Early Chilling Injury Symptom Development and Recovery of Mature Green Banana: Involvement of Ethylene under Different Severities of Chilling Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Procedure
2.3. Respiration Rate and Ethylene Production
2.4. Evaluation of Peel Color and Vascular Browning
2.5. Chlorophyll Fluorescence
2.6. Peel Malondialdehyde (MDA) Content and Electrolyte Efflux (EE)
2.7. Statistical Analysis
3. Results
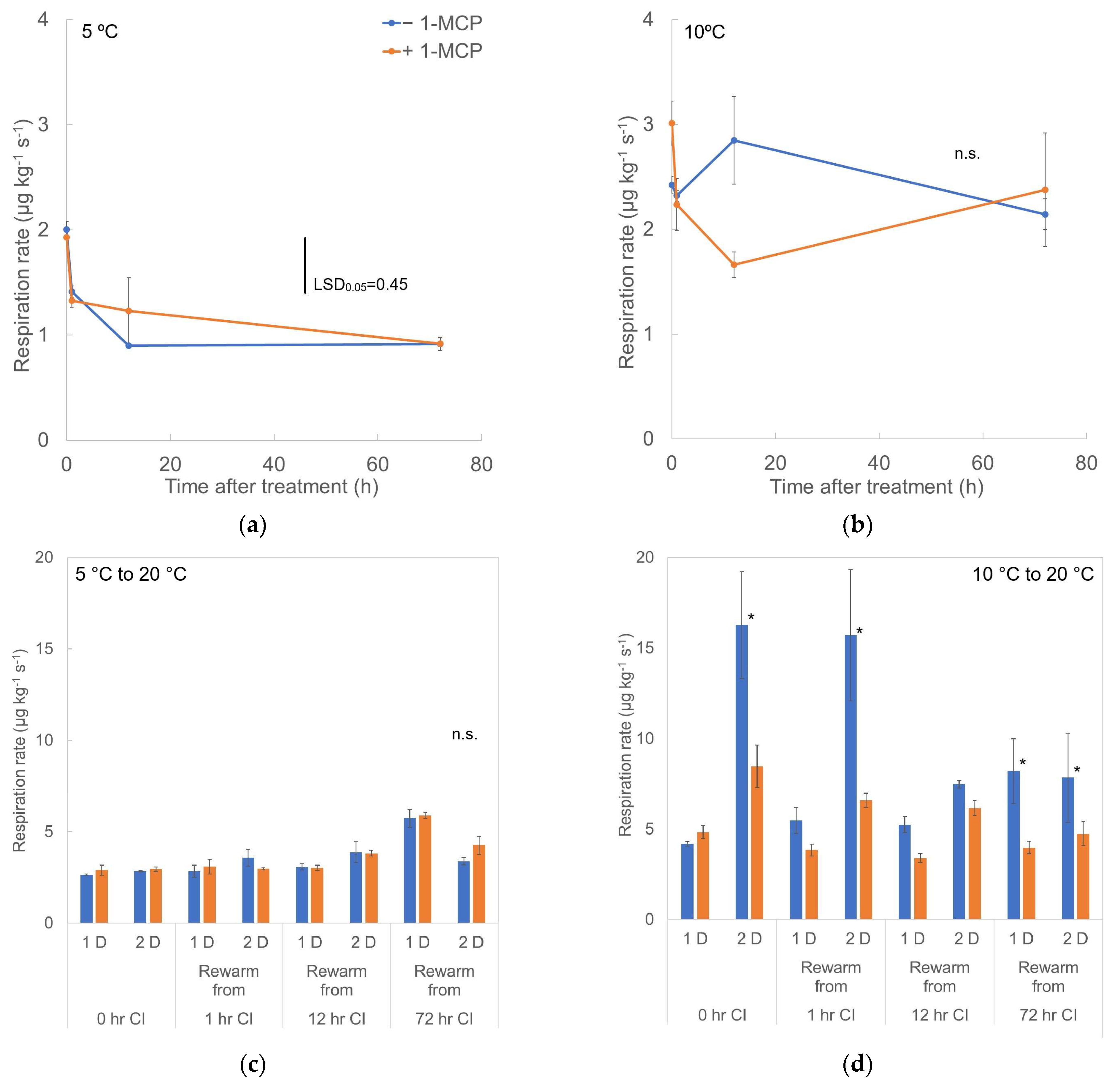
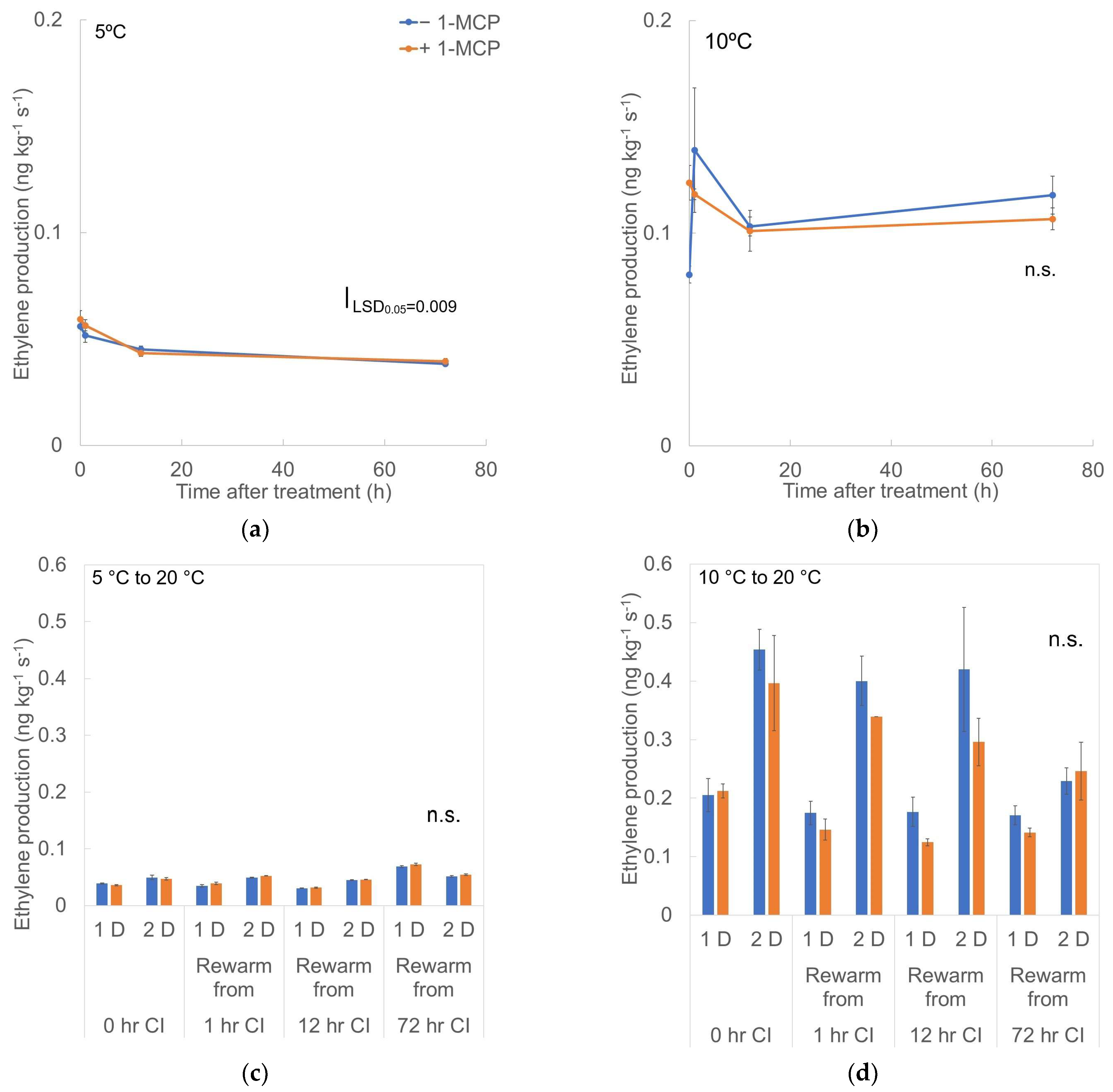
3.1. Respiration Rate and Ethylene Production
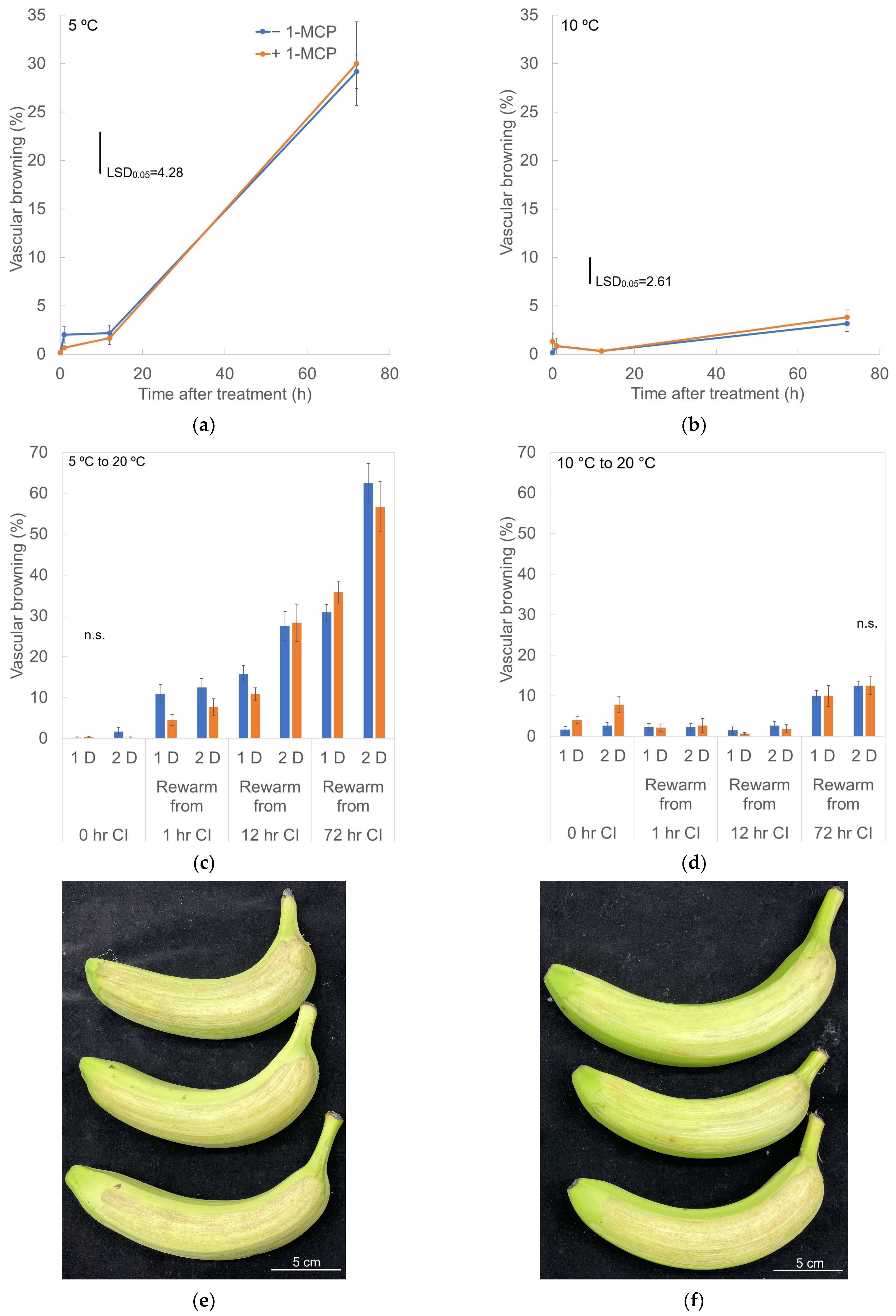
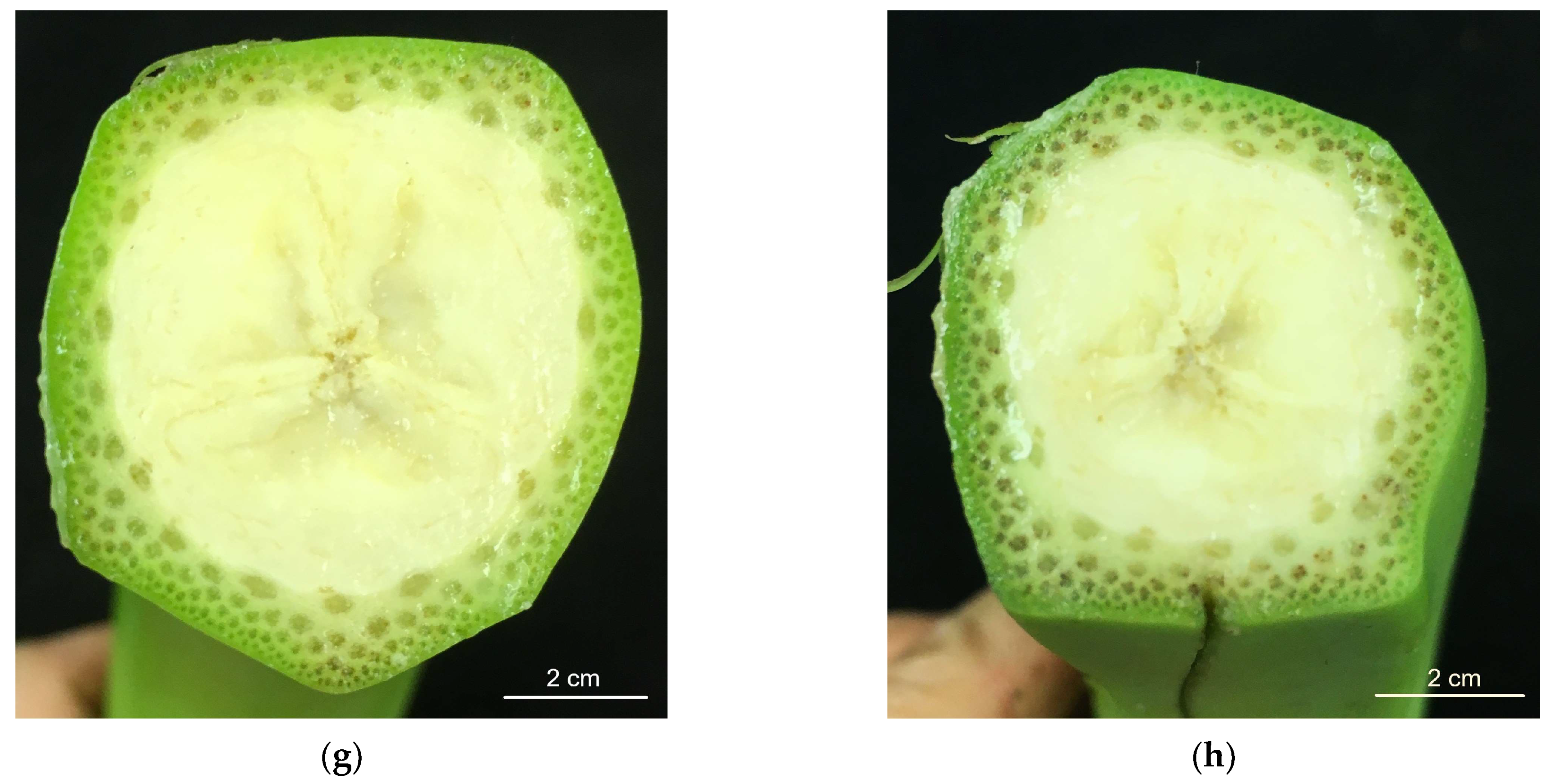
3.2. Vascular Browning (VB)
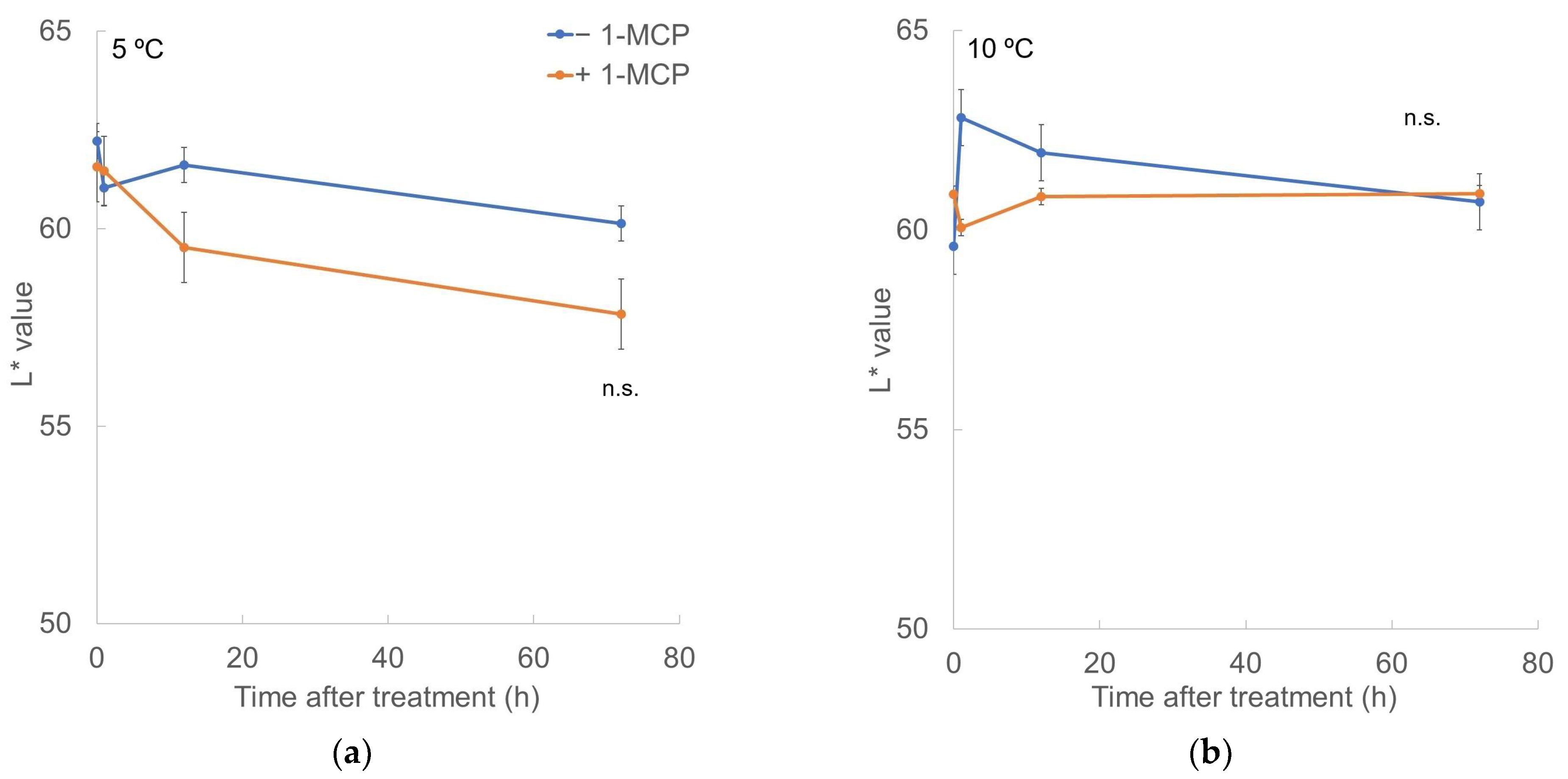
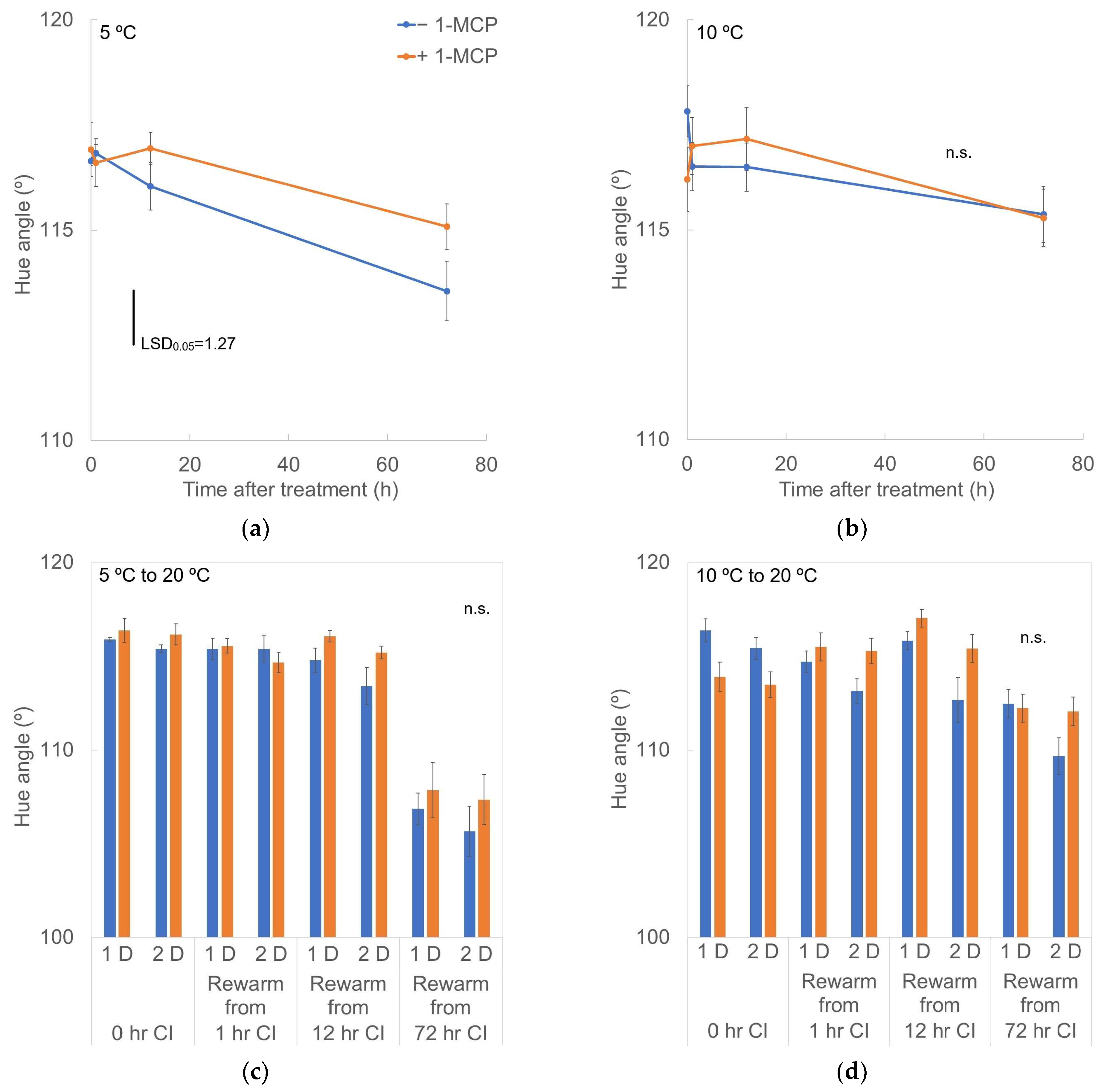
3.3. External Color
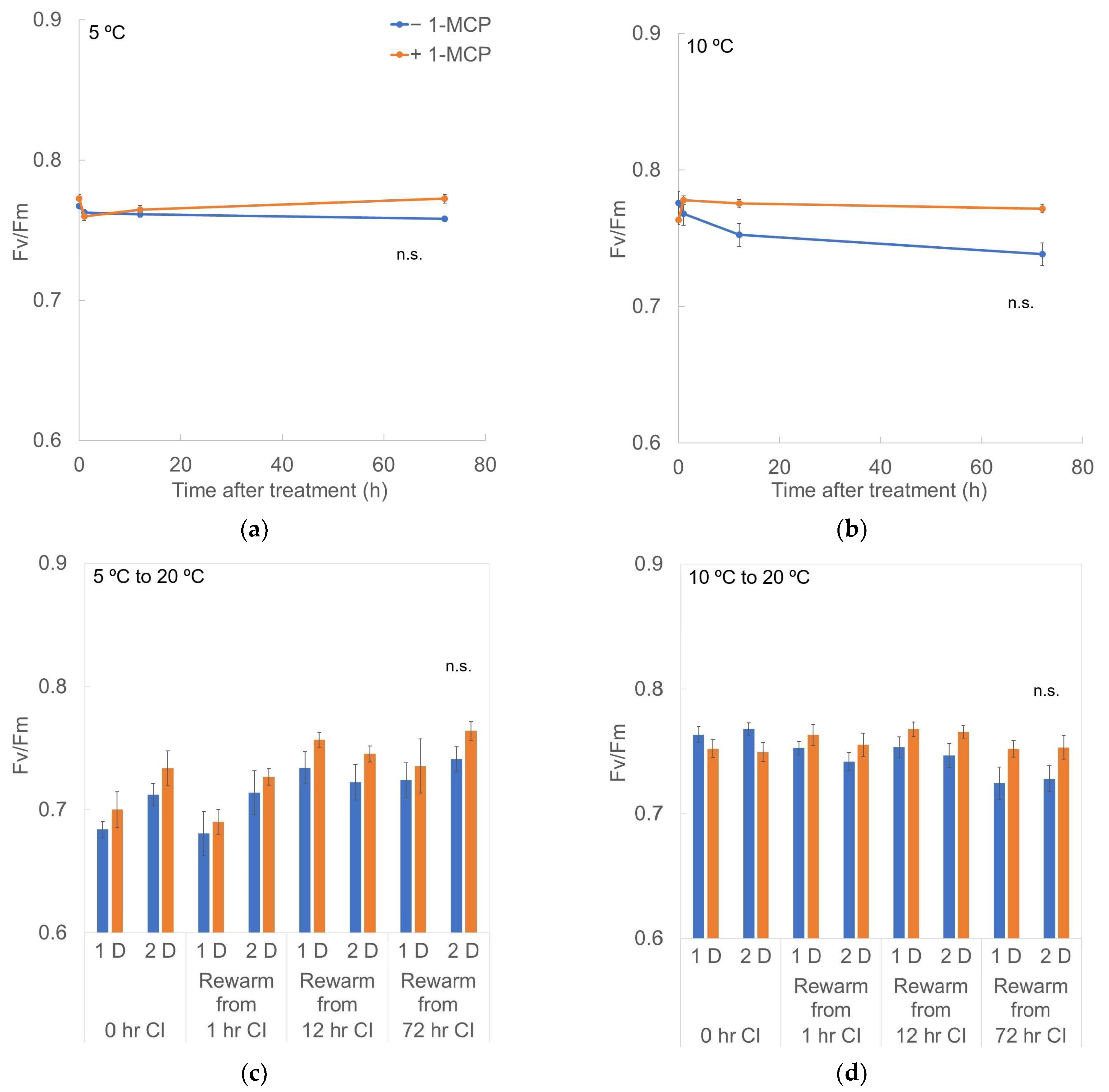
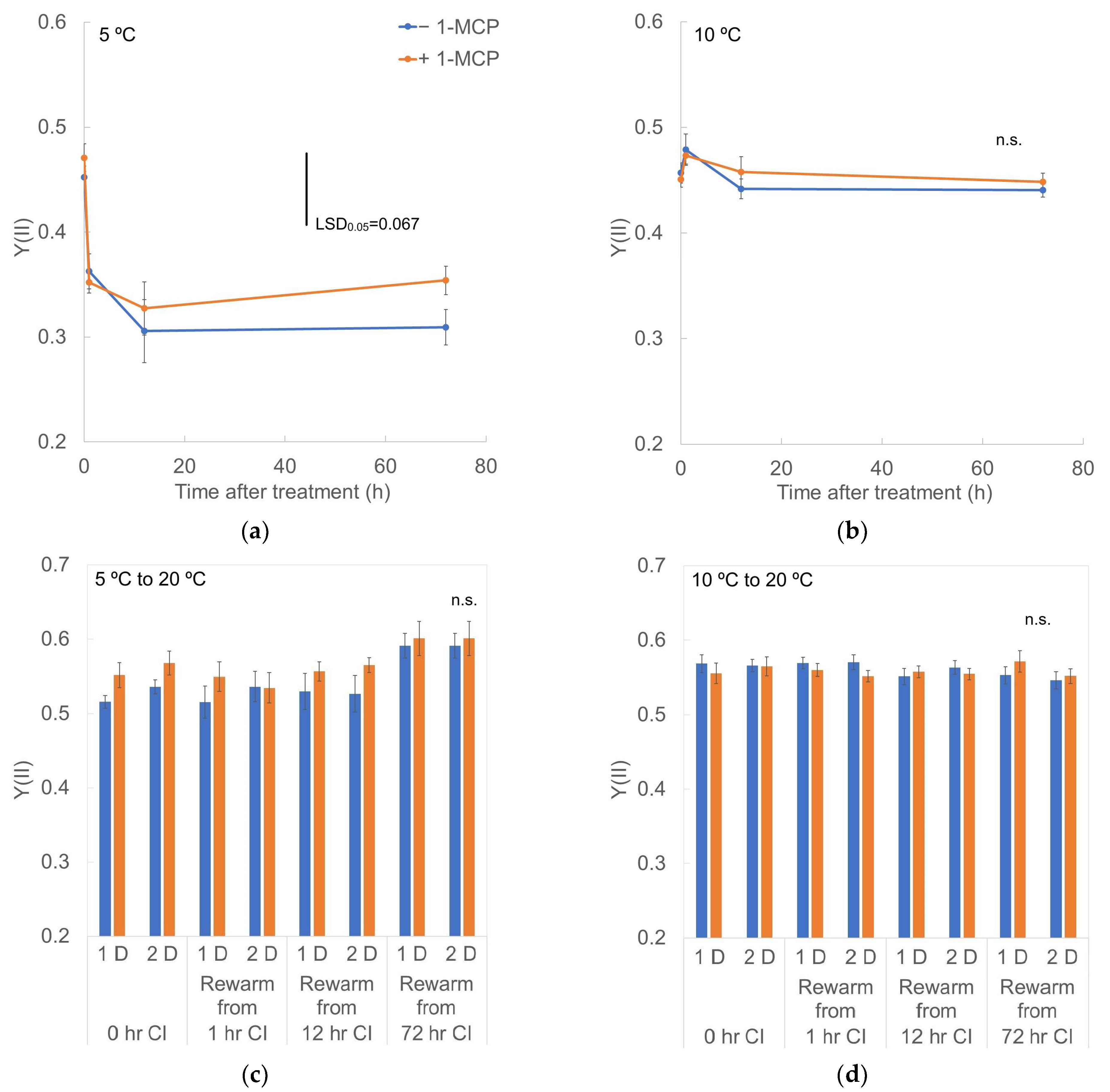
3.4. Chlorophyll Fluorescence
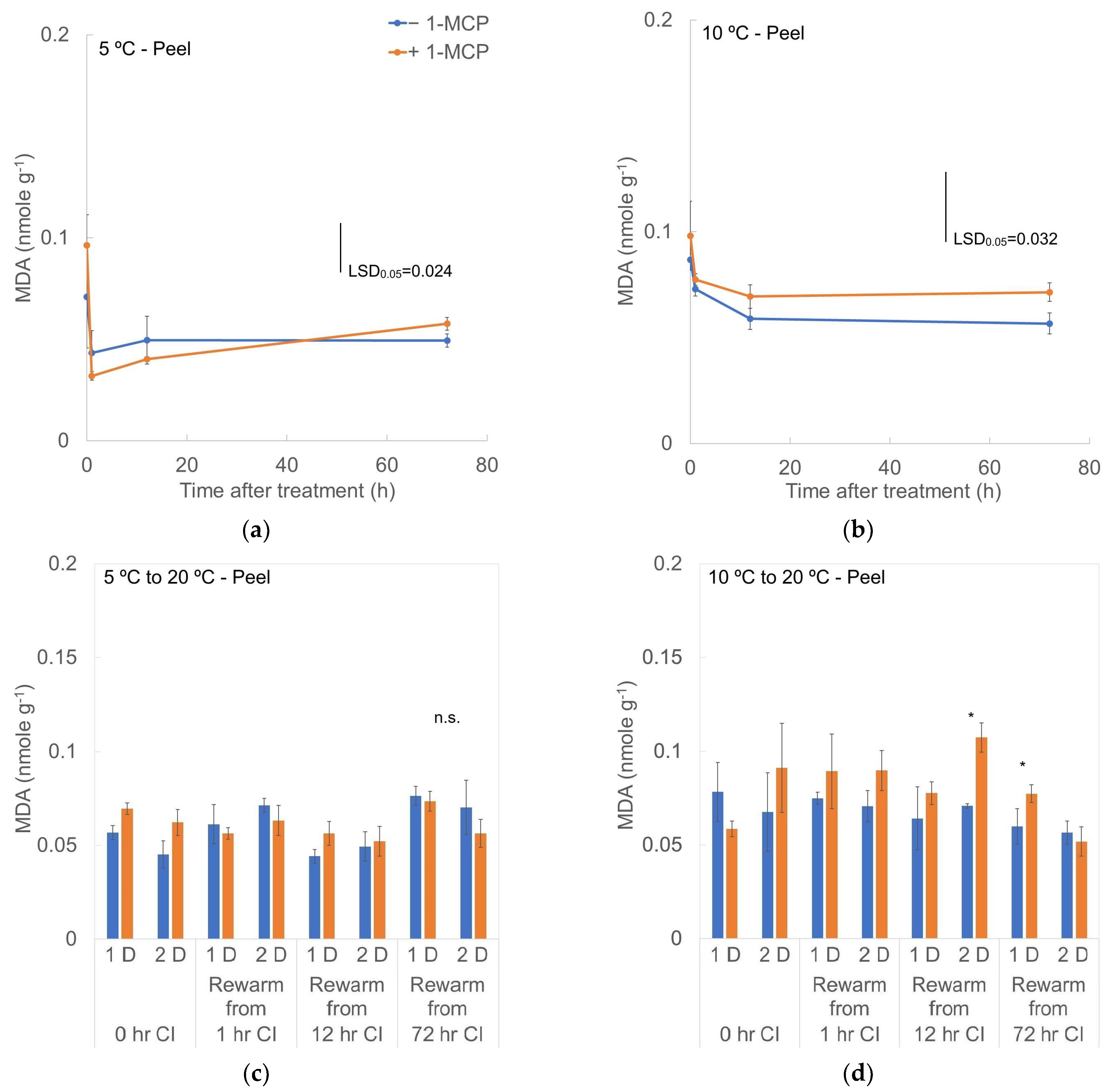
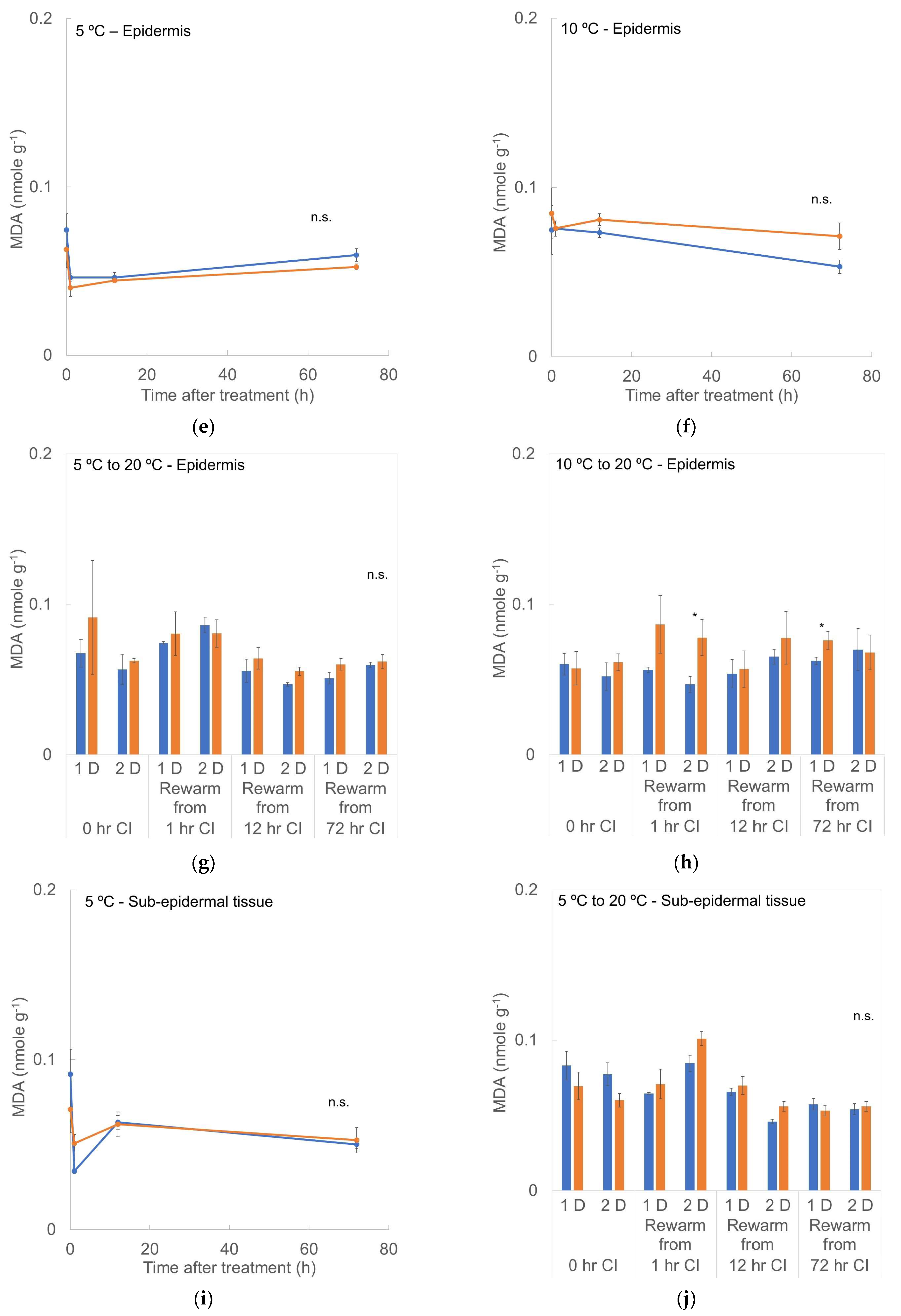
3.5. MDA Content
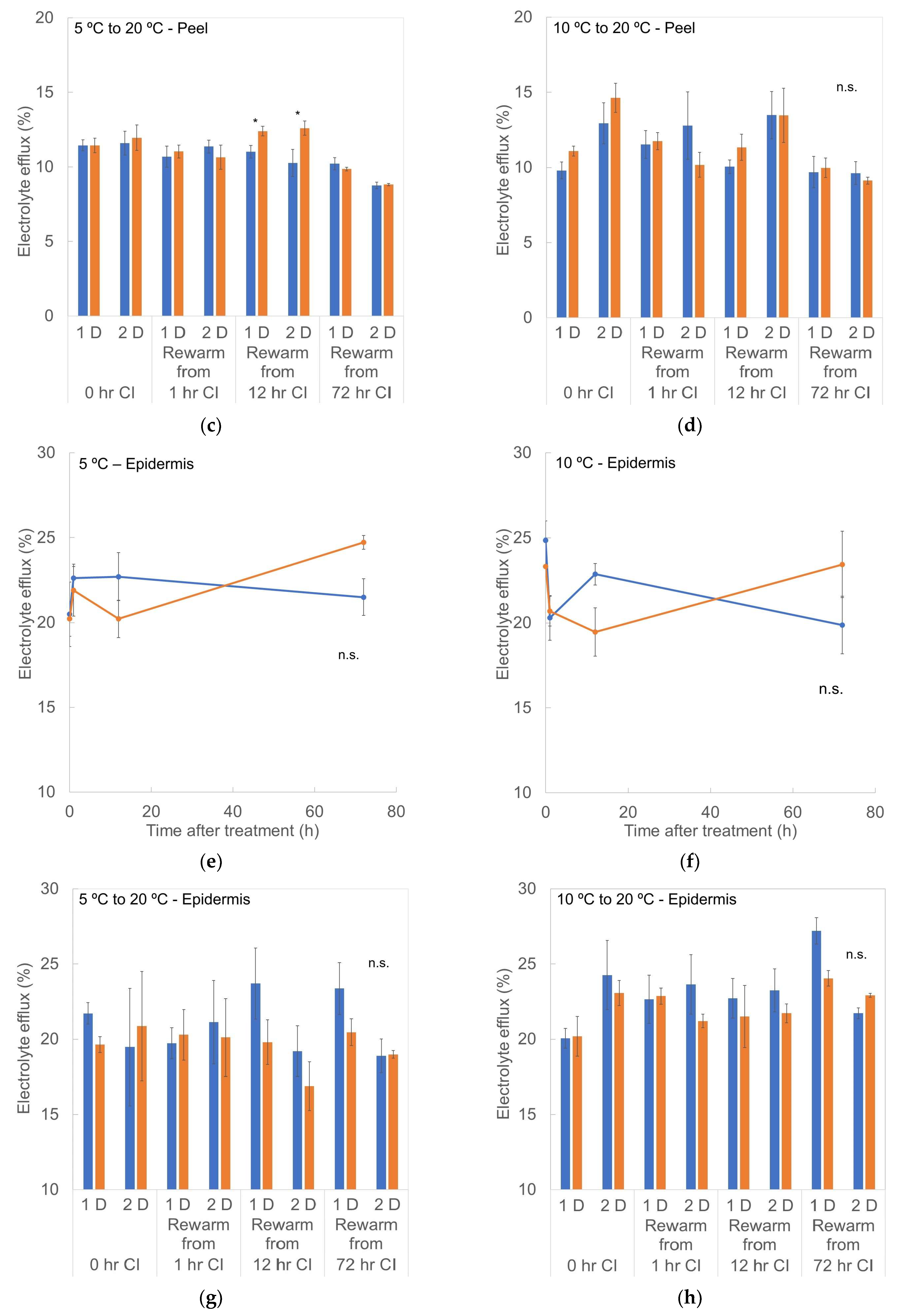
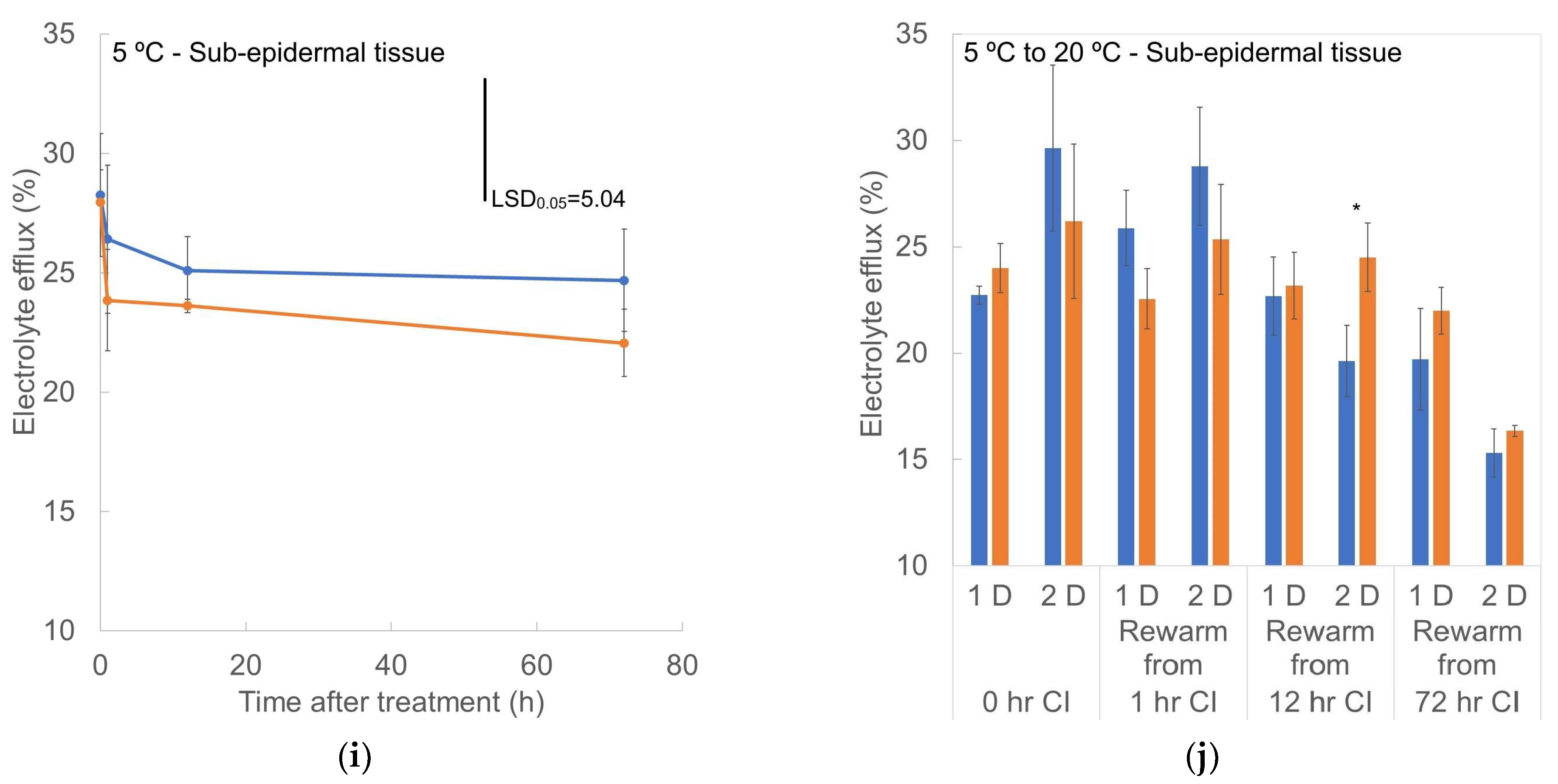
3.6. Electrolyte Efflux (EE)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kader, A.A. Postharvest Biology and Technology: An Overview. In Postharvest Technology of Horticultural Crops, 3rd ed.; Kader, A.A., Ed.; University of California Agriculture & Natural Resources: Oakland, CA, USA, 2002; pp. 39–47. [Google Scholar]
- Lyons, J.M. Chilling injury in plants. Annu. Rev. Plant Physiol. 1973, 24, 445–466. [Google Scholar] [CrossRef]
- Saltveit, M.E., Jr.; Morris, L.L. Overview on Chilling Injury of Horticultural Crops. In Chilling Injury of Horticultural Crops; Wang, C.Y., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 3–16. [Google Scholar]
- Eaks, I.L. Effect of chilling on respiration and volatiles of California lemon fruit. J. Am. Soc. Hortic. Sci. 1980, 105, 865–869. [Google Scholar] [CrossRef]
- Eaks, I.L. Effects of chilling on respiration and ethylene production of ‘Hass’ avocado fruit at 20 °C. HortScience 1983, 18, 235–237. [Google Scholar] [CrossRef]
- Trejo-Márquez, M.A.; Vendrell, M. Physiological response to chilling temperature of banana fruit (Musa acuminata cv. Dwarf cavendish). Acta Hortic. 2010, 864, 387–393. [Google Scholar] [CrossRef]
- Sevillano, L.; Sanchez-Ballesta, M.T.; Romojaro, F.; Flores, F.B. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 2009, 89, 555–573. [Google Scholar] [CrossRef]
- Aroca, R.; Irigoyen, J.J.; Sanchez-Diaz, M. Photosynthetic characteristics and protective mechanisms against oxidative stress during chilling and subsequent recovery in two maize varieties differing in chilling sensitivity. Plant Sci. 2001, 161, 719–726. [Google Scholar] [CrossRef]
- Cabrera, R.M.; Saltveit, M.E.; Owens, K. Sensitivity of cucumber lines to chilling injury. HortScience 1990, 24, 1174. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Wise, R.R. The ultrastructure of chilling stress. Plant Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Smillie, R.M.; Nott, R.; Hetherington, S.E.; Öquist, G. Chilling injury and recovery in detached and attached leaves measured by chlorophyll fluorescence. Physiol. Plant. 1987, 69, 419–428. [Google Scholar] [CrossRef]
- Prasad, T.K.; Anderson, M.D.; Stewart, C.R. Acclimation, hydrogen peroxide, and abscisic acid protect mitochondria against irreversible chilling injury in maize seedlings. Plant Physiol. 1994, 105, 619–627. [Google Scholar] [CrossRef]
- Lurie, S.; Laamin, M.; Zand, L.; Fallik, E. Heat treatments to decrease chilling injury in tomato fruit. Effects on lipids, pericarp lesions and fungal growth. Physiol. Plant. 1997, 100, 297–302. [Google Scholar] [CrossRef]
- Andersen, C.R.; Kent, M.W. Storage of cucumber fruit at chilling temperatures. HortScience 1982, 17, 527. [Google Scholar]
- Anderson, R.E. The influence of storage temperatures and warming during storage on peach and nectarine fruit quality. J. Am. Soc. Hortic. Sci. 1979, 104, 459–461. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Mitchell, F.G.; Ju, Z. Susceptibility to chilling injury of peach, nectarine, and plum cultivars grown in California. HortScience 1999, 34, 1116–1118. [Google Scholar] [CrossRef]
- Groen, S.C.; Whiteman, N.K. The Evolution of Ethylene Signaling in Plant Chemical Ecology. J. Chem. Ecol. 2014, 40, 700–716. [Google Scholar] [CrossRef]
- Hyodo, H. Stress/Wound Ethylene. In The Plant Hormone Ethylene; Mattoo, A.K., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 43–63. [Google Scholar]
- Grichko, V.P.; Glick, B.R. Ethylene and flooding stress in plants. Plant Physiol. Biochem. 2001, 39, 1–9. [Google Scholar] [CrossRef]
- Megías, Z.; Martínez, C.; Manzano, S.; García, A.; del Mar Rebolloso-Fuentes, M.; Valenzuela, J.L.; Garrido, D.; Jamilena, M. Ethylene biosynthesis and signaling elements involved in chilling injury and other postharvest quality traits in the non-climacteric fruit of zucchini (Cucurbita pepo). Postharvest Biol. Technol. 2016, 113, 48–57. [Google Scholar] [CrossRef]
- Pesis, E.; Ackerman, M.; Ben-Arie, R.; Feygenberg, O.; Feng, X.; Apelbaum, A.; Goren, R.; Prusky, D. Ethylene involvement in chilling injury symptoms of avocado during cold storage. Postharvest Biol. Technol. 2002, 24, 171–181. [Google Scholar] [CrossRef]
- Ben-Amor, M.; Flores, B.; Latche, A.; Bouzayen, M.; Pech, J.C.; Fomojaro, F. Inhibition of ethylene biosynthesis by antisense ACC oxidase RNA prevents chilling injury in Charentais cantaloupe melons. Plant Cell Environ. 2002, 22, 1579–1586. [Google Scholar] [CrossRef]
- Palou, L.; Crisosto, C.H.; Garner, D.; Basinal, L.M. Effect of continuous exposure to exogenous ethylene during cold storage on postharvest decay development and quality attributes of stone fruits and table grapes. Postharvest Biol. Technol. 2003, 27, 243–254. [Google Scholar] [CrossRef]
- Wang, C.Y. Approaches to reduce chilling injury of fruits and vegetables. Hortic. Rev. 1993, 15, 63–95. [Google Scholar]
- Li, T.; Yun, Z.; Zhang, D.; Yang, C.; Zhu, H.; Jiang, Y.; Duan, X. Proteomics analysis of differentially expressed proteins involved in ethylene-induced chilling tolerance in harvested banana fruit. Front. Plant Sci. 2015, 6, 845. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Qin, W.; Zhou, Z.; Wu, A.M.; Deng, W.; Li, Z.; Shan, W.; Chen, J.Y.; Kuang, J.F.; Lu, W.J. Banana MaNAC1 activates secondary cell wall cellulose biosynthesis to enhance chilling resistance in fruit. Plant Biotechnol. J. 2024, 22, 413–426. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Y.; Shan, W.; Zhang, H.; Wei, W.; Kuang, J.; Chen, J.; Lu, W. Ethylene attenuates chilling injury of banana fruit via the MabHLH060/183 module in controlling phosphatidic acid formation genes. Postharvest Biol. Technol. 2022, 183, 111724. [Google Scholar] [CrossRef]
- Marriott, J.; Palmer, J.K. Bananas—Physiology and biochemistry of storage and ripening for optimum quality. Crit. Rev. Food Sci. Nutr. 1980, 13, 41–88. [Google Scholar] [CrossRef]
- De Vasconcelos Facundo, H.V.; dos Santos Garruti, D.; dos Sants Dias, C.T.; Cordenunsi, B.R.; Lajolo, F.M. Influence of different banana cultivars on volatile compounds during ripening in cold storage. Food Res. Int. 2012, 49, 626–633. [Google Scholar] [CrossRef]
- De Vasconcelos Facundo, H.V.; Gurak, P.D.; Mercadante, A.Z.; Lajolo, F.M.; Cordenunsi, B.R. Storage at low temperature differentially affects the colour and carotenoid composition of two cultivars of banana. Food Chem. 2015, 170, 102–109. [Google Scholar] [CrossRef]
- Grierson, E.B.; Grierson, W.; Soule, J. Chilling injury in tropical fruit. I. Bananas (Musa paradisiaca var. sapientum cv. Lacatan). Proc. Trop. Reg. Am. Soc. Hortic. Sci. 1967, 11, 82–94. [Google Scholar]
- Jiang, Y.; Joyce, D.C.; Jiang, W.; Lu, W. Effects of chilling temperatures on ethylene binding by banana fruit. Plant Growth Regul. 2004, 43, 109–115. [Google Scholar] [CrossRef]
- Kader, A.A.; Sommer, N.F.; Arpaia, M.L. Postharvest Handling Systems: Tropical Fruits. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California, Agriculture and Natural Resources: Davis, CA, USA, 2002; pp. 385–398. [Google Scholar]
- Mootoo, A.; Murr, D.P. Quality attributes of banana fruit as affected by chilling and non-chilling temperatures. In Proceedings of the 21st Annual Meeting of Caribbean Food Crops Society, Port-of-Spain, Trinidad and Tobago, 8–13 September 1985. [Google Scholar]
- Ratule, A.; Osman, M.T.; Ahmad, S.H.; Saari, N. Development of chilling injury of ‘Berangan’ banana (Musa cv. ‘Berangan’ (AAA)) during storage at low temperature. J. Food Agric Environ. 2006, 4, 128–134. [Google Scholar]
- Von Loesecke, H.W. Bananas, 2nd ed.; Interscience: New York, NY, USA, 1950; pp. 52–66. [Google Scholar]
- Chang, W.H.; Hwang, Y.J. Effect of ethylene treatment on the ripening, polyphenoloxydase activity and water soluble tannin content of Taiwan northern banana at different maturity stages and the stability of banana polyphenoloxydase. Acta Hortic. 1990, 275, 603–610. [Google Scholar] [CrossRef]
- Chang, L.Y.; Sargent, S.A.; Kim, J.; Brecht, J.K. Delaying ripening using 1-MCP reveals chilling injury symptom development at the putative chilling threshold temperature for mature green banana. Front. Plant Sci. 2022, 13, 966789. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bullock, D.A., Jr.; Alonso, J.M.; Stepanova, A.N. To Fight or to Grow: The Balancing Role of Ethylene in Plant Abiotic Stress Responses. Plants 2022, 11, 33. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Ketsa, S.; van Doorn, W.G. Relationship between browning and the activities of polyphenol oxidase and phenylalanine ammonia lyase in banana peel during low temperature storage. Postharvest Biol. Technol. 2003, 30, 187–193. [Google Scholar] [CrossRef]
- Jeong, J.; Brecht, J.; Huber, D.; Sargent, S. Influence of 1-methylcyclopropene (1-MCP) on the shelf life and deterioration of fresh-cut cantaloupe. HortScience 2004, 39, 816. [Google Scholar] [CrossRef]
- Natalini, A.; Martinez-Diaz, V.; Ferrante, A.; Pardossi, A. Ethylene sensitivity regulates the wounding response in wild type and never ripe tomatoes. J. Hortic. Sci. Biotechnol. 2017, 92, 591–597. [Google Scholar] [CrossRef]
- Thompson, J.E.; Mayak, S.; Shinitzky, M.; Halevy, A.H. Acceleration of membrane senescence in cut carnation flowers by treatment with ethylene. Plant Physiol. 1982, 69, 859–863. [Google Scholar] [CrossRef]
- Murata, T. Relation of Chilling Stress to Membrane Permeability. In Chilling Injury of Horticultural Crops; Wang, C.Y., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 202–210. [Google Scholar]
- Mao, L.; Karakurt, Y.; Huber, D.J. Incidence of water-soaking and phospholipid catabolism in ripe watermelon (Citrullus lanatus) fruit: Induction by ethylene and prophylactic effects of 1-methylcyclopropene. Postharvest Biol. Technol. 2004, 33, 1–9. [Google Scholar] [CrossRef]
- Choi, S.T.; Huber, D.J. Influence of aqueous 1-methylcyclopropene concentration, immersion duration, and solution longevity on the postharvest ripening of breaker-turning tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2008, 49, 147–154. [Google Scholar] [CrossRef]
- Hagan, L.L.; Johnson, P.N.T.; Sargent, S.A.; Huber, D.J.; Berry, A. 1-Methylcyclopropene treatment and storage condition delay the ripening of plantain fruit while maintaining sensory characteristics of ampesi, the boiled food product. Int. Food Res. J. 2017, 24, 630–636. [Google Scholar]
- Chang, L.Y.; Brecht, J.K. Responses of 1-methylcyclopropene (1-MCP)-treated banana fruit to pre—And post—Treatment ethylene exposure. Sci. Hortic. 2023, 309, 111636. [Google Scholar] [CrossRef]
- Huang, H.; Jing, G.; Gua, L.; Zhang, D.; Yang, B.; Duan, X.; Ashraf, M.; Jiang, Y. Effect of oxalic acid on ripening attributes of banana fruit during storage. Postharvest Biol. Technol. 2013, 84, 22–27. [Google Scholar] [CrossRef]
- Villalta, A.M.; Sargent, S.A. Response of Beit Alpha-type cucumbers (Cucumis sativus L., ‘Manar’) to continuous ethylene exposure. Proc. Fla. State Hortic. Soc. 2004, 117, 368–372. [Google Scholar]
- Bugaud, C.; Joannès-Dumec, C.; Louisor, J.; Tixier, P.; Salmon, F. Preharvest temperature affects chilling injury in dessert bananas during storage. J. Sci. Food Agric. 2008, 96, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.V. Mild Chilling Injury of Banana (Cavendish cv. Williams) and Its Control in the Field. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2005. [Google Scholar]
- Lizada, M.C. Postharvest physiology of the mango—A review. Acta Hortic. 1991, 291, 437–453. [Google Scholar] [CrossRef]
- Phakawatmongkol, W.; Ketsa, S.; Van Doorn, W.G. Variation in fruit chilling injury among mango cultivars. Postharvest Biol. Technol. 2004, 32, 115–118. [Google Scholar] [CrossRef]
- Suresh Nair, S.; Zora Singh, Z.; Tan, S.C. Chilling injury in relation to ethylene biosynthesis in ‘Kensington Pride’ mango fruit. J. Hortic. Sci. Biotechnol. 2015, 79, 82–90. [Google Scholar] [CrossRef]
- Castaner, M.; Gil, M.I.; Artes, F.; Tomas-Barberan, F.A. Inhibition of browning of harvested head lettuce. J. Food Sci. 1996, 61, 314–316. [Google Scholar] [CrossRef]
- Griffiths, L.A. Detection and identification of polyphenol oxidase substrate of banana. Nature 1959, 184, 58–59. [Google Scholar] [CrossRef]
- Luengwilai, K.; Beckles, D.M.; Siriphanich, J. Postharvest internal browning of pineapple fruit originates at the phloem. J. Plant Physiol. 2016, 202, 121–133. [Google Scholar] [CrossRef]
- Hewage, K.S.; Wainwright, H.; Wijeratnam, R.S.W. Quantitative assessment of chilling injury in bananas using a colorimeter. J. Hortic. Sci. 1996, 71, 135–139. [Google Scholar] [CrossRef]
- Dalal, V.B.; Nagaraja, N.; Thomas, P.; Amla, B.L. Some aspects of storage of dwarf Cavendish bananas at refrigerated temperature for export. Indian Food Pack. 1969, 23, 34. [Google Scholar]
- Couture, R.; Cantwell, M.I.; Ke, D.; Saltveit, M.E., Jr. Physiological attributes related to quality attributes and storage life of minimally processed lettuce. HortScience 1993, 28, 723–725. [Google Scholar] [CrossRef]
- Jin, P.; Shang, H.; Chen, J.; Zhu, H.; Zhao, Y.; Zheng, Y. Effect of 1-methylcyclo-propene on chilling injury and quality of peach fruit during cold storage. J. Food Sci. 2011, 76, S485–S491. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jian, Q.; Jiang, Y.; Duan, X.; Qu, H. Enhanced chilling tolerance of banana fruit treated with malic acid prior to low-temperature storage. Postharvest Biol. Technol. 2016, 111, 209–213. [Google Scholar] [CrossRef]
- Chaiprasart, P.; Gemma, H.; Iwahori, S. Changes in chlorophyll fluorescence and enzyme activity for scavenging of free radicals in banana fruits stored at low temperature. Jpn. J. Trop. Agric. 2001, 45, 181–191. [Google Scholar]
- Dong, Z.; Men, Y.; Liu, Z.; Ji, J. Application of chlorophyll fluorescence imaging technique in analysis and detection of chilling injury of tomato seedlings. Comput. Electron. Agric. 2020, 168, 105109. [Google Scholar] [CrossRef]
- Shewfelt, R.L.; Rosario, B.A. The role of lipid peroxidation in storage disorders of fresh fruits and vegetables. HortScience 2000, 35, 575–579. [Google Scholar] [CrossRef]
- Murata, T. Physiological and biochemical studies of chilling injury in bananas. Physiol. Plant. 1969, 22, 401–411. [Google Scholar] [CrossRef]
- Gemma, H.; Matsuyama, Y.; Wang, H. Ripening characteristics and chilling injury of banana fruit. I. Effect of storage temperature on respiration, ethylene production and membrane permeability of peel and pulp tissues. Jpn. J. Trop. Agric. 1994, 38, 216–220. [Google Scholar]
- Parkin, K.L.; Kuo, S.J. Chilling-induced lipid degradation in cucumber (Cucumis sativa L. cv Hybrid C) fruit. Plant Physiol. 1989, 90, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Z.; Hu, M.; Pan, Y.; Xu, X.; Zhang, Z. Delay of ripening and senescence in mango fruit by 6-benzylaminopurine is associated with inhibition of ethylene biosynthesis and membrane lipid catabolism. Postharvest Biol. Technol. 2022, 185, 111797. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.-Y.; Brecht, J.K. Early Chilling Injury Symptom Development and Recovery of Mature Green Banana: Involvement of Ethylene under Different Severities of Chilling Stress. Horticulturae 2024, 10, 1050. https://doi.org/10.3390/horticulturae10101050
Chang L-Y, Brecht JK. Early Chilling Injury Symptom Development and Recovery of Mature Green Banana: Involvement of Ethylene under Different Severities of Chilling Stress. Horticulturae. 2024; 10(10):1050. https://doi.org/10.3390/horticulturae10101050
Chicago/Turabian StyleChang, Lan-Yen, and Jeffrey K. Brecht. 2024. "Early Chilling Injury Symptom Development and Recovery of Mature Green Banana: Involvement of Ethylene under Different Severities of Chilling Stress" Horticulturae 10, no. 10: 1050. https://doi.org/10.3390/horticulturae10101050
APA StyleChang, L.-Y., & Brecht, J. K. (2024). Early Chilling Injury Symptom Development and Recovery of Mature Green Banana: Involvement of Ethylene under Different Severities of Chilling Stress. Horticulturae, 10(10), 1050. https://doi.org/10.3390/horticulturae10101050





