Influence of Intercropping on Eugenia dysenterica (Mart.) DC. Fruit Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Physical Characterization
2.3. Chemical Characterization
2.4. Total Bioactive Compounds and Antioxidant Activity
2.5. Identification and Quantification of Phenolic Compounds
2.6. Infrared Absorption Spectroscopy
2.7. Statistical Analysis
3. Results and Discussions
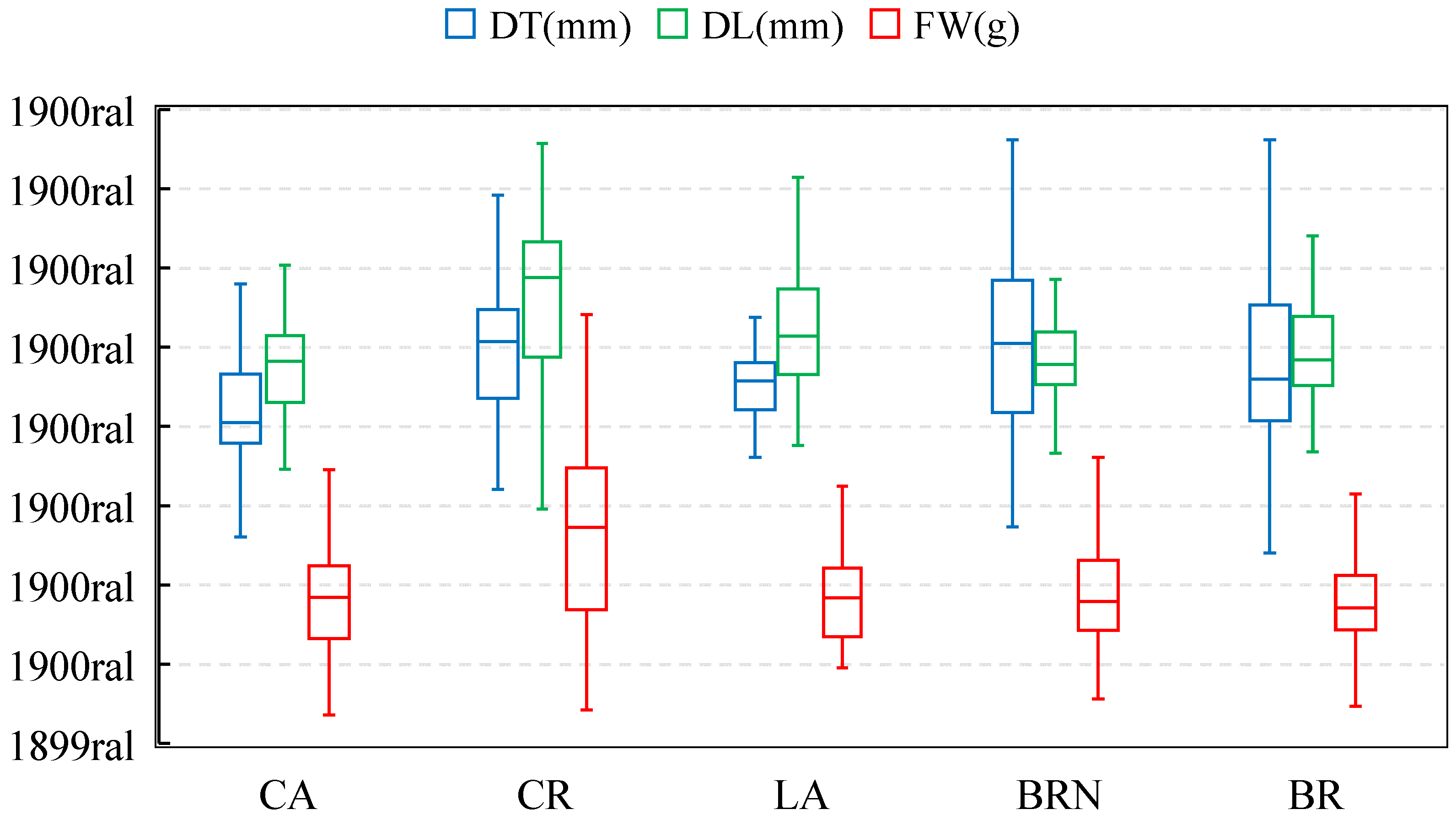
3.1. Physical Parameters and Proximal and Mineral Composition
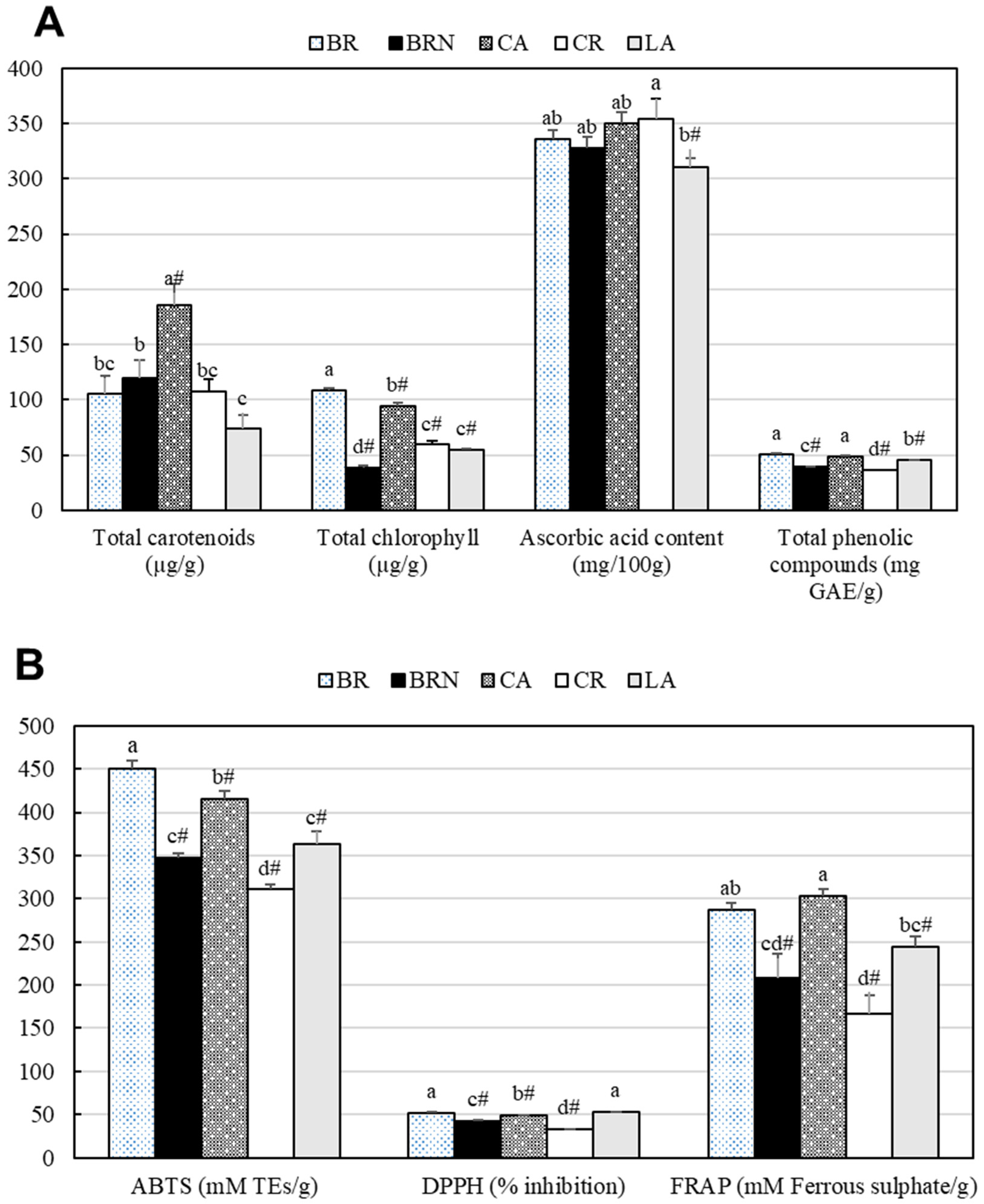
3.2. Bioactive Compounds and Antioxidant Activity
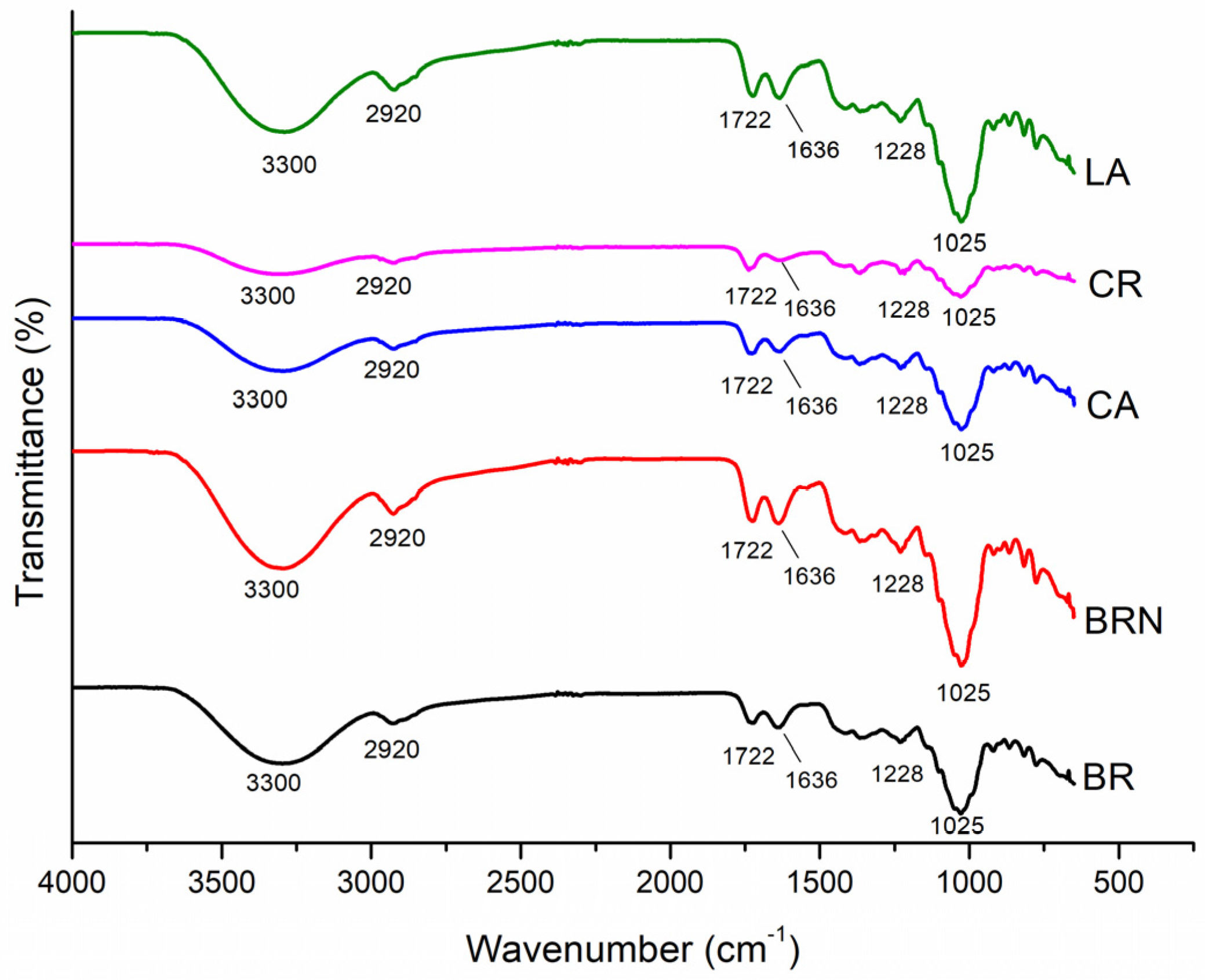
3.3. Fourier Transform Infrared Absorption Spectroscopy (FTIR) Analyze
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brazil. O Bioma Cerrado. MMA (Ministério do Meio Ambiente). Available online: https://www.mma.gov.br/biomas/cerrado> (accessed on 10 October 2023).
- Santos, D.D.; Oliveira Filho, J.d.; Sousa, T.d.; Ribeiro, C.; Egea, M. Ameliorating effects of metabolic syndrome with the consumption of rich-bioactive compounds fruits from Brazilian Cerrado: A narrative review. Crit. Rev. Food Sci. Nutr. 2021, 62, 7632–7649. [Google Scholar] [CrossRef] [PubMed]
- De Morais Cardoso, L.; Martino, H.S.D.; Moreira, A.V.B.; Ribeiro, S.M.R.; Pinheiro-Sant’Ana, H.M. Cagaita (Eugenia dysenterica DC.) of the Cerrado of Minas Gerais, Brazil: Physical and chemical characterization, carotenoids and vitamins. Food Res. Int. 2011, 44, 2151–2154. [Google Scholar] [CrossRef]
- Vieira, R.F.; Agostini-Costa, T.d.S.; Silva, D.d.; Sano, S.M.; Ferreira, F.R. Frutas Nativas da Região Centro-Oeste do Brazil; Embrapa Informação Tecnológica: Brasília, Brazil, 2010. [Google Scholar]
- Genovese, M.I.; Da Silva Pinto, M.; De Souza Schmidt Gonçalves, A.; Lajolo, F.M. Bioactive compounds and antioxidant capacity of exotic fruits and commercial frozen pulps from Brazil. Food Sci. Technol. Int. 2008, 14, 207–214. [Google Scholar] [CrossRef]
- Santana, J.F.D.S.; Hercos, G.F.d.L.; Oliveira Filho, J.G.d.; Santos, D.C.d.; Oliveira, M.S.; Freitas, B.S.M.d.; Silva, F.G.; Egea, M.B. Acetic Fermentation of Cagaita Pulp: Technological and Chemical Characteristics. Beverages 2024, 10, 28. [Google Scholar] [CrossRef]
- Siqueira, E.M.d.A.; Rosa, F.R.; Fustinoni, A.M.; de Sant’Ana, L.P.; Arruda, S.F. Brazilian savanna fruits contain higher bioactive compounds content and higher antioxidant activity relative to the conventional red delicious apple. PLoS ONE 2013, 8, e72826. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, R.C.; dos Passos, A.M.A.; Coelho, A.M.; de Albuquerque Filho, M.R.; de Resende, Á.V.; Neto, M.M.G.; Borghi, E. Manejo do solo com foco em sistemas integrados de produção. In Agricultura de Baixo Carbono Tecnologias e Estratégias de Implantação; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- de Oliveira, F.d.A.; de Oliveira Filho, A.F.; de Medeiros, J.F.; de Almeida Júnior, A.B.; Linhares, P.C.F. Desenvolvimento inicial da mamoneira sob diferentes fontes e doses de matéria orgânica. Rev. Caatinga 2009, 22, 206–211. [Google Scholar]
- Wutke, E.; Ambrosano, E.; Razera, L.; Medina, P.; Carvalho, L.; Kikuti, H. Bancos Comunitários de Sementes de Adubos Verdes: Informações Técnicas; MAPA: Brasília, Brazil, 2007. [Google Scholar]
- Veras, M.D.S.; Ramos, M.L.G.; Oliveira, D.N.S.; Figueiredo, C.C.D.; Carvalho, A.M.D.; Pulrolnik, K.; Souza, K.W.d. Cover crops and nitrogen fertilization effects on nitrogen soil fractions under corn cultivation in a no-tillage system. Rev. Bras. Ciência Solo 2016, 40, e0150092. [Google Scholar] [CrossRef]
- Torres, J.L.R.; Pereira, M.G. Potassium dynamics in crop residues of cover plants in Cerrado. Rev. Bras. Ciência Solo 2008, 32, 1609–1618. [Google Scholar] [CrossRef]
- Calil, F.N.; Lima, N.L.; Silva, R.T.; de Moraes, M.D.A.; Barbosa, P.V.G.; Lima, P.A.F.; Brandao, D.C.; e Silva-Neto, C.d.M.; de Sousa Carvalho, H.C.; dos Reis Nascimento, A. Biomass and nutrition stock of grassland and accumulated litter in a silvopastoral system with Cerrado species. Afr. J. Agric. Res. 2016, 11, 3701–3709. [Google Scholar]
- Abreu, S.A.H.; Arruda, E.M.; Barros, L.R.; de Almeida, R.F.; MaranhÃ, D.D.C.; da Silva, V.L.; Flores, R.A.; Calil, F.N.; Collier, L.S. Chemical attributes of the soil in agroforestry systems subjected to organic fertilizations. Afr. J. Agric. Res. 2016, 11, 2378–2388. [Google Scholar]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.; Livingston Staffier, K.; Lee, H.; Howell, B.; Battaglia, K.; Bell, B.M.; Matteson, J.; McKeown, N.M.; Cash, S.B.; Zhang, F.F. Measurement of diets that are healthy, environmentally sustainable, affordable, and equitable: A scoping review of metrics, findings, and research gaps. Front. Nutr. 2023, 10, 1125955. [Google Scholar] [CrossRef] [PubMed]
- Dornelles, P.; Perin, A.; GuimarÃ, F.; Melo, G.B. Water content and soil nutrient in consortium of native fruit trees with cover crops. Afr. J. Agric. Res. 2016, 11, 4100–4108. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburgs, MD, USA, 2006. [Google Scholar]
- Merrill, A.; Watt, B. Energy Value of Foods: Basis and Derivation; Agriculture handbook; United States Department of Agriculture: Washington, DC, USA, 1973; p. 74.
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef]
- Bruuinsma, J. The quantitative analysis of chlorophylls a and b in plant extracts. Photochem. Photobiol. 1963, 2, 241–249. [Google Scholar] [CrossRef]
- Talcott, S.; Howard, L. Phenolic autoxidation is responsible for color degradation in processed carrot puree. J. Agric. Food Chem. 1999, 47, 2109–2115. [Google Scholar] [CrossRef]
- Gross, J. Pigment in Vegetables: Chlorophyll and Carotenoids; Van Nostrand Reinhold: New York, NY, USA, 1991. [Google Scholar]
- Benassi, M.; Antunes, A. A comparison of metaphosphoric and oxalic acids as extractans solutions for the determination of vitamin C in selected vegetables. Arq. Biol. E Tecnol. 1988, 31, 507–513. [Google Scholar]
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Li, Y.; Li, P.; Wang, H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chem. 2009, 112, 454–460. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.C.d.; Sousa, T.L.d.; Santana, J.F.d.S.; Almeida, A.B.d.; Silva, F.G.; Egea, M.B. Commercial craft beers of midwest Brazil: Biochemical and physicochemical properties and their relationship with its sensory profile. Food Sci. Technol. 2023, 43, e112222. [Google Scholar] [CrossRef]
- Bueno, G.H.; Guedes, M.N.S.; de Souza, A.G.; Madeira, A.P.C.; Garcia, E.M.; Taroco, H.A.; Melo, J.O.F. Caracterização física e físico-química de frutos de Eugenia dysenterica DC originados em região de clima tropical de altitude. Braz. J. Biom. 2017, 35, 515–522. [Google Scholar]
- Guido, F.; Behija, S.E.; Manel, I.; Nesrine, Z.; Ali, F.; Mohamed, H.; Noureddine, H.A.; Lotfi, A. Chemical and aroma volatile compositions of date palm (Phoenix dactylifera L.) fruits at three maturation stages. Food Chem. 2011, 127, 1744–1754. [Google Scholar]
- Fagundes, G.R.; Yamanishi, O.K. Características físicas e químicas de frutos de mamoeiro do grupo‘Solo’comercializados em 4 estabelecimentos de Brasília-DF. Rev. Bras. Frutic. 2001, 23, 541–545. [Google Scholar] [CrossRef]
- Alves, E.M.; Silva, F.G.; Avila, R.G.; Lourenço, L.L.; de Oliveira, T.C.; Custódio, A.M.; Rosa, M.; Pennacchi, J.P.; do Prado Paim, T. Intercropping and environmental seasonality modulate the physiology and growth of Hancornia speciosa (Gomes). CABI Agric. Biosci. 2024, 5, 31. [Google Scholar] [CrossRef]
- Borges, A.; Xavier, F.d.S.; de Carvalho, J. N-verde substitui parte do N-mineral na produção de laranjeira’ Pera´.—Cruz das Almas, BA: Embrapa Mandioca e Fruticultura, 2022. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/doc/1149976/1/BoletimPesquisa139-AnaLucia-2022-AINFO.pdf (accessed on 26 September 2024).
- Sousa, H.A.d.F.; Oliveira Filho, J.G.d.; Egea Mariana, B.; Silva, E.R.d.; Macagnan, D.; Pires, M.; Peixoto, J. Active film incorporated with clove essential oil on storage of banana varieties. Nutr. Food Sci. 2019, 49, 911–924. [Google Scholar] [CrossRef]
- Vianna-Silva, T.; Resende, E.D.d.; Pereira, S.M.d.F.; Viana, A.P.; Rosa, R.C.C.; Carlos, L.d.A.; Vitorazi, L. Influence of the ripening stages on the physical characteristics of the yellow passion fruit. Bragantia 2008, 67, 521–525. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Estevam, E.B.B.; Lemes, R.S.; do Prado, D.M.F.; da Silva, V.P.; dos Santos Silva, L.; Duarte, L.G.R.; Egea, M.B. Acerola (Malpighia emarginata) pulp: Characterization and stability of anthocyanins under different conditions. Food Sci. Technol. 2023, 43, e0000110. [Google Scholar] [CrossRef]
- Freitas, B.; Plácido, G.; Cagnin, C.; Caliari, M.; Silva, R.; Silva, C.; Cavalcante, M.; Souza, J.; Célia, J.; Oliveira, K. Evaluation of the postharvest quality of Cagaita fruits (Eugenia dysenterica DC.) coated with chitosan and associated with refrigeration. Afr. J. Biotechnol. 2015, 14, 2035–2046. [Google Scholar] [CrossRef]
- FDA. Hazard Analysis and Risk-Based Preventive Controls for Human Food: Guidance for Industry. Available online: https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/UCM517610.pdf (accessed on 29 November 2019).
- Schiassi, M.; de Souza, V.; Lago, A.; Campos, L.; Queiroz, F. Fruits from the Brazilian Cerrado region: Physico-chemical characterization, bioactive compounds, antioxidant activities, and sensory evaluation. Food Chem. 2018, 245, 305–311. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.P.; Queiroz, F.; Borges, S.V.; Carneiro, J.D.D.S. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012, 134, 381–386. [Google Scholar] [CrossRef]
- de Morais Cardoso, L.; de Lazzari Reis, B.; da Silva Oliveira, D.; Pinheiro-Sant’Ana, H.M. Mangaba (Hancornia speciosa Gomes) from the Brazilian Cerrado: Nutritional value, carotenoids and antioxidant vitamins. Fruits 2014, 69, 89–99. [Google Scholar] [CrossRef]
- Sacramento, C.d.; Matos, C.; Souza, C.; Barretto, W.; Faria, J. Características físicas, físico-químicas e químicas de cajás oriundos de diversos municípios da região sul da Bahia. Magistra Cruz Almas 2007, 19, 283–289. [Google Scholar]
- Singh, S.K.; Sharma, M.; Singh, P.K. Intercropping-An approach to reduce fruit drop and improve fruit quality in guava. J. Chem. Pharm. Sci. 2016, 9, 3182–3187. [Google Scholar]
- Primavesi, O.; Primavesi, A.; Armelin, M. Qualidade mineral e degradabilidade potencial de adubos verdes conduzidos sobre Latossolos, na região tropical de São Carlos, SP, Brazil. Rev. Agric. 2014, 77, 89–102. [Google Scholar]
- Hanly, J.; Gregg, P. Green-manure impacts on nitrogen availability to organic sweetcorn (Zea mays). N. Z. J. Crop Hortic. Sci. 2004, 32, 295–307. [Google Scholar] [CrossRef]
- Haas, G.; Brand, H.; de la Vega, M.P. Nitrogen from hairy vetch (Vicia villosa Roth) as winter green manure for white cabbage in organic horticulture. Biol. Agric. Hortic. 2007, 25, 37–53. [Google Scholar] [CrossRef]
- Campiglia, E.; Caporali, F.; Radicetti, E.; Mancinelli, R. Hairy vetch (Vicia villosa Roth.) cover crop residue management for improving weed control and yield in no-tillage tomato (Lycopersicon esculentum Mill.) production. Eur. J. Agron. 2010, 33, 94–102. [Google Scholar] [CrossRef]
- Kramberger, B.; Gselman, A.; Kristl, J.; Lešnik, M.; Šuštar, V.; Muršec, M.; Podvršnik, M. Winter cover crop: The effects of grass–clover mixture proportion and biomass management on maize and the apparent residual N in the soil. Eur. J. Agron. 2014, 55, 63–71. [Google Scholar] [CrossRef]
- Carneiro, N.; Alves, C.; Cagnin, C.; Belisario, C.; Silva, M.; Miranda, M.; Oliveira Filho, J.; Alves, J.; Pereira, P.; Silva, F.; et al. Eugenia Klotzschiana O. Berg Fruits as New Sources of Nutrients: Determination of their Bioactive Compounds, Antioxidant Activity and Chemical Composition. Braz. Arch. Biol. Technol. 2019, 62, e19170562. [Google Scholar] [CrossRef]
- Espindola, J.A.A.; Guerra, J.G.M.; Almeida, D.L.d.; Teixeira, M.G.; Urquiaga, S. Decomposição e liberação de nutrientes acumulados em leguminosas herbáceas perenes consorciadas com bananeira. Rev. Bras. Ciência Solo 2006, 30, 321–328. [Google Scholar] [CrossRef]
- Meurer, E.J. Potássio. In Nutrição mineral de plantas; Fernandes, M.S., Ed.; UFV: Viçosa, Brazil, 2006; pp. 281–298. [Google Scholar]
- Araújo, A.; Menezes, E.; Terra, A.; Dias, B.; Oliveira, É.d.; Queiroz, F. Bioactive compounds and chemical composition of Brazilian Cerrado fruits’ wastes: Pequi almonds, murici, and sweet passionfruit seeds. Food Sci. Technol. 2018, 38, 203–214. [Google Scholar] [CrossRef]
- Martins, C.R.; Rodrigues, G.S.; Barros, I.D. Assessment of economic and environmental performance in citrus-based intercropping systems. Rev. Bras. Frutic. 2021, 43, e-463. [Google Scholar] [CrossRef]
- Da Silva, M.M.M.; da Silva, E.P.; da Silva, F.A.; Ogando, F.I.B.; de Aguiar, C.L.; Damiani, C. Physiological development of cagaita (Eugenia dysenterica). Food Chem. 2017, 217, 74–80. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. Rapid kinetic microassay for catalase activity. J. Biomol. Tech. JBT 2007, 18, 185. [Google Scholar]
- IOM. Dietary Reference Intakes: Thiamin R, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, Choline; Institute of Medicine; Food and Nutrition Board; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Morales-Soto, A.; García-Salas, P.; Rodríguez-Pérez, C.; Jiménez-Sánchez, C.; de la Luz Cádiz-Gurrea, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Antioxidant capacity of 44 cultivars of fruits and vegetables grown in Andalusia (Spain). Food Res. Int. 2014, 58, 35–46. [Google Scholar] [CrossRef]
- Baranwal, A.; Aggarwal, P.; Rai, A.; Kumar, N. Pharmacological actions and underlying mechanisms of catechin: A review. Mini Rev. Med. Chem. 2022, 22, 821–833. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.P.A.; Conte-Junior, C.A. Health benefits of phytochemicals from Brazilian native foods and plants: Antioxidant, antimicrobial, anti-cancer, and risk factors of metabolic/endocrine disorders control. Trends Food Sci. Technol. 2021, 111, 534–548. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef]
- Almeida, A.d.; Silva, A.; Lodete, A.; Egea, M.; Lima, M.; Silva, F. Assessment of chemical and bioactive properties of native fruits from the Brazilian Cerrado. Nutr. Food Sci. 2019, 49, 381–392. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Genovese, M.I.; Lajolo, F.M. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J. Agric. Food Chem. 2005, 53, 2928–2935. [Google Scholar] [CrossRef]
- Leong, L.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Santana, L.F.; Sasso, S.; Aquino, D.F.S.; de Cássia Freitas, K.; de Cássia Avellaneda Guimarães, R.; Pott, A.; do Nascimento, V.A.; Bogo, D.; de Oliveira Figueiredo, P.; Hiane, P.A. Nutraceutic potential of bioactive compounds of Eugenia dysenterica DC in metabolic alterations. Molecules 2022, 27, 2477. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.L.A.A.; Brandi, I.V.; Durães, C.A.F.; Lima, J.P.d.; Soares, S.B.; Mesquita, B.M.A.d.C. Chemical characterization and antioxidant potential of native fruits of the Cerrado of northern Minas Gerais. Braz. J. Food Technol. 2020, 23, e2019296. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X. Metal–ion interactions with sugars. The crystal structure and FTIR study of an SrCl2–fructose complex. Carbohydr. Res. 2004, 339, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.H. Biological Applications of Infrared Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- D’Souza, L.; Devi, P.; Divya Shridhar, M.; Naik, C.G. Use of Fourier Transform Infrared (FTIR) spectroscopy to study cadmium-induced changes in Padina tetrastromatica (Hauck). Anal. Chem. Insights 2008, 3, 117739010800300001. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Oliver, A.E.; Tablin, F.; Crowe, J.H. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr. Res. 2004, 339, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Benning, L.G.; Phoenix, V.R.; Ferris, F.G. Characterization of metal−cyanobacteria sorption reactions: A combined macroscopic and infrared spectroscopic investigation. Environ. Sci. Technol. 2004, 38, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Leopold, L.F.; Leopold, N.; Diehl, H.-A.; Socaciu, C. Quantification of carbohydrates in fruit juices using FTIR spectroscopy and multivariate analysis. Spectroscopy 2011, 26, 93–104. [Google Scholar] [CrossRef]
- Fischer, G.; Braun, S.; Thissen, R.; Dott, W. FT-IR spectroscopy as a tool for rapid identification and intra-species characterization of airborne filamentous fungi. J. Microbiol. Methods 2006, 64, 63–77. [Google Scholar] [CrossRef]
- Dumas, P.; Miller, L. The use of synchrotron infrared microspectroscopy in biological and biomedical investigations. Vib. Spectrosc. 2003, 32, 3–21. [Google Scholar] [CrossRef]
| Abbreviation | Description |
|---|---|
| BR | Cagaiteira tree in intercrop with brachiaria (Brachiaria decumbens L.), which was sown only once in October, cut at the beginning of the dry period (July/August) and left on top of the soil for dry matter to decompose. |
| BRN | Cagaiteira tree in intercrop with brachiaria (Brachiaria decumbens L.) was sown only once in October and received nitrogen fertilizer with 220 g of urea/plant in the crown region (0.5 m diameter). They were cut at the beginning of the dry season (July/August) and left on top of the soil to decompose the dry matter. |
| CA | Cagaiteira tree in intercrop with Calopogonium mucunoides Desv., which was sown in October (annually), cut at the beginning of the dry period (July/August), and left on top of the soil for dry matter to decompose. |
| CR | Cagaiteira tree in intercrop with Crotalaria juncea was sown in November (annually), cut at the beginning of the dry season (July/August), and left on top of the soil for dry matter to decompose. |
| LA | Cagaiteira tree in intercrop with Lablab purpureus (L.) Sweet was sown in November (annually), cut at the beginning of the dry period (July/August), and left on top of the soil for dry matter to decompose. |
| Parameters | BR | BRN | CA | CR | LA |
|---|---|---|---|---|---|
| Fruit colorimetric analysis (epicarp) (n = 20) | |||||
| L* | 54.60 ± 6.92 a | 53.91 ± 6.44 ab | 51.30 ± 6.03 b# | 52.85 ± 6.80 ab | 54.57 ± 4.76 a |
| a* | 3.84 ± 6.46 a | 1.11 ± 2.85 bc# | 1.31 ± 3.23 ab# | −1.37 ± 3.42 c# | −0.04 ± 7.67 bc# |
| b* | 47.44 ± 8.70 a | 46.75 ± 7.98 a | 45.73 ± 7.41 a | 43.77 ± 7.91 a# | 45.52 ± 6.77 a |
| C* | 48.26 ± 7.24 a | 46.85 ± 7.96 a | 45.87 ± 7.36 a | 43.93 ± 7.88 a# | 46.22 ± 6.26 a |
| °h | 83.17 ± 10.22 b | 86.98 ± 2.49 a# | 86.82 ± 3.38 a# | 85.97 ± 4.41 a | 84.37 ± 8.67 ab |
| Pulp colorimetric analysis (mesocarp) | |||||
| L* | 43.10 ± 1.25 a | 39.98 ± 1.93 bc# | 38.93 ± 1.91 c# | 40.20 ± 1.02 bc# | 41.63 ± 1.39 ab# |
| a* | −0.95 ± 0.26 d | 2.52 ± 0.20 a# | 0.11 ± 0.15 b# | −0.95 ± 0.15 d | −0.33 ± 0.27 c# |
| b* | 33.00 ± 1.24 a | 30.38 ± 2.33 b# | 31.19 ± 1.71 ab# | 30.49 ± 1.94 b# | 32.99 ± 1.69 a |
| C* | 33.01 ± 1.24 a | 30.48 ± 2.32 b# | 31.19 ± 1.70 ab# | 30.48 ± 1.93 b# | 32.98 ± 1.68 a |
| °h | 91.64 ± 0.41 a | 85.22 ± 0.62 c# | 90.07 ± 0.93 b# | 91.79 ± 0.21 a | 90.65 ± 0.32 b# |
| Physicochemical and chemical composition | |||||
| pH | 3.05 ± 0.01 c | 3.14 ± 0.01 b# | 3.15 ± 0.01 b# | 3.16 ± 0.01 b# | 3.19 ± 0.01 a# |
| Titratable acidity (g citric acid/100 g) | 0.86 ± 0.01 b | 0.97 ± 0.01 c# | 0.89 ± 0.01 d# | 0.86 ± 0.01 b | 1.06 ± 0.02 a# |
| Soluble solids (°Brix) | 8.30 ± 0.10 c | 9.53 ± 0.15 a# | 8.60 ± 0.0 bc# | 8.23 ± 0.1 c | 8.90 ± 0.10 b# |
| Moisture (g/100 g) | 91.87 ± 0.12 ab | 91.17 ± 0.09 bc# | 92.32 ± 0.14 a# | 92.16 ± 0.05 a# | 91.00 ± 0.62 c |
| Lipid (g/100 g) | 4.48 ± 2.92 a | 4.05 ± 2.87 a | 4.66 ± 2.77 a | 3.37 ± 2.21 a | 3.61 ± 1.87 a |
| Ash (g/100 g) | 3.74 ± 0.09 a | 2.68 ± 0.24 c# | 3.12 ± 0.03 b# | 2.87 ± 0.1 bc# | 2.71 ± 0.17 c# |
| Protein (g/100 g) | 5.76 ± 0.42 b | 12.24 ± 2.47 a# | 11.93 ± 0.53 a# | 11.97 ± 1.34 a# | 14.91 ± 3.93 a# |
| Carbohydrate (g/100 g) | 6.99 ± 0.24 ab | 7.15 ± 0.17 a | 6.16 ± 0.25 c# | 6.41 ± 0.28 bc | 7.08 ± 0.17 a |
| Energy (Kcal) | 33.13 ± 1.19 b | 36.17 ± 1.34 a# | 31.54 ± 1.09 b | 31.77 ± 0.87 b | 36.72 ± 0.84 a# |
| Macrominerals | |||||
| Phosphor (mg/100 g) | 172.70 ± 0.01 a | 127.82 ± 0.01 a | 163.15 ± 17.75 a | 150.32 ± 35.43 a | 161.22 ± 17.54 a |
| Potassium (mg/100 g) | 1579.01 ± 0.01 a | 1150.38 ± 36.15 b# | 1305.23 ± 0.01 ab | 1252.64 ± 70.86 b# | 1190.52 ± 140.30 b |
| Sulfur (mg/100 g) | 12.34± 0.01 a | 12.78 ± 0.01 a | 18.82 ± 8.87 a | 18.79 ± 8.86 a | 12.40 ± 0.00 a |
| Calcium (mg/100 g) | 1437.15± 218.07 a | 287.59 ± 63.27 b# | 301.21 ± 70.99 b# | 225.47 ± 17.71 b# | 241.82 ± 8.77 b# |
| Magnesium (mg/100 g) | 376.25 ± 8.72 a | 102.26 ± 36.15 b# | 125.50 ± 17.75 b# | 106.47 ± 26.57 b# | 99.21 ± 17.54 b# |
| Microminerals | |||||
| Copper (mg/100 g) | 0.99 ± 0.17 a | 0.89 ± 0.18 a | 1.00 ± 0.18 a | 1.13 ± 0.35 a | 1.24 ± 0.53 a |
| Iron (mg/100 g) | 9.07 ±1.66 b | 8.56 ± 0.18 b | 13.87 ± 0.44 a | 11.27 ± 1.77 ab | 11.47 ± 0.44 ab |
| Manganese (mg/100 g) | 1.60 ± 0.35 a | 2.75 ± 0.63 a | 2.00 ± 0.18 a | 2.07 ± 0.26 a | 3.84 ± 1.05 a |
| Zinc (mg/100 g) | 1.48 ± 0.35 a | 1.79 ± 0.9 a | 1.82 ± 0.27 a | 2.69 ±1.15 a | 2.54 ± 1.31 a |
| Boron (mg/100 g) | 0.31 ±0.09 a | 0.32 ± 0.09 a | 0.69 ± 0.27 a | 0.31 ± 0.09 a | 0.31 ± 0.09 a |
| Treatments/ Phenolic Compounds | Catequin | Epicatechin | Galic Acid |
|---|---|---|---|
| Mn | 289.07214 | 289.07205 | 169.01343 |
| BR | X | X | X |
| CA | X | - | X |
| CR | X | X | X |
| BRN | X | X | X |
| LA | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, M.J.d.; Dornelles, P.; Rezende, T.A.M.d.; Silva, L.d.L.d.; Silva, F.G.; Duarte, L.G.R.; de Oliveira Filho, J.G.; Egea, M.B. Influence of Intercropping on Eugenia dysenterica (Mart.) DC. Fruit Quality. Horticulturae 2024, 10, 1028. https://doi.org/10.3390/horticulturae10101028
Almeida MJd, Dornelles P, Rezende TAMd, Silva LdLd, Silva FG, Duarte LGR, de Oliveira Filho JG, Egea MB. Influence of Intercropping on Eugenia dysenterica (Mart.) DC. Fruit Quality. Horticulturae. 2024; 10(10):1028. https://doi.org/10.3390/horticulturae10101028
Chicago/Turabian StyleAlmeida, Micael Jose de, Paulo Dornelles, Thaisa Alves Matos de Rezende, Ludiele de Lima da Silva, Fabiano Guimarães Silva, Larissa Graziele Rauber Duarte, Josemar Gonçalves de Oliveira Filho, and Mariana Buranelo Egea. 2024. "Influence of Intercropping on Eugenia dysenterica (Mart.) DC. Fruit Quality" Horticulturae 10, no. 10: 1028. https://doi.org/10.3390/horticulturae10101028
APA StyleAlmeida, M. J. d., Dornelles, P., Rezende, T. A. M. d., Silva, L. d. L. d., Silva, F. G., Duarte, L. G. R., de Oliveira Filho, J. G., & Egea, M. B. (2024). Influence of Intercropping on Eugenia dysenterica (Mart.) DC. Fruit Quality. Horticulturae, 10(10), 1028. https://doi.org/10.3390/horticulturae10101028






