Inheritance of the Flesh Color and Shape of the Tuberous Root of Sweet Potato (Ipomoea batatas [L.] Lam.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Crossing Blocks
2.3. Botanical Seeds and Seedlings
2.4. Characters Investigated
2.5. Morphometric Variables
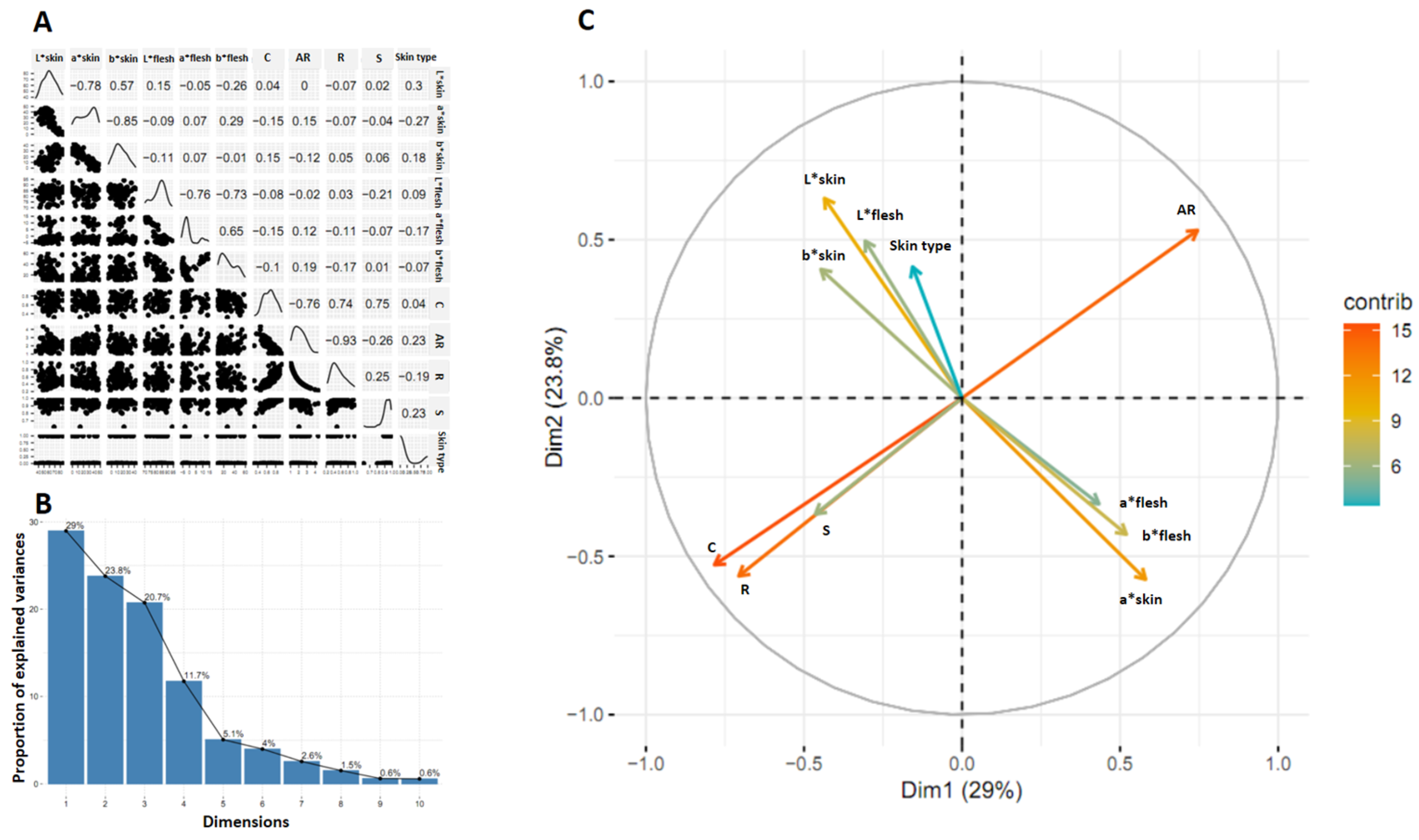
2.6. Statistical Analysis and Visualization
- : phenotypic variance;
- : genotypic variance;
- : environmental variance.
- Fi: term of the data set;
- m: medium;
- n: number of observations.
- r(jk) = correlation between the original variable x(j) and the k-th component.
- l(jk) = j-th element of the k-th eigenvector.
- λ(κ) = k-th eigenvalue.
- S(ij) = variances of the correlation matrix.
3. Results
3.1. Pollinations
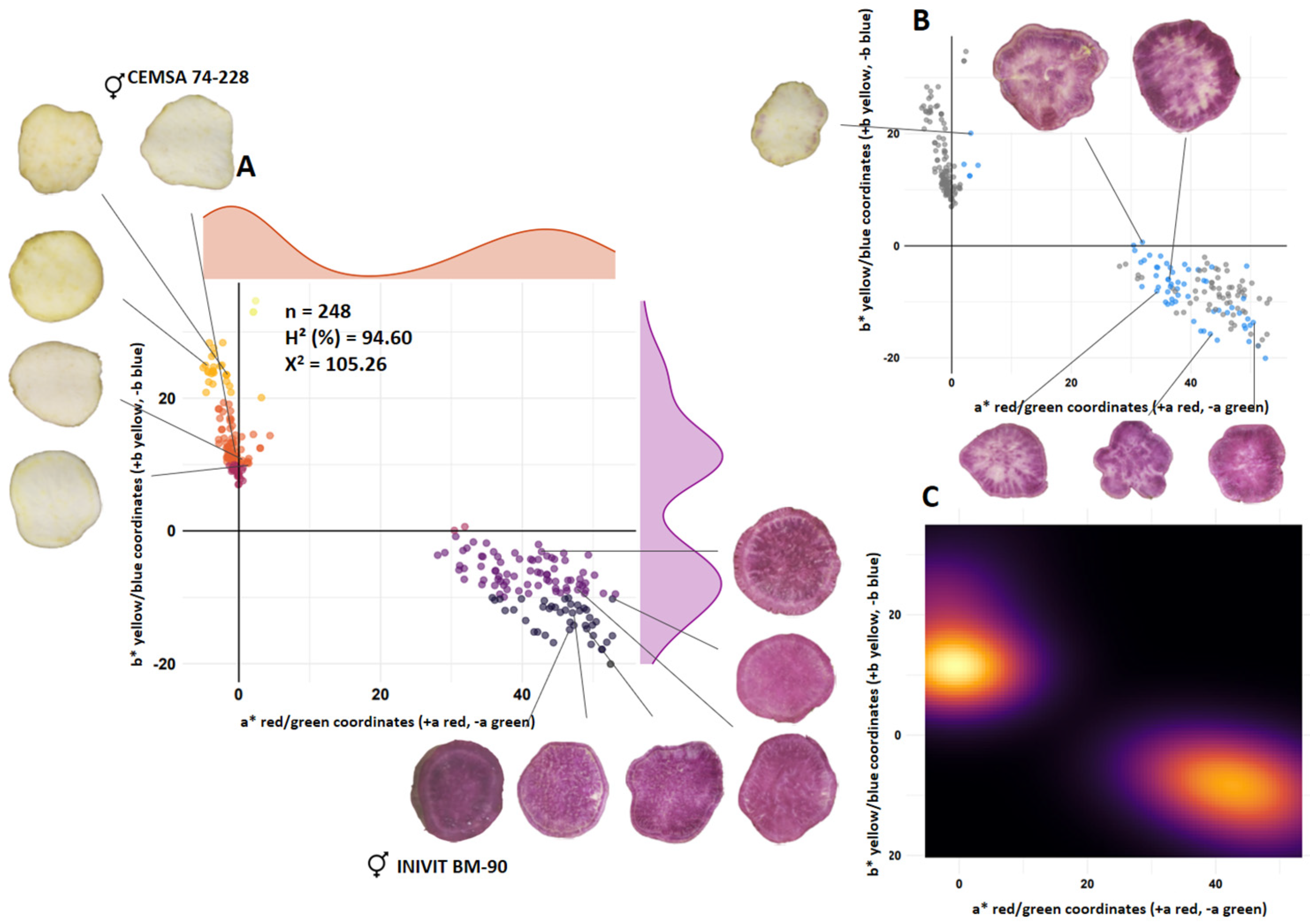
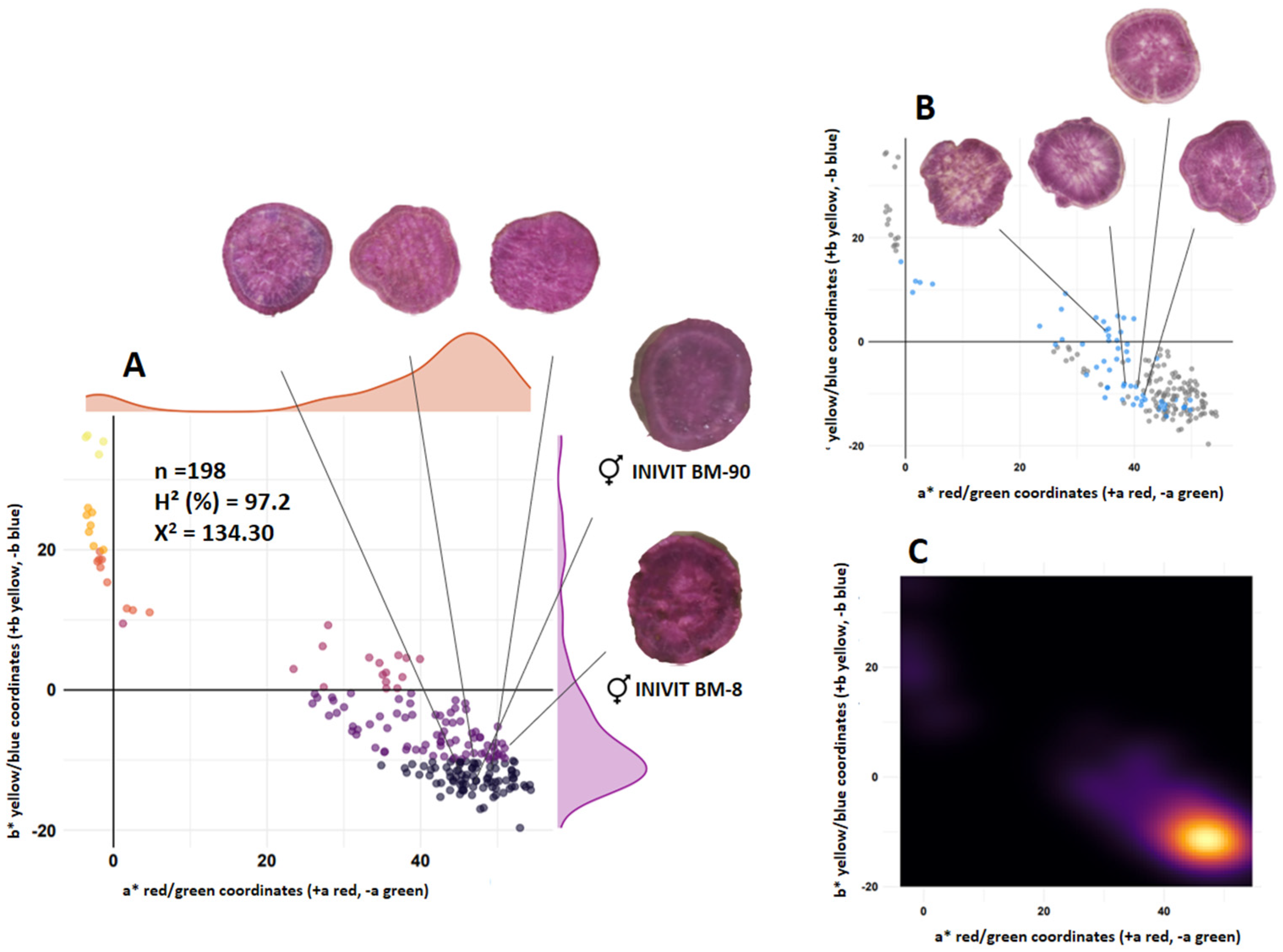
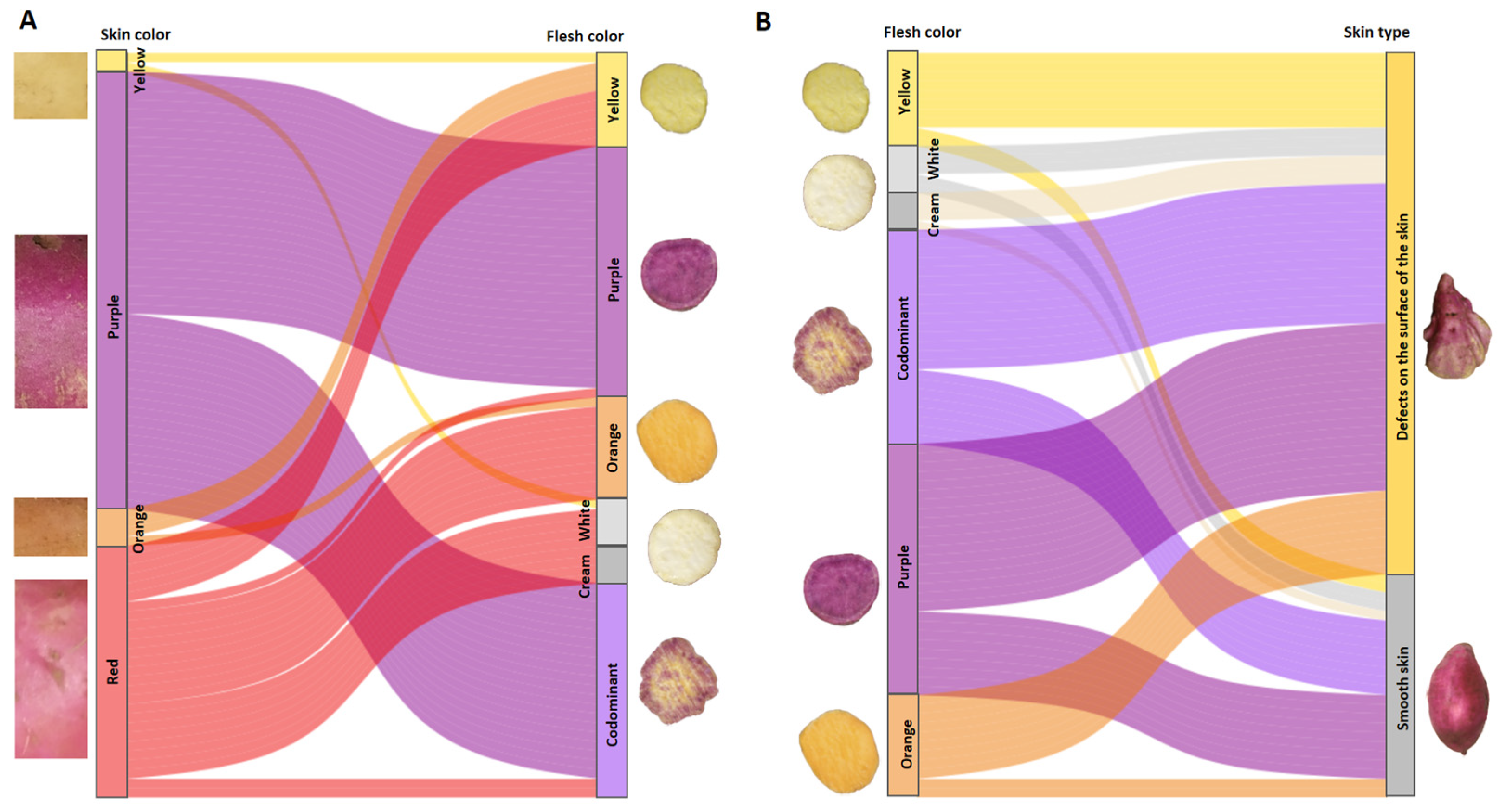
3.2. Flesh Color of Tuberous Roots
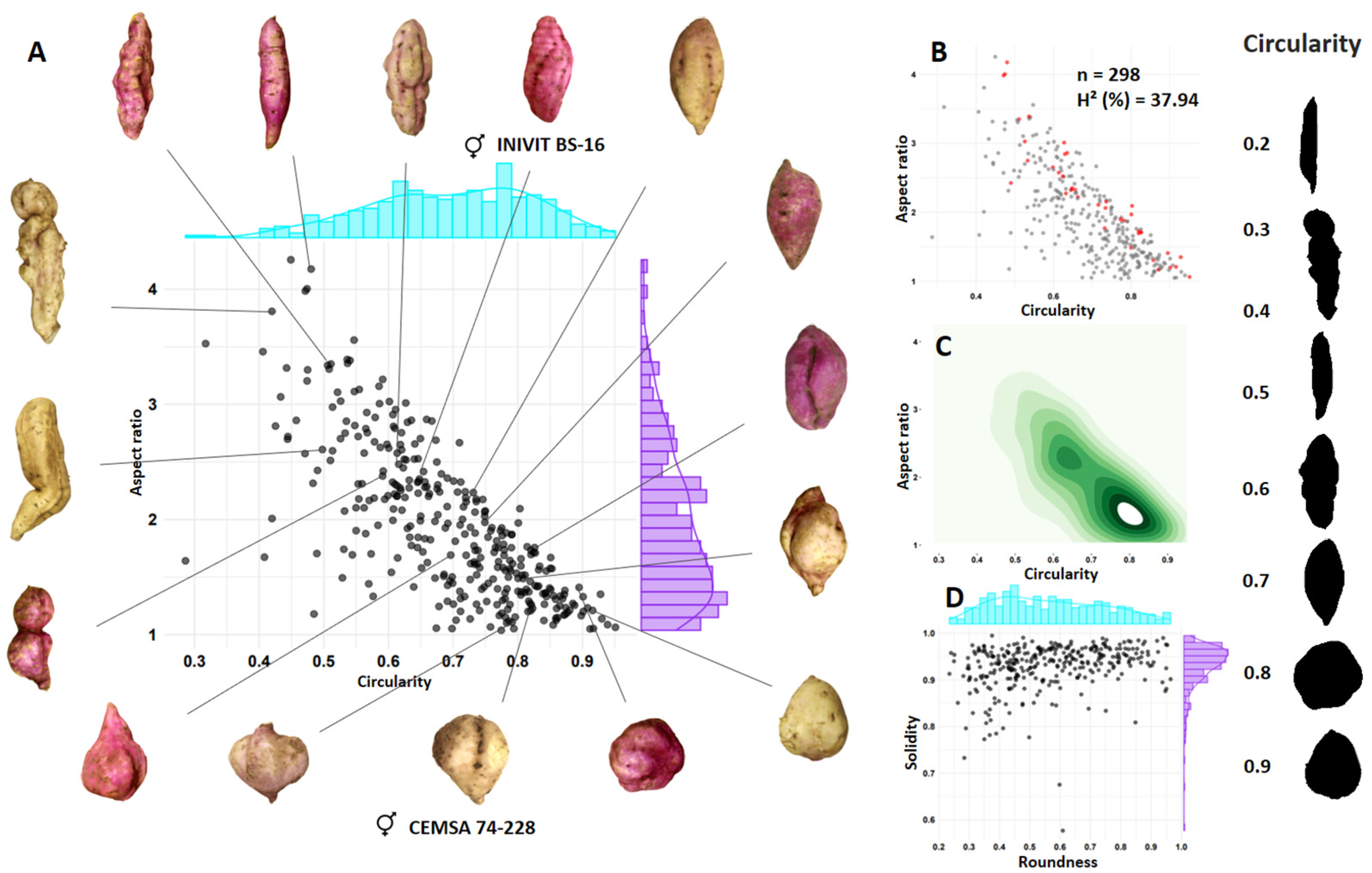
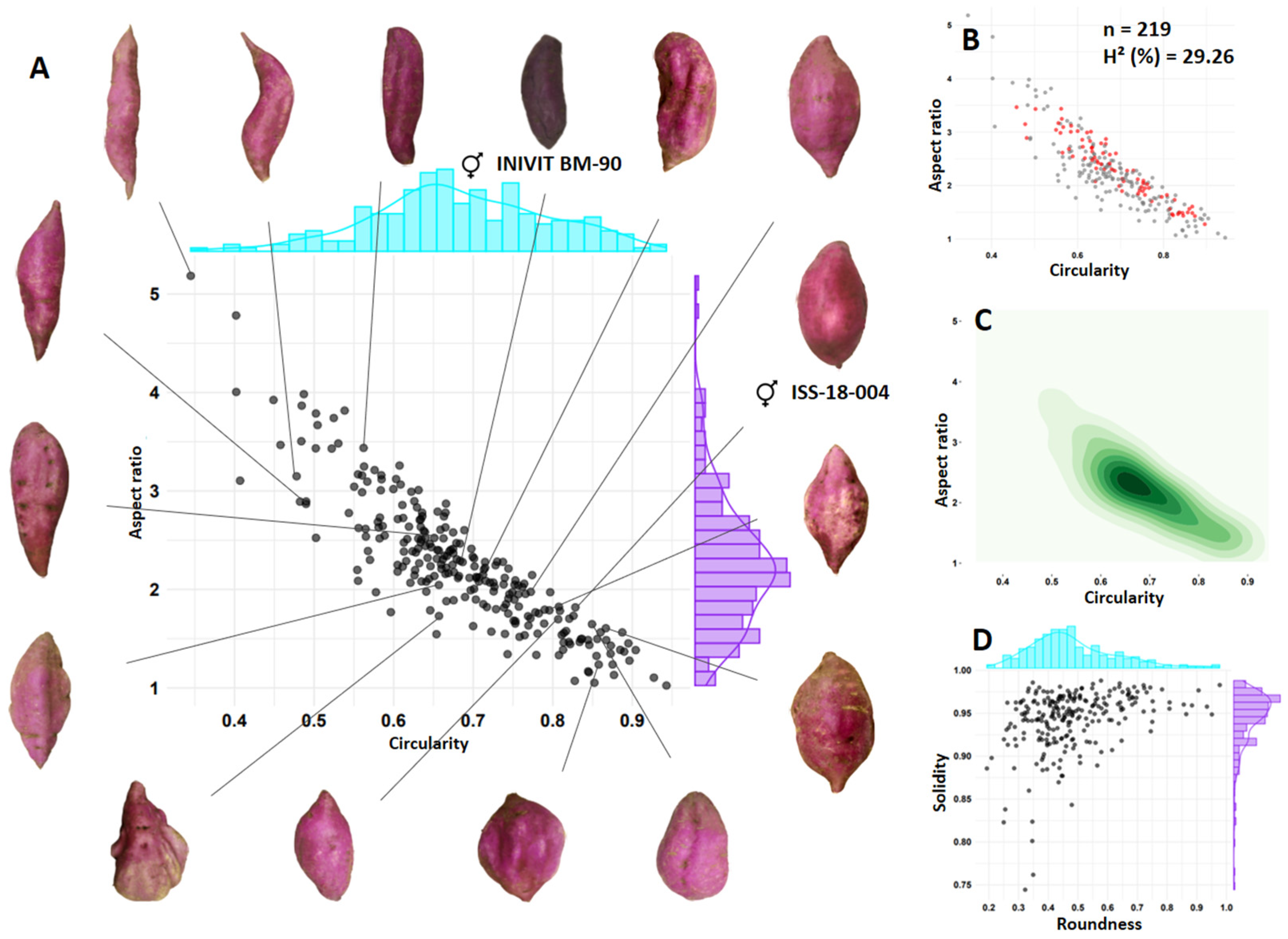
3.3. Shape of Tuberous Roots
3.4. Association between Traits
- PS: Progenies to be selected;
- n: Number of seedlings per family;
- PPDC1: proportion of progeny with the desired color;
- PPDC2: proportion of progeny with the desired color (if necessary, otherwise it will be = 1);
- SSC: Smooth Skin Coefficient.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- BBC News Mundo. Gregor Mendel: Cómo un Monje con un Jardín de Arvejas Descubrió las leyes de la Herencia Genética. 2021. Available online: https://www.bbc.com/mundo/noticias-56719582 (accessed on 18 August 2024).
- Sadurní, J.M. EL MONJE BOTÁNICO. Gregor Mendel, el Padre de la Genética. 2023. Available online: https://historia.nationalgeographic.com.es/a/gregor-mendel-padre-genetica_15509 (accessed on 12 August 2024).
- Isobe, S.; Shirasawa, K.; Hirakawa, H. Current status in whole genome sequencing and analysis of Ipomoea spp. Plant Cell Rep. 2019, 38, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Kriegner, A.; Cervantes, J.C.; Burg, K.; Mwanga, O.M.; Zhang, D. A genetic linkage map of sweetpotato [Ipomoea batatas (L.) Lam.] based on AFLP markers. Mol. Breed. 2003, 11, 169–185. [Google Scholar] [CrossRef]
- Mollinari, M.; Olukolu, B.A.; Pereira, D.S.; Khan, A.; Gemenet, D.; Yencho, G.C.; Zeng, Z.B. Unraveling the Hexaploid Sweetpotato Inheritance Using Ultra-Dense Multilocus Mapping. G3 2020, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, J.C.; Yencho, G.C.; Kriegner, A.; Pecota, K.V.; Faulk, M.A.; Mwanga, R.O.; Sosinski, B.R. Development of a genetic linkage map and identification of homologous linkage groups in sweetpotato using multiple-dose AFLP markers. Mol. Breed. 2008, 21, 511–532. [Google Scholar] [CrossRef]
- Jones, A. Theoretical Segregation Ratios of Qualitatively Inherited Characters for Hexaploid Sweetpotato (Ipomoea batatas L.); Technical Bulletin; Agricultural Research Service US: Washington, DC, USA, 1967; p. 1368. [Google Scholar]
- Shiotani, I. Sweet Potato Evolution. Sweet Potato Research and Development for Small Farmers. In Sweet Potato Research and Development for Small Farmers; Kennet, T., Manuel, K., Rolinda, T., Eds.; SEAMEO-SEARCA: Santa Cruz, Philippines, 1989; pp. 5–15. [Google Scholar]
- Grüneberg, W.J.; Ma, D.; Mwanga, R.; Carey, E.; Huamani, K.; Diaz, F.; Eyzaguirre, R.; Guaf, E.; Jusuf, M.; Karuniawan, A.; et al. Advances in sweet potato breeding from 1992 to 2012. In Potato and Sweetpotato in Africa: Transforming the Value Chains for Food and Nutrition Security; CAB International: Wallingford, UK, 2015; Chapter 1; pp. 3–68. [Google Scholar]
- Rangaswami, A.K.; Sampathkumar, R. Inheritance of leaf shape and colour in sweet potato. Indian J. Genet. Plant Breed. 1978, 38, 262–263. [Google Scholar]
- Vimala, B.; Sreekanth, A.; Hariprakash, B.; Wolfgang, G. Variation in morphological characters and storage root yield among exotic orange-fleshed sweet potato clones and their seedling population. J. Root Crops 2012, 38, 32–37. [Google Scholar]
- Hernandez, T.P. A Study of Some Genetic Characters of the Sweet Potato. Ph.D. Thesis, LSU, Baton Rouge, LA, USA, 1942; p. 66. [Google Scholar]
- Harmon, S.A. Genetic Studies and Compatibilities in the Sweet Potato (Ipomoea batatas). Ph.D. Thesis, LSU, Baton Rouge, LA, USA, 1960. [Google Scholar]
- Poole, C.F. Sweet Potato Genetic Studies; Technical Bulletin; University of Hawaii: Honolulu, HI, USA, 1955; p. 27. [Google Scholar]
- Arizio, C.M.; Manifesto, M.M.; Martí, H.R. Análisis de caracteres relacionados con el color de la raíz engrosada en un cruzamiento de dos clones de Ipomoea batatas L. (Lam.). Hortic. Argent. 2009, 28, 5–13. [Google Scholar]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhu, M.; Gao, S. Genetic and Genomic Research on Sweet Potato for Sustainable Food and Nutritional Security. Genes 2022, 13, 1833. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, M.R.; Sampaio, K.B.; Souza, E.L. Sweet potato roots: Unrevealing an old food as a source of health promoting bioactive compounds. A review. Trends Food Sci. Technol. 2019, 85, 277–286. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Minatel, I.O.; Borges, C.V.; Alonzo, H.; Hector, G.; Gomez, C.O.; Pace, G.; Lima, P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability, Phenolic Compounds—Biological Activity; InTech: London, UK, 2017; pp. 1–24. [Google Scholar]
- Hernandez, T.P.; Hernandez, T.; Constantin, R.J.; Kakar, R.S. Improved techniques in breeding and inheritance of some of the characters In the sweet potato, Ipomoea batatas L. In Proceedings of the International Symposium on Tropical Root Crops, St. Augustine, Trinidad, 2–8 April 1967; Volume 1, pp. 31–49. [Google Scholar]
- Morales, R.A.; Rodríguez, S.D.; Xie, Y.; Guo, X.; Jia, X.; Ma, P.; Bian, X. Self-incompatibility of sweet potato (Ipomoea batatas (L.) Lam.). A review. Rev. Agric. Trop. 2019, 5, 1–8. [Google Scholar]
- Arizio, C.M. Marcadores Funcionales Relacionados con la Síntesis de Pigmentos y su Localización en un Mapa de Ligamiento en Ipomoea batatas L. Lam. Ph.D. Thesis, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, Argentina, 2011; p. 267. [Google Scholar]
- Schroeder, T. Okinawa Sweet Potato: Japan’s Amazing Purple Superfood! 2021. Available online: https://sakura.co/blog/okinawa-sweet-potato-japans-amazing-purple-superfood (accessed on 11 May 2024).
- Morales, R.A.; Rodríguez, S.D.; Rodríguez, M.S.; Rodríguez, G.Y.; Trujillo, O.N.; Jiménez, M.A.; Molina, C.O. Floral biology and phenology of sweet potato (Ipomoea batatas [L.] Lam.) in Cuba: Bases for genetic improvement. Afr. J. Agric. Res. 2023, 19, 1043–1055. [Google Scholar]
- Hernández, A.; Pérez, J.; Bosch, D.; Castro, N. Clasificación de los Suelos de Cuba; Ediciones INCA: La Habana, Cuba, 2015; p. 61. [Google Scholar]
- Ferreira, T.; Rasband, W. ImageJ User Guide. IJ 1.46, Revised ed.; 2012; p. 198. Available online: https://imagej.net/ij/docs/guide/user-guide.pdf (accessed on 18 August 2024).
- X-Rite. Guía para Entender la Comunicación del Color. L10-001-SL 2002, 2, 24. [Google Scholar]
- Popat, R.; Patel, R.; Parmar, D. Variability: Genetic Variability Analysis for Plant Breeding Research, R package version 0.1.0. 2020; 7. Available online: https://cran.r-project.org/web/packages/variability/variability.pdf (accessed on 18 August 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin, Germany, 2016; p. 260. [Google Scholar]
- Harrower, M.; Brewer, C.A. ColorBrewer.org: An Online Tool for Selecting Colour Schemes for Maps. Cartogr. J. 2003, 40, 27–37. [Google Scholar] [CrossRef]
- Garnier, S. Viridis: Default Color Maps from ‘Matplotlib’, R package Version 0.4.0. 2017. Available online: https://CRAN.R-project.org/package=viridis (accessed on 2 August 2024).
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate Data Analysis; MacMillan Publ. Co.: Nueva York, NY, USA, 1992; p. 544. [Google Scholar]
- Pla, L.E. Análisis Multivariado: Método de Componentes Principales; Secretaría General de la Organización de los Estados Americanos (OEA): Washington, DC, USA, 1986; p. 95. [Google Scholar]
- Reynoso, D.; Huaman, Z.; Aguilar, C. Methods to Determine the Fertility and Compatibility of Sweetpotato; Training Manual; International Potato Center: Lima, Peru, 1998; pp. 133–140. [Google Scholar]
- Kambale, H.; Ngugi, K.; Olubayo, F.; Kivuva, B.; Muthomi, J.; Nzuve, F. The Inheritance of Yield Components and Beta Carotene Content in Sweet Potato. J. Agric. Sci. 2018, 10, 2. [Google Scholar]
- Lestari, S.U.; Hapsari, R.I.; Basuki, N. Crossing Among Sixteen Sweet Potato Parents for Establishing Base Populations Breeding. AGRIVITA J. Agric. Sci. 2019, 41, 246–255. [Google Scholar] [CrossRef]
- Rukarwa, R.J.; Mukasa, S.B.; Sefasi, A.; Ssemakula, G.; Mwanga, O.M.; Ghislain, M. Segregation analysis of cry7Aa1 gene in F1 progenies of transgenic and non-transgenic sweetpotato crosses. J. Plant Breed. Crop Sci. 2013, 5, 209–213. [Google Scholar]
- Constantin, R.J. A Study of the Inheritance of Several Characters in the Sweet Potato, (Ipomoea batatas). Ph.D. Thesis, LSU, Baton Rouge, LA, USA, 1964; p. 93. [Google Scholar]
- Hernandez, T.P. A Study of the Inheritance of Skin Color, Total Carotenoid Pigments, Dry Matter, and Techniques in Classifying These Characters in Ipomoea batatas. Ph.D. Thesis, La. State University, Baton Rouge, LA, USA, 1963; p. 67. [Google Scholar]
- Miller, R.M.; Melampy, J.J.; Hernandez, T.P. Effect of storage on the carotene content of fourteen varieties of sweet potatoes. Proc. Am. Soc. Hortic. Sci. 1949, 54, 399–402. [Google Scholar]
- Edmond, J.B. Genetics, breeding behaviour, and development of superior varieties. In Sweet Potatoes: Production, Processing, Marketing; AVI Publishing Co., Inc.: Westport, CT, USA, 1971; pp. 58–80. [Google Scholar]
- Hammett, H.L. A Study of the Inheritance of Root Shape, Skin Color, Total Carotenoid Pigments, Dry Matter, Fiber and Baking Quality in the Sweet Potato (Ipomoea batatas). Ph.D. Thesis, LSU, Baton Rouge, LA, USA, 1965; p. 140. [Google Scholar]
- Thibodeaux, S.D.; Hernandez, T.P.; Hernandez, T. Breeding techniques, combining aility of parents, heritabilities, insect resistance and other factors affecting sweet potato breeding. In Proceedings of the Third International Symposium on Tropical Root Crops, IITA, Ibadan, Nigeria, 2–9 December 1973; Volume 12, pp. 2–9. [Google Scholar]
- Stokdyk, E.A. Selection of sweet potatoes. J. Hered. 1925, 16147–16150. [Google Scholar] [CrossRef]
- Franklin, M.; Jones, A. Plant Breeding Reviews; AVI Publishing Co.: Clark, CO, USA, 1986; Volume 4, pp. 313–345. [Google Scholar]






| Character to Be Investigated | Parents | Parents | Family | |
|---|---|---|---|---|
| Color of the flesh of the tuberous root | INIVIT BM-90 (purple flesh) | × | CEMSA 74-228 (white flesh) | ISS-2305 |
| INIVIT BM-90 (purple flesh) | × | INIVIT BM-8 (purple flesh) | ISS-2306 | |
| INIVIT BS-16 (orange flesh) | × | Español (orange flesh) | ISS-2307 | |
| INIVIT BS-16 (orange flesh) | × | CEMSA 78-326 (white flesh) | ISS-2308 | |
| INIVIT BM-25-19 (purple flesh) | × | INIVIT BS-16 (orange flesh) | ISS-2309 | |
| Shape of tuberous root | INIVIT BS-16 (Skin with horizontal constrictions and longitudinal grooves) | × | CEMSA 74-228 (Skin with longitudinal grooves) | ISS-2310 |
| INIVIT BM-90 (smooth skin) | × | ISS-18-004 (smooth skin) | ISS-2311 |
| Variables | Code | Formula |
|---|---|---|
| Circularity | C | |
| Aspect ratio | AR | |
| Roundness | R | |
| Solidity | S | |
| Luminosity | L* | - |
| a* coordinate | a* | - |
| b* coordinate | b* | - |
| Family | No. of Pollinations | No. of Capsules | Positive Pollinations (%) | No. of Sedes | No. of Seeds/Capsule | Seed Germination (%) | No. of Seedlings |
|---|---|---|---|---|---|---|---|
| ISS-2305 | 416 | 273 | 65.7 | 320 | 1.17 | 77.5 | 248 |
| ISS-2306 | 191 | 138 | 72.47 | 221 | 1.60 | 89.4 | 198 |
| ISS-2307 | 497 | 226 | 45.53 | 279 | 1.23 | 79.2 | 221 |
| ISS-2308 | 273 | 189 | 69.35 | 269 | 1.42 | 93.2 | 251 |
| ISS-2309 | 343 | 155 | 45.06 | 306 | 1.98 | 87.6 | 268 |
| ISS-2310 | 419 | 199 | 47.48 | 366 | 1.84 | 90.8 | 332 |
| ISS-2311 | 280 | 181 | 64.43 | 284 | 1.57 | 86.7 | 246 |
| Average | - | - | 58.57 | - | 1.54 | 86.34 | - |
| Total | 2419 | 1361 | - | 2045 | - | - | 1764 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, A.; Vargas, I.J.P.; del-Sol, D.R.; Portal, O.; García, Y.B.; García, Y.R.; Medina, A.J.; Valdivies, Y.L.; Chávez, V.V. Inheritance of the Flesh Color and Shape of the Tuberous Root of Sweet Potato (Ipomoea batatas [L.] Lam.). Horticulturae 2024, 10, 1032. https://doi.org/10.3390/horticulturae10101032
Morales A, Vargas IJP, del-Sol DR, Portal O, García YB, García YR, Medina AJ, Valdivies YL, Chávez VV. Inheritance of the Flesh Color and Shape of the Tuberous Root of Sweet Potato (Ipomoea batatas [L.] Lam.). Horticulturae. 2024; 10(10):1032. https://doi.org/10.3390/horticulturae10101032
Chicago/Turabian StyleMorales, Alfredo, Iván Javier Pastrana Vargas, Dania Rodríguez del-Sol, Orelvis Portal, Yoel Beovides García, Yuniel Rodríguez García, Alay Jiménez Medina, Yusbiel León Valdivies, and Vaniert Ventura Chávez. 2024. "Inheritance of the Flesh Color and Shape of the Tuberous Root of Sweet Potato (Ipomoea batatas [L.] Lam.)" Horticulturae 10, no. 10: 1032. https://doi.org/10.3390/horticulturae10101032
APA StyleMorales, A., Vargas, I. J. P., del-Sol, D. R., Portal, O., García, Y. B., García, Y. R., Medina, A. J., Valdivies, Y. L., & Chávez, V. V. (2024). Inheritance of the Flesh Color and Shape of the Tuberous Root of Sweet Potato (Ipomoea batatas [L.] Lam.). Horticulturae, 10(10), 1032. https://doi.org/10.3390/horticulturae10101032







