Biobutanol Production from Acetone–Butanol–Ethanol Fermentation: Developments and Prospects
Abstract
:1. Introduction
2. Butanol-Producing Strain
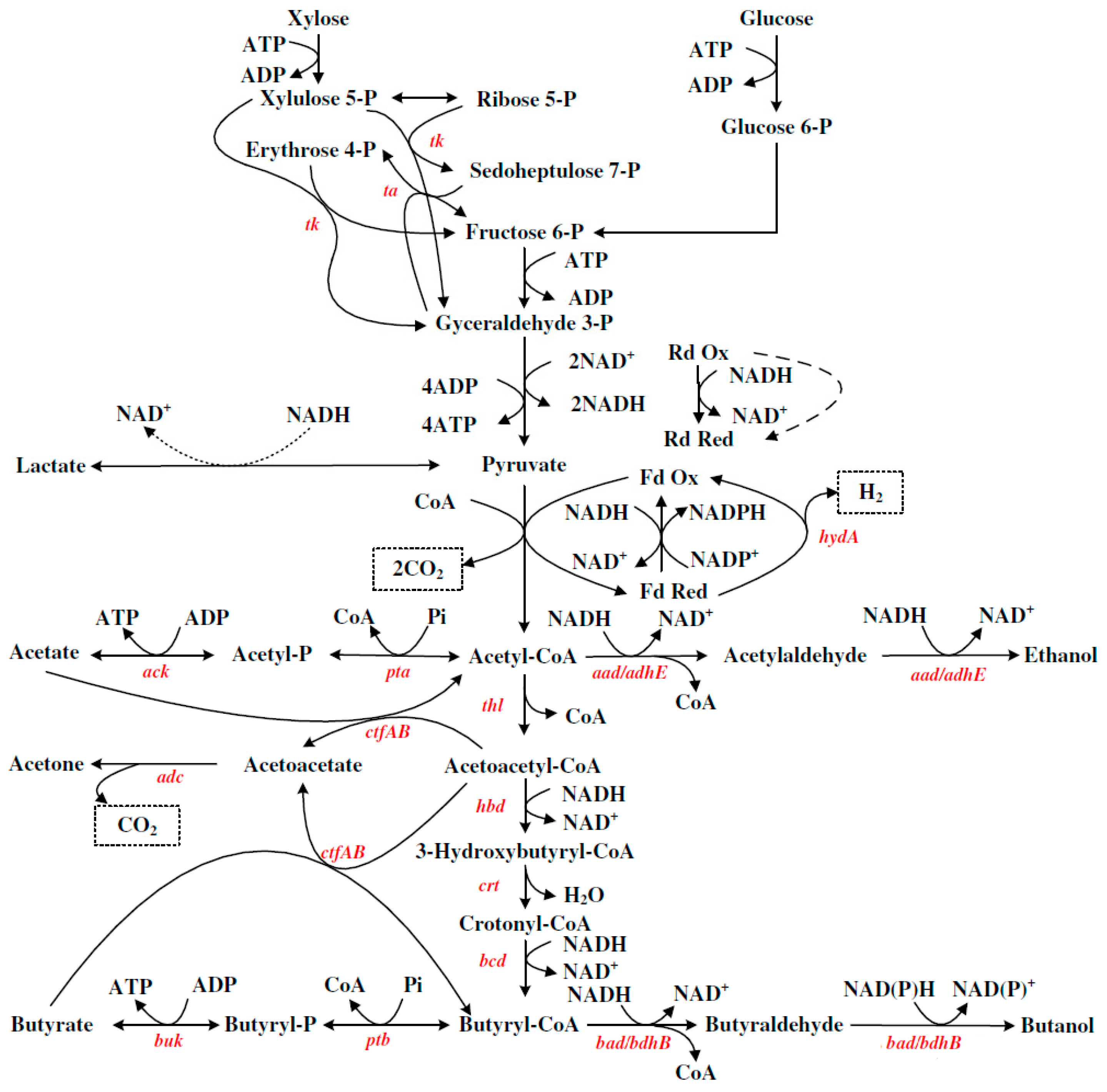
3. ABE Synthetic Metabolic Pathway of Clostridium
4. Raw Materials for Butanol Fermentation
4.1. Starch-Based Raw Materials
4.2. Lignocellulose Raw Materials
4.3. Molasses
5. Research Progress of Butanol Fermentation Technology
5.1. Batch Fermentation
5.2. Continuous Fermentation
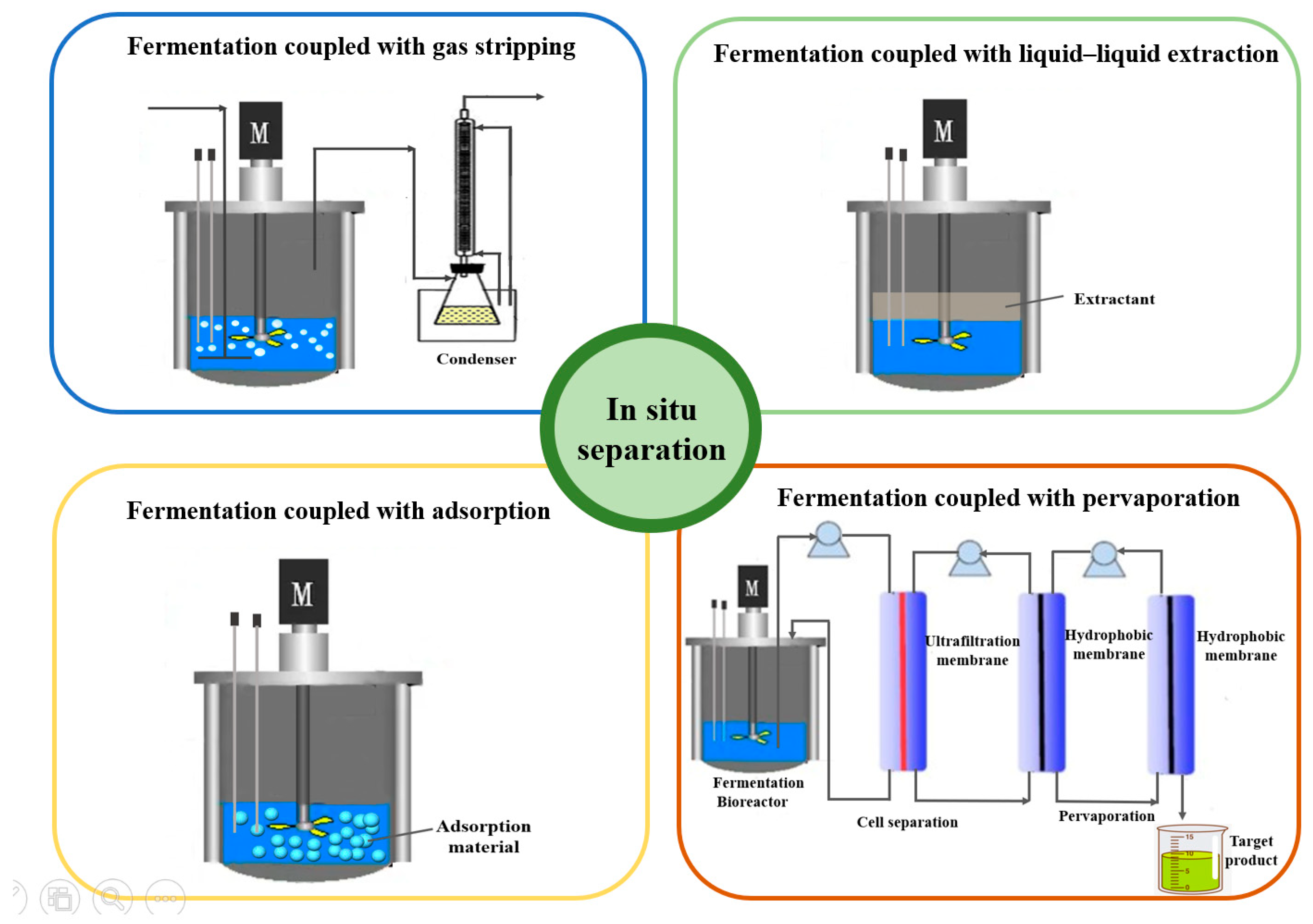
5.3. Fermentation Coupled with In Situ Separation
5.4. Cocultivation
5.4.1. Improving Butanol Production
5.4.2. Synthesis of Butanol from Cellulose-Based Raw Materials
5.4.3. Enhanced Oxygen Tolerance
6. Issues and Challenges
6.1. Toxicity of Butanol to Cells
6.2. High-Cost Raw Materials for Butanol Production
6.3. High Standard of Equipment
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Algayyim, S.J.M.; Yusaf, T.; Hamza, N.H.; Wandel, A.P.; Fattah, I.M.R.; Laimon, M.; Rahman, S.M.A. Sugarcane Biomass as a Source of Biofuel for Internal Combustion Engines (Ethanol and Acetone-Butanol-Ethanol): A Review of Economic Challenges. Energies 2022, 15, 8644. [Google Scholar] [CrossRef]
- Zhou, Z.; Jing, Y.; Wei, S.; Zhang, Q.; Peng, S.; An, X.; Li, H. Enhancement of butanol production in Clostridium acetobutylicum SE25 through oxidation-reduction potential regulation and analysis of its metabolic mechanisms. Fuel 2022, 331, 125708. [Google Scholar] [CrossRef]
- Moon, H.G.; Jang, Y.S.; Cho, C.; Lee, J.; Binkley, R.; Lee, S.Y. One hundred years of clostridial butanol fermentation. Fems. Microbiol. Lett. 2016, 363, fnw001. [Google Scholar] [CrossRef] [PubMed]
- Huesemann, M.H.; Kuo, L.-J.; Urquhart, L.; Gill, G.A.; Roesijadi, G. Acetone-butanol fermentation of marine macroalgae. Bioresour. Technol. 2012, 108, 305–309. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Dien, B.; Hector, R.E.; Cotta, M.A. Production of butanol (a biofuel) from agricultural residues: Part I—Use of barley straw hydrolysate. Biomass Bioenergy 2010, 34, 559–565. [Google Scholar] [CrossRef]
- Thang, V.H.; Kanda, K.; Kobayashi, G. Production of Acetone-Butanol-Ethanol (ABE) in Direct Fermentation of Cassava by Clostridium saccharoperbutylacetonicum N1-4. Appl. Biochem. Biotech. 2010, 161, 157–170. [Google Scholar] [CrossRef]
- Berezina, O.V.; Brandt, A.; Yarotsky, S.; Schwarz, W.H.; Zverlov, V.V. Isolation of a new butanol-producing Clostridium strain: High level of hemicellulosic activity and structure of solventogenesis genes of a new Clostridium saccharobutylicum isolate. Syst. Appl. Microbiol. 2009, 32, 449–459. [Google Scholar] [CrossRef]
- Boumba, V.A.; Economou, V.; Kourkoumelis, N.; Gousia, P.; Papadopoulou, C.; Vougiouklakis, T. Microbial ethanol production: Experimental study and multivariate evaluation. Forensic. Sci. Int. 2012, 215, 189–198. [Google Scholar] [CrossRef]
- Ahn, J.H.; Sang, B.I.; Um, Y. Butanol production from thin stillage using Clostridium pasteurianum. Bioresour. Technol. 2011, 102, 4934–4937. [Google Scholar] [CrossRef]
- Lewis, R.S.; Tanner, R.S.; Huhnke, R.L. Indirect or Direct Fermentation of Biomass to Fuel Alcohol. U.S. Patent US20070275447, 29 November 2007. [Google Scholar]
- Bharathiraja, B.; Jayamuthunagai, J.; Sudharsanaa, T.; Bharghavi, A.; Praveenkumar, R.; Chakravarthy, M.; Yuvaraj, D. Biobutanol—An impending biofuel for future: A review on upstream and downstream processing techniques. Renew. Sust. Energy Rev. 2017, 68, 788–807. [Google Scholar] [CrossRef]
- Pan, H. Study on Extractive Fermentation Conditions and Using Wheat Starch Wastewater to Improve Butanol Production. Master’s Thesis, Jiangnan University, Wuxi, China, 2014. [Google Scholar]
- Annous, B.A.; Blaschek, H.P. Isolation and characterization of Clostridium acetobutylicum mutants with enhanced amylolytic activity. Appl. Environ. Microb. 1991, 57, 2544–2548. [Google Scholar] [CrossRef] [PubMed]
- Syed, Q.; Nadeem, M.; Nelofer, R. Enhanced Butanol Production by Mutant Strains of Clostridium acetobutylicum in Molasses Medium. Türk. Biyokim. Derg. 2008, 33, 25–30. [Google Scholar]
- Li, H.-G.; Luo, W.; Gu, Q.-Y.; Wang, Q.; Hu, W.-J.; Yu, X.-B. Acetone, butanol, and ethanol production from cane molasses using Clostridium beijerinckii mutant obtained by combined low-energy ion beam implantation and N-methyl-N-nitro-N-nitrosoguanidine induction. Bioresour. Technol. 2013, 137, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Blaschek, H.P. Recent advances in ABE fermentation: Hyper-butanol producing Clostridium beijerinckii BA101. J. Ind. Microbiol. Biot. 2001, 27, 287–291. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, J.; Wang, Z.; Yang, S.-T. Stable high-titer n-butanol production from sucrose and sugarcane juice by Clostridium acetobutylicum JB200 in repeated batch fermentations. Bioresour. Technol. 2014, 163, 172–179. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, C.; Dong, F.; Yang, Y.; Jiang, W.; Yang, S.T. Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab. Eng. 2009, 11, 284–291. [Google Scholar] [CrossRef]
- Jang, Y.S.; Lee, J.Y.; Lee, J.; Park, J.H.; Im, J.A.; Eo, M.H.; Lee, J.; Lee, S.H.; Song, H.; Cho, J.H. Enhanced butanol production obtained by reinforcing the direct butanol-forming route in Clostridium acetobutylicum. mBio 2012, 3, 429–493. [Google Scholar] [CrossRef]
- Mann, M.S.; Dragovic, Z.; Schirrmacher, G.; Lutke-Eversloh, T. Over-expression of stress protein-encoding genes helps Clostridium acetobutylicum to rapidly adapt to butanol stress. Biotechnol. Lett. 2012, 34, 1643–1649. [Google Scholar] [CrossRef]
- Scotcher, M.C.; Bennett, G.N. SpoIIE Regulates sporulation but does not directly affect solventogenesis in Clostridium acetobutylicum ATCC 824. J. Bacteriol. 2005, 187, 1930. [Google Scholar] [CrossRef]
- Cong, J. Synergetic Engineering and Metabolic Regulation of Clostridium beijerinckii for Enhancing Xylose Metabolism and Butanol Biosynthesis. Master’s Thesis, Dalian University of Technology, Dalian, China, 2022. [Google Scholar]
- Abdelaal, A.S.; Yazdani, S.S. Engineering E. coli to synthesize butanol. Biochem. Soc. Trans. 2022, 50, 867–876. [Google Scholar] [CrossRef]
- Liao, H.; Ahmad, K.; Ahsan, M.; Hussain, M.I.; Iqbal, M.W.; Aqeel, S.M.; Hussain, A.; Xia, X. Bacterial metabolic engineering for the production of second-generation (2 G) bioethanol and biobutanol: A review. J. Appl. Microbiol. 2022, 134, lxac061. [Google Scholar] [CrossRef]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451, 86–99. [Google Scholar] [CrossRef]
- Eric, J.S.; Rossana, C.; Nilu, P.; Myers, S.; Petzold, C.J.; Redding, A.; Ouellet, M.; Keasling, J.D. Metabolic engineering of Saccharomyces cerevisiae for the production of n-butanol. Microb. Cell Fact. 2008, 7, 36. [Google Scholar]
- Schadeweg, V.; Boles, E. n-Butanol production in Saccharomyces cerevisiae is limited by the availability of coenzyme A and cytosolic acetyl-CoA. Biotechnol. Biofuels 2016, 9, 44. [Google Scholar] [CrossRef]
- Zheng, J.; Tashiro, Y.; Wang, Q.H.; Sonomoto, K. Recent advances to improve fermentative butanol production: Genetic engineering and fermentation technology. J. Biosci. Bioeng. 2015, 119, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dürre, P. Biobutanol: An attractive biofuel. Biotechnol. J. 2007, 2, 1525–1534. [Google Scholar] [CrossRef]
- Grimmler, C.; Janssen, H.; Krausse, D.; Fischer, R.J.; Bahl, H.; Durre, P.; Liebl, W.; Ehrenreich, A. Genome-wide gene expression analysis of the switch between acidogenesis and solventogenesis in continuous cultures of Clostridium acetobutylicum. J. Mol. Microb. Biotech. 2011, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Na, D.; Park, J.M.; Lee, J.; Choi, S.; Lee, S.Y. Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat. Chem. Biol. 2012, 8, 536–546. [Google Scholar] [CrossRef]
- Jin, X.; Wang, G.; He, B. Research progress and high yield strategy of acetone-butanol fermentation. Chem. Ind. Eng. Prog. 2007, 26, 1727–1732. [Google Scholar]
- Li, H.G.; Luo, W.; Wang, Q.; Yu, X.B. Direct fermentation of gelatinized cassava starch to acetone, butanol, and ethanol using Clostridium acetobutylicum mutant obtained by atmospheric and room temperature plasma. App. Biochem. Biotechnol. 2014, 172, 3330–3341. [Google Scholar] [CrossRef]
- Gu, Y.; Hu, S.; Chen, J.; Shao, L.; He, H.; Yang, Y.; Yang, S.; Jiang, W. Ammonium acetate enhances solvent production by Clostridium acetobutylicum EA 2018 using cassava as a fermentation medium. J. Ind. Microbiol. Biot. 2009, 36, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Li, H.G.; Ofosu, F.K.; Li, K.T.; Gu, Q.Y.; Wang, Q.; Yu, X.B. Acetone, butanol, and ethanol production from gelatinized cassava flour by a new isolate with high butanol tolerance. Bioresour. Technol. 2014, 172, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Zheng, J.; Shi, Z.; Li, L. Yeast extract promotes phase shift of bio-butanol fermentation by Clostridium acetobutylicum ATCC824 using cassava as substrate. Bioresour. Technol. 2012, 125, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-G.; Zhang, Q.-H.; Yu, X.-B.; Wei, L.; Wang, Q. Enhancement of butanol production in Clostridium acetobutylicum SE25 through accelerating phase shift by different phases pH regulation from cassava flour. Bioresour. Technol. 2016, 201, 148–155. [Google Scholar] [CrossRef]
- Tran, H.T.M.; Cheirsilp, B.; Hodgson, B.; Umsakul, K. Potential use of Bacillus subtilis, in a co-culture with Clostridium butylicum, for acetone-butanol-ethanol production from cassava starch. Biochem. Eng. J. 2010, 48, 260–267. [Google Scholar] [CrossRef]
- Lépiz-Aguilar, L.; Rodríguez-Rodríguez, C.E.; Arias, M.L.; Lutz, G. Acetone-Butanol-Ethanol (ABE) production in fermentation of enzymatically hydrolyzed cassava flour by Clostridium beijerinckii BA101 and solvent separation. J. Microbiol. Biotechnol. 2013, 23, 1092–1098. [Google Scholar] [CrossRef]
- Li, T.G.; Yan, Y.; He, J.Z. Enhanced direct fermentation of cassava to butanol by Clostridium species strain BOH3 in cofactor-mediated medium. Biotechnol. Biofuels 2015, 8, 166. [Google Scholar] [CrossRef]
- Lin, Z.N.; Liu, H.J.; Wu, J.; Patakova, P.; Branska, B.; Zhang, J.A. Effective continuous acetone-butanol-ethanol production with full utilization of cassava by immobilized symbiotic TSH06. Biotechnol. Biofuels 2019, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Madihah, M.; Ariff, A.; Sahaid, K.; Suraini, A.; Karim, M. Direct fermentation of gelatinized sago starch to acetone-butanol-ethanol by Clostridium acetobutylicum. World J. Microb. Biot. 2001, 17, 567–576. [Google Scholar] [CrossRef]
- Liu, Z.; Ying, Y.; Li, F.; Ma, C.; Xu, P. Butanol production by Clostridium beijerinckii ATCC 55025 from wheat bran. J. Ind. Microbiol. Biotechnol. 2010, 37, 495–501. [Google Scholar] [CrossRef]
- Lin, Y.S.; Jing, W. Optimization of butanol production from corn straw hydrolysate by Clostridium acetobutylicum using response surface method. Chin. Sci. Bull. 2011, 56, 1422–1428. [Google Scholar] [CrossRef]
- Li, D.; Chen, H. Fermentation of Acetone and Butanol Coupled with Enzymatic Hydrolysis of Steam Exploded Cornstalk Stover in a Membrane Reactor. Chin. J. Process Eng. 2007, 7, 1212–1216. [Google Scholar]
- Qureshi, N.; Li, X.L.; Hughes, S.; Saha, B.C.; Cotta, M.A. Butanol production from corn fiber xylan using Clostridium acetobutylicum. Biotechnol. Progr. 2006, 22, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Saha, B.C.; Cotta, M.A. Butanol production from wheat straw hydrolysate using Clostridium beijerinckii. Bioprocess Biosyst. Eng. 2007, 30, 419–427. [Google Scholar] [CrossRef]
- Qureshi, N.; Ezeji, T.C.; Ebener, J.; Dien, B.S.; Cotta, M.A.; Blaschek, H.P. Butanol production by Clostridium beijerinckii. Part I: Use of acid and enzyme hydrolyzed corn fiber. Bioresour. Technol. 2008, 99, 5915–5922. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Hector, R.E.; Cotta, M.A. Removal of fermentation inhibitors from alkaline peroxide pretreated and enzymatically hydrolyzed wheat straw: Production of butanol from hydrolysate using Clostridium beijerinckii, in batch reactors. Biomass Bioenergy 2008, 32, 1353–1358. [Google Scholar] [CrossRef]
- Qureshi, N.; Sahaa, B.C.; Hector, R.E.; Hughes, S.R.; Cotta, M.A. Butanol production from wheat straw by simultaneous saccharification and fermentation using Clostridium beijerinckii: Part I—Batch fermentation. Biomass Bioenergy 2008, 32, 168–175. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Hector, R.E.; Dien, B.; Hughes, S.; Liu, S.; Iten, L.; Bowman, M.J.; Sarath, G.; Cotta, M.A. Production of butanol (a biofuel) from agricultural residues: Part II—Use of corn stover and switchgrass hydrolysates. Biomass Bioenergy 2010, 34, 566–571. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Liu, S.Q.; Ezeji, T.C.; Nichols, N.N. Cellulosic Butanol Biorefinery: Production of Biobutanol from High Solid Loadings of Sweet Sorghum Bagasse-Simultaneous Saccharification, Fermentation, and Product Recovery. Fermention 2021, 7, 310. [Google Scholar] [CrossRef]
- Lin, X.Q.; Liu, Y.; Zheng, X.J.; Qureshi, N. High-efficient cellulosic butanol production from deep eutectic solvent pretreated corn stover without detoxification. Ind. Crops Prod. 2021, 162, 113258. [Google Scholar] [CrossRef]
- Cho, D.H.; Shin, S.J.; Yong, H.K. Effects of acetic and formic acid on ABE production by Clostridium acetobutylicum and Clostridium beijerinckii. Biotechnol. Bioprocess Eng. 2012, 17, 270–275. [Google Scholar] [CrossRef]
- Görke, B.; Stülke, J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef]
- Shaheen, R.; Shirley, M.; Jones, D.T. Comparative fermentations studies of industrial strains belonging to four species of solvent- producing Clostridia. J. Mol. Microb. Biotechnol. 2000, 2, 115–124. [Google Scholar]
- Pei, J.; Zuo, W.; Pang, H.; Huang, Z.M.; Li, Z.C.; Wei, Y.T.; Huang, R.B. Isolation, Screening & Identification of Butanol-producing Strains by Molasses Fermentation. Liquor. Mak. Sci Technol. 2010, 5, 32–35. [Google Scholar]
- Qureshi, N.; Lolas, A.; Blaschek, H.P. Soy molasses as fermentation substrate for production of butanol using Clostridium beijerinckii BA101. J. Ind. Microbiol. Biot. 2001, 26, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Xia, Z.Y.; Wang, Y.; Sun, Z.H. Continuous butanol fermentation from inexpensive sugar-based feedstocks by Clostridium saccharobutylicum DSM 13864. Bioresour. Technol. 2013, 129, 680–685. [Google Scholar] [CrossRef]
- Yi, W.; Li, X.; Blaschek, H.P. Effects of supplementary butyrate on butanol production and the metabolic switch in Clostridium beijerinckii NCIMB 8052: Genome-wide transcriptional analysis with RNA-Seq. Biotechnol. Biofuels 2013, 6, 138. [Google Scholar]
- Zhang, Y.D.; Ma, Y.J.; Yang, F.X.; Zheng, C.H. Continuous acetone-butanol-ethanol production by corn stalk immobilized cells. J. Ind. Microbiol. Biot. 2009, 36, 1117–1121. [Google Scholar] [CrossRef]
- Survase, S.A.; Van Heiningen, A.; Granström, T. Continuous biocatalytic conversion of sugar mixture to acetone-butanol-ethanol by immobilized Clostridium acetobutylicum DSM 792. Appl. Microbiol. Biot. 2012, 93, 2309–2316. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Mashima, H.; Tanaka, H. Scale up of fuel ethanol production from sugar beet juice using loofa sponge immobilized bioreactor. Bioresour. Technol. 2001, 76, 1–8. [Google Scholar] [CrossRef]
- Cheung, I.H.S.; Gishen, M.; Ghosh, P.; Pamment, N.B. Continuous ethanol production in an immobilized whole cell fermenter using untreated sugar cane bagasse as carrier. Appl. Microbiol. Biot. 1986, 23, 413–416. [Google Scholar] [CrossRef]
- Schlote, D.; Gottschalk, G. Effect of cell recycle on continuous butanol-acetone fermentation with Clostridium acetobutylicum, under phosphate limitation. Appl. Microbiol. Biot. 1986, 24, 1–5. [Google Scholar] [CrossRef]
- Tashiro, Y.; Takeda, K.; Kobayashi, G.; Sonomoto, K. High production of acetone-butanol-ethanol with high cell density culture by cell-recycling and bleeding. J. Biotechnol. 2005, 120, 197–206. [Google Scholar] [CrossRef]
- Jang, Y.-S.; Malaviya, A.; Lee, S.Y. Acetone-butanol-ethanol production with high productivity using Clostridium acetobutylicum BKM19. Biotechnol. Bioeng. 2013, 110, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Mutschlechner, O.; Swoboda, H.; Gapes, J.R. Continuous two-stage ABE-fermentation using Clostridium beijerinckii NRRL B592 operating with a growth rate in the first stage vessel close to its maximal value. J. Mol. Microb. Biotech. 2000, 2, 101–105. [Google Scholar]
- Huang, W.-C.; Ramey, D.E.; Yang, S.-T. Continuous production of butanol by Clostridium acetobutylicum, immobilized in a fibrous bed bioreactor. Appl. Biochem. Biotechnol. 2004, 115, 887–898. [Google Scholar] [CrossRef]
- Yen, H.W.; Li, R.J.; Ma, T.W. The development process for a continuous acetone-butanol-ethanol (ABE) fermentation by immobilized Clostridium acetobutylicum. J. Taiwan Inst. Chem. Eng. 2011, 42, 902–907. [Google Scholar] [CrossRef]
- Badr, H.R.; Toledo, R.; Hamdy, M.K. Continuous acetone-ethanol-butanol fermentation by immobilized cells of Clostridium acetobutylicum. Biomass Bioenergy 2001, 20, 119–132. [Google Scholar] [CrossRef]
- Qureshi, N.; Schripsema, J.; Lienhardt, J.; Blaschek, H.P. Continuous solvent production by Clostridium beijerinckii BA101 immobilized by adsorption onto brick. World J. Microb. Biot. 2000, 16, 377–382. [Google Scholar] [CrossRef]
- Malaviya, A.; Jang, Y.S.; Lee, S.Y. Continuous butanol production with reduced byproducts formation from glycerol by a hyper producing mutant of Clostridium pasteurianum. Appl. Microbiol. Biot. 2012, 93, 1485–1494. [Google Scholar] [CrossRef]
- Khedkar, M.A.; Nimbalkar, P.R.; Gaikwad, S.G.; Chavan, P.V.; Bankar, S.B. Solvent extraction of butanol from synthetic solution and fermentation broth: Batch and continuous studies. Sep. Purif. Technol. 2020, 249, 117058. [Google Scholar] [CrossRef]
- Lin, Z.N.; Liu, H.J.; Yan, X.; Zhou, Y.J.; Cheng, K.K.; Zhang, J.A. High-efficiency acetone-butanol-ethanol production and recovery in non-strict anaerobic gas-stripping fed-batch fermentation. Appl. Microbiol. Biot. 2017, 101, 8029–8039. [Google Scholar] [CrossRef]
- Raganati, F.; Procentese, A.; Olivieri, G.; Russo, M.E.; Salatino, P.; Marzocchella, A. Bio-butanol separation by adsorption on various materials: Assessment of isotherms and effects of other ABE-fermentation compounds. Sep. Purif. Technol. 2018, 191, 328–339. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.C.; Wang, N.; Chen, X.M.; Li, B.G.; Shi, J.P.; Li, X. Process optimization of acetone-butanol-ethanol fermentation integrated with pervaporation for enhanced butanol production. Biochem. Eng. J. 2021, 173, 108070. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, J.; Lu, C.; Yang, S.-T.; Bai, F.; Tang, I.-C. High-titer n-butanol production by Clostridium acetobutylicum, JB200 in fed-batch fermentation with intermittent gas stripping. Biotechnol. Bioeng. 2012, 109, 2746–2756. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, H.F.; Liu, D.; Zhao, T.; Shi, X.C.; Cheng, H.; Zhao, N.; Li, Z.J.; Li, B.B.; Niu, H.Q.; et al. Enhancement of n-butanol production by in situ butanol removal using permeating-heating-gas stripping in acetone-butanol-ethanol fermentation. Bioresour. Technol. 2014, 164, 276–284. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Garg, N.P.; Luo, H.; Kontogeorgis, G.M.; Woodley, J.M. Ionic liquid-based in situ product removal design exemplified for an acetone-butanol-ethanol fermentation. Biotechnol. Bioprocess Eng. 2021, 5, e3183. [Google Scholar] [CrossRef]
- Gonzalez-Penas, H.; Eibes, G.; Lu-Chau, T.A.; Moreira, M.T.; Lema, J.M. Altered. Clostridia response in extractive ABE fermentation with solvents of different nature. Biochem. Eng. J. 2020, 154, 107455. [Google Scholar] [CrossRef]
- Xue, C.; Yang, D.; Du, G.; Chen, L.; Ren, J.; Bai, F. Evaluation of hydrophobic micro-zeolite-mixed matrix membrane and integrated with acetone-butanol-ethanol fermentation for enhanced butanol production. Biotechnol. Biofuels 2015, 8, 105. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Y.; Ding, F.Y.; Zhao, T.; Wu, J.L.; Guo, T.; Ren, H.F.; Li, B.B.; Niu, H.Q.; Cao, Z. Biobutanol production in a Clostridium acetobutylicum biofilm reactor integrated with simultaneous product recovery by adsorption. Biotechnol. Biofuels 2014, 7, 5. [Google Scholar] [CrossRef]
- Xue, C.; Liu, F.F.; Xu, M.M.; Tang, I.C.; Zhao, J.B.; Bai, F.W.; Yang, S.T. Butanol production in acetone-butanol-ethanol fermentation with in situ product recovery by adsorption. Bioresour. Technol. 2016, 219, 158–168. [Google Scholar] [CrossRef]
- Zengler, K.; Zaramela, L.S. The social network of microorganisms-how auxotrophies shape complex communities. Nat. Rev. Microbiol. 2018, 16, 383–390. [Google Scholar] [CrossRef]
- Giri, S.; Oña, L.; Waschina, S.; Shitut, S.; Yousif, G.; Kaleta, C.; Kost, C. Metabolic dissimilarity determines the establishment of cross-feeding interactions in bacteria. Curr. Biol. 2021, 31, 5547–5557. [Google Scholar] [CrossRef]
- Fritts, R.K.; McCully, A.L.; McKinlay, J.B. Extracellular Metabolism Sets the Table for Microbial Cross-Feeding. Microbiol. Mol. Biol. Rev. 2021, 85, e00135-20. [Google Scholar] [CrossRef]
- Li, X.L.; Zhou, Z.; Li, W.N.; Yan, Y.J.; Shen, X.L.; Wang, J.; Sun, X.X.; Yuan, Q.P. Design of stable and self-regulated microbial consortia for chemical synthesis. Nat. Commun. 2022, 13, 1554. [Google Scholar] [CrossRef]
- Bergstrom, S.L.; Foutch, G.L. Production of Butanol by Fermentation in the Presence of Cocultures of Clostridium. U.S. Patent US4539293, 3 September 1985. [Google Scholar]
- Lin, L.; Ai, H.X.; Zhang, S.X.; Li, S.; Liang, Z.X.; Wu, Z.Q.; Yang, S.T.; Wang, J.F. Enhanced butanol production by coculture of Clostridium beijerinckii and Clostridium tyrobutyricum. Bioresour. Technol. 2013, 143, 397–404. [Google Scholar]
- Luo, H.Z.; Ge, L.B.; Zhang, J.S.; Zhao, Y.L.; Ding, J.; Li, Z.G.; He, Z.N.; Chen, R.; Shi, Z.P. Enhancing Butanol Production under the Stress Environments of Co-Culturing Clostridium acetobutylicum/Saccharomyces cerevisiae Integrated with Exogenous Butyrate Addition. PLoS ONE 2015, 10, e0141160. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.K.C.; Chan, K.H.; Saddler, J.N. Butanol production from cellulosic substrates by sequential co-culture of Clostridium thermocellum and Clostridium acetobutylicum. Biotechnol. Lett. 1985, 7, 509–514. [Google Scholar] [CrossRef]
- Nakayama, S.; Kosaka, T.; Hirakawa, H.; Matsuura, K.; Yoshino, S.; Furukawa, K. Metabolic engineering for solvent productivity by downregulation of the hydrogenase gene cluster hupCBA in Clostridium saccharoperbutylacetonicum strain N1-4. Appl. Microbiol. Biot. 2008, 78, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Mahadevan, R. Characterizing metabolic interactions in a clostridial co-culture for consolidated bioprocessing. BMC Biotechnol. 2013, 13, 95. [Google Scholar] [CrossRef]
- Wang, G.Y.; Wu, P.F.; Liu, Y.; Mi, S.; Mai, S.; Gu, C.K.; Wang, G.H.; Liu, H.J.; Zhang, J.A.; Borresen, B.T. Isolation and characterisation of non-anaerobic butanol-producing symbiotic system TSH06. Appl. Microbiol. Biot. 2015, 99, 8803–8813. [Google Scholar] [CrossRef]
- Wu, P.F.; Wang, G.Y.; Wang, G.H.; Borresen, B.T.; Liu, H.J.; Zhang, J.A. Butanol production under microaerobic conditions with a symbiotic system of Clostridium acetobutylicum, and Bacillus cereus. Microb. Cell Fact. 2016, 15, 82–89. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Amiri, H.; Asadollahi, M. Efficient butanol production under aerobic conditions by coculture of Clostridium acetobutylicum and Nesterenkonia sp. strain F. Biotechnol. Bioeng. 2020, 117, 392–405. [Google Scholar] [CrossRef]
- Ezeji, T.; Milne, C.; Price, N.D.; Blaschek, H.P. Achievements and perspectives to overcome the poor solvent resistance in acetone and butanol-producing microorganisms. Appl. Microbiol. Biot. 2010, 85, 1697–1712. [Google Scholar] [CrossRef]
- Yamauchi, S.; Kawakita, M.; Takeuchi, J. Comparative economic assessment of ABE fermentation based on cellulosic and non-cellulosic feedstocks. Appl. Energ. 2012, 93, 193–204. [Google Scholar]
- Kortei, N.K.; Dzogbefia, V.P.; Obodai, M. Assessing the Effect of Composting Cassava Peel Based Substrates on the Yield, Nutritional Quality, and Physical Characteristics of Pleurotus ostreatus (Jacq. ex Fr.) Kummer. Biotech. Res. Intern. 2014, 2014, 571520. [Google Scholar] [CrossRef]

| Strain | Raw Material | Main Product | Butanol Titer (g/L) | Reference |
|---|---|---|---|---|
| Clostridium acetobutylicum | Glucose | Butanol, acetone, ethanol | 10.4 | [4] |
| Clostridium beijerinckii | Glucose | Butanol, isopropyl alcohol | 15.2 | [5] |
| Clostridium saccharoperbutylacetonicum | Glucose | Butanol, acetone, ethanol | 16.2 | [6] |
| Clostridium saccharoperbutylicum | Glucose | Butanol, acetone, ethanol | 9.7 | [7] |
| Clostridium sporogenes | Glucose | Butanol, ethanol, propyl alcohol, isobutanol, methyl butanol | 0.12 | [8] |
| Clostridium perfrigens | Glucose | Butanol, ethanol, propyl alcohol, isobutanol, methyl butanol | 0.02 | [8] |
| Clostridium pasteurianum | Glycerinum | Butanol, 1, 3-propylene glycol | 6.5 | [9] |
| Clostridium carboxidivorus | CO | Butanol, ethanol | 0.37 | [10] |
| Strain | Pretreatment | Butanol Titer (g/L) | ABE Titer (g/L) | Butanol Productivity (g/L·h) | ABE Productivity (g/L·h) | Reference |
|---|---|---|---|---|---|---|
| C.acetobutylicum SE25 | Enzymolysis | 16.2 | -- | 0.23 | -- | [37] |
| C. butylicum TISTR1032 | Gelatinization | -- | 8.9 | -- | 0.12 | [38] |
| C. beijerinckii BA101 | Enzymolysis | 25.7 | 37 | 0.31 | 0.45 | [39] |
| C. saccharoperbutylace-tonicum N1–4 | Gelatinization | 16.9 | 21 | 0.23 | 0.29 | [6] |
| C. acetobutylicum PW12 | Gelatinization | 15.8 | 23.2 | 0.19 | 0.28 | [33] |
| C. species strain BOH3 | Gelatinization | 17.8 | 24.2 | 0.25 | 0.34 | [40] |
| C. acetobutylicum | Gelatinization | 16.1 | 24.9 | 0.20 | 0.31 | [35] |
| Symbiotic system TSH06 | Non | 13.3 | 20 | 0.18 | 0.27 | [41] |
| Strain | Dilution Rate (h−1) | Raw Material | ABE Titer (g/L) | Yield (g/g) | Productivity (g/(L·h)) | Strategy | Reference |
|---|---|---|---|---|---|---|---|
| C. acetobutylicum ATCC 55025 | 1.0 | Glucose | 11.3 | 0.53 | 5.6 | Cell immobilization | [69] |
| C. aetobutylicum BCRC 10639 | 0.05 | Glucose | 14.3 | 0.24 | -- | Cell immobilization | [70] |
| C. acetobutylicum P-262 | 0.13 | Potato | 7.7 | 0.19 | 1.0 | Cell immobilization | [71] |
| C. beijerinckii BA 101 | 2.0 | Glucose | 7.9 | 0.38 | 15.8 | Cell immobilization | [72] |
| C. acetobutylicum DSM 792 | 1.9 | Glucose | 7.2 | 0.40 | 13.66 | Cell immobilization | [62] |
| C. acetobutylicum DSM 792 | 1.5 | Mix sugar | 8.1 | 0.28 | 12.14 | Cell immobilization | [62] |
| C. pasteurianum MSEL-GLY2 | 0.9 | Glycerinum | 8.6 | -- | 7.8 | Cell recycling | [73] |
| C. saccharoperbuty- lacetonicum N1–4 | 0.9 | Glucose | 8.6 | -- | 7.6 | Cell recycling | [66] |
| Symbiotic system TSH06 | 0.2 | Glucose | 16.8 | -- | 1.12 | Cell immobilization | [41] |
| Separation Method | Titer (g/L) | Products Titer in the Condensate (g/L) | Strategy | Reference | ||
|---|---|---|---|---|---|---|
| Butanol | ABE | Butanol | ABE | |||
| Gas stripping | 113.2 | 172.1 | 150.5 | 195.9 | C. acetobutylicum JB200 | [78] |
| Gas stripping | 185.7 | 267.2 | 624.1 | 665 | Symbiotic system TSH06 | [75] |
| Gas stripping | 66.1 | 106.3 | 150.6 | ~200 | C. acetobutylicum B3 | [79] |
| Liquid–liquid extraction | 36.7 | -- | -- | -- | C. acetobutylicum; Oleyl alcohol; Ionic liquid | [80] |
| Liquid–liquid extraction | 21.4 | 31.6 | -- | -- | C. acetobutylicum ATCC 824; 2- butyl-1-octanol | [81] |
| Pervaporation | 34.5 | 54.9 | 169.6 | 253.3 | C. acetobutylicum ATCC 55025; Hydrophobic micro-zeolite-mixed matrix membrane | [82] |
| Pervaporation | 18.95 | 30.83 | -- | -- | C. beijerinckii ZL01; PDMS-PVDF composite membrane | [77] |
| Adsorption | 92.6 | 130.7 | -- | -- | C. acetobutylicum B3; Macroporous adsorption resin KA-I | [83] |
| Adsorption | 54.6 | -- | 167.1 | 190.4 | C. acetobutylicum JB200; Norit ROW0.8 | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Cong, W.; Zhang, J. Biobutanol Production from Acetone–Butanol–Ethanol Fermentation: Developments and Prospects. Fermentation 2023, 9, 847. https://doi.org/10.3390/fermentation9090847
Lin Z, Cong W, Zhang J. Biobutanol Production from Acetone–Butanol–Ethanol Fermentation: Developments and Prospects. Fermentation. 2023; 9(9):847. https://doi.org/10.3390/fermentation9090847
Chicago/Turabian StyleLin, Zhangnan, Wei Cong, and Jian’an Zhang. 2023. "Biobutanol Production from Acetone–Butanol–Ethanol Fermentation: Developments and Prospects" Fermentation 9, no. 9: 847. https://doi.org/10.3390/fermentation9090847
APA StyleLin, Z., Cong, W., & Zhang, J. (2023). Biobutanol Production from Acetone–Butanol–Ethanol Fermentation: Developments and Prospects. Fermentation, 9(9), 847. https://doi.org/10.3390/fermentation9090847






