Optimizing Biochemical and Phytochemical Attributes in Peaches through Foliar Applications of Silicon and Zinc
Abstract
1. Introduction
2. Materials and Methods
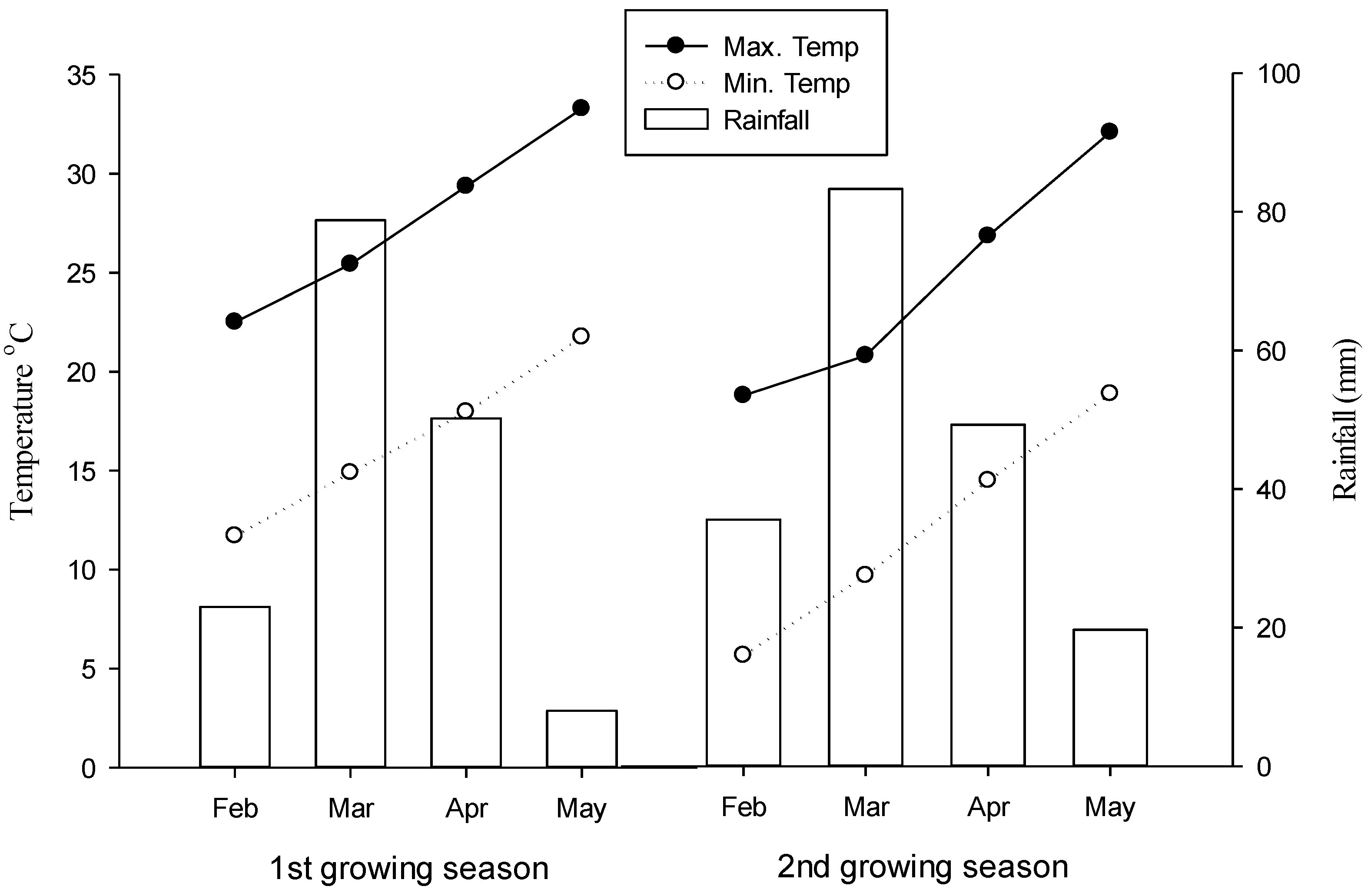
2.1. Experimental Site and Plant Materials
2.2. Experimental Design and Treatment Combinations
2.3. Preparation and Application of Silicon and Zinc Sprays
2.4. Harvesting and Postharvest Procedure
2.5. Data Collection
2.5.1. Fruit Growth and Yield Attributes
2.5.2. Pulp Stone Ratio
2.5.3. Biochemical Attributes
2.6. Sample Preparation and Quantification of DPPH Free Radicals Antioxidant Activity (%), Flavonoid Content (mg g−1 FW), and Proline Content (μg g−1 FW)
2.7. Enzymatic Activities
2.8. Statistical Analysis and Data Visualization
3. Results
3.1. Fruit Growth and Yield Attributes
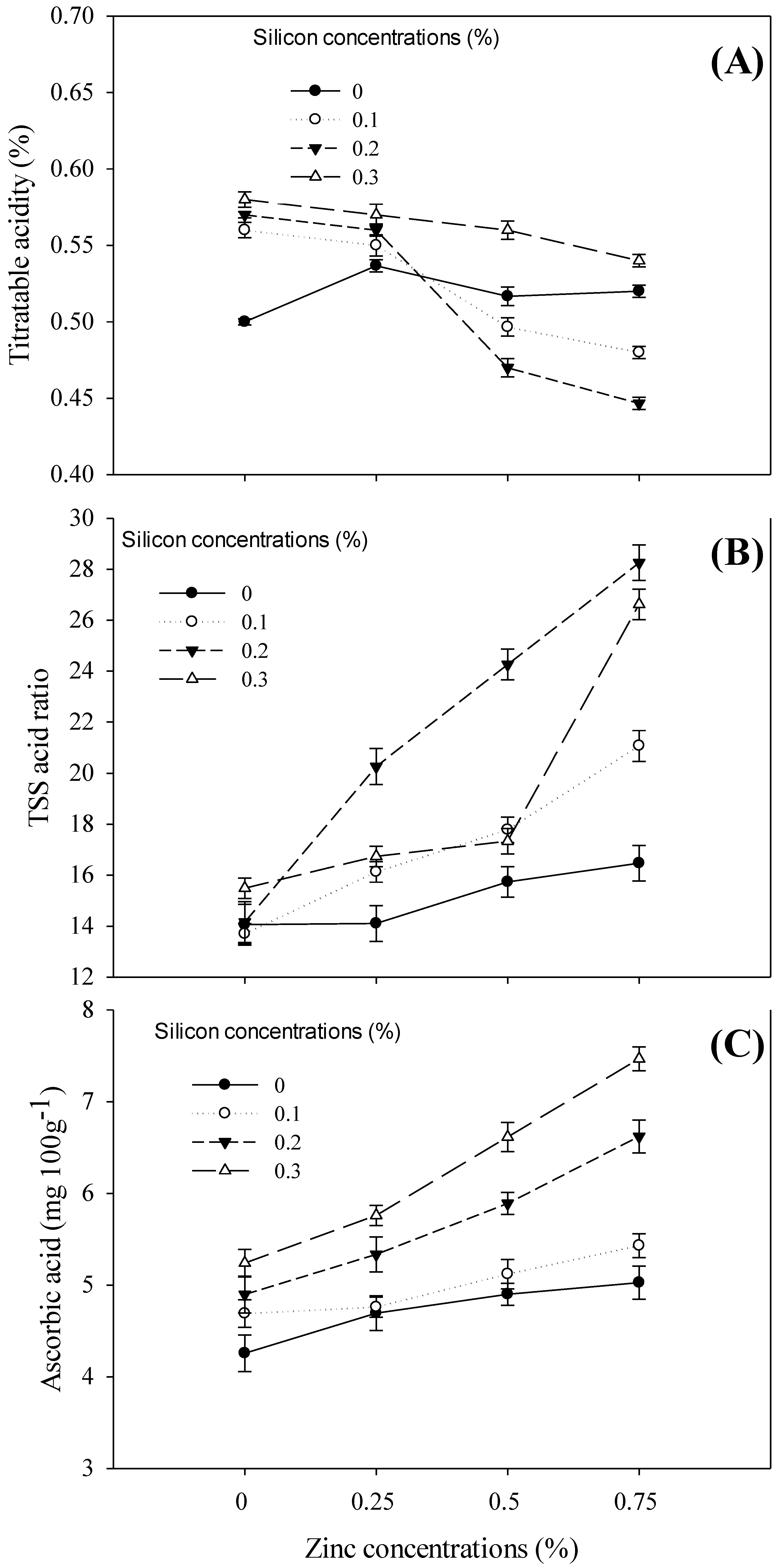
3.2. Quality or Biochemical Attributes
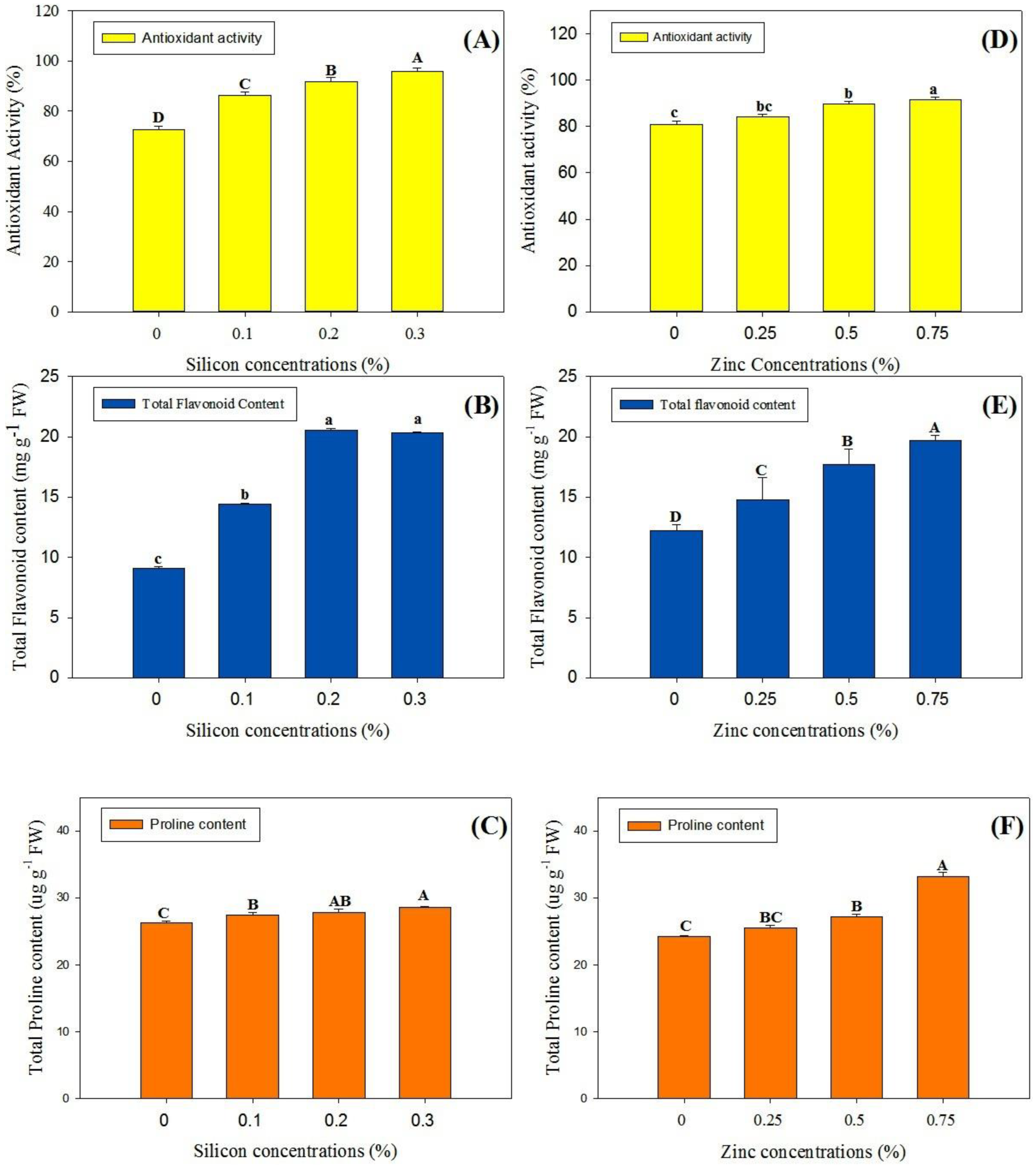
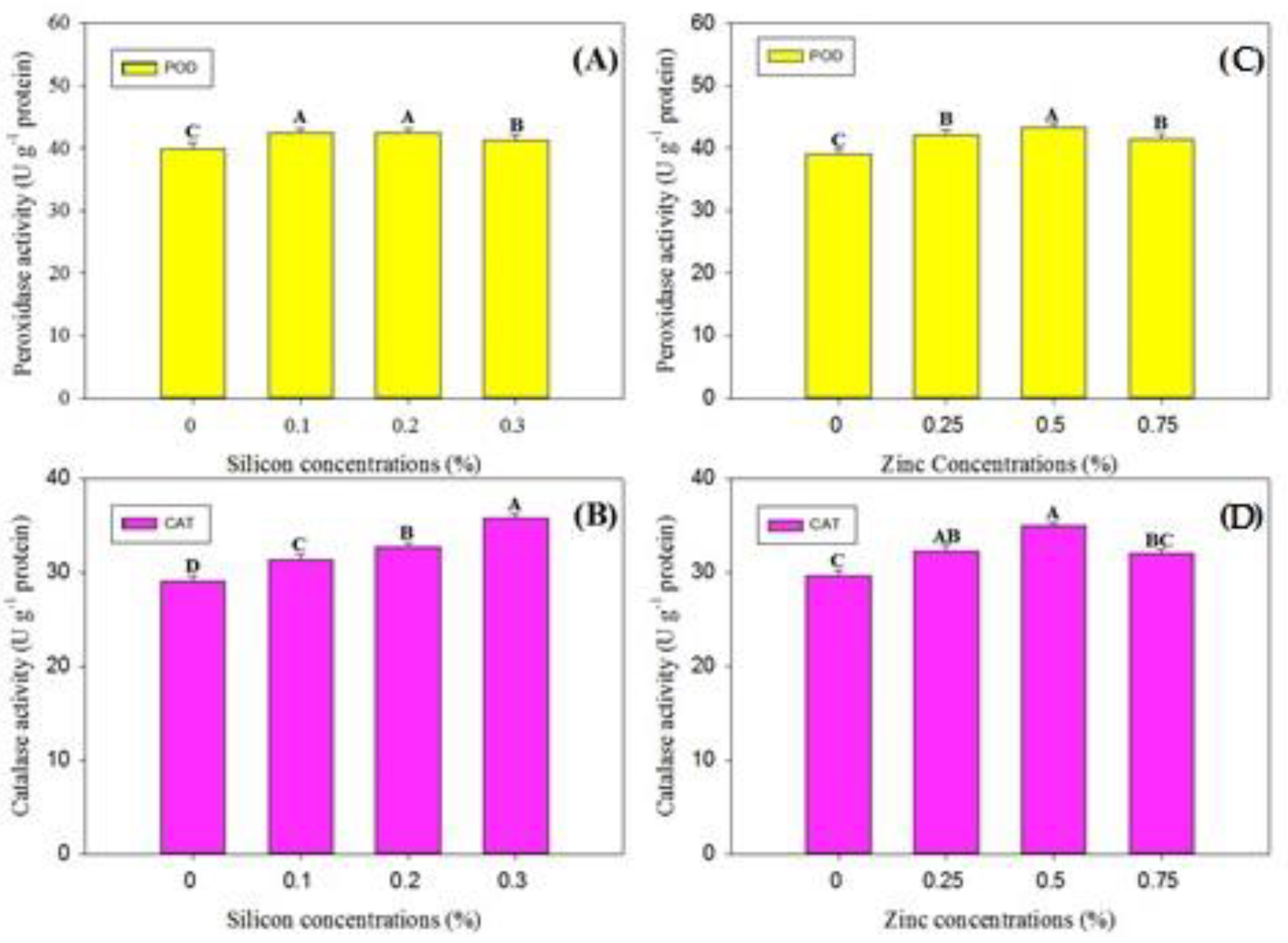
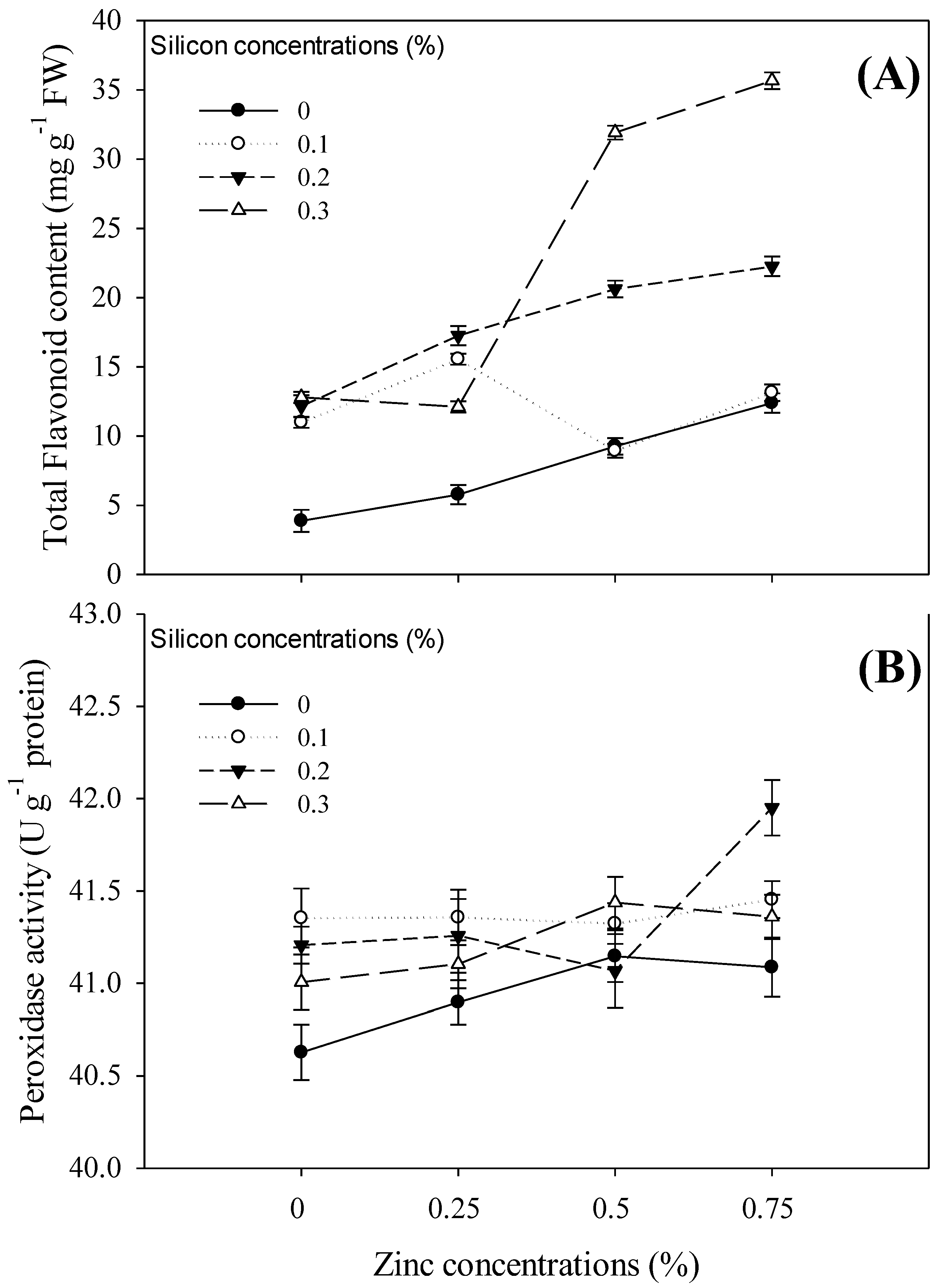
3.3. Phytochemical Analysis and Enzymatic Activities
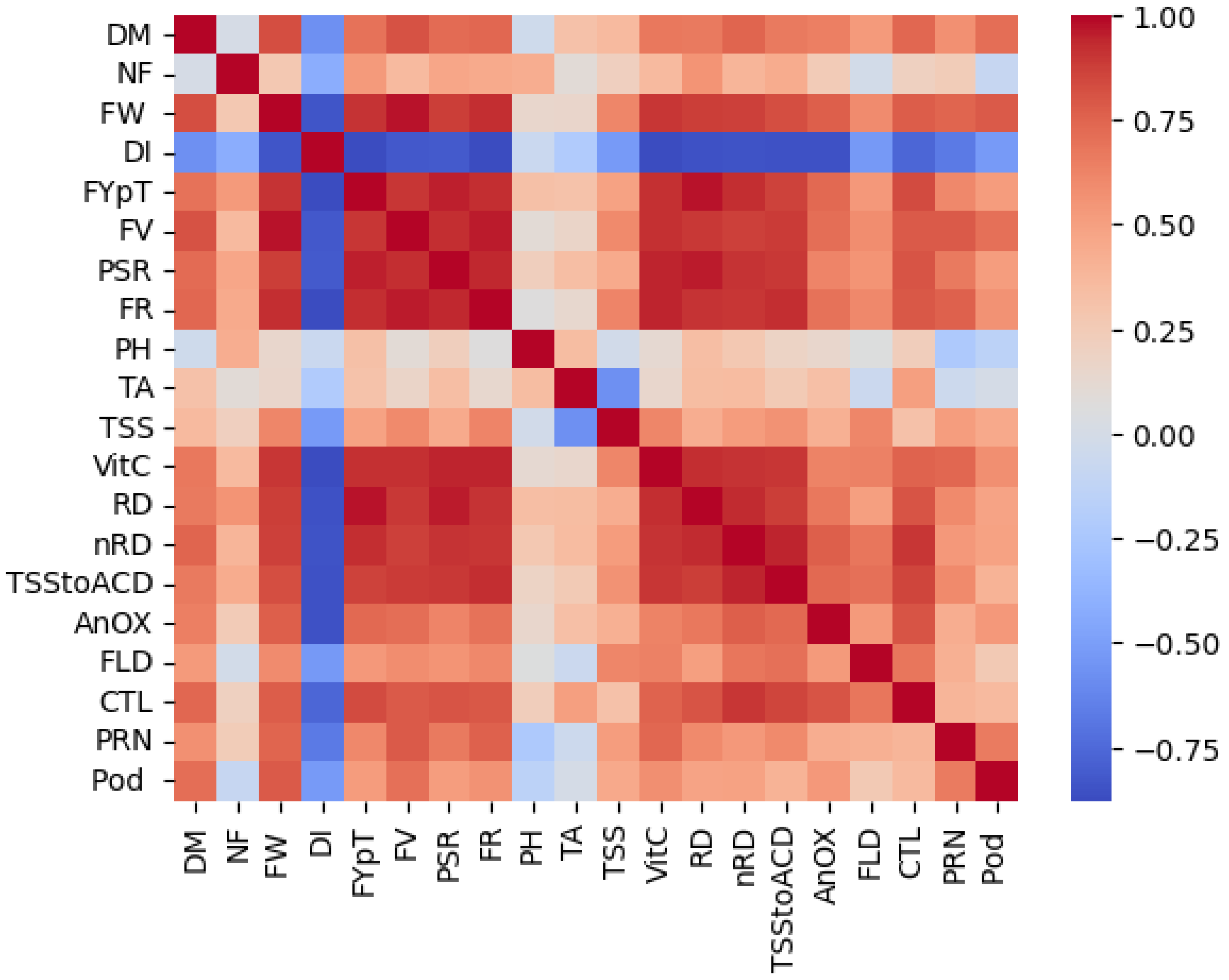
3.4. Correlation Analysis
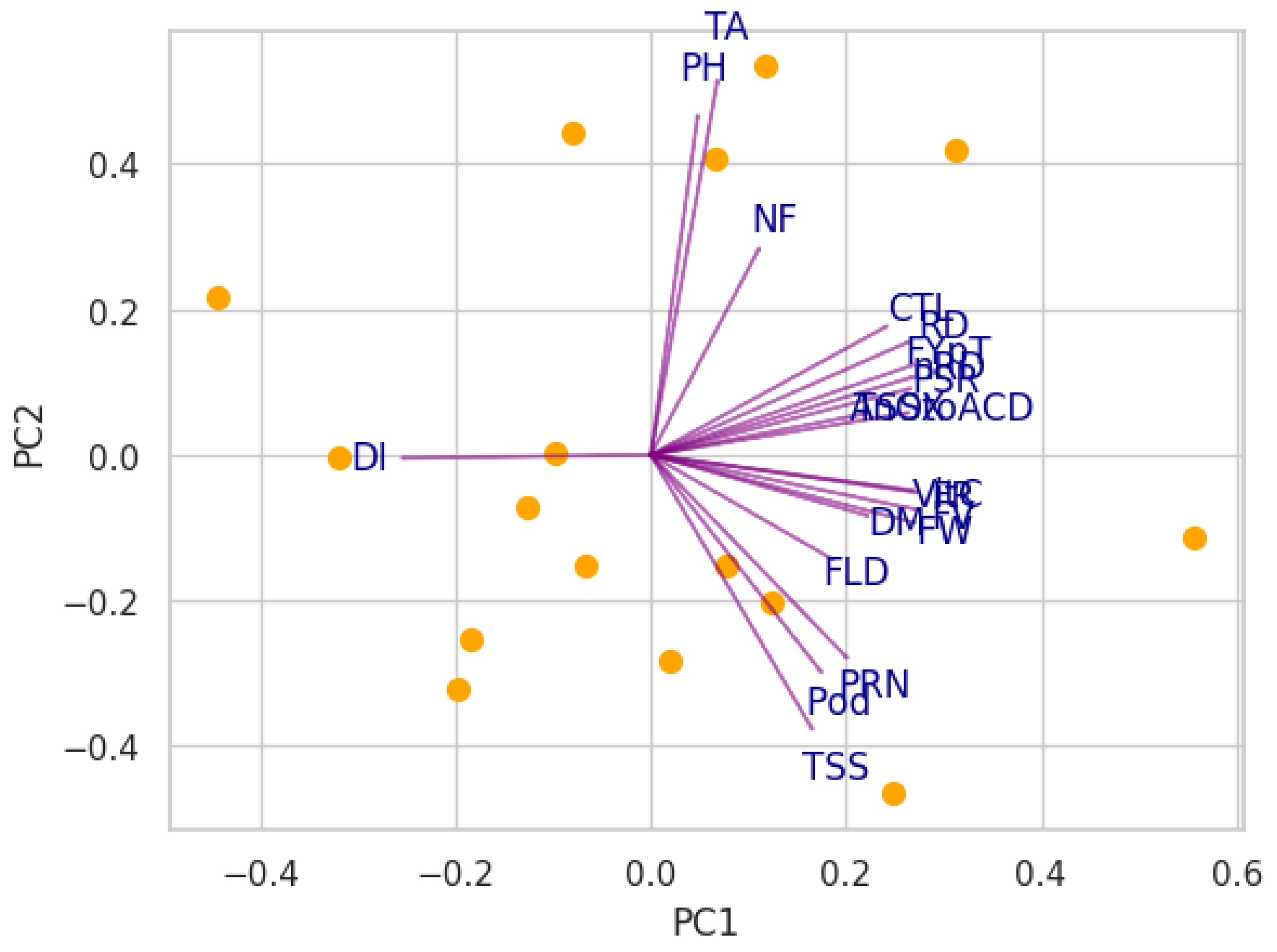
3.5. Principal Component Analysis
4. Discussion
4.1. Fruit Growth and Yield Attributes
4.2. Pulp-to-Stone Ratio
4.3. Biochemical Attributes
4.4. Phytochemical Attributes
4.5. Enzymatic Activities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A.; Perveen, S.; Shah, S.N.M.; Zhang, Z.; Wahid, F.; Shah, M.; Bibi, S.; Majid, A. Effect of foliar application of micronutrients on fruit quality of peach. Amer. J. Plant Sci. 2014, 5, 1258–1264. [Google Scholar] [CrossRef]
- Habib, S. Peach: Queen of fruit. Pak. J. Food Sci. 2015, 26–27. Available online: https://foodjournal.pk/2015/September-October-2015/PDF-September-October-2015/Exclusive-on-Peach.pdf (accessed on 24 September 2024).
- MNFS&R. Statistical data of Peach for 2022–2023. Ministry of National Food Security and Research; Government of Pakistan: Islamabad, Pakistan, 2023; pp. 1–5. [Google Scholar]
- Hartman, J.R. Peach fruit diseases. In Plant Pathology Fact Sheet; UK Cooperative Extension Service; College of Agri PPFS-FR-T-09; University of Kentucky: Lexington, KY, USA, 2007. [Google Scholar]
- Crisosto, C.H.; Day, K.R.; Johnson, R.S.; Garner, D. Influence of in season foliar calcium sprays on fruit quality and surface discoloration incidence of peaches and nectarines. J. Amer. Pomol. Soc. 2000, 54, 118–122. [Google Scholar]
- Neo, G.M. Control of post-harvest pericarp browning of litchi (Litchi chinensis). J. Food Sci. Technol. 2010, 47, 100–104. [Google Scholar]
- Lurie, S.; Crisosto, C.H. Chilling injury in Peach and Nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Van-Bockhaven, J.; Vleesschauwer, D.; Hofte, M. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 128–129. [Google Scholar] [CrossRef]
- Meena, V.D.; Dotaniya, M.L.; Coumar, V.; Rajendiran, S.; Kundu, A.S.; Rao, A.S. A case for silicon fertilization to improve crop yields in tropical soils. Proc. Natl. Acad. Sci. India Sect. B. Biol. Sci. 2014, 84, 505–518. [Google Scholar] [CrossRef]
- Kanai, S.; Ohkura, K.; Adu-gyamfi, J.J.; Mohapatra, P.K.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Depression of sink activity precedes the inhibition of biomass production in tomato plants subjected to potassium deficiency stress. J. Exp. Bot. 2007, 58, 2917–2928. [Google Scholar] [CrossRef]
- Swietlik, D. Zinc nutrition in horticultural crops. Hort. Rev. 1999, 23, 109–180. [Google Scholar]
- Cakmak, I. Role of zinc in protecting plant cells from reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Rosato, A. Zinc through the three domains of life. J. Proteome Res. 2006, 12, 3173–3178. [Google Scholar] [CrossRef]
- Coleman, J.E. Zinc enzymes. Curr. Opin. Chem. Biol. 1998, 2, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liu, Q. Oxidative metabolism-related changes during germination of mono maple (Acer mono Maxim.) seeds under seasonal frozen soil. Ecol. Res. 2010, 25, 337–345. [Google Scholar] [CrossRef]
- Altaf, N.; Khan, A.R. Variation within Kinnow (Citrus reticulata) and rough lemon (Citrus jambheri). Pak. J. Bot. 2008, 40, 589–598. [Google Scholar]
- Basit, A.; Khan, S.; Shah, A.A. Morphological features of various selected tree species on the greater university campus Peshawar, Pakistan. J. Bot. Stud. 2019, 4, 92–97. [Google Scholar]
- Basit, A.; Hassnain, M.; Shah, S.T.; Ullah, S.A. Quality indices of tomato plant as affected by water stress conditions and chitosan application. Pure App. Biol. 2020, 9, 1364–1375. [Google Scholar] [CrossRef]
- Pocharski, W.J.; Konopacka, D.; Zwierz, J. Comparison of Magness-Taylor pressure test with mechanical, nondestructive methods of apple and pear firmness measurements. Int. Agrophy. 2000, 14, 311–331. [Google Scholar]
- AOAC. Official Methods of Analysis. Analytical Chemist, 15th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Ahmad, N.; Abbasi, B.H.; Fazal, H.; Khan, M.A.; Afridi, M.S. Effect of reverse photoperiod on in vitro regeneration and piperine production in Piper nigrum L. J. C. R. Biol. 2014, 337, 19–28. [Google Scholar] [CrossRef]
- Sanchez, E.; Lopez-Lefebre, L.R.; Garcia, P.C.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Proline metabolism in response to highest nitrogen dosages in green bean plants (Phaseolus vulgaris L. cv. Strike). J. Plant Physiol. 2001, 158, 593–598. [Google Scholar] [CrossRef]
- Abbasi, N.A.; Kushad, M.M.; Endress, A.G. Active Oxygen-scavenging enzymes activities in developing apple flowers and fruits. Sci. Hort. 1998, 74, 183–194. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dukey, D. Principles and Procedure of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book Company: New York, NY, USA, 1997. [Google Scholar]
- Nawaz, M.A.; Ahmad, W.; Ahmad, S.; Khan, M.M. Role of growth regulators on preharvest fruit drop, yield and quality in Kinnow mandarin. Pak. J. Bot. 2008, 40, 1971–1981. [Google Scholar]
- Wang, M.; Nie, L.; Xu, R.; Wang, S. Effects of foliar application of silicon on accumulation of sugar and vitamin C and related enzymes in cucumber fruit. J. Acta Hort. Sini. 2018, 45, 351–358. [Google Scholar]
- Smith, A. Silicon’s key role in plant growth. Aust. Grain 2011, 35. [Google Scholar]
- Laane, H.M. The effects of foliar sprays with different silicon compounds. Plants 2018, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tanabe, K.; Wang, S.; Tamura, F.; Yoshida, A.; Sumoto, K.M. The Impact of Cell Division and Cell Enlargement on the Evolution of Fruit Size in Pyrus pyrifolia. Ann. Bot. 2006, 98, 537–543. [Google Scholar] [CrossRef]
- Ravishankar, M.P. Studies on Effect of Pre-Harvest Bunch Treatment and Bagging on Yield and Post-Harvest Quality of Banana. Ph.D. Thesis, KRC College of Horti, Bagalkot, India, 2016; p. 261. [Google Scholar]
- Jarosz, Z. The effect of silicon application and type of medium on yielding and chemical composition of tomato. Acta Sci. Pol. Hortorum Cultus. 2014, 13, 171–183. [Google Scholar]
- Haripriya, P.; Stella, P.M.; Anusuya, S. Foliar Spray of Zinc Oxide Nanopartcles Improves Salt Tolerance in Finger Millet Crops under Glasshouse Conditon. J. Scio. Biotechnol. 2018, 1, 20–29. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Mishra, S.; Chauhan, D.K.; Dubey, N.K. Micronutrients and their diverse role in agricultural crops: Advances and future prospective. Acta Physiol. Plantarum. 2015, 37, 1–14. [Google Scholar] [CrossRef]
- Dhotra, B.; Bakshi, P.; Jeelani, M.I.; Vikas, V. Influence of foliar application of micronutrients on fruit growth, yield and quality of peach cv. Shan-e-Punjab. Indian Res. J. Genetics Biotechnol. 2018, 10, 105–112. [Google Scholar]
- Sourour, M.M. Effect of some micronutrients forms on growth, yield, fruit quality and leaf mineral composition of Valencia orange trees grown in North Sinai. Alex. J. Agri. Res. 2000, 45, 269–285. [Google Scholar]
- Shehata, M.N.; Abdelgawad, K.F. Potassium Silicate and Amino Acids improve growth, flowering and productivity of Summer Squash under high Temperature condition. Am.-Eurasian J. Agri. Environ. Sci. 2019, 19, 74–86. [Google Scholar]
- Rawat, V.; Tomar, Y.K.; Rawat, J.M.S. Influence of foliar application of micronutrients on the fruit quality of guava cv. Lucknow-49. J. Hill Agri. 2010, 1, 75–78. [Google Scholar]
- Gurjar, M.K.; Kaushik, R.A.; Baraily, P. Effect of zinc and boron on the growth and yield of Kinnow mandarin. Int. J. Sci. Res. 2015, 4, 21–25. [Google Scholar]
- Cronje, R.B.; Sivakumar, D.; Mostert, P.G.; Korsten, L. Effect of different pre harvest treatment regimens on fruit quality of litchi cultivar “Maritius”. J. Plant Nutri. 2009, 32, 19–29. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop. Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Vijayan, A.; Sriramachandrasekharan, M.V.; Manivannan, R.; Shakila, A. Effect of silicon through potassium silicate on yield, nutrientuptake and quality of grand naine banana. Asian J. Agri. Food Sci. 2021, 9, 91–98. [Google Scholar]
- Fiori, M.P. Comportamento de Cultivares de Tomateiro Quanto à Utilização de Escórias Siderúrgicas em Ambiente Protegido. Ph.D. Thesis, Universidade De Marília, Marília, Brazil, 2006. Volume 54, pp.155–157. [Google Scholar]
- Lalithya, K.A.; Bhagya, H.P.; Choudhary, R. Response of silicon and micronutrients on fruit character and nutrient content in leaf of sapota. Biolife 2014, 2, 593–598. [Google Scholar]
- Broadley, M.; White, P.; Hammond, J.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 17, 677–702. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kumar, S.; Verma, S.; Dubey, S.; Dubey, A.K. Effect of zinc sulphate, boric acid and iron sulphate on vegetative growth, yield and quality of strawberry (Fragaria × Ananassa Duch.) cv. Chandler. Bioscan 2016, 11, 2222–2225. [Google Scholar]
- Singh, S.; Parekh, N.S.; Patel, H.R.; Kore, P.N.; Vasara, R.P. Effect of soil and foliar application of multi micronutrients on fruit yield and physical parameters of fruit of mango (Mangifera indica L.) var. Amrapali. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3495–3499. [Google Scholar] [CrossRef]
- Roshdy, K.H.A. Effect of spraying silicon and seaweed extract on growth and fruiting of Grand Naine banana. Egypt. J Agri. Res. 2014, 92, 979–991. [Google Scholar]
- Chandra, R.; Singh, K.K. Foliar application of zinc sulphate, magnesium sulphate and copper sulphate on the yield and quality of aonla (Emblica officinallis Gaerth L.) cv.“NA-7” under Garhwal Himalaya. Himalaya J. Med. Plants Stud. 2015, 3, 42–45. [Google Scholar]
- Xie, R.; Zhao, J.; Lu, L.; Brown, P.; Guo, J.; Tian, S. Penetration of foliar-applied Zn and its impact on apple plant nutrition status: In vivo evaluation by synchrotron-based X-ray fluorescence microscopy. Hort. Res. 2020, 7, 147. [Google Scholar] [CrossRef]
- Shukla, H.S.; Kumar, V.; Tripathi, V.K. Effect of gibberellic acid and boron on development and quality of aonla fruit ‘Banarasi’. Acta Hort. 2011, 890, 375–380. [Google Scholar] [CrossRef]
- Weerahewa, D.; David, D. Effect of silicon and potassium on tomato anthracnose and on the postharvest quality of tomato fruit (Lycopersicon esculentum Mill.). J. Nat. Sci. Found. Sri Lanka. 2015, 43, 273–280. [Google Scholar] [CrossRef]
- Matas, M.A.; Gonzalez-Fontes, A.; Camacho-Cristobal, J.J. Effect of boron supply on nitrate concentration and its reduction in roots and leaves of tobacco plants. Biol. Plantarum. 2009, 53, 120–124. [Google Scholar] [CrossRef]
- Shireen, F.; Jaskani, M.J.; Nawaz, M.A.; Hayat, F. Exogenous application of naphthalene acetic acid improves fruit size and quality of Kinnow mandarin (Citrus reticulata) through regulating fruit load. J. Anim. Plant Sci. 2018, 28, 1080–1084. [Google Scholar]
- Martichenkov, V.V.; Calvert, D.V. Prospective of silicon fertilization for citrus in Florida. Proc. Soil Crop Sci. Soc. Fla. 2002, 5, 137–141. [Google Scholar]
- Zhang, Y.Z.; Li, P.M.; Cheng, L.L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 4, 1013–1018. [Google Scholar] [CrossRef]
- Khalaj, K.; Ahmadi, N.; Souri, M.K. Improvement of postharvest quality of Asian pear fruits by foliar application of boron and calcium. Horticulturae 2017, 3, 15. [Google Scholar] [CrossRef]
- El-Kholy, M.F.; Mahmoud, A.A.; Mehaisen, S.M.A. Impact of potassium silicate sprays on fruiting, fruit quality and fruit storability of loquat trees. Middle East J. Agri. Res. 2018, 7, 139–153. [Google Scholar]
- Anees, M.; Tahir, H.M.; Shahzad, J.; Mahmood, N. Effect of foliar application of micronutrients on the quality of mango (Mangifera indica L.) cv. Dusehri fruit. Mycopathologia 2011, 9, 25–28. [Google Scholar]
- Kumari, U.; Prahlad, D. Effect of foliar application of zinc and boron on quality of pineapple cv. Mauritius. J. Pharmacol. Phytochem. 2018, 7, 1166–1168. [Google Scholar]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutri. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Xue, G.; Zhang, G.; Sun, Y.; Liao, S.; Chen, Y. Influences of spraying two different forms of silicon on plant growth and quality of tomato in solar greenhouse. China Agric. Sci Bull. 2012, 28, 272–276. [Google Scholar]
- Su, X.W.; Wei, S.C.; Jiang, Y.M.; Huang, Y.Y. Effects of silicon on quality of apple fruit and Mn content in plants on acid soils. Shandong Agric. Sci. 2011, 6, 59–61. [Google Scholar]
- Alloway, B.J. Zinc in Soils and Crop Nutrition; International Zinc Association: Brussels, Belgium, 2008; p. 135. [Google Scholar]
- Rasouli, M.; Saba, M.K. Pre-harvest zinc spray impact on enzymatic browning and fruit flesh colour changes in two apple cultivars. Sci. Hort. 2018, 240, 318–325. [Google Scholar] [CrossRef]
- Aglar, E.; Yildiz, K.; Ozkan, Y.; Ozturk, B.; Erdem, H. The effects of aminoethoxyvinylglycine and foliar zinc treatments on pre-harvest drops and fruit quality attributes of Jersey Mac apples. Sci. Hort. 2016, 213, 173–178. [Google Scholar] [CrossRef]
- Ahmed, F.F.; Gad El-Kareem, M.R.; Oraby-Mona, M.M. Response of Zaghloul date palms to spraying boron, silicon and glutathione. Stem Cell 2013, 4, 29–34. [Google Scholar]
- Duraes, T.; Azevedo, M.; Cosme, F.; Nunes, F.M. Enzymatic Reduction of Sugar Content in Sucrose-Rich Fruit Products. Med. Sci. Forum. 2023, 23, 6. [Google Scholar]
- Thibodeaux, C.J.; Melançon, C.E.; Liu, H. Natural Product Sugar Biosynthesis and Enzymatic Glycodiversification. Angew. Chem. Int. Ed. Engl. 2008, 47, 9814–9859. [Google Scholar] [CrossRef]
- Singh, D.M.; Singh, H.K.; Singh, B. Effect of foliar spray of zinc sulphate, urea, muriate of potash on yield and quality and physico-chemical composition of aonla fruit. Ann. Hort. 2009, 2, 95–97. [Google Scholar]
- Wang, H.; Jin, J.Y. Photosynthetic rate, chlorophyll fuorescence parameters and lipid peroxidation of maize leaves as affected by zinc defciency. Photosynthetica 2005, 43, 591–596. [Google Scholar] [CrossRef]
- Vinha, A.F.; Alves, R.C.; Barreira, S.V.P.; Castro, A.; Costa, A.S.G.; Oliveira, M.B.P.P. Effect of peel and seed removal on the nutritional value and antioxidant activity of tomato (Lycopersicon esculentum L.) fruits. LWT Food Sci. Technol. 2014, 55, 197–202. [Google Scholar] [CrossRef]
- Lin, C.C.; Kao, C.H. Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Reg. 2000, 30, 151–155. [Google Scholar] [CrossRef]
- Hernandez, M.; Fernandez-Garcia, N.; Diaz-Vivancos, P.; Olmos, E. Different role for hydrogen peroxide and the antioxidative system under short and long salt stress in Brassica oleracea roots. J. Exp. Bot. 2010, 61, 521–535. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chen, Q.; Liu, Q.; Zhang, W.H.; Ding, R.X. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; Mazrou, Y.S.; Hafez, Y.M. Silicon foliar application mitigates salt stress in sweet pepper plants by enhancing water status, photosynthesis, antioxidant enzyme activity and fruit yield. Plants 2020, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Badakhshan, H. Effects of zinc application on growth, absorption and distribution of mineral nutrients under salinity stress in soybean (Glycine max L.). J. Plant Nutri. 2014, 37, 2255–2269. [Google Scholar] [CrossRef]
- Shahriaripour, R.; Pour, A.T.; Mozaffari, V.; Dashti, H.; Adhami, E. Effects of salinity and soil zinc application on growth and chemical composition of pistachio seedlings. J. Plant Nutri. 2010, 33, 1166–1179. [Google Scholar] [CrossRef]
- Salim, B.B.M.; Abou El-Yazied, A.; Salama, Y.A.M.; Raza, A.; Osman, H.S. Impact of silicon foliar application in enhancing antioxidants, growth, flowering and yield of squash plants under deficit irrigation condition. Ann. Agri. Sci. 2021, 66, 176–183. [Google Scholar] [CrossRef]
- Hossain, Z.; Yasmeen, F.; Komatsu, S. Nanoparticles: Synthesis, Morpho-physiological Effects, and Proteomic Responses of Crop Plants. Int. J. Mol. Sci. 2020, 21, 3056. [Google Scholar] [CrossRef] [PubMed]
- Mateus, M.P.B.; Tavanti, R.F.R.; Tavanti, T.R.; Santos, E.F.; Jalal, A.; Reis, A.R. Selenium biofortification enhances ROS scavenge system increasing yield of coffee plants. Ecotoxicol. Environ. Saf. 2021, 209, 111772. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Guerrero, Z.H.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; González-Morales, S.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Form of silica improves yield, fruit quality and antioxidant defence system of tomato plants under salt stress. Agriculture 2020, 10, 367. [Google Scholar] [CrossRef]
- Zafar, H.; Ali, A.; Ali, J.S.; Haq, I.U.; Zia, M. Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: Growth dynamics and antioxidative response. Front. Plant. Sci. 2016, 7, 535. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, S.; Zhu, H.; Colvin, V.I.; Alvarez, P.J. Quantum dot weathering results in microbial toxicity. Environ. Sci. Technol. 2008, 42, 9424–9430. [Google Scholar] [CrossRef] [PubMed]
- Zaman, L.; Shafqat, W.; Qureshi, A.; Sharif, N.; Raza, K.; Ud Din, S.; Kamran, M. Effect of foliar spray of zinc sulphate and calcium carbonate on fruit quality of Kinnow mandarin (Citrus reticulata Blanco). J. Glob. Innov. Agric. Soc. Sci. 2019, 7, 157–161. [Google Scholar] [CrossRef]
- Dawood, M.G.; Taie, H.A.A.; Nassar, R.M.A.; Abdelhamid, M.T.; Schmidhalter, U. The changes induced in the physiological, biochemical and anatomical characteristics of Vicia faba by the exogenous application of proline under seawater stress. S. Afr. J. Bot. 2014, 93, 54–63. [Google Scholar] [CrossRef]
- Shahid, M.; Balal, R.; Pervez, M.; Abbas, T.; Aqeel, M.; Javaid, M.; Garcia-Sanchez, F. Foliar spray of phyto-extracts supplemented with silicon: An efficacious strategy to alleviate the salinity-induced deleterious effects in pea (Pisum sativum L.). Turk. J. Bot. 2015, 39, 408–419. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Li, J.; Tanaka, K.; Oka, M. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiol. Plants 2013, 35, 3099–3107. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Perveen, S.; Mahmood, S. Dynamic Proline Metabolism: Importance and Regulation in Water-Limited Environments. In Plant Metabolites and Regulation under Environmental Stress; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 323–336. [Google Scholar] [CrossRef]
- Menon, G.S.; Kamthe, S.A.; Gharge, S. Research Article Effect of Metal on Germination and Proline Accumulation in Spinacea Oleracea. Int. J. Curr. Res. Life Sci. 2018, 7, 1376–1380. [Google Scholar]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in DiseasePrevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Kosar, F.; Akram, N.A.; Ashraf, M. Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. S. Afr. J. Bot. 2015, 96, 71–77. [Google Scholar] [CrossRef]
- Mo, Y.; Gong, D.; Liang, L.; Han, R.; Xie, J.; Li, W. Enhanced preservation effects of sugar apple fruits by salicylic acid treatment during post-harvest storage. J. Sci. Food Agri. 2008, 88, 2693–2699. [Google Scholar] [CrossRef]
- Patil, H.; Tank, R.V.; Manoli, P. Significance of silicon in fruit crops-A review. Plant Arch. 2017, 17, 769–774. [Google Scholar]
- Emamverdian, A.; Ding, Y.; Xie, Y.; Sangari, S. Silicon Mechanisms to Ameliorate Heavy Metal Stress in Plants. BioMed Res. Inter. 2018, 8492898. [Google Scholar] [CrossRef]
- Vieira, D.L.; Oliveira, V.B.; Souza, W.C.O.; Silva, J.G.; Malaquias, J.B.; Luna, J.B. Potassium silicate induced resistance against blackfly in seedlings of Citrus reticulata. Fruits 2016, 71, 49–55. [Google Scholar] [CrossRef]


| Treatments | Parameters | |
|---|---|---|
| Silicon (S) Levels (%) | Fruit Firmness | Vitamin C |
| 0 | 0.95 C | 4.72 C |
| 0.1 | 1.18 B | 5.00 BC |
| 0.2 | 1.25 B | 5.69 AB |
| 0.3 | 1.53 A | 6.27 A |
| LSD (p ≤ 0.01) | 0.14 | 0.72 |
| Zinc (Z) concentrations (%) | ||
| 0 | 0.99 C | 4.77 C |
| 0.25 | 1.17 B | 5.14 BC |
| 0.50 | 1.26 B | 5.63 AB |
| 0.75 | 1.50 A | 6.14 A |
| LSD (p ≤ 0.01) | 0.14 | 0.72 |
| Interaction (p ≤ 0.01) | ||
| S × Z | NS | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.T.; Ahmad, N.; Basit, A.; Sajid, M.; Jamal, A.; Saeed, M.F.; Iqbal, W.; Seleiman, M.F.; Radicetti, E.; Mancinelli, R. Optimizing Biochemical and Phytochemical Attributes in Peaches through Foliar Applications of Silicon and Zinc. Horticulturae 2024, 10, 1031. https://doi.org/10.3390/horticulturae10101031
Shah ST, Ahmad N, Basit A, Sajid M, Jamal A, Saeed MF, Iqbal W, Seleiman MF, Radicetti E, Mancinelli R. Optimizing Biochemical and Phytochemical Attributes in Peaches through Foliar Applications of Silicon and Zinc. Horticulturae. 2024; 10(10):1031. https://doi.org/10.3390/horticulturae10101031
Chicago/Turabian StyleShah, Syed Tanveer, Naseer Ahmad, Abdul Basit, Muhammad Sajid, Aftab Jamal, Muhammad Farhan Saeed, Waleed Iqbal, Mahmoud F. Seleiman, Emanuele Radicetti, and Roberto Mancinelli. 2024. "Optimizing Biochemical and Phytochemical Attributes in Peaches through Foliar Applications of Silicon and Zinc" Horticulturae 10, no. 10: 1031. https://doi.org/10.3390/horticulturae10101031
APA StyleShah, S. T., Ahmad, N., Basit, A., Sajid, M., Jamal, A., Saeed, M. F., Iqbal, W., Seleiman, M. F., Radicetti, E., & Mancinelli, R. (2024). Optimizing Biochemical and Phytochemical Attributes in Peaches through Foliar Applications of Silicon and Zinc. Horticulturae, 10(10), 1031. https://doi.org/10.3390/horticulturae10101031












