Abstract
Background: Viticulture bioclimatic indexes like the Heliothermal Index (HI), Cool Night Index (CI), and Dryness Index (DI), can be used to assess the influence of climate on grapes’ quality. Methods: HI, CI, and DI + total seasonal irrigation were utilized to assess the effect of climate on the flavonoids content and composition of two Vitis vinifera seedless varieties, ‘Summer Royal’ and ‘Crimson Seedless’, both grown in Apulia (Southern Italy). Results: The flavonoids content was significantly affected by variety and climate conditions on the base of HI, CI, and DI + total seasonal irrigation. Factor analysis applied to climate indexes and flavonoids showed that anthocyanins and flavonols were negatively and positively correlated to CI in both varieties, respectively. Additionally, warmer night temperatures determined higher fla-van-3-ols. HI increase promoted anthocyanins, flavonols, and flavan-3-ols content in Crimson Seedless, whilst it induced an opposite trend in Summer Royal. Finally, DI + total seasonal irrigation showed to be positively linked to flavonols content and negatively linked to anthocyanins content just in the case of Crimson Seedless. Significant regression models were also determined between climate indexes and productive parameters (i.e., yield, TSS, TA, pH, bunch, and berry weight). Conclusions: Climate indexes HI, CI, and DI + total seasonal irrigation showed an effect on quality grape parameters like flavonoids and contributed to building predictive models when new climatic zones are going to be evaluated for the production of table grapes.
1. Introduction
Flavonoids are a group of polyphenolic compounds, different in chemical structure and characteristics, which are biosynthesized through the shikimic acid pathway. Flavonols, flavan-3-ols, and anthocyanins are among the principal flavonoid classes present in fruits and vegetables [1]. Grapes, which are economically the most important fruit species in the world, represent one of the major sources of flavonoid compounds [2]. The cultivation of grapes is widely spread around the world [3]. In 2018, the global production of table grapes was estimated to be 27 million tons [4]. Italy is the eighth highest producing country, with 1.04 million tons, after China (9.19 million tons). Italy is also in second place in terms of exports, with 450 thousand tons of table grapes, for a value of EUR 550 million. Apulia, in Southern Italy, is the leading Italian region, with approximately 24 thousand hectares and a yearly production of over 0.6 million tons [4,5].
Anthocyanins are pigments that give the color of black and red grape varieties; they are mainly present as 3-O-monoglucosides, 3-O-acetylglucosides, and 3-O-p-cinnamoyl-monoglucosides [6]. Flavonols are represented mainly by kaempferol, quercetin, and myricetin, which accumulate in grapes as glycosides [7]. Flavan-3-ols can be present as monomers, (+)-catechin and (−)-epicatechin, and proanthocyanidins, composed of flavan-3-ols units linked together through C(4)–C(6) and C(4)–C(8) interflavonoid bonds [8].
In vine cycle growth, grape flavonoids carry out multiple functions: they protect against predation and UV damage and serve as attractants for pollinators or foraging animals to aid in seed dispersion [9,10]. Flavonoids are also of interest in human nutrition because of their multiple biological properties that are beneficial for health [1,11]. Due to the abundant roles of flavonoids in plants and humans, many studies to determine factors influencing their metabolism were carried out.
It has long been known that the biosynthetic pathways involved in flavonoid production in grape berries are greatly influenced by environmental conditions [12,13,14], such as sunlight. In this regard, it would be normally expected that grapes highly exposed to daylight are capable of increased flavonoid biosynthesis [7]. Also, temperature influences the composition and quality of grapes. Indeed, during the ripening period, the air day temperature plays a determinant role in grape maturation, including the aroma and the coloration, and the effect of cool night temperatures on them is even stronger [15]. The different water level in the soil affects grape quality and flavonoid contents and in regions with high rainfall, the ripening capacity of grapes together with their phenols bioaccumulation is lower than that predicted by the climatic thermal indexes [16]. Within wine grape research, the primary focus has been on the production of anthocyanins and other flavonoids, seen as critical to wine quality [10]. Research has helped to uncover the effects of environmental factors such as sunlight exposure and temperature on the accumulation of these compounds, as well as the biosynthetic pathways involved in their biosynthesis [13,17]. However, to our knowledge, detailed long-term studies focusing on the effect of Mediterranean growth conditions described by climate factor indexes on flavonoid contents in table grapes are absent, there being, on the other hand, some research available dealing with wine grapes [6]. Moreover, in most cases, in order to make it possible to study the light exposure and/or temperature of the fruit in the vineyard, investigators have trained shoots using artificial shading or leaf removal [18,19]; however, these reports were not able to differentiate the effects of light from those of temperature on grape composition. Eventually, experiments were also conducted in phytotrons, growth chambers, or greenhouses, even though the ripening process and final grape composition differ from field conditions [20,21].
Thus, the objective of the present study was to analyze yield parameters and characterize the composition of flavonoid compounds by HPLC-DAD-ESI-MS of two seedless table grape varieties (Summer Royal and Crimson Seedless), in three consecutive harvesting years (2014–2016) under field conditions. Several bioclimatic indexes based on temperature are available: Growing Season Temperature (GST) [22]; Heliothermic Huglin Index (HI) [23]; Winkler Degree Days (WI) [24], Biologically Effective Degree Days (BEDD) [25] Cool Night Index (CI) [26]. Moreover, at least two based on precipitation, like the Growing Season Precipitation Index (GSP) and the Spring Rain Index (SprR), are known [27].
Furthermore, according to a literature study that tested their pertinence in characterizing different viticulture areas [26], HI related to thermal conditions, CI related to night temperature, and the Dryness Index (DI) related to water balance; these were calculated all along the three years of study to determine their influence on the berry compositional parameters. It is worth noting that these indexes in more recent research studies have been also utilized to describe different future scenarios due to climate changes [28].
2. Materials and Methods
2.1. Plant Material and Growth Condition
The experiment was conducted along three consecutive harvesting years (2014–2016). ‘Summer Royal’ and ‘Crimson Seedless’ (Vitis vinifera L.), two seedless black and red table grape varieties, respectively, were grown both in the same trial site (long. 40.56° E, lat. 17.12° N) in the Apulian region (Southern Italy) on a sandy-clay soil composed of 50% sand, 12% silt and 38% clay, with a root zone depth of 1 m. All vines were grafted in 1999 on 140-RU Ruggeri (Vitis berlandieri × Vitis rupestris) rootstocks, 2.5 × 2.5 m spaced and with a 1600 vines/ha planting density. Vines were fertilized once a year just before bud break with 400 kg/ha of a complex fertilizer (Nitrophoska blu spezial 12.12.17.2) and trained to a “tendone” trellis system, widely used in the Apulian region.
Similar to previous descriptions [29], the “tendone” allows controlling cluster temperature (grape berries were on average 2 °C below ambient), because it improves the canopy management protecting the grapes from direct UV radiation and avoids the sun-burning of berries. Moreover, during the ripening stage, the grapes experience diffuse light that avoids the different coloring between the shaded and the exposed parts of the bunches. As regards the climate of the experimental site, it is classified as “Mediterranean semi-arid”, characterized by hot dry summers and mild rainy winters. The warmest month is August with a mean temperature of 24.9 °C, while the coldest one is January with a mean temperature of 6.3 °C. The mean annual rainfall is 640 mm, while the mean summer precipitation is 150 mm [30]. The irrigation treatments were imposed counterbalancing the total annual reintegration and maintaining it (Net Irrigation Requirements NIR = ETc − effective rainfall) fixed over the years of experimentation, thus eventually amplifying yearly climate variations. Also, the vineyards, similarly to the most part of the commercial vineyards present in the area, were covered by an anti-hail net (Kristal—Retilplast, Salerno, Italy). This covering net allows a slight reduction (15–20%) of vineyard crop evapotranspiration (ETc) due to the moderate shading and wind reduction effect [31]. In particular, ETc was estimated using varying crop coefficients (kc) (ETc = ET0 × kc) based on those proposed by FAO and adjusted for the Mediterranean area. ET0 was calculated from the mean values of the preceding 6 years (2005–2013) using the daily climate data collected by a set of climatic sensors located 2.1 km from the experimental vineyard and were extrapolated from the databank of the Ministry of Defense, Air Force weather station. The applied kc values were 0.35 in April, 0.45 in May, 0.5 in June, 0.75 from July to mid-August, and 0.60 from August to the end of September [32]. In the three seasons, a fixed irrigation volume of 216 mm/Ha was imposed considering the ETc reduction due to anti-hail net and the averaged total ETc calculated in the earlier six years. The vines were drip irrigated by means of irrigation lines installed 180 cm above the soil surface with drippers spaced 70 cm apart and set to supply water at constant pressure with two 8 L/h drippers per vine. The time between irrigation cycles was approximately 15 days starting 20 days after flowering until harvest.
2.2. Climate Data
Climate raw data were taken from a meteorological station situated close to the vineyards (6 km). The daily Tmax, Tmin, Tmean, Rainfall (mm), Radiation (kJ/m2), and ET0 (mm) as reference evapotranspiration of the ground cover were collected during the growth periods of the two grape varieties. ET0 data were used to calculate ETc (mm) by means of grape kc (monthly viticulture coefficient). Finally, data were used to calculate the following climate indexes (HI, CI, and DI) with some modifications as reported below:
where Tmean and Tmax are the mean and maximum monthly temperature (°C), respectively, and d is the length of day coefficient ranging from 1.02 to 1.06 between 40° and 50° of latitude, 1.00 for latitudes below 40°. Given the latitude of the trial field, 1.02 was adopted. The HI was partially modified since the period considered for its calculation was from 1 April until the harvest date, instead of the original formula for which the period goes from 1 April to 30 September [23]. This index, largely used in world viticulture, provides information regarding the level of heliothermal potential, includes the day temperature of the period when photosynthesis is active, providing a better idea of the potential sugar, and is well-correlated with the Thermal Index of Winkler, but more pertinent to the qualitative factors (e.g., berry sugar potential).
The CI is the night coolness that normally considers the average of Tmin during the month of September, when normally grape ripeness occurs. In this case, for both varieties the harvest month was considered, August for Summer Royal and September for Crimson Seedless. The purpose of this index is to improve the assessment of the qualitative potentials of wine-growing regions, notably in relation to secondary metabolites (polyphenols and aroma) and grape color.
For what concerns the DI formula, it has to be underlined that it does not consider irrigation, whilst in our case, being table grapes, the total seasonal irrigation component was added. Therefore, we calculated an integrated Dryness Index as follows:
DI + total seasonal irrigation
The DI is the Dryness Index calculated as follows:
where W0 is the initial soil water reserve, which can be accessed by the vine roots (the initial W0 was taken as 200 mm); P is the total monthly rainfalls (mm); Tv is the potential monthly transpiration of the vineyard (mm); and Es is the monthly direct evaporation from the soil (mm).
Tv was calculated using the expression:
where ETp is the monthly reference evapotranspiration given by the local weather station using a Class A Evaporation Pan and k is the coefficient of radiation absorption by the vine plant (k = 0.1 April, 0.3 May and 0.5 June-July-August).
Tv = ETp × k
Es was calculated using the expression:
where N is the number of days in the month, and JPm is the number of days of effective evaporation from the soil per month, which was estimated by dividing P per 5 and should be <N.
Es = (ETp N) × (1 − k)JPm
2.3. Chemicals
Formic acid, hydrochloric acid, and HPLC grade water were purchased from J.T. Baker (Deventer, Holland). LC-MS grade solvent acetonitrile was purchased from Riedelde Haën (Steinheim, Germany). Ethanol RPE was purchased from Carlo Erba (Milano, Italy). The anthocyanins delphinidin-3-O-glucoside chloride, cyanidin-3-O-glucoside chloride, peonidin-3-O-glucoside chloride, and malvidin-3-O-glucoside chloride, the flavonols quercetin-3-O-rutinoside, quercetin-3-O-glucoside, kaempferol-3-O-glucoside, and the flavan-3-ols procyanidin B1, procyanidin B2 and catechin were purchased from Extrasynthese (Genay, France) and used as HPLC reference standards.
2.4. Sampling and Chemical Analysis
The criterion used to determine when grapes were ready for harvest was their technology maturity, when the amount of sugar in the pulp was at its highest, while the acidity was low and the sugar/acid ratio was high [33]; therefore, comparable harvesting periods for each cultivar were guaranteed in the three years. Specifically, in 2014, 2015, and 2016, Summer Royal grapes were harvested on 6th, 20th, and 30th of August, respectively, whilst Crimson Seedless on 30 September, 21 and 5 October, respectively. Randomized blocks with three replicates for each year were used. From ten different vines per replicate, 1 bunch (by taking bunches in the middle of the fruit canes) was picked up. A total of 10 berries from each bunch were collected and immediately frozen at −20 °C for HPLC flavonoids analysis. From the remaining berries, the juice was extracted by gentle pressure and was centrifuged for 5 min at 4000× g. Over the supernatant, the pH was determined using an equilibrated pH meter (CRISON BASIC 20, Barcelona, Spain), total soluble solids (TSS) were measured as °Brix using a portable refractometer (Atago PR32, Norfolk, VA, USA), and titratable acidity was determined by titration with 0.1 N of NaOH to a pH 7 endpoint and was expressed as grams of tartaric acid per liter.
2.5. Extraction and HPLC-DAD-MS Analysis of Flavonoids
According to our previous report [2], the frozen 10-berries samples were manually separated from the pulp and freeze-dried skin samples were extracted with 25 mL of 70% ethanol containing 1% hydrochloric acid. The solution was kept overnight in the dark. The extracts were centrifuged at 4000× g for 3 min in an EPPENDORF centrifuge 5810R (Hamburg, Germany), filtered through a 0.45 µm syringe cellulose filter, and analyzed by HPLC.
The HPLC-DAD-MS system adopted in this work consisted of a capillary HPLC 1100 (Agilent Technologies, Palo Alto, CA, USA) equipped with a degasser, binary pump solvent delivery, thermostated column compartment, diode array detector, and an XCT-trap Plus mass detector (Agilent Technologies, Palo Alto, USA) coupled with a pneumatic nebulizer assisted electrospray LC-MS interface. The reversed stationary phase employed was a Gemini C18 110A (Phenomenex) 5 µm (150 × 2 mm i.d.) with a pre-column Gemini C18 5 µm (4 × 2 mm i.d., Phenomenex). The following gradient system was used with acetonitrile (solvent A) and water/formic acid (99:1, v/v) (solvent B): 0 min, 5% A-95%B; 10 min, 13% A-87% B; 20 min, 15% A-85% B; 30 min, 22% A-78% B; 50 min 22% A-78%B; 55 min 5% A-95% B; stop time to 70 min. The flow was maintained at 0.2 mL/min; sample injection was 3 µL. Diode array detection was between 250 and 650 nm, and absorbance was recorded at 520, 360, and 280 nm.
Both positive and negative electrospray modes were used for the ionization of molecules with capillary voltage at 4000 V and skimmer voltage at 30 V. The nebulizer pressure was 40 psi and the nitrogen flow rate was 9 L/min. The temperature of the drying gas was 350 °C. In the full scan (MS) and daughter (MS/MS) modes, the monitored mass range was from m/z 100 to 1200. Collision-induced fragmentation experiments were performed in the ion trap using helium as the collision gas, and the collision energy was set at 50%. Mass spectrometry data were acquired in the positive ionization mode for anthocyanins and in the negative ionization mode for flavonols and flavan-3-ols. All data were acquired and processed using ChemStation software (Agilent Technologies, Palo Alto, USA).
Compound identification was achieved by combining different information: positions of absorption maxima (λmax), the retention times, mass spectra, and the corresponding daughter MS-MS fragments were compared with those from pure standards and/or interpreted with the help of structural models already hypothesized in the literature [2,34]. Quantification of flavonoids was made by using the calibration curves of pure standards, malvidin-3-O-glucoside, quercetin-3-O-glucoside, and catechin with R2 = 0.9918, 0.9921, and 0.9992, respectively. Anthocyanins were quantified at 520 nm as malvidin-3-O-glucoside, flavonols at 360 nm as quercetin-3-O-glucoside, and flavan-3-ols at 280 nm as catechin equivalents.
2.6. Experimental Design and Statistical Analyses
All data were analyzed statistically by the STATISTICA 12.0 software package (StatSoft Inc., Tulsa, OK, USA). Three randomized replicates for variety were imposed as experimental design. A PCA and a successive factor analysis (FA) with orthogonal rotation of axes (varimax rotation) were carried out on yield parameters and flavonoids together with climatic indexes (HI, CI, IDI). Moreover, a one-way analysis of variance (ANOVA) together with a Tukey’s HSD post hoc test for determining significance at p < 0.05 was performed assuming years as additional replicates.
3. Results and Discussion
3.1. Climate Indexes
According to Table 1, the highest HI occurred in 2015 for both varieties with values ranging from 2320 to 2689. Therefore, 2015 was the year with the warmest climatic conditions, and this happened for both varieties though they had different growth cycle durations. As regards the CI index, for ‘Summer Royal’ the warmest nights were registered during the 2015 ripening period (August). In contrast, for ‘Crimson Seedless’ the highest CI in the ripening period (September) occurred in 2014. Finally, a moderately dry condition was generally observed along the years, and this was reasonably due to the irrigation practice providing a fixed integration of water irrigation all along the three years of the trial. However, 2014 represented an exception as DI + total seasonal irrigation registered a high variation ranging from 14 to 87.5, even though an irrigation water supply was provided. Surely, it was due to an arid August and rainy September in the case of the Summer Royal and Crimson Seedless ripening periods, respectively, as usually happens in Mediterranean climate conditions.

Table 1.
Climate indexes of two seedless table grape varieties, ‘Summer Royal’ and ‘Crimson Seedless’, during a three-year trial (2014–2016) grown in the Apulian region (Southern Italy).
3.2. Yield and Chemical Parameters
As reported in Table 2, a significant influence of the year on yield was found in the case of Summer Royal ranging from 12.3 to 13.0 kg/vine (p < 0.05) in 2014 and 2015, respectively. In Crimson Seedless, the yield ranged from 9.7 in 2015 to 14.9 kg/vine (p < 0.001) in 2016. Summer Royal bunch weight was significantly higher in 2016 (1010 g) than in 2014 (730 g) and 2015 (810), whilst in Crimson Seedless the highest bunch weight was registered in 2015 (910 g). Berry weight did not show differences within the years in Summer Royal, whilst 3.4 g in 2015 and 4.8 g in 2016 as minimum and maximum values, respectively, were registered in Crimson Seedless.

Table 2.
Effect of the year on productive parameters of two seedless table grape varieties, ‘Summer Royal’ and ‘Crimson Seedless’, during a three-year trial (2014–2016) grown in the Apulian region (Southern Italy).
Chemical parameters (TSS, pH, and TA) showed significant variations over the years in both varieties. They showed higher TSS contents (17.3 and 20.4 °Brix) in 2015. Despite the different ripening periods, TA contents also reacted over the years in the same way in the two varieties appearing more acid in 2014 and 2016 than in 2015. Finally, pH was statistically influenced by the year only in the case of Crimson Seedless (Table 2).
3.3. Anthocyanins, Flavonols and Flavan-3-ols Content in the Grape Cultivars
Nineteen anthocyanins were identified (Table 3) and three main groups were clearly distinguished: the monoglucosides of five anthocyanidins (delphinidin, cyanidin, petunidin, peonidin, and malvidin), the acylated anthocyanins, and the cinnamoyl (coumaroyl and caffeoyl) derivatives.

Table 3.
Anthocyanin compounds in the two varieties over the three years (2014–2016).
The only difference in the anthocyanin profile was the absence of the acylated derivatives as well as the cis isomer of petunidin and malvidin-3-O-p-coumaroyl-glucoside and the trans isomer of delphinidin-3-O-p-coumaroyl-glucoside in Crimson Seedless. However, there were significant quantitative differences in the anthocyanin content according to cultivar and study year (year x cultivar, F = 7730; p < 0.01). The analytical data showed that the content of anthocyanins varied between 880 and 1200 mg/kg and 36 and 90 mg/kg of fresh weight grapes (fw) in Summer Royal and Crimson Seedless, respectively. As expected, the concentrations were higher in the berries from the black variety, irrespective of the year.
Similar to wine grapes [35], the main pigments in the black cultivar were the trihydroxylated anthocyanins, malvidin-3-O-glucoside and its trans-p-coumaroyl derivative, with values ranging from 320 to 430 mg/kg and from 135 to 182 mg/kg of fw, respectively. The other most abundant compounds were peonidin-3-O-glucoside and its trans-p-coumaroyl derivative, petunidin-3-O-glucoside and delphinidin-3-O-glucoside that occurred in higher amounts in 2014 grape extracts. The above quantitative profile of anthocyanins was not accomplished by Crimson Seedless which mainly contained dihydroxylated anthocyanins, peonidin-3-O-glucoside and cyanidin-3-O-glucoside, of which the highest amount was observed in grapes from the 2015 vintage (Table 3).
As in previously published studies [36,37], the concentrations of the different pigments recorded in this experiment depended on the year and cultivar, while the ratio between them remained relatively constant. Indeed, in Summer Royal, for instance, the contribution of delphinidins, petunidins, and malvidins to the total anthocyanins, was always around 12, 11 and 53%, respectively. Otherwise, the percentages of cyanidins and peonidins in Crimson Seedless were more variable along the seasons, with values between 4 and 10% or 75 and 89%, respectively.
Table 4 shows the total flavonols and flavan-3-ols, together with the amounts of procyanidins B1 and B2, (+)-catechin, and quercetins, kaempferols and isorhamnetins glycoside isomers in the skins of table grapes. Not all the phenolics were identified, and the structure of some of them is the object of further investigation due to the similarity of their structure and the consequent little differences in their spectra. Nevertheless, the unidentified compounds were clearly distinguishable based on their specific retention times. Although the phenolic profiles of table grapes are less complex than those of wine grapes, highly significant differences in the flavonols and flavan-3-ols composition and amounts were found among the investigated cultivars and seasons (year x cultivar, F = 124; p < 0.01). Apart from the absence of quercetin-3-O-galactoside in Crimson Seedless, the other quercetins were identified in both varieties with higher contents in black grapes. In particular, quercetin-3-O-glucoronide and quercetin-3-O-rutinoside (they coeluted in a single peak under these chromatographic conditions and thus they were quantified as a single peak) were the dominant flavonols in Summer Royal, with higher concentrations in the 2015 and 2016 vintages. Kaempferol was completely undetected in Crimson Seedless as already reported in other research [34]; furthermore, higher concentrations of isorhamnetin-3-O-galactoside and isorhamnetin-3-O-glucoside were measured in Summer Royal from the 2015 vintage. As regards flavan-3-ols, very high levels of procyanidin B1 and B2, and (+)-catechin were found in Summer Royal, especially in 2015 (Table 4).

Table 4.
Effect of the year on flavan-3-ols and flavonol compounds of the two varieties.
3.4. Relationship between Yield Paramenters, Flavonoids, and Climate Indexes
The behavior of the determined flavonoids and yield parameters was significantly different in the three years of the experiments, suggesting that for the two grape varieties grown using the same culture practices, the climate conditions during the annual growing season exerted a decisive influence on the quantities produced. It is known that the temperature of a grape berry or cluster is a function of its energy balance that involves the exchange of energy between the cluster and its environment, which generally is dominated by shortwave (i.e., solar) radiation and convection, and the transfer of heat between the cluster and moving air. Accordingly, the effects of thermal conditions on grape berry composition have been studied extensively, primarily in growth chambers, glasshouses, and phytotrons to compare constant day and/or night temperatures, but altering, in this way, the effective field influence on grape ripening [20,21]. On the contrary, in this research, viticultural practices (such as a training system and adequate irrigation to optimize canopy management and control berry temperature with minimal compromise to the cluster microclimate) have been developed, that could exclude the effects of direct solar radiation from confounding the assessment of those related to thermal conditions alone.
A PCA score plot (Figure 1) was obtained considering the whole variability due to yield parameters and flavonoids measured in the two table grapes along the three years of study. The separation between Summer Royal and Crimson Seedless with respect to PC1 (accounting for 58.95% of the experimental variance) was more evident than the clustering of the vintages along PC2 (accounting for 17.50% of the experimental variance). This finding suggested to separately analyze the influence of the climate indexes for each variety investigating the effects of the years. Therefore, in order to tentatively discover a possible correlation between yield parameters, as well as flavonoids and climate indexes, a factorial analysis (FA) was performed. A maximum extraction of three factors was imposed from data (based on Kaiser and scree rules) and comprising the maximum cumulative variance of 94.1% and 95.5% for Summer Royal and Crimson Seedless, respectively (Figure 2 and Figure 3). Specifically, an orthogonal axes rotation (varimax normalized rotation) was performed to enhance the interpretability of the results; this rotation did not affect the sum of eigenvalues but by changing the axes, the eigenvalues of factors can be changed, as well as the factor loadings. In this way, a clearer pattern of loadings was determined by providing higher loadings of variables on a single factor, finding the best fit of the three extracted factors with the variables, thus avoiding sizeable loadings of the same variable on different factors contemporarily and, finally, improving the understanding of the variables’ associations and rules.
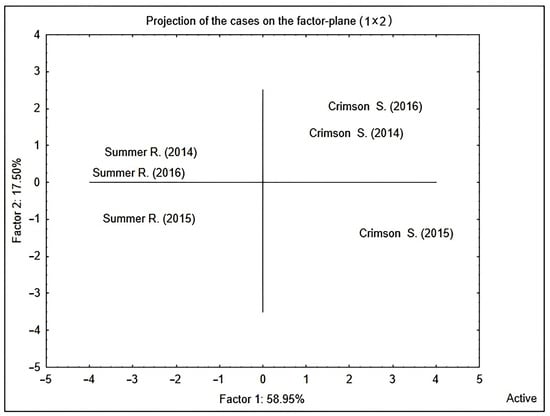
Figure 1.
PCA score plot of cv. Summer Royal and cv. Crimson Seedless grouping the two varieties on the basis of total variability due to yield parameters and flavonoids over the three years of the study.
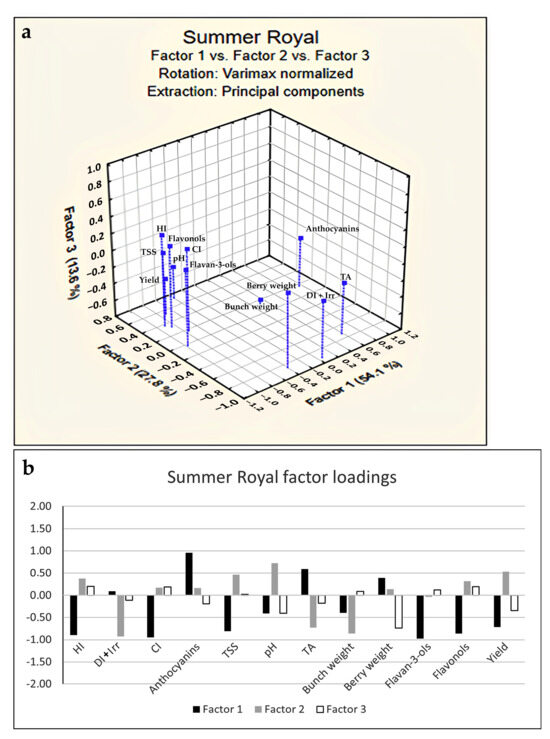
Figure 2.
Factor analysis score plot (a) and factor loadings (b) relative to flavonoids, yield parameters, and climate indexes of cv. Summer Royal.
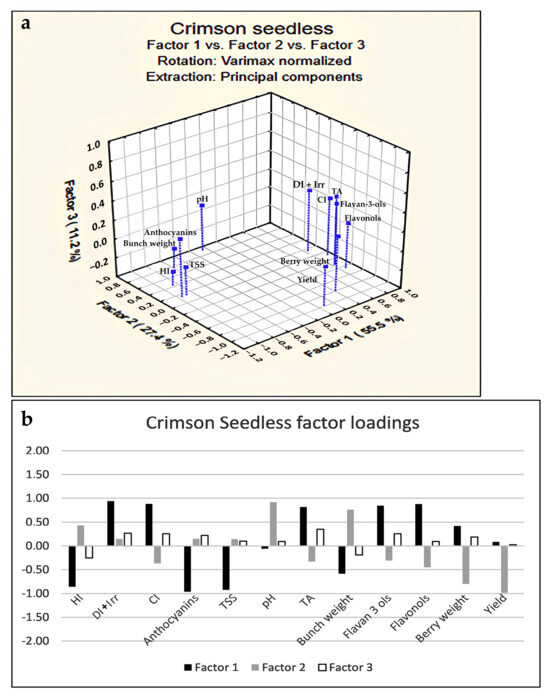
Figure 3.
Factor analysis score plot (a) and factor loadings (b) relative to flavonoids, yield parameters, and climate indexes of cv. Crimson Seedless.
Firstly, it is worth noting that in both varieties, as confirmed by the negative loadings on Factor 1 (Figure 2a and Figure 3a), TSS accumulation was favored by an increase in environmental energy as measured by the HI index despite the different growth cycle and climate conditions which occurred in the ripening stage. After all, HI has the advantage of integrating day length and temperature, having a strong influence on grape development and quality [26,38]; indeed, previous literature reports have already proved how HI was positively correlated to late season phenological events (véraison and harvest) and provided a better assessment of the sugar potential of vine varieties [39,40]. On the contrary, HI inversely influenced TA as confirmed by FA score plots (Figure 3).
In addition, HI was also positively linked to the yield but only for Summer Royal (Figure 2), while the yield as well as bunch and berry weight of Crimson Seedless (having higher loadings on Factor 2) seemed to not be linked to climatic indexes (Figure 3).
Summer Royal positively reacted to the higher environmental energy registered by the HI index and this was likely due to its genetic predisposition represented by its higher cluster-to-shoot ratio. Summer Royal showed to favorably absorb more energy for its grape ripening as compared with Crimson Seedless, mostly considering that it completed its growth cycle within August and in a shorter time, with more yield per vine and with this yield being more stable over the years (Table 4). Accordingly, Ponti et al. found that in the future the temperature increase will generally determine higher yield levels except for countries with already high availability of environmental energy and located at very lower latitudes (i.e., Egypt) [41].
Regarding the effect of the Cool Night Index (CI), opposite behavior was registered on the two varieties; in particular, CI was differently related to TSS (directly and inversely for Summer Royal and Crimson Seedless, respectively). This was likely due to the different ripening period of the two varieties, with Summer Royal characterized by very high night temperatures (from the second half of July to the first half of August). Important processes such as plant respiration, repair of damaged photosystems, and carbohydrate translocation occurs during the night; the lack of photosyntate translocation can influence photosynthetic efficiency of the leaves in the subsequent day. Therefore, night temperature can play an important role at respiration level influencing the carbohydrate translocation [42,43]. Indeed, in the case of Summer Royal (Figure 2), CI and TSS were directly related (both with high negative loadings on Factor 1) probably because the greater amount of sunlight during the day, which was available in the ripening of this variety, needs a much better efficiency in the photosyntate translocation process favored by the higher night temperatures. Conversely, it is worth pointing out that this process did not seem to happen for Crimson Seedless (having CI and TSS inversely related on Factor 1), due to the lower diurnal temperatures of its ripening period because they are not capable to compensate for the negative effect of the higher temperature of the night on the sugar accumulation (Figure 3).
As regards TA content, a significant decrease in the presence of a higher CI value was registered in Summer Royal, denoting an opposite trend with respect to Crimson Seedless. However, this can be explained by the different ranges of temperatures that occurred: 17.2–17.8 °C and 13.4–15.4 °C in the ripening of Summer Royal and Crimson Seedless, respectively. Indeed, the temperature ranges highlighted for both varieties that the maximum TA content was to be reached when CI tended to be around 15–16 °C. Even though the bunch is generally connected with the berry weight increase, in Summer Royal CI appeared not related to berry and bunch weight, showing instead a positive link to yield and TSS. This was clearly due to the fact that the number, and consequently the weight of berries, originated from environmental conditions that occurred during the fruit set and were completely unrelated to CI variations over the ripening period. DI + total seasonal irrigation was positively related to the TA of grapes even though their higher loadings were revealed on Factor 1 and Factor 2 for Summer Royal and Crimson Seedless, respectively.
For what concerns DI, in this paper we provided for the first time an integrated bioclimatic index. Indeed, the original DI, born to characterize the suitability of an area to host wine grape which is generally cultivated without irrigation, does not contemplate irrigation in its formula. In our case, the total seasonal irrigation amount (mm) was added to the traditional formula because it represents a significant input to achieve qualitative and quantitative yields for table grapes cultivation.
In Summer Royal, the relationship of the DI + total seasonal irrigation with yield was not significant confirming the mitigating effect of the irrigation already highlighted in Crimson Seedless. However, in this variety, the DI + total seasonal irrigation was shown to be negatively related to the TSS level with the same strength as the HI Index and, conversely, to positively be linked to TA content, even if, in a weaker way. It means that, despite the water irrigation amount applied, the different climate conditions over the years did not completely cancel their consequences on TA contents. Indeed, grapes with higher acidic content were produced during the rainy years, with the opposite happening in case of drought years.
In the Crimson Seedless variety, the bunch growth, as well as the yield and the pH were found not linked to the DI + total seasonal irrigation index. This was likely due to the compensating effect exerted by irrigation water amounts and by the different ripening periods, the fall season, that occurred in the case of Crimson Seedless and which was generally characterized by more rainfall amounts than August. With regard to flavonoids, anthocyanins content in both varieties was negatively related to higher CI to emphasize that the concentration of these compounds, and consequently the grape color, was primarily influenced by the mean minimum night temperature during the ripening period. These results agreed with other published data where total anthocyanins were highest in artificial cooler berries of either wine grapes [22,44] or table grapes [45]. Notably, HI was differently related to the amount of these pigments in the two grapes.
Indeed, in the presence of a higher HI, they increase or decrease in Crimson Seedless and Summer Royal, respectively (Figure 2 and Figure 3). June, July, and August are determinant months for the HI accumulation, and with these months being already characterized by high daily average temperatures, it can be expected that even more heat could be definitely deleterious for anthocyanins.
Conversely to anthocyanins, flavonols and flavanols-3-ols resulted more concentrated in grapes from vintage with higher CI values as shown by FA and factor loadings plots (Figure 2 and Figure 3). The level of flavonols and flavan-3-ols in grapes was also connected to HI values (Figure 2 and Figure 3); in particular, they were positively or negatively correlated with HI in Summer Royal and Crimson seedless, respectively. These observations were in contrast with previous studies which reported that the temperature regimes after veraison had little effect on the proanthocyanins and flavonols concentration and composition [10,21]. The absence of seeds in the analyzed grapes could justify the discrepancy of our data with those reported in the literature; however, further research is in progress to confirm this speculation and generally to assess the impact of thermal indexes on the phenolic metabolism of seedless table grapes. Similar to anthocyanins, DI + total seasonal irrigation was found to be linked to these metabolites only in the case of Crimson Seedless but this time they were directly related (positive loadings on Factor 1, Figure 3), meaning that the dryer growing period also determined an increment of flavonols and flavan-3-ols concentration in grapes.
4. Conclusions
In this work, we reported some data on the effects of climate indexes HI, CI and DI + total seasonal irrigation calculated during the vegetative cycle, over field grown grape berries of two seedless varieties (Summer Royal and Crimson Seedless) for three consecutive years (2014–2016), with a focus on the flavonoids content and composition. The relationships between these indexes and the berry characteristics of table grapes represent one of the novelties of the present work, since the use of bioclimatic indexes in viticulture has been considered mainly in the characterization of the suitability of an area for viticulture. So far, little attention has been paid to table grapes, which also represent one of the most important agricultural sectors in the world. As expected, climatic conditions have shown a great relationship with the quantitative–qualitative characteristics of grapes, especially with the content of sugars, flavonoids, anthocyanins, flavan-3-ols and flavonols.
In particular, the Cool Night Index (CI) was related to TSS and TA, differently between the two cultivars; then, in contrast to anthocyanins, flavanols and flavanol-3-ols were more concentrated when CI values were higher.
Despite the mitigating effect of irrigation water, DI + irrigation was positively related to TA in both varieties.
In Summer Royal, DI + irrigation was negatively related to TSS and in Crimson Seedless, it was positively linked only to the concentration of flavonols and flavan-3-ols.
Finally, the most effective relationship via HI was found with anthocyanins, which were increased or decreased in the presence of higher HI in Crimson Seedless and Summer Royal, respectively.
The programmatic choices made by growers should consider the different variables that can influence the quantitative–qualitative aspects of production. To this end, the methodology used in this study provides useful information to elucidate the environmental influences on the development and composition of the berries, on the basis of objective parameters that, as such, characterize the climatic potential of a viticultural area and can influence the qualitative and quantitative aspects of production. However, further research is needed to confirm these results on other seeded and seedless grape varieties.
Author Contributions
Conceptualization, P.C., G.F. and A.C.; methodology, P.C., V.A. and G.G.; software, A.C., V.A. and G.G.; validation, P.C., G.F. and A.C.; formal analysis, P.C., V.A., M.G. and A.M.; investigation, P.C.; resources, A.C.; data curation: P.C., V.A., G.G. and A.C.; writing—original draft preparation, P.C., A.C. and V.A.; writing—review and editing, P.C., M.G. and A.M.; supervision, A.C., P.C. and G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Antonacci, D.; Savino, M.; Genghi, R.; Perniola, R.; Coletta, A. Girdling and gibberellic acid effects on yield and quality of a seedless red table grape for saving irrigation water supply. Europ. J. Agronomy 2016, 80, 21–31. [Google Scholar] [CrossRef]
- Roselli, L.; Casieri, A.; De Gennaro, B.C.; Sardaro, R.; Russo, G. Environmental and economic sustainability of table grape production in Italy. Sustainability 2020, 12, 3670. [Google Scholar] [CrossRef]
- OIV. Statistical Report on World Vitiviniculture. 2019. Available online: https://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 14 November 2023).
- ISTAT. 2020. Available online: http://dati.istat.it/Index.aspx?QueryId=33706 (accessed on 14 November 2023).
- Theodorou, N.; Nikolaou, N.; Zioziou, E.; Kyraleou, M.; Kallithraka, S.; Kotseridis, Y.; Koundouras, S. Anthocyanin content and composition in four red winegrape cultivars (Vitis vinifera L.) under variable irrigation: Anthocyanin content and composition under variable irrigation. Oeno One 2019, 53, e59956. [Google Scholar] [CrossRef]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Comp. Anal. 2006, 19, 396–404. [Google Scholar] [CrossRef]
- De Freitas, V.A.P.; Glories, Y.; Bourgeois, G.; Virty, C. Characterization of oligomeric and polymeric procyanidins from grape seeds by liquid secondary ion mass spectrometry. Phytochemistry 1998, 49, 1435–1441. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food. Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Cohen, S.D.; Tarara, J.M.; Kennedy, J.A. Assessing the impact of temperature on grape phenolic metabolism. Anal. Chim. Acta 2008, 621, 57–67. [Google Scholar] [CrossRef]
- Milella, R.A.; Antonacci, D.; Crupi, P.; Incampo, F.; Carrieri, C.; Semeraro, N.; Colucci, M. Skin extracts from 2 Italian table grapes (Italia and Palieri) inhibit tissue factor expression by human blood mononuclear cells. J. Food Sci. 2012, 77, H154–H159. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espìn, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food. Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Derlot, S.; Geros, H. Biochemical changes throughout grape berry development and fruit and wine quality. Food 2007, 1, 1–22. [Google Scholar]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Gaiotti, F.; Pastore, C.; Filippetti, I.; Lovat, L.; Belfiore, N.; Tomasi, D. Low night temperature at veraison enhances the ac-cumulation of anthocyanins in Corvina grapes (Vitis vinifera L.). Sci. Rep. 2018, 8, 8719. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, C.; Destrac-Irvine, A. Modified grape composition under climate change conditions requires adaptations in the vineyard. OENO One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Fujita, A.; Soma, N.; Goto-Yamamoto, N.; Mizuno, A.; Kiso, K.; Hashizume, K. Effect of shading on proanthocyanidin biosynthesis in the grape berry. J. Jpn. Hort. Sci. 2007, 76, 112–119. [Google Scholar] [CrossRef]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight exposure and temperature effects on berry growth and composition of Cabernet Sauvignon and Grenache in the central San Joaquin valley of California. Am. J. Enol. Vitic. 2001, 52, 1–7. [Google Scholar] [CrossRef]
- Iacono, F.; Bertamini, M.; Scienza, A.; Coombe, B.G. Differential effects of canopy manipulation and shading of Vitis vinifera L. cv Cabernet Sauvignon. Leaf gas exchange, photosynthetic electron transport rate and sugar accumulation in berries. Vitis 1995, 34, 201–206. [Google Scholar]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Goto-Yamamoto, N.; Mori, K.; Numata, M.; Koyama, K.; Kitayama, M. Effects of temperature and water regimes on flavonoid contents and composition in the skin of red-wine grapes. J. Int. Sci. Vigne Vin 2009, 43, 75–80. [Google Scholar]
- Jones, G.V.; Duff, A.A.; Hall, A.; Myers, J.W. Spatial analysis of climate in winegrape growing regions in the Western United States. Am. J. Enol. Vitic. 2010, 61, 313–326. [Google Scholar] [CrossRef]
- Huglin, P. Comptes Rendus de l’Académie d’Agriculture; Académie d’Agriculture de France: Paris, France, 1978; pp. 1117–1126. [Google Scholar]
- Amerine, M.A.; Winkler, A.J. Composition and quality of musts and wines of California grapes. Hilgardia 1944, 15, 493–675. [Google Scholar] [CrossRef]
- Gladstones, J. Viticulture and Environment; Winetitles: Adelaide, Australia, 1992. [Google Scholar]
- Tonietto, J.; Carbonneau, A. A multicriteria climatic classification system for grape-growing regions worldwide. Agric. For. Meteorol. 2004, 124, 81–97. [Google Scholar] [CrossRef]
- Badr, G.; Hoogenboom, G.; Abouali, M.; Moyer, M.; Keller, M. Analysis of several bioclimatic indices for viticultural zoning in the Pacific Northwest. Clim. Res. 2018, 76, 203–223. [Google Scholar] [CrossRef]
- Blanco-Ward, D.; Monteiro, A.; Lopes, M.; Borrego, C.; Silveira, C.; Viceto, C.; Rocha, A.; Ribeiro, A.J.; Feliciano, M.; Castro, J.; et al. Analysis of climate change indices in relation to wine production. A case study in the Douro region (Portugal). In Proceedings of the 40th World Vine and Wine Congress, BIO Web of Conferences 2017, Sofia, Bulgaria, 29 May–2 June 2017; Volume 9, p. 01011. [Google Scholar] [CrossRef]
- Alba, V.; Natrella, G.; Gambacorta, G.; Crupi, P.; Coletta, A. Effect of over crop and reduced yield by cluster thinning on phenolic and volatile compounds of grapes and wines of Sangiovese trained to Tendone. J. Sci. Food Agric. 2022, 102, 7155–7163. [Google Scholar] [CrossRef] [PubMed]
- Mattia, C.; Bischetti, G.B.; Gentile, F. Biotechnical characteristics of root systems of typical Mediterranean species. Plant Soil 2005, 278, 23–32. [Google Scholar] [CrossRef]
- Rana, G.; Nader, K.; Introna, M.; Hammami, A. Microclimate and plant water relationship of the overhead table grape vineyard managed with three different covering techniques. Sci. Horti. 2004, 102, 105–120. [Google Scholar] [CrossRef]
- Romero, P.; Fernández-Fernández, J.I.; Martinez-Cutillas, A. Physiological thresholds for efficient regulated deficit-irrigation management in winegrapes grown under semiarid conditions. Am. J. Enol. Vitic. 2010, 61, 300–312. [Google Scholar] [CrossRef]
- Rousseau, J.; Delteil, D. Présentation d’une méthode d’analyse sensorielles des raisins: Principe, méthodes et grille d’interprétation. Rev. Fr. Oenol. 2000, 183, 10–13. [Google Scholar]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Varietal differences among the polyphenols profiles of seven table grape cultivars studied by LC-DAD-MS-MS. J. Agric. Food Chem. 2002, 50, 5691–5696. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef]
- Crupi, P.; Coletta, A.; Milella, R.A.; Perniola, R.; Gasparro, M.; Genghi, R.; Antonacci, D. HPLC-DAD-ESI-MS analysis of fla-vonoid compounds in 5 seedless table grapes grown in Apulian region. J. Food Sci. 2012, 77, C174–C181. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.A.; Villanueva, M.J.; Lissarague, J.R. Effect of irrigation on changes in the anthocyanin composition of the skin of cv Tempranillo (Vitis vinifera L) grape berries during ripening. J. Sci. Food Agric. 2001, 81, 409–420. [Google Scholar] [CrossRef]
- Hidalgo, H.G.; Alfaro, E.J.; Quesada-Montano, B. Observed (1970–1999) climate variability in Central America using a high-resolution meteorological dataset with implication to climate change studies. Clim. Chang. 2017, 141, 13–28. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Carbonneau, A.; Ojeda, H.; Samson, A.; Pacos, J.; Jolivot, A.; Heywang, M. Methodological chain for analysis of quality: An overall example for vitiviniculture of studies of training of Syrah in dry areas at the Pech Rouge experimental unit. In Proceedings of the XIV International GESCO Viticulture Congress, Geisenheim, Germany, 23–27 August 2005; pp. 326–334. [Google Scholar]
- Ponti, L.; Gutierrez, A.P.; Boggia, A.; Neteler, M. Analysis of Grape Production in the Face of Climate Change. Climate 2018, 6, 20. [Google Scholar] [CrossRef]
- Tombesi, S.; Cincera, I.; Frioni, T.; Ughini, V.; Gatti, M.; Palliotti, A.; Poni, S. Relationship among night temperature, carbohydrate translocation and inhibition of grapevine leaf photosynthesis. Environ. Exp. Bot. 2019, 157, 293–298. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Sarah, G.; Ardisson, M.; Brillouet, J.M.; Romieu, C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 2016, 16, 164. [Google Scholar] [CrossRef] [PubMed]
- Spayd, S.E.; Tarara, J.M.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar] [CrossRef]
- Serrano-Megías, M.; Nùñez-Delicado, E.; Pérez-López, A.J.; López-Nicolás, J.M. Study of the effect of ripening stages and climatic conditions on the physicochemical and sensorial parameters of two varieties of Vitis vinifera L. by principal component analysis: Influence on enzymatic browning. J. Sci. Food Agric. 2006, 86, 592–599. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
