1. Introduction
Steel slag is the molten slag produced during the steelmaking process [
1]. With the rapid development of the steel industry, the production of steel and steel slag has continuously increased. Currently, steel slag is only used for road construction and other construction-related fields, but consumption cannot keep up with production. One important reason for this is its very high transportation cost. However, much steel slag is piled up on land, which leads to a serious waste of land resources and the risk of heavy metal pollution at the stacking site [
2]. Previous studies have shown that steel slag can improve soil and increase crop yields because it contains high levels of calcium (Ca), magnesium (Mg), iron (Fe), silicon (Si), and other medium and trace elements. For example, Ca is an essential element for plant growth and development. It not only plays an important role in the stability of the plant cell wall and cell membrane but also acts as a major signal molecule in the cytoplasm to regulate plant growth and development [
3]. Mg is an important component of chlorophyll [
4], and it plays an important role in photosynthesis, fat metabolism, carbohydrate synthesis, enzyme activation, and protein synthesis in plants [
5]. Fe is one of the most required elements in plants, and it participates in chlorophyll synthesis, plant photosynthesis, respiration, and electron transfer [
6]. In addition, the rich Si in steel slag can be made into silicon fertilizer to promote plant growth. After high-temperature calcination, the slag’s solubility is increased, making it easy for plants to absorb. Therefore, steel slag has a high potential for agricultural use [
7].
However, due to its high pH and bulk density, steel slag cannot be widely and directly used in field crops. When steel slag is used in field crops for the long term, it may lead to an imbalance of soil acidity and basicity because of increased pH value. Previous studies have shown that when steel slag was used directly as silicon fertilizer in fields, a high content of heavy metals was examined [
8]. Therefore, if the steel slag is not carefully selected, it will cause soil hardening and contamination. However, potted flowers are only used as ornaments, which can prevent large soil alkalization and hardening areas, which may be a novel way to use steel slag.
Hydrangea macrophylla ((Thunb.) Ser.) is a deciduous shrub of the Hydrangeaceae family and the Hydrangea genus. Its colorful and large inflorescences have high ornamental value and can be used as cut, potted, and garden greening. Therefore, hydrangea is called one of the world’s three major garden plants [
9,
10]. In recent years, the consumption of hydrangea potted flowers has increased daily. Cutting propagation is an essential and primary method to rapidly obtain high-quality seedlings in many plants including hydrangea [
11]. At present, the substrates used to cut hydrangea are mainly peat, perlite, and vermiculite [
12]. However, these substrates are non-renewable resources with a high cost [
13]. Therefore, the present study uses steel slag mixed with the above substrates to cut hydrangea seedlings. The aim is to find a new way to utilize the rich mineral elements in steel slag and reduce the production cost of hydrangea seedlings. The result is meaningful to protect the ecological environment and promote the healthy development of the flower industry.
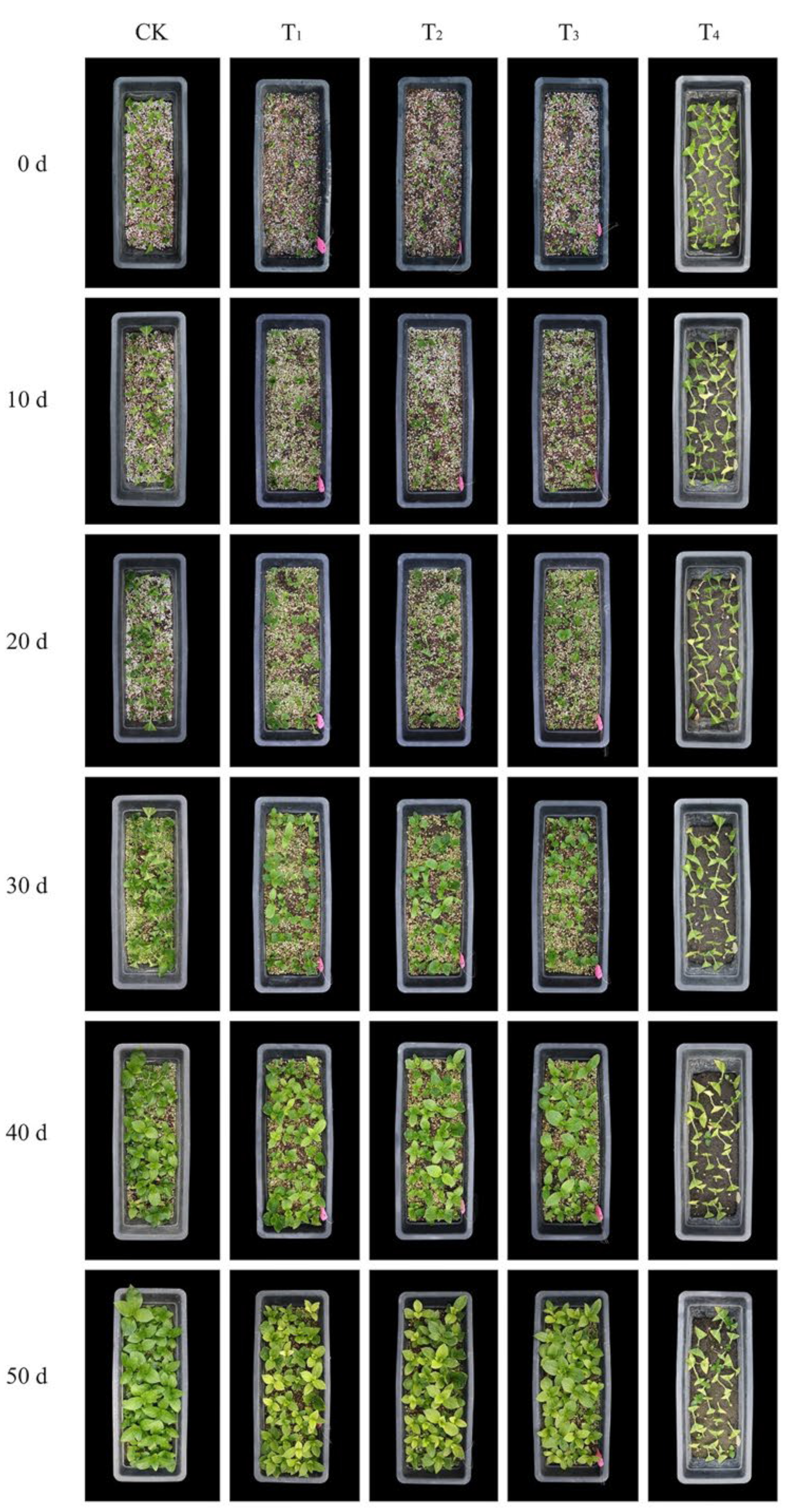
4. Discussion
The physical and chemical properties are essential indexes reflecting the seedling substrates’ structural characteristics and nutrient status [
21,
22]. The results of this study show that the addition of steel slag increased the substrate pH, EC, and bulk density to some extent and reduced the substrate’s porosity. The pure steel slag (T
4)‘s pH value and bulk density were 11.96 and 1.50 g·cm
−3, respectively. This indicated that it was not suitable for cutting hydrangea. However, when mixed with perlite, vermiculite, and peat, the pH value and bulk density were significantly reduced. The growth of cuttings in T
1, T
2, and T
3 treatments was better than in T
4, indicating that the reduced pH values of the mixed substrates were suitable for hydrangea. The EC values of all treatments in this experiment were lower than the limited EC value of substrates (2.6 mS·cm
−1) [
16]. The EC values of mixed treatments were significantly increased compared with CK and T
4. The reason may be that some oxides (such as free calcium oxide, etc.) and silicates in steel slag were dissolved in soil aqueous medium, and silicate ions could also release phosphate ions fixed in the soil [
23,
24,
25]. Therefore, many ions were released, increasing the treatment’s EC value. With the increase of steel slag content, the pH values of mixed treatments gradually improved. Some metal ions in the steel slag formed hydroxide precipitate, which could adsorb silicate or form silicate precipitate [
26,
27]. The ion content decreased, and the EC value decreased gradually. The large bulk weight of the substrate reduced its water-holding and fertilizer-holding capacity, which is harmful to the growth of plant roots. The small bulk density was not conducive to the growth and development of plant roots. The total porosity reflects the permeability and water retention of the substrate. According to a former study [
28], the total porosity and bulk density of an ideal growing medium for plants were appropriate at 0.1–0.8 g·cm
−3 and 54–96%, respectively. In the present study, the bulk density and total porosity of T
4 were out of the range, while other treatments were all in the range. Thus, pure steel slag was not suitable as a growing medium. However, steel slag reduced the substrate’s aeration and increased water retention. He et al. [
29] found that adding fine sand would increase the matrix water content and decrease the porosity and air permeability. Our study was consistent with that conclusion.
The plant height, stem diameter, and fresh and dry weights of plants are the primary growth indicators that reflect the quality of seedlings [
30,
31]. According to the present study, the plant height, fresh weight, and dry weight of T
3 were higher than other treatments. He et al. [
29] found that adding fine sand increased the bulk density of soil and the contact area between roots and soil, which was conducive to nutrient absorption and promoted above-ground parts’ growth. Appropriately increasing the proportion of steel slag was conducive to developing the above-ground parts.
The growth and distribution of roots result from a plant’s interaction with the environment during its development and reflect the ability of the plant to absorb and transfer water and nutrients [
32,
33,
34]. Root activity is a physiological index to judge the growth and development characteristics of plants and reveal their adaptability, biosynthesis ability, and health status [
35]. In this study, root length, root surface area, root volume, root tip number, and root activity in T
3 were significantly higher than those in other treatments. Carvalho Pupatto et al. [
36] found that steel slag could promote plant root growth and distribution in soil profiles. Islam et al. [
37] studied the addition of steel slag, which improved soil fertility and promoted plant growth and nutrient absorption. Combined with previous studies and our results, we speculated that a certain proportion of steel slag in the substrate could promote the growth and development of plant roots and the absorption of nutrients. It is preliminarily hypothesized that this enhancement may be linked to the bulk density of the substrate. Ola et al. [
38] concluded that bulk density significantly influences plant root development by promoting elongation of root cells. Plants must withstand greater substrate reaction forces in substrates with higher bulk density, potentially enhancing root activity. Wang et al. [
39] thought that the appropriate matrix compactness was conducive to the growth of root diameter. Therefore, root length, surface area, and root volume increased significantly in the T3 treatment, and it was the most suitable mixed substrate for cuttings.
Malonaldehyde (MDA) is the end product of membrane lipid peroxidation, and its content will reflect the degree of environmental persecution of the organism [
40]. In this study, the content of malondialdehyde in CK and T4 was significantly higher than in other treatments. The content of malondialdehyde in mixed treatments improved with increased steel slag content. The content of soluble silicon in steel slag was higher, and the leaves of plants became thicker after absorbing soluble silicon, which can induce plant photosynthesis to become stronger while increasing biomass [
41,
42]. The mechanical or physical barrier provided by Si deposition in the cell wall helps to enhance resistance and improve seedling roots [
43]. In the same growth conditions, the photosynthesis of leaves in the treatment group was enhanced, and the water loss due to transpiration was reduced compared with CK [
44]. Therefore, the malondialdehyde content in CK was higher than that of T
1, T
2, and T
3. The results showed that the steel slag and conventional cutting substrate mixture significantly reduced environmental stress on cuttings. The reason may be consistent with the study of Chen et al. [
45]. The low content of steel slag did not induce oxidative stress in plants, reduced membrane lipid peroxidation, and improved antioxidant properties to some extent. Studies have shown that adding steel slag could improve soil enzyme activity and fertility [
42,
46]. Therefore, adding steel slag reduced the environmental stress of the substrate to the root system. The malondialdehyde content in T
3 was higher than that of T
1 and T
2, but the growth performance was better than others. The reason can be inferred from two aspects: on the one hand, the high content of calcium and silicon in steel slag made the root system stronger and promoted the growth of plants, nutrient absorption, and biomass increase [
44,
47]; on the other hand, plant roots were significantly affected by the bulk density of the substrate. The appropriate substrate compactness (bulk weight) could promote the coarse growth of plant roots [
48]. Therefore, the growth performance of T
3 was better than that of T
1 and T
2.
Root primordium induction, elongation, and growth of adventitious roots in the rooting process of plant spike cuttings require a large amount of nutrients. Furthermore, plants enhance their stress resistance by accumulating soluble protein during stress [
49,
50]. In this study, the soluble protein content of T
3 was significantly higher than that of other treatments. Different contents of steel slag had significant effects on soluble protein in leaves. With the increase of steel slag content, the soluble protein content first increased and then decreased; the overall trend was gradually upward. Our results were consistent with the results of Zheng et al. [
51]; they found that the soluble protein content of plants in different ratios of yellow sand and slag first increased and then decreased, and the overall trend was upward. The result was inconsistent with the study of Zhou et al. [
52]. The reason may be that the physical and chemical properties of the substitute substrate were quite different from those of steel slag, which resulted in various plant growth environments, and therefore the ability to accumulate soluble protein was different.