Abstract
The effects of home cooking methods (e.g., boiling, steaming, oven, and microwave) on the contents phenolic compounds, biogenic amines, and precursor amino acids in colored-fleshed sweet potatoes were investigated in this study. Sixteen genotypes of colored sweet potatoes (cream/white, yellow, orange, and purple pulp) were analyzed using spectrophotometry for their total phenolic compounds, total flavonoids, antioxidant capacity (DPPH and MDA) and pigments. Of these, five genotypes with orange and purple pulps were investigated using HPLC-DAD for their polyphenols, biogenic amines and precursor amino acids. The results revealed that orange-fleshed sweet potatoes contain higher amounts of phenolic compounds, carotenoids, amino acids, and beneficial amines, especially when cooked in a microwave or in an oven, wrapped in aluminum foil. For the purple sweet potatoes, superior quantities of bioactive were found after cooking with steam, microwave, and in the oven with aluminum foil protection. In general, the colored genotypes showed a superior phytochemical profile than the traditionally commercialized ones before and after heat treatments, characterizing them as richer sources of the bioactive compounds of interest for producers, consumers, and industry.
1. Introduction
Sweet potato (Ipomoea batatas Lam.) is a dicotyledon of the Convolvulaceae family. Due to its ability to adapt to a wide range of climatic conditions, it has been cultivated in tropical, subtropical, and temperate regions [1]. In developing countries, colored flesh and skin genotypes are used in biofortification programs, with a particular emphasis on orange sweet potatoes [2]. In Brazil, cream/white-pulp sweet potato genotypes are traditionally cultivated and marketed in all producing regions [3]. However, with the increasing interest in a diversified and healthy diet in the population, colored genotypes of sweet potato have been more extensively investigated due to their claimed nutritional benefits. In this sense, orange-fleshed sweet potatoes, in addition to demonstrating high nutritional value, are also sources of β-carotene, a precursor of vitamin A in the body [2]. Purple-fleshed sweet potatoes also contain interesting bioactive compounds in their composition, especially phenolic acids and anthocyanins, which, among their several properties, improve cardiovascular health, cognitive function, and eliminate free radicals, preventing the onset of cancer [3].
Phenolic compounds, such as phenolic acids and flavonoids, constitute the antioxidant matrices of colored sweet potatoes and may exhibit different concentrations after cooking. Sweet potatoes are an excellent source of these compounds, and some of the most common phenolic acids found in these tubers are chlorogenic acid and ferulic acid [4,5]. Chlorogenic acid demonstrates several beneficial properties for health, including antioxidant properties, anti-inflammatory effects, potential blood sugar regulation, and a neuroprotective effect [4,6]. Similarly, ferulic acid possesses a high antioxidant capacity and contributes to health. In purple-fleshed sweet potatoes, these compounds also play a crucial role in stabilizing the color of anthocyanins [5,7]. In addition to phenolic compounds, biogenic amines and amino acids are noteworthy molecules in the antioxidant composition of sweet potatoes, as they play important metabolic and physiological roles in human health [8].
Biogenic amines and their precursor amino acids are prevalent compounds in both animal and plant foods, with notable antioxidant capabilities. These compounds occur in both raw and processed foods [3,9]. The content of amines in foods is not organoleptically identifiable, making it important to understand their content. Some biogenic amines serve as valuable indicators of food safety, such as histamine and tyramine, particularly in food items susceptible to anti-nutritional factors, as exemplified by sweet potatoes. These amines can be associated with health hazards [10]. However, amines such as serotonin and melatonin, and polyamines such as spermidine and spermine, act as antioxidants, protecting membranes, and are related to various metabolic processes, such as hormone synthesis and DNA replication [11]. Additionally, they are associated with the maintenance of blood pressure [12], and some of them have been proposed as markers of the progression of dementia (Alzheimer’s) [13]. Therefore, knowledge of the amine content in foods is fundamental, especially if the type of processing influences consumer health. The calculation of the biogenic amine index in foods, structured to indicate the balance between health-promoting biogenic amines and harmful biogenic amines [3], is noteworthy in ensuring food safety, helping to prevent risks to public health, and maintaining the quality of the products available for consumption.
Sweet potatoes, when raw, contain high levels of antinutritional compounds (e.g., oxalates, tannins, phytates, saponins, alkaloids, and hydrogen cyanide), although these levels may vary due to genetic and environmental factors. These compounds can hinder the bioavailability and bioaccessibility of the nutrients consumed [14]. Therefore, cooking the roots is essential to eliminate the unwanted effects of those compounds, which can be achieved through traditional cooking methods such as boiling, steaming, roasting, and frying, or by modern appliances such as microwaves and air fryers [15]. Additionally, the choice of cooking method may influence the extraction of the compounds of interest (e.g., nutrients, minerals, and phytochemicals) from the plant cell matrix, leading to either a better extraction or losses due to leaching [15].
This study unveils not only the content of bioactive compounds and the antioxidant activity of colored sweet potato genotypes in their raw and cooked forms, but also the effect of thermal processes on their polyphenol (e.g., phenolic acids, flavonoids, and anthocyanins), amino acid, and biogenic amine profiles.
2. Materials and Methods
2.1. Plant Material
The purple-fleshed sweet potato genotypes ‘JNRX1’, ‘JNRX2’, ‘JNRX7’, ‘JNRX12’, and ‘Trabuca’ (Figure 1A) and the orange-fleshed ‘LLR15’ were obtained from producers in Presidente Prudente (São Paulo state, southeastern Brazil—latitude: 22°7′39″ S, longitude: 51°23′8″ W, altitude: 475 m, humid tropical climate). The orange-fleshed sweet potato genotypes ‘2913’, ‘3418’, ‘3513’, ‘5202’, ‘2603’, ‘5623’, ‘Beaudegard’, and the yellow-fleshed genotype ‘1603’ (Figure 1B), were cultivated in Ilha Solteira (São Paulo state, southeastern Brazil—latitude: 20°25′52″ S, longitude: 51°20′17″ W, altitude: 366 m, humid tropical climate). The ‘Canadense’ and ‘Coquinho’ white-fleshed sweet potatoes (Figure 1C) were purchased at a local market.
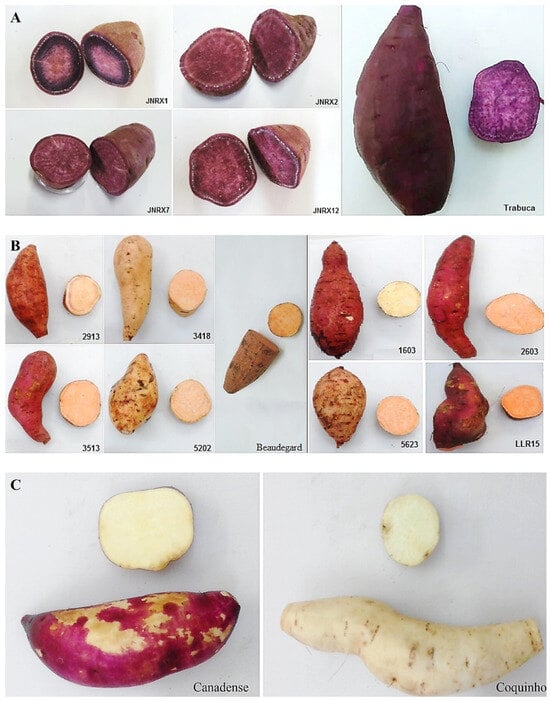
Figure 1.
Details of the peel and pulp colors of some of the sweet potato genotypes used in this study: (A) purple-flesh and -skin genotypes; (B) orange- and yellow-fleshed genotypes; (C) white-fleshed genotypes.
2.2. Cooking Analysis
After cleaning in running water, transverse cuts were made in the tubers, approximately 1.5 cm thick, and the most extreme parts were discarded. The skins were not removed for processing. Five cooking methods were used: microwave cooking in polypropylene plastic bags (15 × 35 cm), free of bisphenol-A (Mi), steaming (S), boiling in water (B), baking in a conventional oven and wrapped in aluminum foil (Op), and baking in a conventional oven without packaging (Owp). Raw biomass of all sweet potato genotypes was considered the control treatment.
Cooking time was defined as the point at which the material did not show resistance to being punctured by a fork, i.e., 3 min for microwave cooking, 12 min for boiling and steam cooking, and 24 min for oven cooking at 230 °C (wrapped or not in aluminum foil). After cooking, or not (control), the roots were macerated in liquid nitrogen and stored at −80 °C for the analysis of bioactive compounds, performed in triplicate (n = 3).
2.3. Phytochemical Analysis and Antioxidant Activity
In all genotypes, the total content of phenolic and flavonoid compounds was determined, as well as the antioxidant activity, using the diphenylpicrylhydrazyl (DPPH) and malondialdehyde (MDA) methods. In tubers with orange, yellow, and white pulps, the total content of carotenoids was analyzed, while in purple-fleshed tubers, the total monomeric anthocyanins were measured.
The total phenolic content was spectrophotometrically determined using the Folin–Ciocalteu reagent [16]. The absorbance reading was performed at 725 nm and the results were expressed in mg gallic acid equivalent (GAE) per 100 g−1. The total flavonoid content was analyzed according to the method adapted by Pękal et al. [17], and the values expressed in mg equivalent of rutin (RE) per 100 g−1 after reading the absorbance at 510 nm.
The DPPH analysis was performed as described by Brand-Williams et al. [18] with a UV-vis spectrophotometer (λ = 517 nm). The absorbance decay of the samples is correlated to the absorbance decay of the control (blank), resulting in the percentage of free radical scavenging, which is then converted into mg of Trolox per 100 g of fresh sample. The MDA assay was carried out using the methodology described by Heath and Packer [19] and the results were expressed in nmol TBARS g−1 (thiobarbituric acid reactive substances).
The total monomeric anthocyanin concentration was determined by the differential pH method [20], with the result expressed in mg cyanidin-3-O-glycoside 100 g−1. The total carotenoid content was measured according to Lichtenthaler [21], determining, via a UV-vis spectrophotometer, the amounts of the chlorophylls a and b, and total carotenoids, expressed in mg 100 g−1.
2.4. Profile of Phenolic Compounds
The samples referring to the five genotypes with purple pulp (‘JNRX1’, ‘JNRX2’, ‘JNRX7’, ‘JNRX12’, and ‘Trabuca’) and orange pulp (‘LLR15’, ‘5603’, ‘2306’, ‘Beaudegard’, and ‘3513’) were the most representative in terms of their total content of phenolic compounds, and their antioxidant activity was analyzed via high-performance liquid chromatography (HPLC). The profile of the phenolic compounds was analyzed as described by Borges et al. [9]. For that, the samples were extracted in mobile phase (50% solvent A, acidified water: trifluoroacetic acid (99.9: 0.1, v/v), and 50% solvent B (100% acetonitrile)) and centrifuged (5 min, 5000× g). The supernatant was recovered, and an aliquot (20 µL) was injected into the HPLC system (Ultimate 3000, Thermo Scientific, Bremen, Germany) coupled to a DAD and C18 column (150 × 4.6 mm, Kinetex® 2.6 µm F5 100 Å; Phenomenex, Torrance, CA, USA). The gradient used was 0–7 min, 90% A + 10% B; 7–9 min, 60% A + 40% B; 9–10.5 min, 50% A + 50% B; 10.5–12 min, 45% A + 55% B; 12–15 min, 40% A + 60% B; 15–17 min, 60% A + 40% B; and 17–22 min, 90% A + 10% B, at a flow rate of 0.75 mL/min (25 °C). The phenolic compounds were quantified at 270 nm (gallic acid, t-cinnamic acid, catechin), 320 nm (chlorogenic acid, caffeic acid, p-coumaric acid, t-ferulic acid), 360 nm (quercetin, rutin, 3-O-methylquercetin, luteolin, kaempferol), and 520 nm (anthocyanins). Calibration curves were built using known amounts of analytical standards (99.98% purity; Sigma-Aldrich Co., St. Louis, MO, USA). The results were expressed as mean ± standard deviation (SD) in mg 100 g−1.
2.5. Profile of Biogenic Amines, Amino Acids, and the Amine Index (AI)
Biogenic amine profiles were chromatographically (i.e., via HPLC) determined according to the method described by Diamante et al. [8]. The mobile phases used were 50% acetonitrile (A) and 100% acetonitrile (B), in a gradient of 0–2 min, 40% A + 60% B; 2–4 min, 60% A + 40% B; 4–8 min, 65% A + 35% B; 8–12 min, 85% A + 15% B; 12–15 min, 95% A + 5% B; 15–21 min, 85% A + 15% B; 21–22 min, 75% A + 25% B; 22–25 min, 40% A + 60% B. The identification of biogenic amines and amino acids was based on the retention times of the analytical standards (Sigma-Aldrich, St. Louis, MO, USA) at 225 nm. The concentration of the amines serotonin, dopamine, agmatine, tryptamine, putrescine, cadaverine, spermidine, spermine, histamine, and tyramine and the amino acids 5-hydroxytryptophan and tryptophan were expressed in mg 100 g−1, while the amounts of L-dopa were expressed in µg 100 g−1. Finally, the amine index (AI) was analyzed as described by Basílio et al. [3] and expressed by the equation
2.6. Statistical Analysis
For the phytochemical analysis and antioxidant activity, the 16 roots studied were randomly processed, and analyzes were performed in triplicate (n = 3). The HPLC analysis of the ten samples (5 purple-fleshed sweet potatoes and 5 orange-fleshed ones) was performed in duplicate for each of the three replications (n = 6). Data were submitted to analysis of variance (ANOVA), followed by the Scott-Knott test (p < 0.05), with the aid of SISVAR software (Version 5.6, 2006; Lavras, Brazil). Importantly, the phytochemical dataset of the white, yellow, and orange genotypes was separately analyzed from the that of the purple ones. The values were expressed as mean ± SD. Additionally, a principal component analysis (PCA) was performed using the XLSTAT software (Version 12017; Addinsoft, Paris, France). To better adjust the data, optimizing the total variance, a Gaussian Filter with 5 points was used for PCA analysis of the phenolic, amine, and amino acid profiles of the purple and orange sweet potatoes.
3. Results and Discussion
In the total phenolic content (TPC) analysis, the highest concentrations were found in the ‘JNRX12’ and ‘Trabuca’ purple-pulp sweet potatoes subjected to cooking in microwaves (432.6 and 374.8 mg GAE 100 mg−1, respectively), which differed statistically (p < 0.05) from all other genotypes (Table 1). Microwave cooking proved to be the best method for phenolic extraction for the purple genotypes, a result also found for the total flavonoid (TF) content. With regard to TF levels, microwaved ‘JNRX12’ showed the highest content (366.5 mg RE 100 mg−1), statistically differing (p < 0.05) from the other genotypes. Regarding the anthocyanin content, the microwave-cooked ‘JNRX2’ genotype presented the highest level (245.5 mg 100 mg−1), statistically differing (p < 0.05) from the others. Additionally, that purple genotype, when oven cooked without protection, showed the highest MDA (245.5 nmol TBARS g−1), statistically differing (p < 0.05) from the others. The type of cooking impacted the MDA and the DPPH antioxidant activity of the sweet potato genotypes in this study. Thus, the genotypes JNRX1 (Mi), JNRX2 (Owp), JNRX7 (Mi), and JNRX12 (Mi, S, B, Op, and Owp) showed the highest mean values for those variables, differing from the other genotypes (Table 1).

Table 1.
Total contents of phenolic compounds (TPC—mg GEA 100 mg−1) and flavonoids (TF—mg RE 100 mg−1). Antioxidant activity assays—MDA, nmol TBARS g−1 and DPPH, mg Trolox 100 mg−1—of purple-, white-, yellow-, and orange-fleshed sweet potato genotypes. Total contents of anthocyanins (TA, purple sweet potato genotypes—mg 100 mg−1) and carotenoids (TC, white-, yellow-, and orange-fleshed sweet potato genotypes—mg 100 mg−1). Values are expressed as mean ± SD.
The orange-fleshed sweet potatoes showed a higher content of bioactive compounds compared to white- and yellow-fleshed ones (Table 1). Microwave cooking allowed for higher TPC and TF contents in those sweet potatoes, mainly in the LLR15 (109.7 mg GAE 100 mg−1 and 88.3 mg RE 100 mg−1), 2603 (104.0 mg GAE 100 mg−1 and 86.6 mg RE 100 mg−1), Beauregard (109.4 mg GAE 100 mg−1 and 94.7 mg RE 100 mg−1), and 3513 (104.2 mg GAE 100 mg−1 and 90.8 mg RE 100 mg−1) genotypes. In turn, the ‘LLR15’ and ‘2603’ genotypes showed higher carotenoid contents when steamed and also when cooked unprotected in an oven.
Regardless of coloration and genotypes, thermal processing improves the availability of the bioactive compounds analyzed by UV-vis spectrophotometry. This may be related to the fact that heat treatment can cause cell rupture and the dissociation of some of the phenolic compounds from their biological structures, releasing them from the food matrix. Besides, heat treatments can also cause a change in their chemical structure, enabling the conversion of insoluble phenolics into more soluble forms [22,23]. According to Burgos et al. [24], cooking can cause the hydrolysis of different components, releasing phenolic compounds and making them more available for extraction. Furthermore, each food matrix contains different compounds that are more or less thermally labile; consequently, the same cooking technique can imply different effects, depending on the plant species [25].
In order to establish a descriptive model of the grouping genotypes according to their bioactive compounds and thermal processing, principal component analysis (PCA) was applied to the data on the composition (phenolics, flavonoids, anthocyanins, and antioxidant activity—DPPH and MDA) of the purple-fleshed sweet potato genotypes. The PCA of purple-fleshed sweet potato genotypes explained 93.38% of the dataset’s variability. All genotypes, both in natura and oven-processed without packaging (grouped in PC1−) showed the smallest contents of bioactive compounds (Figure 2; Table 1), as well as the lowest values of activity against the radical DPPH. The ‘JNRX2’ and ‘Trabuca’ genotypes showed higher MDA values when cooked in an oven without packaging and were also the ones with the lowest contents of phenolics, flavonoids, and anthocyanins. With the exception of ‘JNRX7’, the other genotypes, when boiled, also showed values of total phenolics, flavonoids, and anthocyanins similar to those found when baked in an oven without packaging (PC1− and PC2+).
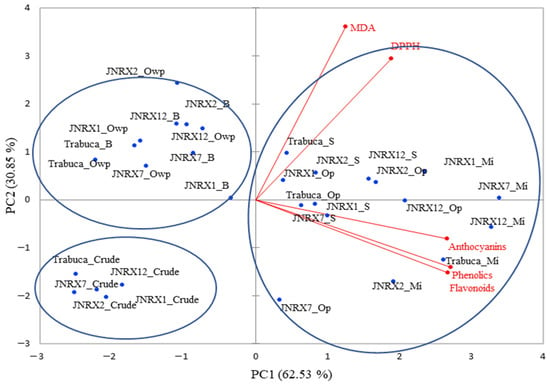
Figure 2.
Two-dimensional projection and scores of total phenolic compounds (mg GAE 100 mg−1), flavonoids (mg RE 100 mg−1), monomeric anthocyanins (mg 100 mg−1), and antioxidant activity determined by the MDA (nmol TBARS g−1) and DPPH (mg Trolox 100 mg−1) assays of raw purple-fleshed sweet potato genotypes submitted to heat treatments. Mi = microwave; S = steam; B = boil; Op = oven with aluminum foil protection; Owp = oven without protection.
In purple sweet potatoes, anthocyanins are the major pigments present in pulps and skins. Anthocyanins are sensitive to temperature changes, in addition to being highly water soluble [26], which may explain the lower content of these compounds when cooked in boiling water and in an oven without protection. A decrease in the anthocyanin contents (51–81% in relation to the raw sample) of the purple-fleshed Korean sweet potatoes ‘Sinjami’ and ‘Yeonjami’ was also observed after baking and boiling, with changes in color seen after the heat treatments [1].
The PCA applied to the contents of the phenolic compounds, flavonoids, carotenoids, and antioxidant activity (DPPH and MDA) of the orange-, yellow-, and white-pulp genotypes explained 77.89% of the total variance of the data. The ‘LLR15’, ‘3513’, ‘2603’, and ‘Beauregard’ orange-pulp genotypes were grouped in PC1+, showing the highest carotenoid contents after thermal processing, reflecting their high antioxidant activity, detected via the DPPH assay (Figure 3; Table 1).
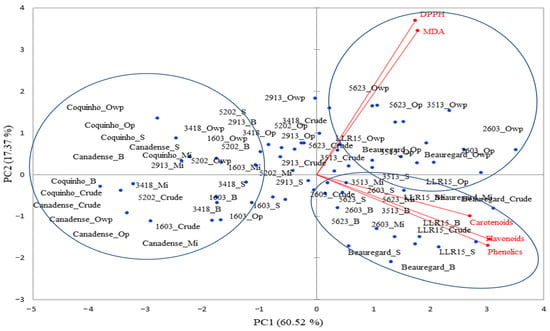
Figure 3.
Two-dimensional projection and scores of total phenolic compounds (mg GAE 100 mg−1), flavonoids (mg RE 100 mg−1), monomeric anthocyanins (mg 100 mg−1), and antioxidant activity determined by the MDA (nmol TBARS g−1) and DPPH (mg Trolox 100 mg−−1) assays of raw purple-fleshed sweet potato genotypes submitted to heat treatments. Mi = microwave; S = steam; B = boil; Op = oven with aluminum foil protection; Owp = oven without protection.
‘Beauregard’ is an American cultivar recognized for its high levels of β-carotene and great potential in the manufacture of functional foods [27], which was confirmed by its clustering in PC1+. However, even though the orange color is associated with a higher carotenoid content in sweet potato and despite the attractive and intense color of the ‘Beauregard’ genotype, the carotenoid content in this cultivar was lower than that found in the ‘2603’ and ‘LLR15’ samples (Table 1), regardless of the processing method. These results demonstrate the potential of these cultivars as a source of carotenoids. Contrarily, the ‘Coquinho’ and ‘Canadense’ cultivars were grouped in PC1− due to their low content of the bioactives investigated (Table 1). The total carotenoids of the ‘Coquinho’ and ‘Canadense’ cultivars also show low levels, regardless of thermal processing.
In most genotypes, oven cooking without packaging did not change the total quantities of phenolics and flavonoids. On the other hand, microwave cooking was effective in promoting the detection of those secondary metabolites in both cream- and orange-fleshed sweet potatoes. The ‘2603’, LLR15’, ‘3513’, and ‘Beauregard’ genotypes showed the highest amounts of total phenolics and flavonoids after microwave thermal processing. This result can be attributed to the cell disruption and dissociation of some phenolic compounds, including flavonoids, from their biological structures, thus facilitating their release from the matrix [28].
Orange-fleshed sweet potatoes that were steamed, microwaved, and boiled were grouped in PC1+ (Figure 3), as they were shown to contain higher concentrations of bioactives (total phenolics, flavonoids, and carotenoids—Table 1). The increased availability of these compounds, noted in all genotypes, is reflected in their high antioxidant activities, measured through the DPPH assay. This finding was more pronounced in the ‘BL2603’, ‘BL3513’, ‘BL2913’, ‘BL5202’, and ‘BL 3623’ genotypes, which, despite having an intense orange color, showed higher antioxidant activity compared to the LR15 genotype, which has an even darker orange color than the others.
The tuber samples that were oven-cooked, whether with packaging or without it, were grouped in PC1−, with an emphasis on the samples cooked without packaging, which showed higher lipid peroxidation (MDA) (Table 1; Figure 3), such as the ‘BL3418’, ‘BL5623’, and ‘BL5202’ genotypes. Interestingly, although the ‘2603’ genotype has shown significant lipid peroxidation (MDA), it also presented higher amounts of total carotenoids (Table 1). MDA is a method used to estimate the lipid peroxidation in biological systems and membranes and is related to the quality, loss of nutritional value, and safety of foods [29].
In a second set of experiments, and based on the results described above, the phenolic compounds of the purple-fleshed potatoes and orange-fleshed ones were profiled via liquid chromatography, due to their phenolic contents and antioxidant activity. The results showed a great variation in the chemical profiles between the purple and the orange genotypes (Table 2 and Table 3). In the former, higher amounts of catechin, t-cinnamic acid, rutin, luteolin, quercetin, 3-O-methylquercetin, and kaempferol were detected, while in the orange pulps, superior amounts of gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, and t-ferulic acid were noted. Chlorogenic acid and t-ferulic acid were the ones that presented higher averages than the others in both types of sweet potatoes. Thus, in the purple-pulp genotypes 1899.30 mg 100 g−1 of chlorogenic acid was found, in comparison to 2030.83 mg 100 g−1 for the orange-pulp genotypes. For t-ferulic acid, the purple and orange genotypes presented 702.81 mg 100 g−1 and 1372.93 mg 100 g−1, respectively. The chlorogenic acid found in sweet potato has demonstrated antibacterial and anti-inflammatory activities, in addition to antitumor, antihypertensive, and liver-protective properties [6]. Trans-ferulic acid stands out for its capacity to protect the organs, tissues, and cells, mainly cardiovascular, neurological, cutaneous, and hematopoietic, due to its antimicrobial, anti-inflammatory, anticancer, and antidiabetic activities [30]. In purple-fleshed sweet potatoes, t-ferulic acid has the interesting feature of acting on anthocyanin copigmentation [7]. A large variation was observed between the genotypes of each sweet potato and between heat treatments, that is, there was a matrix effect between the genotypes and heat treatments (Table 2 and Table 3).

Table 2.
Concentrations (mg 100 g−1) of phenolic acids in orange- and purple-fleshed sweet potatoes, raw and heat-treated samples. Values are expressed as mean ± SD.

Table 3.
Concentration (mg 100 g−1) of flavonoids in orange- and purple-fleshed sweet potatoes, raw and heat-treated samples. Values are expressed as mean ± SD.
Anthocyanins, phenolic compounds belonging to the flavonoid group, were found only in purple-fleshed sweet potato samples, and they were cyanidin-3-O-glucoside, cyanidin-3,5-diglucoside, delphinidin-3-O-glucoside, malvidin-3,5-diglucoside, perlagonidin-3-O-glucoside, malvidin-3-O-glucoside, and peonidin-3-O-glucoside. In addition to being highly antioxidant compounds, anthocyanins are high-value natural dyes [7]. The highest general concentrations were related to perlagonidin-3-O-glucoside and malvidin-3-O-glucoside, at 214.6 mg 100 g−1 and 276.8 mg 100 g−1, respectively. Interestingly, perlagonidin-3-O-glucoside was not recorded in the ‘JNRX1’, ‘JNRX7’, and ‘JNRX12’ genotypes, contrary to the quite relevant concentrations found especially in the ‘JNRX2’ genotype, where its contents ranged from 246.6 mg 100 g−1 (microwave) to 673.3 mg 100 g−1 (crude). In general, the highest concentrations of cyanidin-3-O-glucoside, cyanidin-3,5-diglucoside, delphinidin-3-O-glucoside, perlagonidin-3-O-glucoside, and peonidin-3-O-glucoside can be attributed to the “JNRX2” genotype when crude. Malvidin-3,5-diglucoside was detected in higher amounts in the uncooked ’JNRX1’ (645.0 mg 100 g−1) genotype, as was malvidin-3-O-glucoside in the ‘JNRX12’ genotype, also when uncooked (1490.4 mg 100 g−1).
When PCA was applied to the dataset of the purple-fleshed sweet potatoes’ phenolic compounds, PC1 and PC2 summed up 66,96% of the total variance of the data (Figure 4). A greater similarity was noted between the genotypes’ phenolic composition with respect to the sample grouping resulting from the heat treatments. A clustering of the raw and cooked-in-microwave genotypes was identified in PC1−, apparently related to their anthocyanin contents. Cooking ‘JNRX2’, ‘JNRX12’, and ‘Trabuca’ sweet potatoes caused them to be grouped into PC1+/PC2+ due to their phenolic acid and flavonol concentrations. Genetic variability may influence different responses regarding the availability of phenolic compounds after cooking, including phenolic acids that are found in their free forms in vacuoles and apoplasts [15].
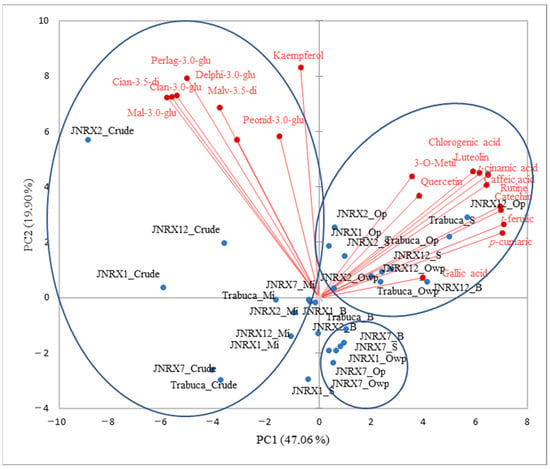
Figure 4.
Two-dimensional projection and phenolic compound profile scores of the raw and heat-treated purple-fleshed sweet potato genotypes. Mi = microwave; S = steam; B = boil; Op = oven with aluminum foil protection; Owp = oven without protection.
In a similar approach, a descriptive model was built by applying PCA to the chemical profile of the data on the phenolic compounds of the orange sweet potatoes. The results revealed that PC1 and PC2 summed up 57.8% of the data’s variance (Figure 5). The control treatments, i.e., samples without cooking, were united in PC2−. In turn, the orange-pulp genotypes submitted to heat treatment in the microwave and oven with aluminum foil protection were grouped in PC2+, because of their higher phenolic acid contents (e.g., gallic acid, chlorogenic acid, p-coumaric acid, and t-ferulic acid), flavonols (e.g., kaempferol and 3-O-methylquercetin), and catechin. A similar factor occurred after the roasting in the oven (240 °C—30, 35, and 40 min) of the orange-fleshed sweet potato genotypes ‘California’, ‘Beauregard’, and ‘Covington’, which showed higher amounts of caffeoylquinic derivatives (e.g., chlorogenic acid) as their cooking time increased [31].
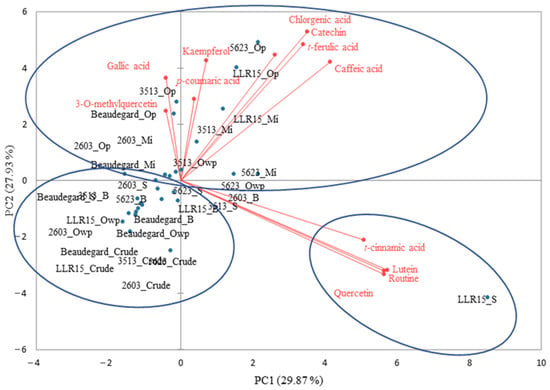
Figure 5.
Two-dimensional projection and phenolic compound profile scores of the raw and heat-treated orange-fleshed sweet potato genotypes. Mi = microwave; S = steam; B = boil; Op = oven with aluminum foil protection; Owp = oven without protection.
The results shown herein are important, as the consumption of polyphenols from plant matrices is associated with a better quality of life [32]. In the young population, usually influenced by poor eating habits, these compounds act to improve neuronal plasticity through the CREB (Camp Response Element Binding) protein in the hippocampus, which is responsible for maintaining cognitive functions [33]. Trans-cinnamic acid, for example, is a powerful anti-inflammatory and antioxidant phenolic acid [31], just as luteolin is considered to have anticancer properties by inducing apoptosis (programmed cell death) and inhibiting cell proliferation [34]. Rutin and quercetin, in turn, have proven antioxidant effects against the damage caused by excess reactive oxygen species in patients with sickle cell anemia [35].
Based on the findings of the phytochemical and antioxidant analysis, a second set of experiments was carried out, aiming to investigate the biogenic amines in sweet potatoes with orange and purple flesh, both raw and subjected to heat treatments.
The results have shown that among the orange- and purple-fleshed sweet potato genotypes distinct amine profiles were identified after heat treatments. The cooking method and the time of exposure affected the biogenic amine concentrations in different ways, even though those metabolites are not considered thermostable [36].
Regarding orange pulp genotypes, microwave thermal processing increased their histamine level, except for in the ‘Beauregard’ genotype, in which it was higher without thermal treatment. This genotype was also the only presenting higher putrescine content in raw samples, since the other genotypes investigated showed augmented amounts after cooking. Practically all orange-pulp genotypes showed higher levels of tyramine following oven cooking without protection (Table 4). Histamine and cadaverine concentrations varied over the purple sweet potatoes’ genotypes according to the thermal treatment, and were higher when boiled in water (‘JNRX1’ and ‘JNRX7’), microwaved (‘JNRX12’), and steamed (‘Trabuca’), clearly revealing the effect of the thermal treatment on the amounts of those biogenic amines. Importantly, cadaverine was not detected in the ‘JNRX2’ genotype, regardless of the cooking method applied to the samples. Additionally, putrescine quantities were increased in all sweet potato genotypes after boiling. Histamine and tyramine are amines related to food allergies, while putrescine and cadaverine have been associated with product quality [37]. Those metabolites have been considered harmful amines, as adverse reactions and even intoxication can occur if consumed at high levels by human beings [37]. However, tyramine is also a precursor of dopamine, an important catecholamine in several physiological processes both in plants, such as disease resistance, and in animals, acting as a neurotransmitter with an essential role in cognitive activities, including learning. Another pathway for dopamine formation is through the decarboxylation of L-dopa, through the action of dopa decarboxylase [38].

Table 4.
Biogenic amines (mg 100 g−1) and the amine index (AI) of raw and heat-treated orange- and purple-fleshed sweet potatoes.
Boiling, a thermal process commonly used at home, did not interfere with the general concentrations of biogenic amines found in orange pulp samples. In these genotypes, microwave and steam cooking increased the serotonin and/or dopamine levels in the ‘LLR15’, ‘5623’, and ‘2306’ genotypes, while in the ‘Beauregard’ (Owp) and ‘3513’ (Op) genotypes and treatments roasting in an oven caused a similar effect. In purple-pulp genotypes, oven cooking with protection (Op) increased the level of dopamine in all genotypes, while serotonin was increased by steam treatments (‘JNRX2’, ‘JNRX12’, and ‘Trabuca’) and oven roasting with protection (‘JNRX1’ and ‘JNRX7’). Serotonin and dopamine are important neurotransmitters that act in the regulation of specific pathways and are responsible for feelings of pleasure and satisfaction, as unwanted neurobiological changes (e.g., depression, anxiety, bipolarity) can occur in the absence of such molecules [34].
The contents of spermine and spermidine in the orange genotypes varied according to the genotype, with no relationship to the cooking method. Microwave cooking increased the spermidine concentration in most purple samples. In foods of plant origin, it is common for spermidine levels to be higher than spermine ones. Both are amines related to the growth and proliferation of human cells and the consumption of these amines is considered toxic at levels >60 mg/100 g [39], which were not detected in the genotypes studied herein (Table 4).
Tryptamine concentrations were elevated after thermal processing in orange- and purple-pulp genotypes (Table 4). There were no meaningful differences (p < 0.05) in tryptamine contents determined to be due to the heat treatments for the purple genotype ‘JNRX12’ and the orange genotypes ‘LLR15’, ‘2306’, ‘Beauregard’, and ‘Trabuca’. Microwave thermal processing, steam, and cooking in an oven without protection provided higher levels of tryptamine in ‘5623’ and ‘3513’, while steam cooking and/or cooking in an oven with aluminum foil protection increased the content of these compounds in the purple genotypes ‘JNRX1’, ‘JNRX2’, and ‘JNRX7’. The biological role of tryptamine in plants is unclear and it has been suggested that, in addition to being present in the metabolic route of serotonin and melatonin, this molecule plays a role similar to that of auxin and accumulates more in fruits than in other vegetative parts [40].
These results highlight the diversity of the plant matrices herein studied, according to their major pigments, showing a greater or lesser impact of the thermal treatment on the extraction of sweet potatoes’ bioactive compounds.
Most of the amine quality indexes have been elaborated while trying to unravel the content of those bioactive compounds in foods of animal origin, which have a much higher level of toxicity than plant samples. In general, values up to 50 mg/L of amines and amino acids are potentially toxic [37]. The amine index (AI) suggests that values close to 1.0 are acceptable, while values <1.0 are desirable [4]. In this study, with the exception of the microwaved (1.24)- and boiled (1.01) ‘JNRX1’ samples, all AI found were considered desirable, showing that the balance between commonly harmful amines and health-promoting amines was favorable for the sweet potatoes (Table 4).
In a final experiment, an analysis of the amino acids 5-hydroxytryptophan, tryptophan, and L-dopa was carried out in the orange- and purple-flesh sweet potato genotypes (Table 5). With regard to 5-hydroxytryptophan for orange-fleshed sweet potatoes, the highest concentrations were linked to the ‘LLR15’, ‘2306’, ‘3513’, ‘Beaudegard’, and ‘5623’ genotypes in the crude state, a result not found for the purple-fleshed ones, where a great variation of contents was detected among the genotypes and heat treatments. However, the overall average of the contents of those metabolites was higher (360.2 mg/100 g) for the purple-fleshed samples when compared to the orange-fleshed ones (149.2 mg/100 g).

Table 5.
Concentrations of 5-hydroxytryptophan (mg/100 g), tryptophan (mg/100 g), and L-dopa (ug/100 g) in raw and heat-treated orange- and purple-fleshed sweet potato genotypes.
The tryptophan levels in orange-fleshed sweet potatoes were higher in raw and microwave- and/or oven-baked genotypes without foil protection. It is worth noting that in a general comparison, the tryptophan levels were higher in orange-fleshed than in purple-fleshed sweet potatoes, which have a higher 5-OH-tryptophan content. For the purple-pulp genotypes, the contents of this amino acid were superior in the raw and microwave-treated ‘JNRX1’, ‘JNRX2’, and ‘JNX7’ genotypes and only in the cooked ‘JNX12’ and ‘Trabuca’ ones. Tryptophan and 5-hydroxytryptophan are serotonin precursors in the human body [41] and the consumption of these compounds is associated with improvements in cognitive function, memory, attention, emotional processing, and psychomotor performance [42]. About 40.7% of sweet potatoes’ protein is formed from amino acids essential to human beings, including tryptophan [43]. The sweet potatoes evaluated in this study proved to be potential sources of these amino acids, and mainly tryptophan, an amino acid that is not synthesized in humans, but is essential for several metabolic processes [42].
The contents of L-dopa were also higher in the orange- and purple-pulp genotypes after cooking, with an emphasis on thermal processing in a microwave, steam, and oven roasting with aluminum foil protection (purple genotypes) and without it (orange genotypes). When cooking food wrapped in aluminum foil, the dull side emits the radiation flowing from the flame to the food, compared to the shiny side. Purple-fleshed sweet potatoes baked in the oven and wrapped in aluminum foil showed higher dopamine levels compared to other heat treatments. This same result is found in most orange-fleshed sweet potato genotypes. Thus, the use of aluminum foil protection in roasting contributed to the compounds of interest remaining in purple sweet potatoes, even at exposure to higher temperatures.
Applying PCA to the dataset of biogenic amines and amino acids of the orange-fleshed sweet potato genotypes revealed that PC1 and PC2 summed up 71.12% of the data’s variance (Figure 6). The ‘Beauregard’ sweet potato’s treatments were grouped in PC1+/PC2− due to their amounts of the amines spermine, spermidine, and dopamine, as well as the amino acid L-dopa. The ‘3513’, ‘2606’, and ‘5623’ genotypes were clustered in PC1+/PC2+ because of their putrescine and histamine levels after cooking treatments. In turn, the ‘LLR15’ genotype samples were grouped in PC2−, as they showed higher contents of tryptophan, 5-hydroxytryptophan, and the amine serotonin. In sweet potatoes, about 40.7% of the protein is formed from essential amino acids for human beings, including tryptophan [43].
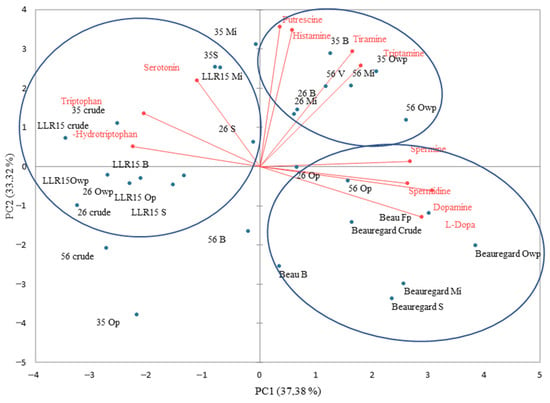
Figure 6.
Two-dimensional projection and biogenic amine and amino acid scores of the raw and heat-treated orange-fleshed sweet potato genotypes. Mi = microwave; S = steam; B = boil; Op = oven with aluminum foil protection; Owp = oven without protection.
In PC2−, the genotypes ‘Trabuca’, ‘JNRX1’, and ‘JNRX7’ were grouped together when raw and baked in the oven, regardless of the protection of the samples with aluminum foil during cooking, since these treatments presented lower levels of biogenic amines and amino acids. In PC2+, the ‘JNRX12’, ‘JNRX1’, and ‘JNRX7’ samples were grouped after boiling and steaming, due to their similar amounts of tryptophan, 5-hydroxytryptophan, and cadaverine. Interestingly, the quantities of the biogenic amines commonly considered harmful to human health (e.g., histamine, putrescine, cadaverine, and tyramine) were found to be low (Figure 7). The lowest adverse effect level (LOAEL) of histamine and tyramine are reported at 500 mg/kg and 301.8 mg/kg, respectively, while putrescine and cadaverine have larger LOAEL values, e.g., 881.50 mg/kg and 510.89 mg/kg, respectively [37,41,44].
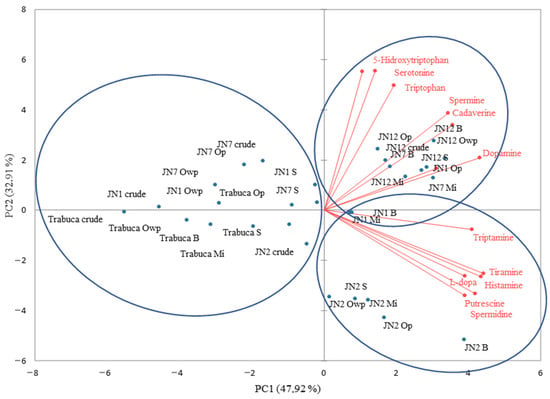
Figure 7.
Two-dimensional projection and biogenic amine and amino acid scores of the raw and heat-treated purple-fleshed sweet potato genotypes. Mi = microwave; S = steam; B = boil; Op = oven with aluminum foil protection; Owp = oven without protection.
As demonstrated, the consumption of sweet potato genotypes after cooking, when observing their content of biogenic amines and amino acids, may play an interesting role as they introduce antioxidants into the body [8].
4. Conclusions
The genotypes and the cooking method effectively influenced the biochemical composition of sweet potatoes with different flesh colors. Chlorogenic and t-ferulic acids were the phenolics detected in greater amounts in the evaluated genotypes. Regarding the anthocyanins, purple-fleshed sweet potatoes revealed superior contents, mostly of cyanidin-3-O-glucoside and malvidin-3-O-glucoside. Interestingly, a higher concentration of tryptophan and its 5-OH derivative were found when the genotypes, regardless of their pulp color, did not undergo thermal processing, indicating the thermal sensitivity of those amino acids in the samples investigated.
The use of boiling water, a common practice when cooking at home, did not determine an increase in any of the bioactive compounds studied. Among the different genotype’s pulp colors, the cream/white ones showed the lowest antioxidant activity. Contrarily, the purple-pulp genotypes revealed higher amounts of bioactive compounds and also antioxidant activity after thermal processing (e.g., steam, microwave, and in the oven with aluminum foil protection). In turn, for orange-fleshed sweet potatoes, microwave and oven cooking with or without aluminum foil protection provided better phytochemical extraction (e.g., phenolic acids, flavonols, and biogenic amines) and greater antioxidant activity.
Sweet potatoes with colored pulp, following distinct cooking processes, are important sources of nutrients that are beneficial to human health. A thermal process promotes beneficial changes in the levels of polyphenols and health-promoting biogenic amines and amino acids available in sweet potatoes.
Author Contributions
L.S.P.B. and G.P.P.L. contributed to the conceptualization, investigation, data curation, and writing the original draft. L.S.P.B., A.N. and G.P.P.L. contributed to the supervision, investigation, data curation, and writing—review and editing. L.S.P.B., M.M. and G.P.P.L. contributed to the project administration and funding acquisition. L.S.P.B., A.N., I.O.M. and F.V. contributed to the investigation, methodology, writing, and editing of the original draft. P.F.V., M.S.D., A.C.A.F.e.S. and C.B.D.L. contributed to the investigation and data curation. I.O.M. and F.V. contributed to the data curation, investigation, and methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the São Paulo Research Foundation (FAPESP—Brazil), grants 2019/27227-1 (G.P.P.L. and M.M.), 2021/03537-1 (P.F.V.) and 2023/03886-1 (A.N.); by the National Council for Scientific and Technological Development, Brazil (CNPq), grants 307571/2019-0 (G.P.P.L.), 201218/2020-8 (L.S.P.B.) and 405949/2022-7 (M.M.); and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, grant 88882.433049/2019-01 (L.S.P.B.).
Data Availability Statement
All data are included and available in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hong, K.H.; Koh, E. Effects of Cooking Methods on Anthocyanins and Total Phenolics in Purple-Fleshed Sweet Potato: Cooking Effects on Anthocyanins. J. Food Process. Preserv. 2016, 40, 1054–1063. [Google Scholar] [CrossRef]
- Oloniyo, R.O.; Omoba, O.S.; Awolu, O.O.; Olagunju, A.I. Orange-fleshed Sweet Potatoes Composite Bread: A Good Carrier of Beta (β)-carotene and Antioxidant Properties. J. Food Biochem. 2021, 45, e13423. [Google Scholar] [CrossRef] [PubMed]
- Basílio, L.S.P.; Vanz Borges, C.; Minatel, I.O.; Vargas, P.F.; Tecchio, M.A.; Vianello, F.; Lima, G.P.P. New Beverage Based on Grapes and Purple-Fleshed Sweet Potatoes: Use of Non-Standard Tubers. Food Biosci. 2022, 47, 101626. [Google Scholar] [CrossRef]
- Guclu, G.; Dagli, M.M.; Aksay, O.; Keskin, M.; Kelebek, H.; Selli, S. Comparative Elucidation on the Phenolic Fingerprint, Sugars and Antioxidant Activity of White, Orange and Purple-Fleshed Sweet Potatoes (Ipomoea Batatas L.) as Affected by Different Cooking Methods. Heliyon 2023, 9, e18684. [Google Scholar] [CrossRef] [PubMed]
- Chintha, P.; Sarkar, D.; Pecota, K.; Dogramaci, M.; Hatterman-Valenti, H.; Shetty, K. Phenolic Bioactive-Linked Antioxidant, Anti-Hyperglycemic, and Antihypertensive Properties of Sweet Potato Cultivars with Different Flesh Color. Hortic. Environ. Biotechnol. 2023, 64, 877–893. [Google Scholar] [CrossRef]
- Gomez-Gomez, H.A.; Borges, C.V.; Minatel, I.O.; Luvizon, A.C.; Lima, G.P.P. Health benefits of dietary phenolic compounds and biogenic amines. In Bioactive Molecules in Food; Springer: Cham, Switzerland, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Lv, X.; Mu, J.; Wang, W.; Liu, Y.; Lu, X.; Sun, J.; Wang, J.; Ma, Q. Effects and mechanism of natural phenolic acids/fatty acids on copigmentation of purple sweet potato anthocyanins. Curr. Res. Food Sci. 2022, 5, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Diamante, M.S.; Borges, C.V.; da Silva, M.B.; Minatel, I.O.; Corrêa, C.R.; Gomez Gomez, H.A.; Lima, G.P.P. Bioactive Amines Screening in Four Genotypes of Thermally Processed Cauliflower. Antioxidants 2019, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.V.; Maraschin, M.; Coelho, D.S.; Leonel, M.; Gomez, H.A.G.; Belin, M.A.F.; Diamante, M.S.; Amorim, E.P.; Gianeti, T.; Castro, G.R.; et al. Nutritional value and antioxidant compounds during the ripening and after domestic cooking of bananas and plantains. Food Res. Int. 2020, 132, 109061. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic Amines: Formation, Action and Toxicity—A Review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef]
- Til, H.P.; Falke, H.E.; Prinsen, M.K.; Willems, M.I. Acute and Subacute Toxicity of Tyramine, Spermidine, Spermine, Putrescine and Cadaverine in Rats. Food Chem. Toxicol. 1997, 35, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, Z.; Podolsky, R.; Nir, A.; Yu, J.; Nir, R.; Halvorsen, S.W.; Quinn, J.F.; Kaye, J.; Kolb, C. Elevated Spermidine Serum Levels in Mild Cognitive Impairment, a Potential Biomarker of Progression to Alzheimer Dementia, a Pilot Study. J. Clin. Neurosci. 2022, 100, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Seidler, A.; Hönow, R. Oxalate-Rich Foods. Food Sci. Technol. 2021, 41, 169–173. [Google Scholar] [CrossRef]
- Nicoletto, C.; Vianello, F.; Sambo, P. Effect of Different Home-cooking Methods on Textural and Nutritional Properties of Sweet Potato Genotypes Grown in Temperate Climate Conditions. J. Sci. Food Agric. 2018, 98, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, 1–13. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; pp. 350–382. [Google Scholar] [CrossRef]
- Gahler, S.; Otto, K.; Böhm, V. Alterations of Vitamin C, Total Phenolics, and Antioxidant Capacity as Affected by Processing Tomatoes to Different Products. J. Agric. Food Chem. 2003, 51, 7962–7968. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. Effect of Industrial and Domestic Processing on Antioxidant Properties of Pumpkin Pulp. LWT Food. Sci. Technol. 2013, 53, 382–385. [Google Scholar] [CrossRef]
- Burgos, G.; Amoros, W.; Muñoa, L.; Sosa, P.; Cayhualla, E.; Sanchez, C.; Díaz, C.; Bonierbale, M. Total Phenolic, Total Anthocyanin and Phenolic Acid Concentrations and Antioxidant Activity of Purple-Fleshed Potatoes as Affected by Boiling. J. Food Compost. Anal. 2013, 30, 6–12. [Google Scholar] [CrossRef]
- Bernhardt, S.; Schlich, E. Impact of Different Cooking Methods on Food Quality: Retention of Lipophilic Vitamins in Fresh and Frozen Vegetables. J. Food Eng. 2006, 77, 327–333. [Google Scholar] [CrossRef]
- Gomez-Gomez, H.A.; Minatel, I.O.; Borges, C.V.; Marques, M.O.M.; da Silva, E.T.; Monteiro, G.C.; da Silva, M.J.R.; Tecchio, M.A.; Lima, G.P.P. Phenolic Compounds and Polyamines in Grape-Derived Beverages. J. Agric. Sci. 2018, 10, 65. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Schneider, J.K.; Leal, I.L.; de Abreu Barreto, G.; Batista, T.; Machado, B.A.S.; Druzian, J.I.; Krause, L.C.; da Costa Mendonça, M.; et al. Physicochemical and Sensory Profile of Beauregard Sweet Potato Beer. Food Chem. 2020, 312, 126087. [Google Scholar] [CrossRef] [PubMed]
- Murador, D.; Braga, A.R.; Da Cunha, D.; De Rosso, V. Alterations in Phenolic Compound Levels and Antioxidant Activity in Response to Cooking Technique Effects: A Meta-Analytic Investigation. Crit. Rev. Food Sci. Nutr. 2018, 58, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.L.; Huang, Z.Y. Reactive oxygen species. In Free Radical in Medical and Agricultural Science; Zheng, R.L., Huang, Z.Y., Eds.; China Higher Education Press: Beijing, China; Springer Press: Beijing, China, 2001; pp. 17–27. [Google Scholar]
- Oliveira Silva, E.; Batista, R. Ferulic Acid and Naturally Occurring Compounds Bearing a Feruloyl Moiety: A Review on Their Structures, Occurrence, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef]
- Carrera, C.; Zelaya-Medina, C.F.; Chinchilla, N.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. How Different Cooking Methods Affect the Phenolic Composition of Sweet Potato for Human Consumption (Ipomea batata (L.) Lam). Agronomy 2021, 11, 1636. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Carrillo, J.Á.; Zafrilla, M.P.; Marhuenda, J. Cognitive Function and Consumption of Fruit and Vegetable Polyphenols in a Young Population: Is There a Relationship? Foods 2019, 8, 507. [Google Scholar] [CrossRef]
- Conio, B.; Martino, M.; Magioncalda, P.; Escelsior, A.; Inglese, M.; Amore, M.; Northoff, G. Opposite Effects of Dopamine and Serotonin on Resting-State Networks: Review and Implications for Psychiatric Disorders. Mol. Psychiatry 2020, 25, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Henneberg, R.; Otuki, M.F.; Furman, A.E.F.; Hermann, P.; do Nascimento, A.J.; Leonart, M.S.S. Protective Effect of Flavonoids against Reactive Oxygen Species Production in Sickle Cell Anemia Patients Treated with Hydroxyurea. Rev. Bras. Hematol. Hemoter. 2013, 35, 52–55. [Google Scholar] [CrossRef]
- Borges, C.V.; Belin, M.A.F.; Amorim, E.P.; Minatel, I.O.; Monteiro, G.C.; Gomez Gomez, H.A.; Monar, G.R.S.; Lima, G.P.P. Bioactive Amines Changes during the Ripening and Thermal Processes of Bananas and Plantains. Food Chem. 2019, 298, 125020. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature Update of Analytical Methods for Biogenic Amines Determination in Food and Beverages. Trends Analyt. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, T.; Liu, W.; Liu, Y.; Zhao, Y.; Liu, Y.; Li, W.; Ding, K.; Ma, F.; Li, C. Functions of Dopamine in Plants: A Review. Plant Signal. Behav. 2020, 15, 1827782. [Google Scholar] [CrossRef]
- Kalač, P.; Krausová, P. A Review of Dietary Polyamines: Formation, Implications for Growth and Health and Occurrence in Foods. Food Chem. 2005, 90, 219–230. [Google Scholar] [CrossRef]
- Negri, S.; Commisso, M.; Avesani, L.; Guzzo, F. The Case of Tryptamine and Serotonin in Plants: A Mysterious Precursor for an Illustrious Metabolite. J. Exp. Bot. 2021, 72, 5336–5355. [Google Scholar] [CrossRef]
- Linares, D.M.; del Rio, B.; Redruello, B.; Ladero, V.; Martin, M.C.; Fernandez, M.; Ruas-Madiedo, P.; Alvarez, M.A. Comparative Analysis of the in Vitro Cytotoxicity of the Dietary Biogenic Amines Tyramine and Histamine. Food Chem. 2016, 197, 658–663. [Google Scholar] [CrossRef]
- Silber, B.Y.; Schmitt, J.A.J. Effects of Tryptophan Loading on Human Cognition, Mood, and Sleep. Neurosci. Biobehav. Rev. 2010, 34, 387–407. [Google Scholar] [CrossRef]
- Musilova, J.; Lidikova, J.; Vollmannova, A.; Frankova, H.; Urminska, D.; Bojnanska, T.; Toth, T. Influence of heat treatments on the content of bioactive substances and antioxidant properties of sweet potato (Ipomoea batatas L.) tubers. J. Food Qual. 2020, 2020, 8856260. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The Biogenic Amines Putrescine and Cadaverine Show in Vitro Cytotoxicity at Concentrations That Can Be Found in Foods. Sci. Rep. 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).