Enhancing Soil Fertility and Elevating Pecan Fruit Quality through Combined Chemical and Organic Fertilization Practices
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental
2.3. Laboratory Analysis
2.4. Data Analysis
3. Results
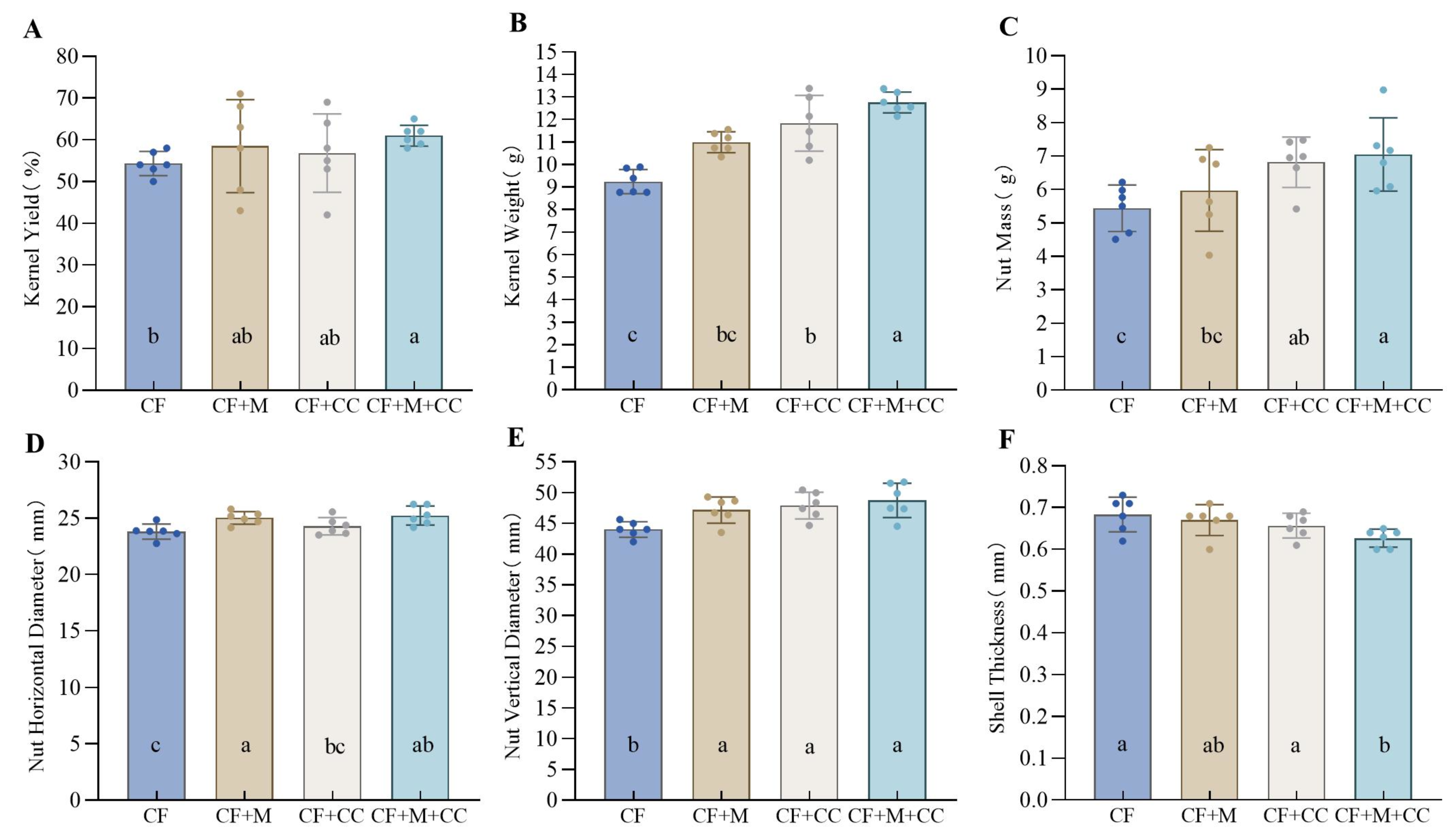
3.1. Subsection Effects of Different Fertilization Treatments on Pecan Yield
3.2. Effects of Different Fertilization Treatments on Fruit Traits of Pecan Nuts
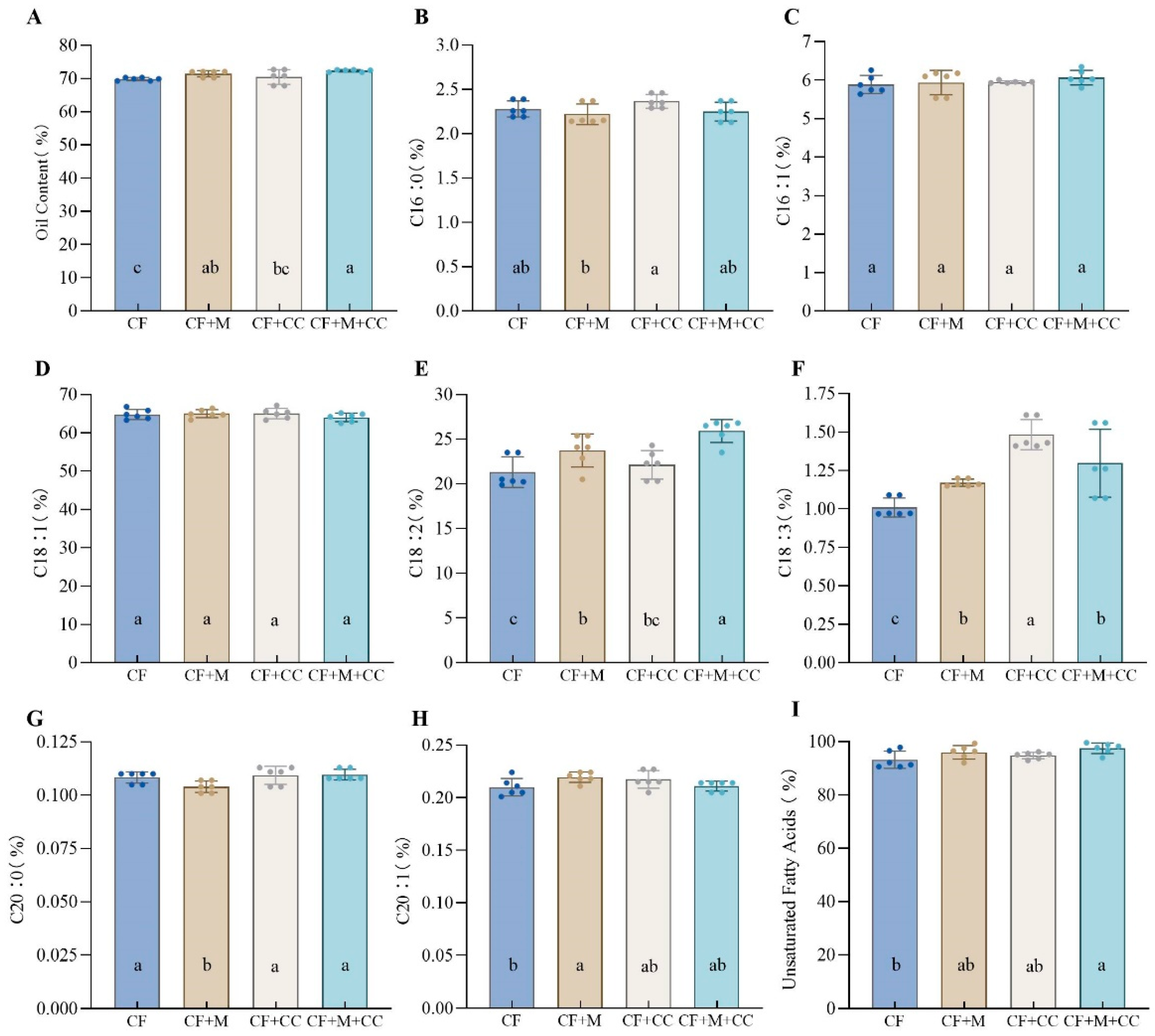
3.3. Effects of Different Fertilization Treatments on Nucleolar Nutritional Quality
3.4. Effects of Different Fertilization Treatments on Soil pH, EC, and SOM
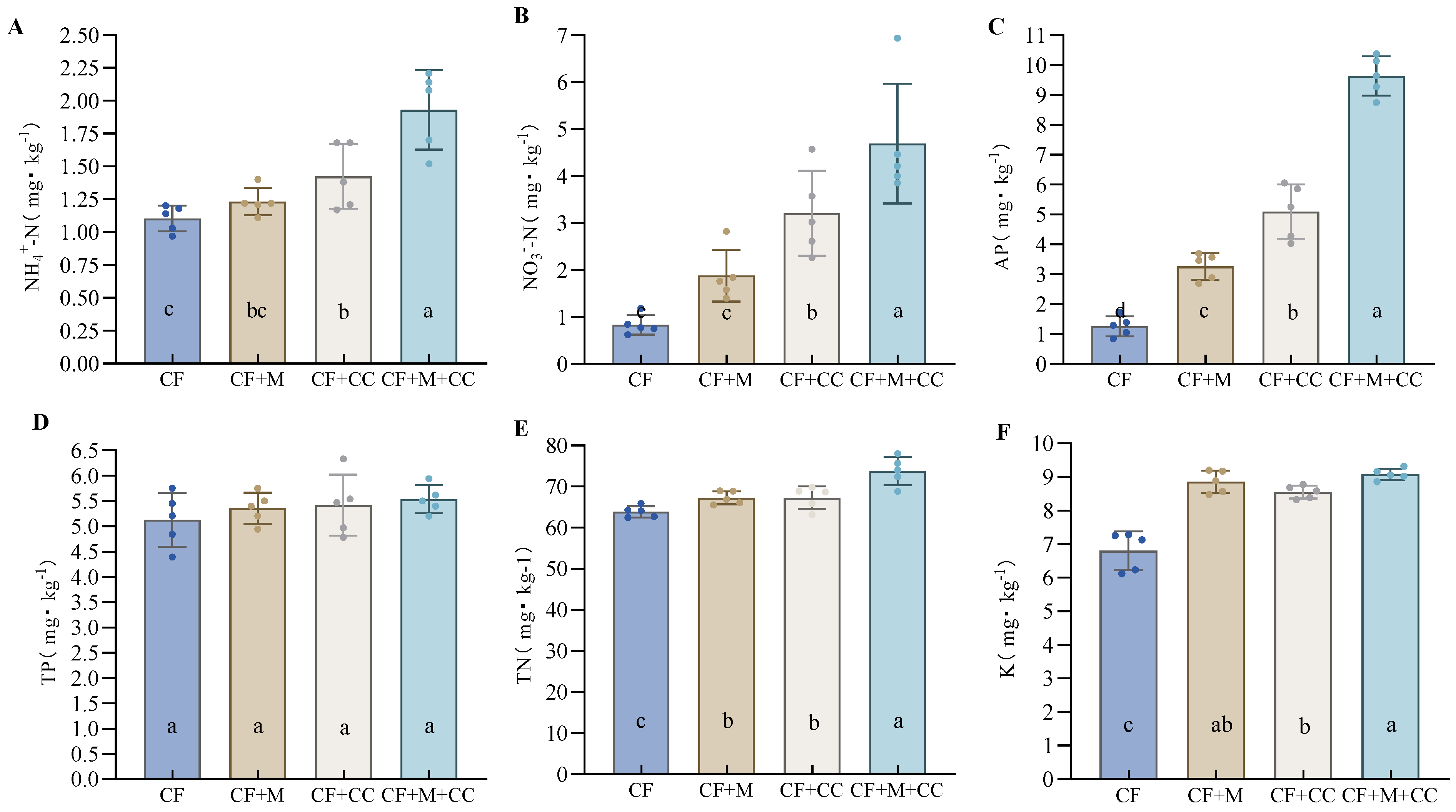
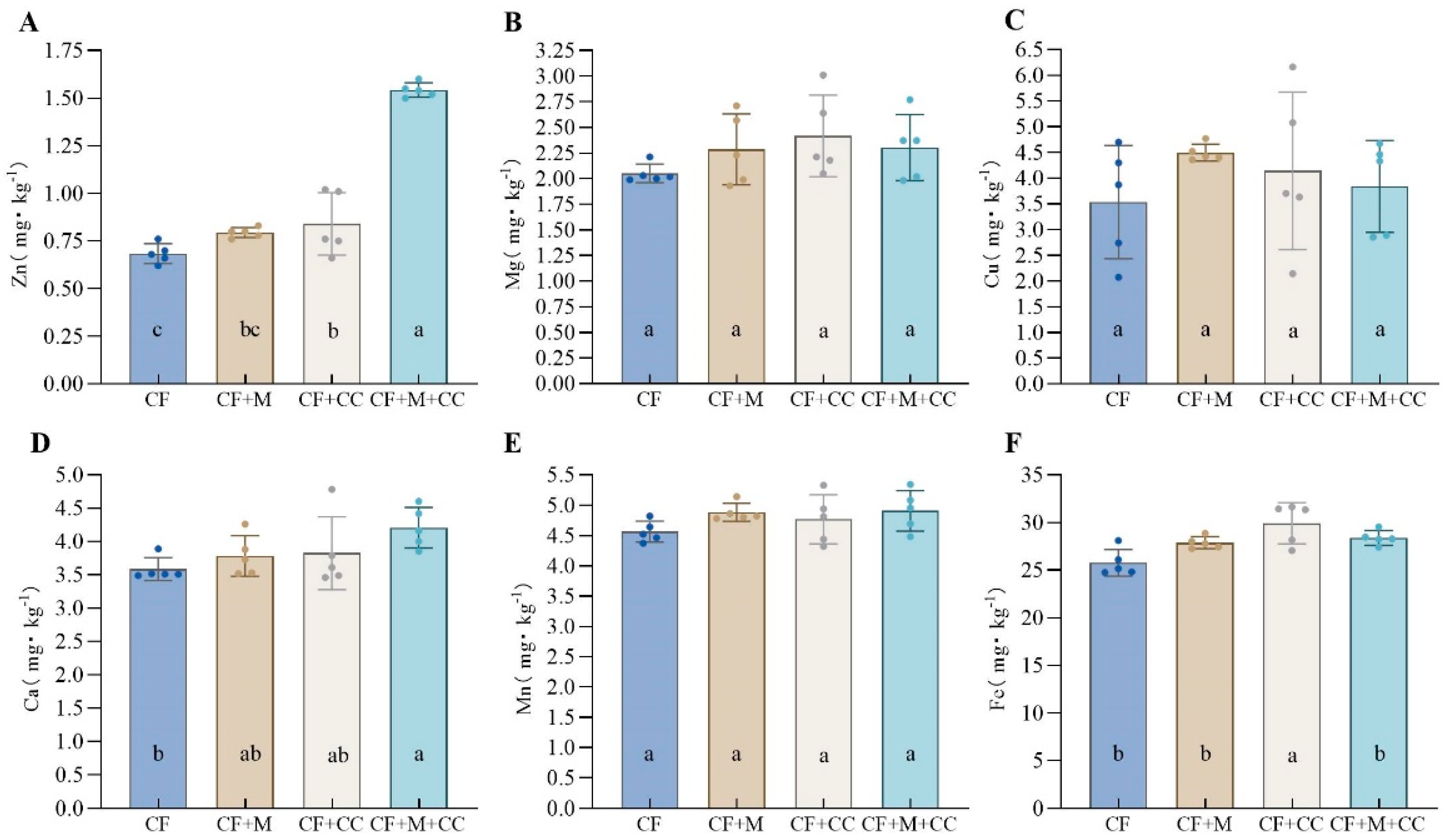
3.5. Effects of Different Fertilization Treatments on Soil Nutrients
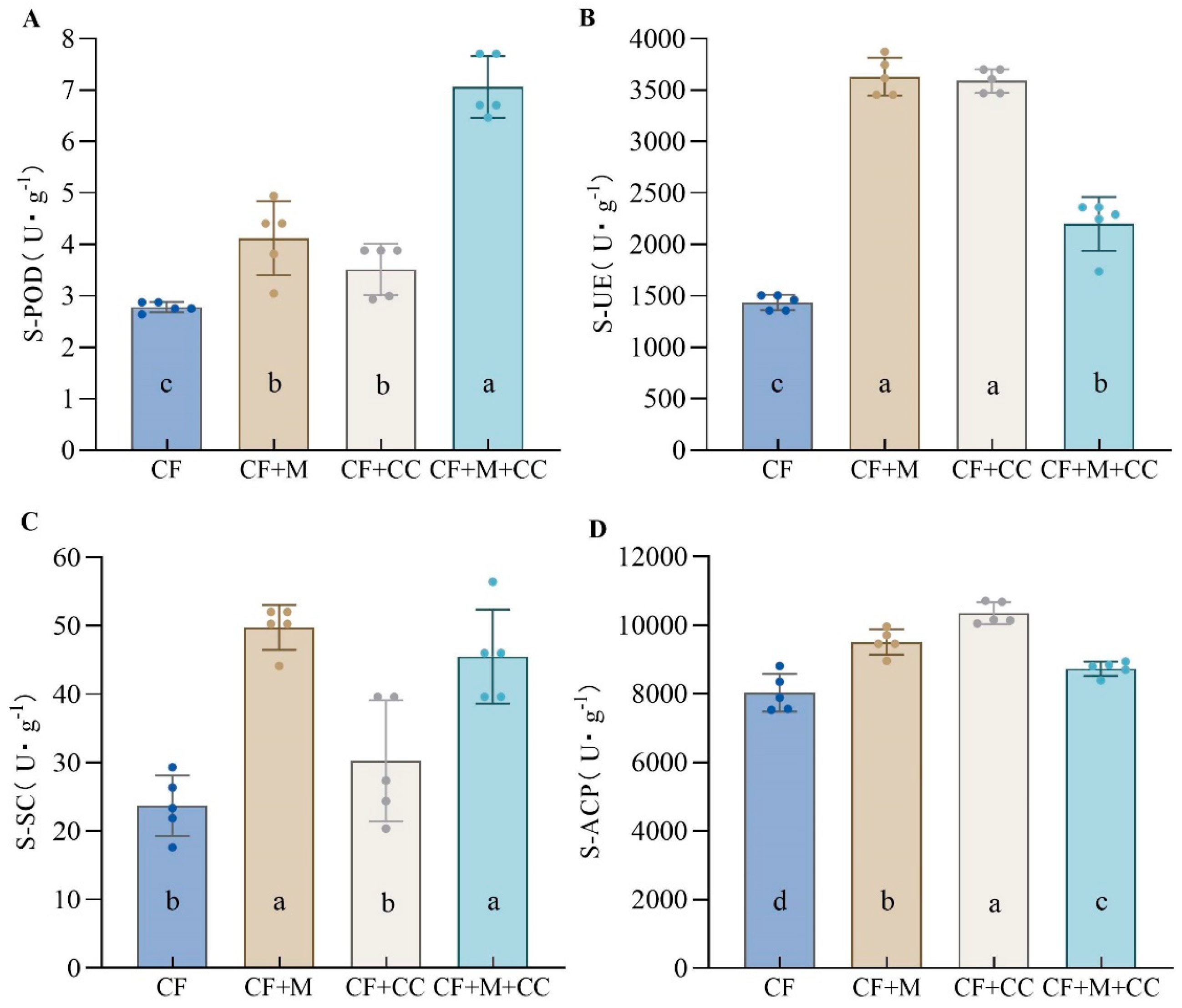
3.6. Effects of Different Fertilization Treatments on Soil Enzyme Activity
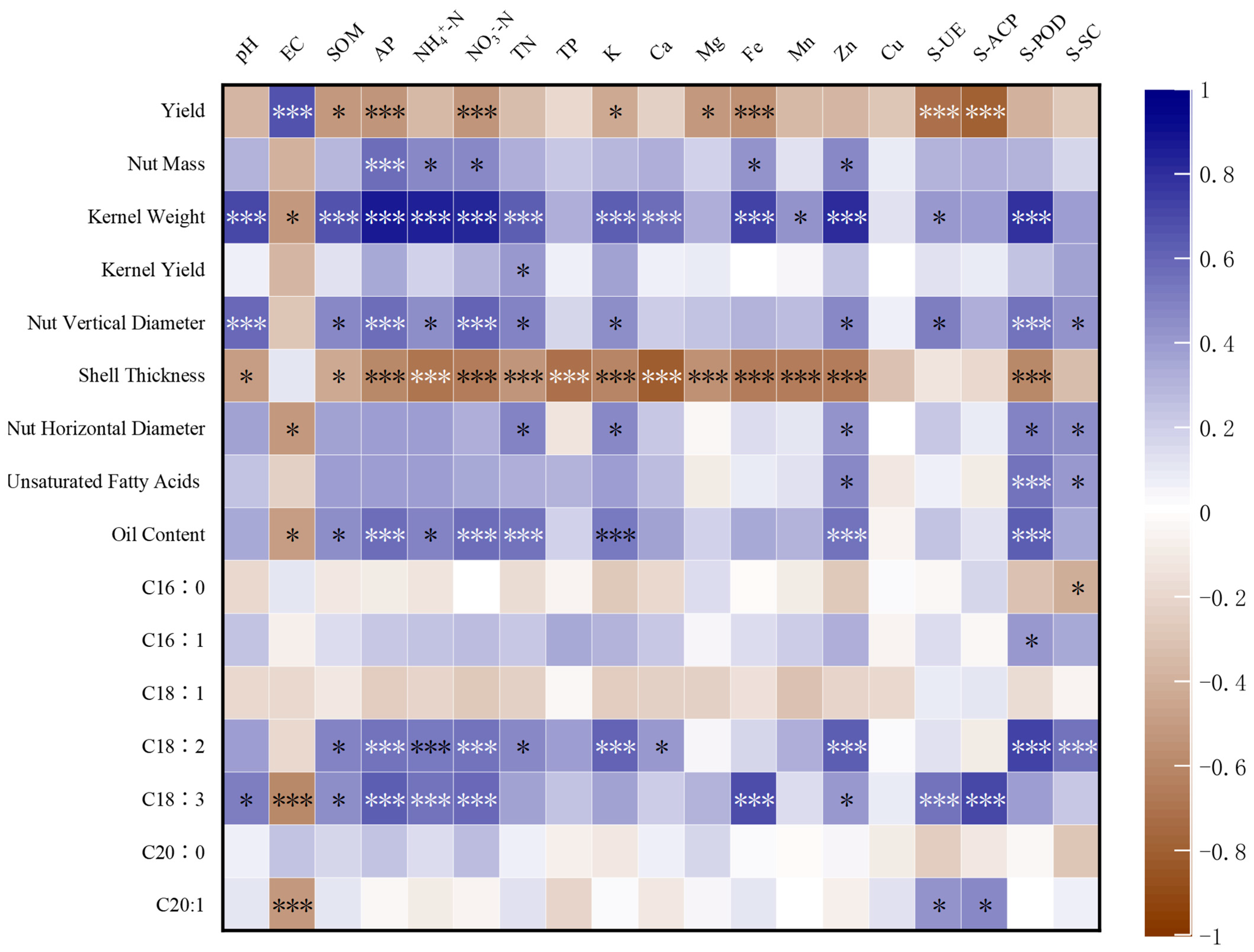
3.7. Correlation Analysis of Soil Nutrient and Enzyme Activities in Pecan
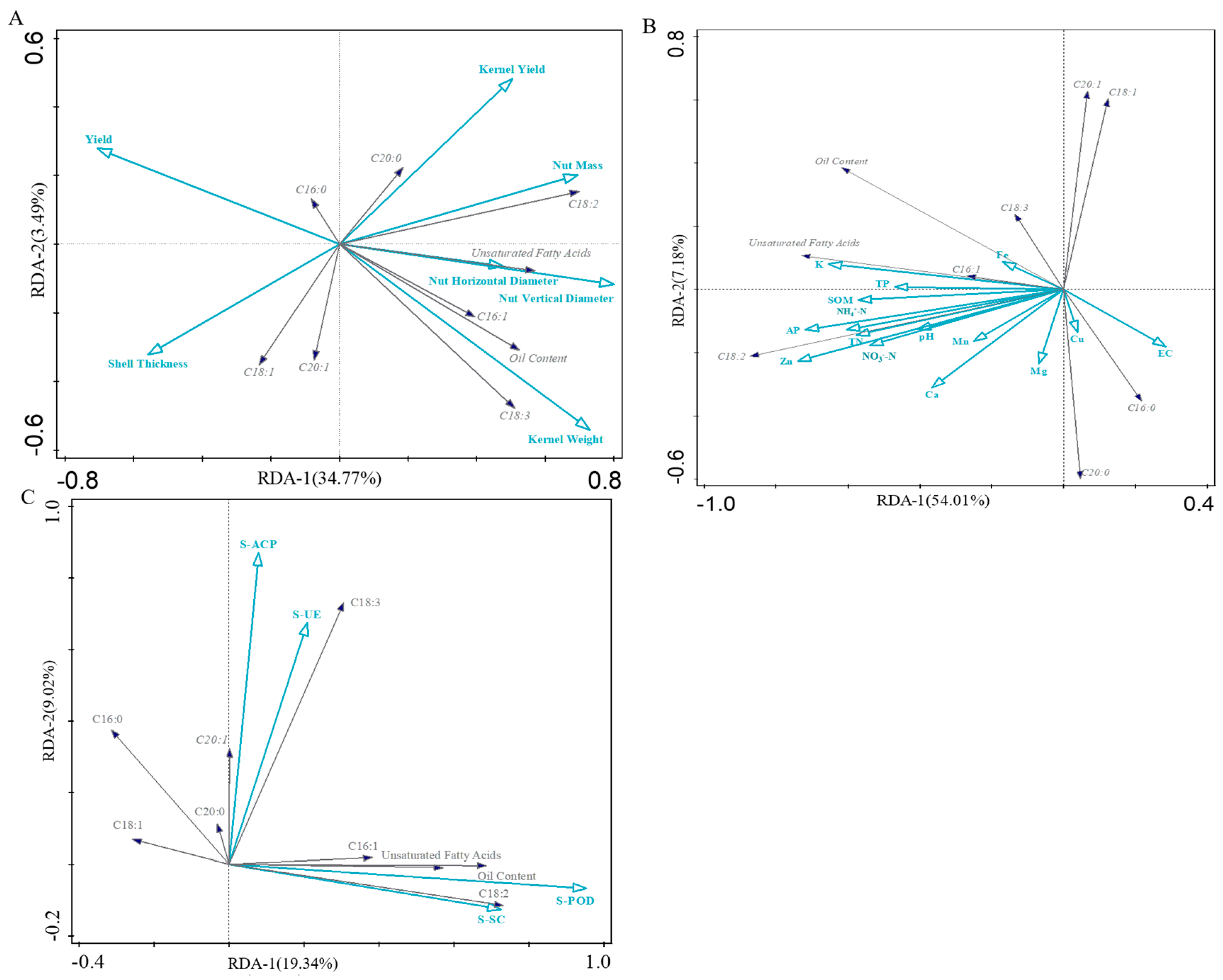
3.8. RDA Analysis of Soil and Quality of Pecan
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Zhang, D.; Wang, C.; Li, X.-L.; Yang, X.-S.; Zhao, L.-B.; Xia, S.-J. Correlation of production constraints with the yield gap of apple cropping systems in Luochuan County, China. J. Integr. Agric. 2019, 18, 1714–1725. [Google Scholar] [CrossRef]
- Conijn, J.G.; Bindraban, P.S.; Schröder, J.J.; Jongschaap, R.E.E. Can our global food system meet food demand within planetary boundaries? Agric. Ecosyst. Environ. 2018, 251, 244–256. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Wakelin, S.A.; Condron, L.M.; Gerard, E.; Dignam, B.E.A.; Black, A.; O’Callaghan, M. Long-term P fertilisation of pasture soil did not increase soil organic matter stocks but increased microbial biomass and activity. Biol. Fertil. Soils 2017, 53, 511–521. [Google Scholar] [CrossRef]
- Ge, S.; Zhu, Z.; Jiang, Y. Long-term impact of fertilization on soil pH and fertility in an apple production system. J. Soil Sci. Plant Nutr. 2018, 18, 282–293. [Google Scholar] [CrossRef]
- Wang, N.; Wolf, J.; Zhang, F.-S. Towards sustainable intensification of apple production in China—Yield gaps and nutrient use effi ciency in apple farming systems. J. Integr. Agric. 2016, 15, 716–725. [Google Scholar] [CrossRef]
- Gu, X.B.; Cai, H.J.; Du, Y.D.; Li, Y.N. Effects of film mulching and nitrogen fertilization on rhizosphere soil environment, root growth and nutrient uptake of winter oilseed rape in northwest China. Soil Tillage Res. 2019, 187, 194–203. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, N.; Wu, L.; Xu, M.; Bingham, I.J.; Li, Z. Effects of enhancing soil organic carbon sequestration in the topsoil by fertilization on crop productivity and stability: Evidence from long-term experiments with wheat-maize cropping systems in China. Sci. Total Environ. 2016, 562, 247–259. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Wang, H.; Li, F.; Pan, H.; Yang, Q.; Li, J.; Zhang, J. The Effects of Natural Humus Material Amendment on Soil Organic Matter and Integrated Fertility in the Black Soil of Northeast China: Preliminary Results. Agronomy 2023, 13, 794. [Google Scholar] [CrossRef]
- Mohamed, H.; Popov, A.I.; Mohamed, R. Integrated use of bio-organic fertilizers for enhancing soil fertility-plant nutrition, germination status and initial growth of corn (Zea mays L.). Environ. Technol. Innov. 2021, 21, 101329. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, W.; Yu, X.; Huang, Q. Effects of long-term fertilization on corn productivity and its sustainability in an Ultisol of southern China. Agric. Ecosyst. Environ. 2010, 138, 44–50. [Google Scholar] [CrossRef]
- Ananthi, T.; Vennila, C. Influence of Organic Manures and Inorganic Fertilizers on Growth and Yield of Fodder Maize (Zea mays L.) Grown in North Eastern Zone of Tamil Nadu. Curr. J. Appl. Sci. Technol. 2021, 40, 70–78. [Google Scholar] [CrossRef]
- Ye, G.; Lin, Y.; Liu, D.; Chen, Z.; Luo, J.; Bolan, N.; Fan, J.; Ding, W. Long-term application of manure over plant residues mitigates acidification, builds soil organic carbon and shifts prokaryotic diversity in acidic Ultisols. Appl. Soil Ecol. 2018, 133, 24–33. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, J.; Shi, A.; Yao, S.; Zhang, B. Manure substitution of mineral fertilizers increased functional stability through changing structure and physiology of microbial communities. Eur. J. Soil Biol. 2016, 77, 34–43. [Google Scholar] [CrossRef]
- Zou, C.; Li, Y.; Huang, W.; Zhao, G.; Pu, G.; Su, J.; Coyne, M.S.; Chen, Y.; Wang, L.; Hu, X.; et al. Rotation and manure amendment increase soil macro-aggregates and associated carbon and nitrogen stocks in flue-cured tobacco production. Geoderma 2018, 325, 49–58. [Google Scholar] [CrossRef]
- Shiwakoti, L.D.; Chalise, K.; Dahal, P.; Shiwakoti, R.; Katuwal, N.; Kc, Y. Effect of fertilizer application on tea plant productivity and phytochemicals in prepared black tea. Cogent Food Agric. 2023, 9, 2184013. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef]
- Wang, Y.; Heerema, R.J.; Walworth, J.L.; Dungan, B.; VanLeeuwen, D.; Holguin, F.O. Nutraceutical Properties of Pecan Kernels Are Affected by Soil Zinc Fertilizer Application. Hortscience 2020, 55, 2001–2007. [Google Scholar] [CrossRef]
- Zhao, Z.-B.; He, J.-Z.; Quan, Z.; Wu, C.-F.; Sheng, R.; Zhang, L.-M.; Geisen, S. Fertilization changes soil microbiome functioning, especially phagotrophic protists. Soil Biol. Biochem. 2020, 148, 107863. [Google Scholar] [CrossRef]
- Dinca, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Gao, M.; Yang, J.; Liu, C.; Gu, B.; Han, M.; Li, J.; Li, N.; Liu, N.; An, N.; Dai, J.; et al. Effects of long-term biochar and biochar-based fertilizer application on brown earth soil bacterial communities. Agric. Ecosyst. Environ. 2021, 309, 107285. [Google Scholar] [CrossRef]
- Wardle, D.A. A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol. Rev. 1992, 67, 321–358. [Google Scholar] [CrossRef]
- Lori, M.; Hartmann, M.; Kundel, D.; Mayer, J.; Mueller, R.C.; Mäder, P.; Krause, H.M. Soil microbial communities are sensitive to differences in fertilization intensity in organic and conventional farming systems. FEMS Microbiol. Ecol. 2023, 99, fiad046. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bauddh, K.; Barman, S.C.; Singh, R.P. Amendments of microbial biofertilizers and organic substances reduces requirement of urea and DAP with enhanced nutrient availability and productivity of wheat (Triticum aestivum L.). Ecol. Eng. 2014, 71, 432–437. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Scharfy, D.; Güsewell, S.; Gessner, M.O.; Venterink, H.O. Invasion of Solidago gigantea in contrasting experimental plant communities: Effects on soil microbes, nutrients and plant-soil feedbacks. J. Ecol. 2010, 98, 1379–1388. [Google Scholar] [CrossRef]
- Jalilian, J.; Modarres-Sanavy, S.A.M.; Saberali, S.F.; Sadat-Asilan, K. Effects of the combination of beneficial microbes and nitrogen on sunflower seed yields and seed quality traits under different irrigation regimes. Field Crops Res. 2012, 127, 26–34. [Google Scholar] [CrossRef]
- Noperi-Mosqueda, L.C.; Soto-Parra, J.M.; Sánchez, E.; Piña-Ramírez, F.J.; Pérez-Leal, R.; Flores-Córdova, M.A.; Salas-Salazar, N.A. Impact of Organic and Mineral Fertilization in Pecan Nut on Production, Quality and Antioxidant Capacity. Agric. Sci. 2019, 10, 227–240. [Google Scholar] [CrossRef][Green Version]
- Atanasov, A.G.; Sabharanjak, S.M.; Zengin, G.; Mollica, A.; Szostak, A.; Simirgiotis, M.; Huminiecki, L.; Horbanczuk, O.K.; Nabavi, S.M.; Mocan, A. Pecan nuts: A review of reported bioactivities and health effects. Trends Food Sci. Technol. 2018, 71, 246–257. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Alvarez-Parrilla, E.; López-Díaz, J.A.; Maldonado-Mendoza, I.E.; Gómez-García, M.d.C.; de la Rosa, L.A. The pecan nut (Carya illinoinensis) and its oil and polyphenolic fractions differentially modulate lipid metabolism and the antioxidant enzyme activities in rats fed high-fat diets. Food Chem. 2015, 168, 529–537. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Vazquez-Flores, A.A.; Alvarez-Parrilla, E.; Rodrigo-García, J.; Medina-Campos, O.N.; Ávila-Nava, A.; González-Reyes, S.; Pedraza-Chaverri, J. Content of major classes of polyphenolic compounds, antioxidant, antiproliferative, and cell protective activity of pecan crude extracts and their fractions. J. Funct. Foods 2014, 7, 219–228. [Google Scholar] [CrossRef]
- Reisner, Y.; de Filippi, R.; Herzog, F.; Palma, J. Target regions for silvoarable agroforestry in Europe. Ecol. Eng. 2006, 29, 401–418. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N. Soil fertility: Plant nutrition vis-à-vis fruit yield and quality of stone fruits. Fruit Crops 2020, 45, 583–606. [Google Scholar] [CrossRef]
- Zhu, M.; Long, Y.; Chen, Y.; Huang, Y.; Tang, L.; Gan, B.; Yu, Q.; Xie, J. Fast determination of lipid and protein content in green coffee beans from different origins using NIR spectroscopy and chemometrics. J. Food Compos. Anal. 2021, 102, 104055. [Google Scholar] [CrossRef]
- El Riachy, M.; Hamade, A.; Ayoub, R.; Dandachi, F.; Chalak, L. Oil Content, Fatty Acid and Phenolic Profiles of Some Olive Varieties Growing in Lebanon. Front. Nutr. 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feng, C.; Ma, Y.; Wang, W.; Huang, C.; Qi, C.; Fu, S.; Chen, H.Y. Transition from N to P limited soil nutrients over time since restoration in degraded subtropical broadleaved mixed forests. For. Ecol. Manag. 2021, 494, 119298. [Google Scholar] [CrossRef]
- Soltanpour, P.N.; Schwab, A.P. A new soil test for simultaneous extraction of macro- and micro-nutrients in alkaline soils. Commun. Soil Sci. Plant Anal. 1977, 8, 195–207. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, L.; Wang, J.; Wang, J.; Su, B.; Liu, T.; Zhang, C.; Gao, C.; Shao, Y. Toxic effects of ionic liquid 1-octyl-3-methylimidazolium tetrafluoroborate on soil enzyme activity and soil microbial community diversity. Ecotoxicol. Environ. Saf. 2017, 135, 201–208. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Taylor, J.P.; Wilson, B.; Mills, M.S.; Burns, R.G. Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol. Biochem. 2002, 34, 381–401. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.Q.; Sun, J.W.; Wang, X.B.; Zhou, W. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 2012, 173, 330–338. [Google Scholar] [CrossRef]
- Somenahally, A.C.; McLawrence, J.; Chaganti, V.N.; Ganjegunte, G.K.; Obayomi, O.; Brady, J.A. Response of soil microbial Communities, inorganic and organic soil carbon pools in arid saline soils to alternative land use practices. Ecol. Indic. 2023, 150, 110227. [Google Scholar] [CrossRef]
- Kumar, T.; Swaminathan, V.; Kumar, S.N. Influence of nitrogen, phosphorus and biofertilizers on growth, yield and essential oil constituents in ratoon crop of davana (Artemisia pallens Wall.). Electron. J. Environ. Agric. Food Chem. 2009, 8, 86–95. [Google Scholar]
- Chen, L.; Wang, X.; Zhou, W.; Guo, S.; Zhu, R.; Qin, Y.; Sun, J. Responses of crop yields, soil enzymatic activities, and microbial communities to different long-term organic materials applied with chemical fertilizer in purple soil. Eur. J. Soil Biol. 2021, 105, 103319. [Google Scholar] [CrossRef]
- Moeskops, B.; Sukristiyonubowo; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; Neve, S.D. Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Luo, X.; Wang, Z.; Xing, B. Biochar-induced negative carbon mineralization priming effects in a coastal wetland soil: Roles of soil aggregation and microbial modulation. Sci. Total Environ. 2018, 610–611, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Deljooy-e-Tohidi, T.; Torkashv, A.M.; Hashemabadi, D. The possibility using some organic wastes as growth medium and nutrition method on the growth of English daisy (Bellis perennis). Eur. J. Exp. Biol. 2013, 3, 139–147. [Google Scholar]
- Pardon, P.; Mertens, J.; Reubens, B.; Reheul, D.; Coussement, T.; Elsen, A.; Nelissen, V.; Verheyen, K. Juglans regia (walnut) in temperate arable agroforestry systems: Effects on soil characteristics, arthropod diversity and crop yield. Renew. Agric. Food Syst. 2019, 35, 1–17. [Google Scholar] [CrossRef]
- Verardo, V.; Riciputi, Y.; Sorrenti, G.; Ornaghi, P.; Marangoni, B.; Caboni, M.F. Effect of nitrogen fertilisation rates on the content of fatty acids, sterols, tocopherols and phenolic compounds, and on the oxidative stability of walnuts. LWT Food Sci. Technol. 2013, 50, 732–738. [Google Scholar] [CrossRef]
- Du, Y.; Cui, B.; Zhang, Q.; Wang, Z.; Sun, J.; Niu, W. Effects of manure fertilizer on crop yield and soil properties in China: A meta-analysis. Catena 2020, 193, 104617. [Google Scholar] [CrossRef]
- Krzyszczak, J.; Brodowska, M.; Bednarek, W.; Dresler, S.; Tkaczyk, P.; Krzyszczak, J.; Baranowski, P. Content of certain macro- and microelements in orchard soils in relation to agronomic categories and reaction of these soils. J. Elem. 2018, 23, 1361–1372. [Google Scholar] [CrossRef]
- Wang, G.-Y.; Zhang, X.-Z.; Wang, Y.; Xu, X.-F.; Han, Z.-H. Key minerals influencing apple quality in Chinese orchard identified by nutritional diagnosis of leaf and soil analysis. J. Integr. Agric. 2015, 14, 864–874. [Google Scholar] [CrossRef]
- Cai, A.D.; Xu, M.G.; Wang, B.R.; Zhang, W.J.; Liang, G.P.; Hou, E.Q.; Luo, Y.Q. Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Tillage Res. 2019, 189, 168–175. [Google Scholar] [CrossRef]
- Choudhary, M.; Panday, S.C.; Meena, V.S.; Singh, S.; Yadav, R.P.; Mahanta, D.; Mondal, T.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A. Long-term effects of organic manure and inorganic fertilization on sustainability and chemical soil quality indicators of soybean-wheat cropping system in the Indian mid-Himalayas. Agric. Ecosyst. Environ. 2018, 257, 38–46. [Google Scholar] [CrossRef]
- Jones, D.L.; Darrah, P.R. Re-sorption of organic compounds by roots of Zea mays L. and its consequences in the rhizosphere: III. Characteristics of sugar influx and efflux. Plant Soil 1996, 178, 153–160. [Google Scholar] [CrossRef]
- Ni, H.; Su, W.; Fan, S.; Chu, H. Effects of intensive management practices on rhizosphere soil properties, root growth, and nutrient uptake in Moso bamboo plantations in subtropical China. For. Ecol. Manag. 2021, 493, 119083. [Google Scholar] [CrossRef]
- Sun, B.; Gao, Y.; Wu, X.; Ma, H.; Zheng, C.; Wang, X.; Zhang, H.; Li, Z.; Yang, H. The relative contributions of pH, organic anions, and phosphatase to rhizosphere soil phosphorus mobilization and crop phosphorus uptake in maize/alfalfa polyculture. Plant Soi 2020, 447, 117–133. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X. Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, e0211163. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Feng, X.; Ling, N.; Chen, H.; Zhu, C.; Duan, Y.; Peng, C.; Yu, G.; Ran, W.; Shen, Q.; Guo, S. Soil ionomic and enzymatic responses and correlations to fertilizations amended with and without organic fertilizer in long-term experiments. Sci. Rep. 2016, 6, 24559. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.-C.; Gao, P.-D.; Wang, B.-Q.; Lin, W.-P.; Jiang, N.-H.; Cai, K.-Z. Impacts of chemical fertilizer reduction and organic amendments supplementation on soil nutrient, enzyme activity and heavy metal content. J. Integr. Agric. 2017, 16, 1819–1831. [Google Scholar] [CrossRef]
- Cheng, J.; Jin, H.; Zhang, J.; Xu, Z.; Yang, X.; Liu, H.; Xu, X.; Min, D.; Lu, D.; Qin, B. Effects of Allelochemicals, Soil Enzyme Activities, and Environmental Factors on Rhizosphere Soil Microbial Community of Stellera chamaejasme L. along a Growth-Coverage Gradient. Microorganisms 2022, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Gu, C.; Shen, C.; Xu, L.; Zhao, Y.; Yi, S.; Zuo, W.; Shan, Y.; Zhang, Z.; et al. Differential Effects of Organic Ameliorants on the Reassembly of Bacterial Communities in Newly Amended Coastal Mudflat Salt-Affected Soil. Agronomy 2022, 12, 2525. [Google Scholar] [CrossRef]
- Parlak, K.U.; Yilmaz, D.D. Response of antioxidant defences to Zn stress in three duckweed species. Ecotoxicol. Environ. Saf. 2012, 85, 52–58. [Google Scholar] [CrossRef]
- Duff, S.M.G.; Sarath, G.; Plaxton, W.C. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant. 1994, 90, 791–800. [Google Scholar] [CrossRef]
- Sannino, F.; Gianfreda, L. Pesticide influence on soil enzymatic activities. Chemosphere 2001, 45, 417–425. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
| Treatment | Chemical Fertilizer (kg·hm−2) | Cake Fertilizer (kg·hm−2) | Manure Fertilizer (kg·hm−2) | ||
|---|---|---|---|---|---|
| N | P2O5 | K2O | |||
| CF | 150 | 150 | 150 | 0 | 0 |
| CF+CC | 75 | 75 | 75 | 720 | 0 |
| CF+M | 75 | 75 | 75 | 0 | 1350 |
| CF+M+CC | 75 | 75 | 75 | 360 | 675 |
| Treatment | 2018 (kg·hm−2) | 2020 (kg·hm−2) | 2022 (kg·hm−2) | 2022 Comparison with CF | |
|---|---|---|---|---|---|
| Production Increase (kg·hm−2) | Yield Increase Rate (%) | ||||
| CF | 756.51 ± 4.82 a | 839.96 ± 4.78 a | 1083.36 ± 10.42 d | - | - |
| CF+M | 597.14 ± 3.99 bc | 768.67 ± 3.19 c | 1195.48 ± 4.63 b | 112 | 10.34 |
| CF+CC | 587.07 ± 2.58 c | 782.14 ± 5.58 b | 1170.8 ± 4.39 c | 87 | 8.03 |
| CF+M+CC | 606.1 ± 6.03 b | 792.53 ± 2.93 b | 1224.97 ± 5.91 a | 141 | 13.02 |
| Treatment | pH | EC (ds·m−1) | SOM (g·kg−1) |
|---|---|---|---|
| CF | 6.93 ± 0.08 b | 1.35 ± 0.02 a | 12.67 ± 1.42 b |
| CF+M | 8.23 ± 0.06 a | 1.10 ± 0.03 b | 21.09 ± 3.68 a |
| CF+CC | 8.35 ± 0.06 a | 1.14 ± 0.03 b | 21.24 ± 1.25 a |
| CF+M+CC | 8.34 ± 0.10 a | 1.16 ± 0.03 b | 28.33 ± 2.52 a |
| Name | Explains % | Contribution % | Pseudo-F | p |
|---|---|---|---|---|
| Zn | 30.3 | 46.9 | 9.6 | 0.002 |
| S-POD | 18.1 | 52.5 | 4.9 | 0.002 |
| Nut Vertical Diameter | 22.3 | 55.8 | 6.3 | 0.006 |
| Nut Mass | 8.9 | 21.9 | 2.7 | 0.064 |
| S-UE | 7.8 | 22.5 | 2.2 | 0.07 |
| NO3−-N | 7.3 | 11.2 | 2.5 | 0.088 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Y.; Wang, Z.; Gong, D.; Huang, C.; Ma, X.; Ma, X.; Yuan, F.; Fu, S.; Feng, C. Enhancing Soil Fertility and Elevating Pecan Fruit Quality through Combined Chemical and Organic Fertilization Practices. Horticulturae 2024, 10, 25. https://doi.org/10.3390/horticulturae10010025
Tong Y, Wang Z, Gong D, Huang C, Ma X, Ma X, Yuan F, Fu S, Feng C. Enhancing Soil Fertility and Elevating Pecan Fruit Quality through Combined Chemical and Organic Fertilization Practices. Horticulturae. 2024; 10(1):25. https://doi.org/10.3390/horticulturae10010025
Chicago/Turabian StyleTong, Yinhao, Zhaocheng Wang, Duxin Gong, Cheng Huang, Xiaomin Ma, Xiaoxiang Ma, Feiyang Yuan, Songling Fu, and Chun Feng. 2024. "Enhancing Soil Fertility and Elevating Pecan Fruit Quality through Combined Chemical and Organic Fertilization Practices" Horticulturae 10, no. 1: 25. https://doi.org/10.3390/horticulturae10010025
APA StyleTong, Y., Wang, Z., Gong, D., Huang, C., Ma, X., Ma, X., Yuan, F., Fu, S., & Feng, C. (2024). Enhancing Soil Fertility and Elevating Pecan Fruit Quality through Combined Chemical and Organic Fertilization Practices. Horticulturae, 10(1), 25. https://doi.org/10.3390/horticulturae10010025









