D1 | D2 | D | |
| control | 25.91 ± 6.26 a | 40.97 ± 7.11 c | 15.06 ± 5.52 c | 3.05 ± 0.58 a | 3.10 ± 0.46 a | 0.35 ± 0.38 a |
| mild | 25.94 ± 6.34 a | 48.86 ± 9.93 ab | 22.92 ± 9.90 a | 3.02 ± 0.65 a | 3.33 ± 0.73 a | 0.51 ± 0.39 a |
| moderate | 27.31 ± 5.69 a | 49.50 ± 8.79 a | 22.19 ± 8.28 ab | 2.96 ± 0.66 a | 3.24 ± 0.50 a | 0.51 ± 0.36 a |
| severe | 26.42 ± 4.59 a | 44.87 ± 7.99 bc | 18.35 ± 8.19 bc | 2.90 ± 0.45 a | 3.08 ± 0.49 a | 0.44 ± 0.32 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, Y.; Wan, Z.; Zhao, C.; Su, X.; Liu, X. Effects of Water-Deficit Stress on the Growth and Physiological Characteristics of Chloranthus spicatus Seedlings. Horticulturae 2024, 10, 1054. https://doi.org/10.3390/horticulturae10101054
Shang Y, Wan Z, Zhao C, Su X, Liu X. Effects of Water-Deficit Stress on the Growth and Physiological Characteristics of Chloranthus spicatus Seedlings. Horticulturae. 2024; 10(10):1054. https://doi.org/10.3390/horticulturae10101054
Chicago/Turabian StyleShang, Yangjuan, Zhibing Wan, Changheng Zhao, Xing Su, and Xinyi Liu. 2024. "Effects of Water-Deficit Stress on the Growth and Physiological Characteristics of Chloranthus spicatus Seedlings" Horticulturae 10, no. 10: 1054. https://doi.org/10.3390/horticulturae10101054
APA StyleShang, Y., Wan, Z., Zhao, C., Su, X., & Liu, X. (2024). Effects of Water-Deficit Stress on the Growth and Physiological Characteristics of Chloranthus spicatus Seedlings. Horticulturae, 10(10), 1054. https://doi.org/10.3390/horticulturae10101054





