Identifying Fire Blight-Resistant Malus sieversii Rootstocks Grafted with Cultivar ‘Aport’ Using Monitoring Data
Abstract
1. Introduction
2. Materials and Methods
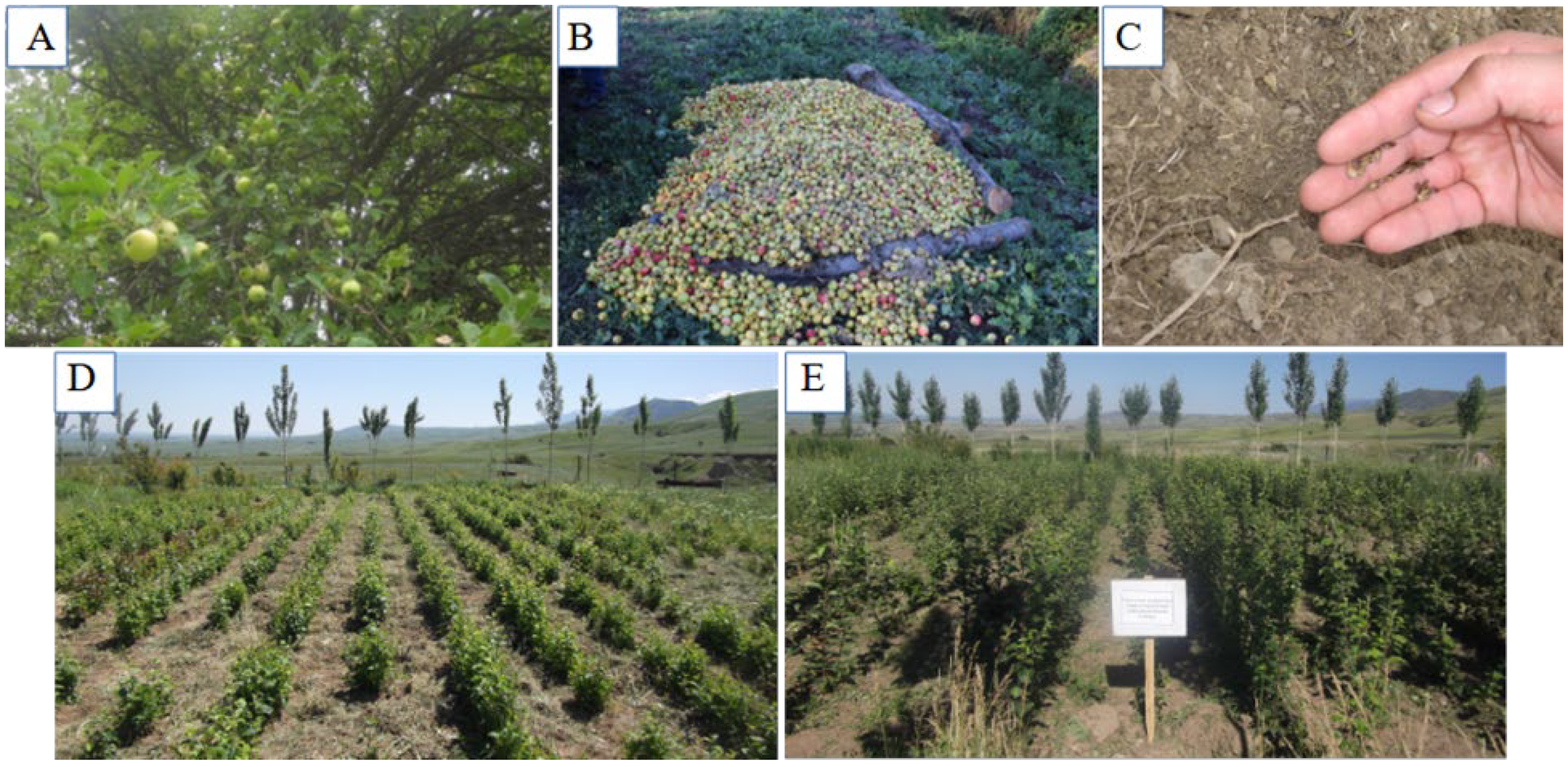
2.1. Collecting Wild Apple Seeds and Grafting
2.2. Planting of Grafted Trees and Disease Monitoring
2.3. qPCR Detection of Pathogen
2.4. Statistical Analysis
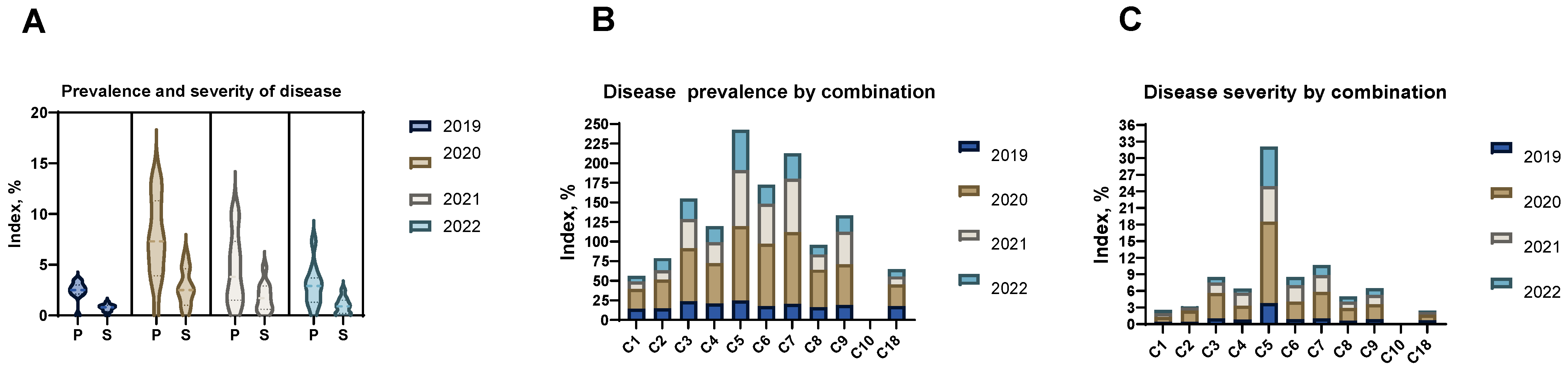
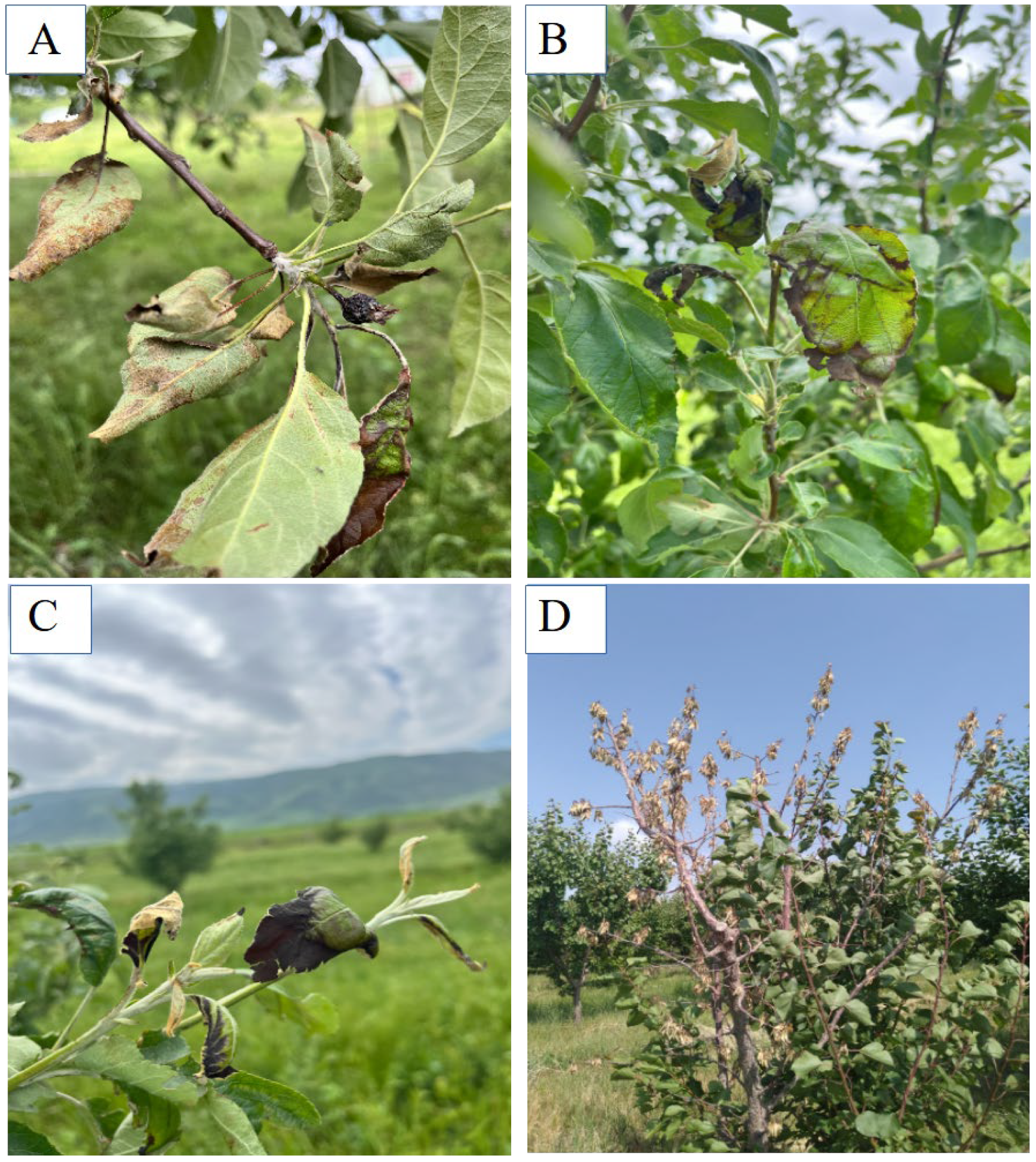
3. Results
Fire Blight Monitoring and Pathogen Identification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gritsenko, D.; Pozharskiy, A.; Dolgikh, S.; Aubakirova, K.; Kenzhebekova, R.; Galiakparov, N.; Karimov, N.; Sadykov, S.; Hortic, E.J. Apple Varieties from Kazakhstan and Their Relation to Foreign Cultivars Assessed with RosBREED 10K SNP Array. Eur. J. Hortic. Sci. 2022, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Omasheva, M.E.; Pozharsky, A.S.; Smailov, B.B.; Ryabushkina, N.A.; Galiakparov, N.N. Genetic Diversity of Apple Cultivars Growing in Kazakhstan. Russ. J. Genet. 2018, 54, 176–187. [Google Scholar] [CrossRef]
- Umiraliyeva, Z.Z.; Kopzhassarov, B.K.; Jaimurzina, A.A.; Niyazbekov, Z.B.; Issenova, G.Z.; Tursunova, A.K.; Berganayeva, G.E. Epidemiology of Fire Blight in Fruit Crops in Kazakhstan. AGRIVITA J. Agric. Sci. 2021, 43, 273–284. [Google Scholar] [CrossRef]
- Djaimurzina, A.; Umiralieva, Z.; Zharmukhamedova, G.; Born, Y.; Bühlmann, A.; Rezzonico, F. Detection of the Causative Agent of Fire Blight—Erwinia Amylovora (Burrill) Winslow et al.—In the Southeast of Kazakhstan. Acta Hortic. 2014, 1056, 129–132. [Google Scholar] [CrossRef]
- Maltseva, E.R.; Zharmukhamedova, G.A.; Jumanova, Z.K.; Naizabayeva, D.A.; Berdygulova, Z.A.; Dmitriyeva, K.A.; Soltanbekov, S.S.; Argynbayeva, A.M.; Skiba, Y.A.; Malakhova, N.P.; et al. Assessment of Fire Blight Introduction in the Wild Apple Forests of Kazakhstan. Biodiversity 2022, 23, 123–128. [Google Scholar] [CrossRef]
- Kolchenko, M.; Nurtaza, A.; Pozharskiy, A.; Dyussembekova, D.; Kapytina, A.; Nizamdinova, G.; Khusnitdinova, M.; Taskuzhina, A.; Kakimzhanova, A.; Gritsenko, D. Wild Malus Niedzwetzkyana Dieck Ex Koehne as a Genetic Resource for Fire Blight Resistance. Horticulturae 2023, 9, 1066. [Google Scholar] [CrossRef]
- Ha, Y.H.; Oh, S.H.; Lee, S.R. Genetic Admixture in the Population of Wild Apple (Malus Sieversii) from the Tien Shan Mountains, Kazakhstan. Genes 2021, 12, 104. [Google Scholar] [CrossRef]
- Forsline, P.L.; Aldwinckle, H.S.; Dickson, E.E.; Luby, J.J.; Hokanson, S.C. Collection, Maintenance, Characterization, and Utilization of Wild Apples of Central Asia. Hortic. Rev. 2010, 29, 1–61. [Google Scholar] [CrossRef]
- Cornille, A.; Giraud, T.; Smulders, M.J.M.; Roldán-Ruiz, I.; Gladieux, P. The Domestication and Evolutionary Ecology of Apples. Trends Genet. 2014, 30, 57–65. [Google Scholar] [CrossRef]
- Omasheva, M.Y.; Flachowsky, H.; Ryabushkina, N.A.; Pozharskiy, A.S.; Galiakparov, N.N.; Hanke, M.V. To What Extent Do Wild Apples in Kazakhstan Retain Their Genetic Integrity? Tree Genet. Genomes 2017, 13, 52. [Google Scholar] [CrossRef]
- Dougherty, L.; Wallis, A.; Cox, K.; Zhong, G.Y.; Gutierrez, B. Phenotypic Evaluation of Fire Blight Outbreak in the USDA Malus Collection. Agronomy 2021, 11, 144. [Google Scholar] [CrossRef]
- Aldwinckle, H.S.; Gustafson, H.L.; Forsline, P.L. Evaluation of the Core Subset of the Usda Apple Germplasm Collection for Resistance to Fire Blight. Acta Hortic. 1999, 489, 269–272. [Google Scholar] [CrossRef]
- Luby, J.J.; Alspach, P.A.; Bus, V.G.M.; Oraguzie, N.C. Field Resistance to Fire Blight in a Diverse Apple (Malus Sp.) Germplasm Collection. J. Am. Soc. Hortic. Sci. 2002, 127, 245–253. [Google Scholar] [CrossRef]
- Harshman, J.M.; Evans, K.M.; Allen, H.; Potts, R.; Flamenco, J.; Aldwinckle, H.S.; Wisniewski, M.E.; Norelli, J.L. Fire Blight Resistance in Wild Accessions of Malus Sieversii. Plant Dis. 2017, 101, 1738–1745. [Google Scholar] [CrossRef]
- Desnoues, E.; Norelli, J.L.; Aldwinckle, H.S.; Wisniewski, M.E.; Evans, K.M.; Malnoy, M.; Khan, A. Identification of Novel Strain-Specific and Environment-Dependent Minor QTLs Linked to Fire Blight Resistance in Apples. Plant Mol. Biol. Report. 2018, 36, 247–256. [Google Scholar] [CrossRef]
- Khan, M.A.; Han, Y.; Zhao, Y.F.; Troggio, M.; Korban, S.S. A Multi-Population Consensus Genetic Map Reveals Inconsistent Marker Order among Maps Likely Attributed to Structural Variations in the Apple Genome. PLoS ONE 2012, 7, e47864. [Google Scholar] [CrossRef]
- Malnoy, M.; Martens, S.; Norelli, J.L.; Barny, M.A.; Sundin, G.W.; Smits, T.H.M.; Duffy, B. Fire Blight: Applied Genomic Insights of the Pathogen and Host. Annu. Rev. Phytopathol. 2012, 50, 475–494. [Google Scholar] [CrossRef]
- Le Roux, P.M.F.; Khan, M.A.; Broggini, G.A.L.; Duffy, B.; Gessler, C.; Patocchi, A. Mapping of Quantitative Trait. Loci for Fire Blight Resistance in the Apple Cultivars “Florina” and “Nova Easygro”. Genome 2010, 53, 710–722. [Google Scholar] [CrossRef]
- Khan, M.A.; Durel, C.E.; Duffy, B.; Drouet, D.; Kellerhals, M.; Gessler, C.; Patocchi, A. Development of Molecular Markers Linked to the “Fiesta” Linkage Group 7 Major QTL for Fire Blight Resistance and Their Application for Marker-Assisted Selection. Genome 2007, 50, 568–577. [Google Scholar] [CrossRef]
- Gardiner, S.E.; Norelli, J.L.; Silva, N.d.; Fazio, G.; Peil, A.; Malnoy, M.; Horner, M.; Bowatte, D.; Carlisle, C.; Wiedow, C.; et al. Putative Resistance Gene Markers Associated with Quantitative Trait Loci for Fire Blight Resistance in Malus “Robusta 5” Accessions. BMC Genet. 2012, 13, 25. [Google Scholar] [CrossRef]
- Durel, C.E.; Denancé, C.; Brisset, M.N. Two Distinct Major QTL for Resistance to Fire Blight Co-Localize on Linkage Group 12 in Apple Genotypes “Evereste” and Malus Floribunda Clone 821. Genome 2009, 52, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Calenge, F.; Drouet, D.; Denancé, C.; Van De Weg, W.E.; Brisset, M.N.; Paulin, J.P.; Durel, C.E. Identification of a Major QTL Together with Several Minor Additive or Epistatic QTLs for Resistance to Fire Blight in Apple in Two Related Progenies. Theor. Appl. Genet. 2005, 111, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Dzhangaliev, A.D.; Salova, T.N.; Turekhanova, P.M. The Wild Fruit and Nut Plants of Kazakhstan. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 305–371. [Google Scholar]
- Dzhangaliev, A.D. Wild Apple and Fruit Trees of Central Asia; Wiley: Hoboken, NJ, USA, 2003; Volume 29, ISBN 9780471219682. [Google Scholar]
- Rasool, A.; Mansoor, S.; Bhat, K.M.; Hassan, G.I.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms Underlying Graft Union Formation and Rootstock Scion Interaction in Horticultural Plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef]
- Garner, R.J. The Grafter’s Handbook; Chelsea Green Publishing: White River Junction, VT, USA, 2013. [Google Scholar]
- Chertov, O.G.; Nadporozhskaya, M.A. Humus Forms in Forest Soils: Concepts and Classifications. Eurasian Soil. Sci. 2018, 51, 1142–1153. [Google Scholar] [CrossRef]
- DuPont, S.T.; Munir, M.; Cox, K.; Johnson, K.; Peter, K.; Baro, A. Evaluation of Pruning Therapies in Apple Trees with Fire Blight. J. Plant Pathol. 2023, 105, 1695–1709. [Google Scholar] [CrossRef]
- Holb, I.J. Fungal Disease Management in Organic Apple Orchards: Epidemiological Aspects and Management Approaches. In Recent Developments in Management of Plant Diseases; Springer: Dordrecht, The Netherlands, 2010; pp. 163–177. [Google Scholar] [CrossRef]
- Maltseva, E.R.; Zharmukhamedova, G.A.; Jumanova, Z.K.; Naizabayeva, D.A.; Berdygulova, Z.A.; Dmitriyeva, K.A.; Tezekbayeva, B.; Khassein, A.; Skiba, Y.A.; Malakhova, N.P.; et al. Fire Blight Cases in Almaty Region of Kazakhstan in the Proximity of Wild Apple Distribution Area. J. Plant Pathol. 2023, 1, 3. [Google Scholar] [CrossRef]
- Gottsberger, R.A. Development and Evaluation of a Real-Time PCR Assay Targeting Chromosomal DNA of Erwinia Amylovora. Lett. Appl. Microbiol. 2010, 51, 285–292. [Google Scholar] [CrossRef]
- Bubán, T.; Orosz-Kovács, Z.; Farkas, Á. The Nectary as the Primary Site of Infection by Erwinia amylovora (Burr.) Winslow et al.: A Mini Review. Plant Syst. Evol. 2003, 238, 183–194. [Google Scholar] [CrossRef]
- Pusey, P.L. The Role of Water in Epiphytic Colonization and Infection of Pomaceous Flowers by Erwinia amylovora. Phytopathology 2000, 90, 1352–1357. [Google Scholar] [CrossRef]
- Pusey, P.L.; Curry, E.A. Temperature and Pomaceous Flower Age Related to Colonization by Erwinia amylovora and Antagonists. Phytopathology 2004, 94, 901–911. [Google Scholar] [CrossRef]
- Santander, R.D.; Biosca, E.G. Erwinia amylovora psychrotrophic Adaptations: Evidence of Pathogenic Potential and Survival at Temperate and Low Environmental Temperatures. PeerJ 2017, 2017, 901–911. [Google Scholar] [CrossRef]
- Norelli, J.L.; Holleran, H.T.; Johnson, W.C.; Robinson, T.L.; Aldwinckle, H.S. Resistance of Geneva and Other Apple Rootstocks to Erwinia amylovora. Plant Dis. 2007, 87, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Isin, M.; Zhumanova, Z.; Zharmuhamedova, G.; Kulanbai, K. Sortovaya Ustojchivost’ Yabloni i Grushi k Bakterial’nomu Ozhogu (In Russian). Available online: https://elibrary.ru/item.asp?id=36498177 (accessed on 26 September 2023).
- Jensen, P.J.; Halbrendt, N.; Fazio, G.; Makalowska, I.; Altman, N.; Praul, C.; Maximova, S.N.; Ngugi, H.K.; Crassweller, R.M.; Travis, J.W.; et al. Rootstock-Regulated Gene Expression Patterns Associated with Fire Blight Resistance in Apple. BMC Genomics 2012, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Peil, A.; Hanke, M.V.; Flachowsky, H.; Richter, K.; Garcia-Libreros, T.; Celton, J.M.; Gardiner, S.; Horner, M.; Bus, V. Confirmation of the Fire Blight QTL of Malus × Robusta 5 on Linkage Group 3. Acta Hortic. 2008, 793, 297–304. [Google Scholar] [CrossRef]
- Emeriewen, O.; Malnoy, M.; Kilian, A.; Richter, K.; Hanke, M.V.; Peil, A. Evidence of a Major QTL for Fire Blight Resistance in the Apple Wild Species Malus Fusca. Acta Hortic. 2014, 1056, 289–294. [Google Scholar] [CrossRef]
- Thomson, S.V. Epidemiology of Fire Blight. In Fire Blight: The Disease and Its Causative Agent, Erwinia amylovora; CABI Publishing: Wallingford, UK, 2000; pp. 9–36. [Google Scholar] [CrossRef]
- Forsline, P.L.; Luby, J.J.; Aldwinckle, H.S. Fire Blight Incidence on Malus sieversii Grown in New York and Minnesota. Acta Hortic. 2008, 793, 345–350. [Google Scholar] [CrossRef]
- Zhu, Y.; Fazio, G.; Mazzola, M. Elucidating the Molecular Responses of Apple Rootstock Resistant to ARD Pathogens: Challenges and Opportunities for Development of Genomics-Assisted Breeding Tools. Hortic. Res. 2014, 1, 14043. [Google Scholar] [CrossRef]
- Norelli, J.; Aldwinckle, H.; Momol, T.; Johnson, B.; Demarree, A.; Reddy, M.V.B. Fire Blight of Apple Rootstocks. Plants 2024, 13, 5–8. [Google Scholar]
- Singh, J.; Fabrizio, J.; Desnoues, E.; Silva, J.P.; Busch, W.; Khan, A. Root System Traits Impact Early Fire Blight Susceptibility in Apple (Malus × Domestica). BMC Plant Biol. 2019, 19, 579. [Google Scholar] [CrossRef]
- Russo, N.L.; Robinson, T.L.; Fazio, G.; Aldwinckle, H.S. Field Evaluation of 64 Apple Rootstocks for Orchard Performance and Fire Blight Resistance. HortScience 2007, 42, 1517–1525. [Google Scholar] [CrossRef]


| № | Location and Altitude of the Population | Seedling Population |
|---|---|---|
| Dzungarian Alatau | ||
| 1 | Steep Tract (Genetic reserve), 1457 masl | C1 |
| 2 | Chernova River (Genetic reserve), 1329 masl | C2 |
| 3 | Chernova River (Genetic reserve), 1470 masl | C3 |
| 4 | Chernova River (Genetic reserve), 1350 masl | C4 |
| 5 | Chernova River (Genetic reserve), 1190 masl | C5 |
| 6 | Bulenka River, 1237 masl | C6 |
| 7 | Bulenka River, 1121 masl | C7 |
| 8 | Population of Malus niedzwetzkyana, 1370 masl | C8 |
| 9 | Form 28 of wild apple; the altitude is not available | C18 |
| Ile Alatau | ||
| 10 | Talgar, 1340 masl | C9 |
| 11 | Turgen, 1250 masl | C10 |
| Prevalence by Years | Severity by Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Seedling Population | Number of Trees | 2019 | 2020 | 2021 | 2022 | 2019 | 2020 | 2021 | 2022 |
| C1 | 25 | 14.1 | 25.3 | 9.9 | 7.0 | 0.4 | 0.9 | 0.7 | 0.6 |
| C2 | 25 | 14.8 | 36.6 | 12.0 | 15.5 | 0.4 | 2.0 | 0.6 | 0.2 |
| C3 | 25 | 23.9 | 67.6 | 37.3 | 26.0 | 1.0 | 4.6 | 1.9 | 1.0 |
| C4 | 30 | 21.1 | 51.4 | 26.7 | 20.4 | 0.8 | 2.5 | 2.4 | 0.7 |
| C5 | 30 | 24.6 | 95.0 | 71.8 | 51.4 | 3.8 | 14.7 | 6.4 | 7.2 |
| C6 | 25 | 17.6 | 79.5 | 51.4 | 23.9 | 0.9 | 3.2 | 2.9 | 1.5 |
| C7 | 25 | 20.4 | 91.5 | 68.3 | 32.4 | 1.0 | 4.8 | 3.1 | 1.8 |
| C8 | 25 | 16.2 | 47.9 | 19.7 | 12.0 | 0.6 | 2.3 | 1.2 | 0.9 |
| C9 | 28 | 19.0 | 52.1 | 41.5 | 21.1 | 0.9 | 2.7 | 1.7 | 1.2 |
| C10 | 28 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C18 | 25 | 17.6 | 27.4 | 10.6 | 9.2 | 0.7 | 1.0 | 0.5 | 0.2 |
| Dependent Variable | Factor | SS (Type III) | DF | MS | F (DFn, DFd) | p Value |
|---|---|---|---|---|---|---|
| Prevalence | Seedling population | 12893 | 10 | 1289 | F (10, 30) = 9.351 | <0.0001 |
| Year | 8374 | 3 | 2791 | F (3, 30) = 20.25 | <0.0001 | |
| Residual | 4136 | 30 | 137.9 | |||
| Severity | Seedling population | 187.4 | 10 | 18.74 | F (10, 30) = 10.44 | <0.0001 |
| Year | 41.39 | 3 | 13.80 | F (3, 30) = 7.686 | 0.0006 | |
| Residual | 53.85 | 30 | 1.795 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taskuzhina, A.; Pozharskiy, A.; Jumanova, Z.; Soltanbekov, S.; Issina, Z.; Kerimbek, N.; Kapytina, A.; Khusnitdinova, M.; Sagitov, A.; Darubayev, A.; et al. Identifying Fire Blight-Resistant Malus sieversii Rootstocks Grafted with Cultivar ‘Aport’ Using Monitoring Data. Horticulturae 2024, 10, 1052. https://doi.org/10.3390/horticulturae10101052
Taskuzhina A, Pozharskiy A, Jumanova Z, Soltanbekov S, Issina Z, Kerimbek N, Kapytina A, Khusnitdinova M, Sagitov A, Darubayev A, et al. Identifying Fire Blight-Resistant Malus sieversii Rootstocks Grafted with Cultivar ‘Aport’ Using Monitoring Data. Horticulturae. 2024; 10(10):1052. https://doi.org/10.3390/horticulturae10101052
Chicago/Turabian StyleTaskuzhina, Aisha, Alexandr Pozharskiy, Zhulduzay Jumanova, Sagi Soltanbekov, Zhanna Issina, Nazym Kerimbek, Anastasiya Kapytina, Marina Khusnitdinova, Abay Sagitov, Alibi Darubayev, and et al. 2024. "Identifying Fire Blight-Resistant Malus sieversii Rootstocks Grafted with Cultivar ‘Aport’ Using Monitoring Data" Horticulturae 10, no. 10: 1052. https://doi.org/10.3390/horticulturae10101052
APA StyleTaskuzhina, A., Pozharskiy, A., Jumanova, Z., Soltanbekov, S., Issina, Z., Kerimbek, N., Kapytina, A., Khusnitdinova, M., Sagitov, A., Darubayev, A., Seisenova, A., Omarov, Y., & Gritsenko, D. (2024). Identifying Fire Blight-Resistant Malus sieversii Rootstocks Grafted with Cultivar ‘Aport’ Using Monitoring Data. Horticulturae, 10(10), 1052. https://doi.org/10.3390/horticulturae10101052






